Abstract
Geckos owe their remarkable stickiness to millions of dry, hard setae on their toes. In this study, we discovered that gecko setae stick more strongly the faster they slide, and do not wear out after 30 000 cycles. This is surprising because friction between dry, hard, macroscopic materials typically decreases at the onset of sliding, and as velocity increases, friction continues to decrease because of a reduction in the number of interfacial contacts, due in part to wear. Gecko setae did not exhibit the decrease in adhesion or friction characteristic of a transition from static to kinetic contact mechanics. Instead, friction and adhesion forces increased at the onset of sliding and continued to increase with shear speed from 500 nm s−1 to 158 mm s−1. To explain how apparently fluid-like, wear-free dynamic friction and adhesion occur macroscopically in a dry, hard solid, we proposed a model based on a population of nanoscopic stick–slip events. In the model, contact elements are either in static contact or in the process of slipping to a new static contact. If stick–slip events are uncorrelated, the model further predicted that contact forces should increase to a critical velocity (V*) and then decrease at velocities greater than V*. We hypothesized that, like natural gecko setae, but unlike any conventional adhesive, gecko-like synthetic adhesives (GSAs) could adhere while sliding. To test the generality of our results and the validity of our model, we fabricated a GSA using a hard silicone polymer. While sliding, the GSA exhibited steady-state adhesion and velocity dependence similar to that of gecko setae. Observations at the interface indicated that macroscopically smooth sliding of the GSA emerged from randomly occurring stick–slip events in the population of flexible fibrils, confirming our model predictions.
Keywords: gecko adhesion, friction, tribology, adhesives
1. Introduction
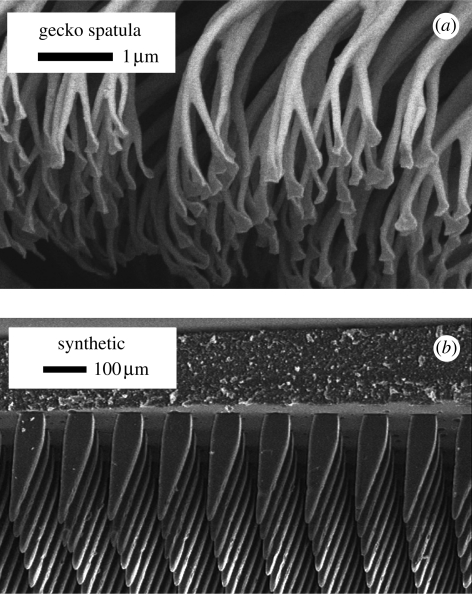
Geckos are the world's supreme climbers, capable of attaching and detaching their adhesive toes in milliseconds while running with reckless abandon on vertical and inverted surfaces (Autumn 2006). Over two millennia ago, Aristotle commented on the ability of geckos to ‘run up and down a tree in any way’ (Aristotle 350 BCE 1918). We know now that it is the micro- and nanostructures on their toes that enable geckos’ exceptional climbing performance (Maderson 1964; Russell 1975; Pianka & Sweet 2005; Autumn 2006). Gecko toes bear arrays of millions of angled, branched, hair-like setae (110 µm long by 2.1 µm radius) formed of stiff, hydrophobic β-keratin (elastic modulus, E = 1.5 GPa; Alibardi 2003; Autumn et al. 2006c; Rizzo et al. 2006; Peattie et al. 2007). Setae act as a bed of springs to conform to a variety of surface profiles (Persson 2003; Sitti & Fearing 2003; Autumn et al. 2006c). Each seta branches into a nanoarray of hundreds of smaller stalks (800 nm long, 25 nm radius) ending in thin spatular structures (200 nm wide, 5 nm thick) that make intimate contact with the substrate (Ruibal & Ernst 1965; Arzt et al. 2003; Huber et al. 2005a; Eimuller et al. 2008; Zhao et al. 2008).
While gecko setae can meet the challenges of dynamic climbing (Autumn et al. 2006b), and last for months under real-world conditions, conventional pressure-sensitive adhesives (PSAs) do not. PSAs, such as those of adhesive tapes, are fabricated from soft, viscoelastic materials (Pocius 2002; Creton 2003) that (i) become dirty, (ii) self-adhere, (iii) attach accidentally to inappropriate surfaces, (iv) wear out, and (v) detach at the onset of sliding. In previous studies, we explained how gecko setae resist fouling by becoming cleaner with repeated use (Hansen & Autumn 2005; Hui et al. 2006; Lamb & Bauer 2006), and how a non-adhesive default state prevents self-adherence and inappropriate attachment (Autumn & Hansen 2006). This enabled the development of gecko-like synthetic adhesives (GSAs) with a non-adhesive default state (Lee et al. 2008; Qu et al. 2008; Schubert et al. 2008) and a self-cleaning function (Lee & Fearing 2008; Sethi et al. 2008). This study focuses on how the related phenomena of wear and sliding affect adhesion and friction in natural and synthetic gecko setae.
1.1. Kinetic friction and adhesion: why do geckos not fall when they slip?
Contact forces between dry solids (Bhushan 2002) typically decrease at the onset of sliding, and as velocity increases, force continues to decrease because of a reduction in the interfacial contact fraction, due in part to wear. This would represent an unstable dynamic condition for a climbing gecko: any slip would likely result in a fall (Autumn et al. 2006a). Yet, geckos do not fall when their feet slide (Huber et al. 2007; Jusufi et al. 2008). Indeed, gecko setae require shear displacement to initiate adhesion (Autumn et al. 2000, 2006a).
The application of a vertical preload (20–200 kPa), followed by a small drag in shear (5–10 µm; figure 1), brings the seta and its 100–1000 spatulae into sub-nanometre contact with the substrate (Autumn et al. 2000; Chen et al. 2008, 2009). Adhesion in gecko setae requires maintenance of a shear force (as described by the frictional adhesion model; Autumn & Hansen 2006; Autumn et al. 2006a; Tian et al. 2006). Force measurements over shear displacements from 20 to 500 µm in length showed that initially, frictional–adhesion forces increase linearly with shear displacement (Autumn et al. 2000; Gravish et al. 2007; Lee et al. 2008; Zhao et al. 2008), and approach steady-state values only after the setal tip begins to slide (approx. 5–10 µm) and reorient (Autumn et al. 2006a; Zhao et al. 2008) in the drag step (figure 1). Maximum friction and adhesion forces occur only after the onset of interfacial sliding (Autumn et al. 2007; Gravish et al. 2007; Lee et al. 2008; Zhao et al. 2008), suggestive of a viscous, fluid-like system (Bullock et al. 2008; Eimuller et al. 2008) but inconsistent with the well-documented rigid, dry structure of gecko setae (Ruibal & Ernst 1965; Russell 1975; Williams & Peterson 1982; Autumn et al. 2002, 2006c; Huber et al. 2005b, 2007; Rizzo et al. 2006; Peattie et al. 2007).
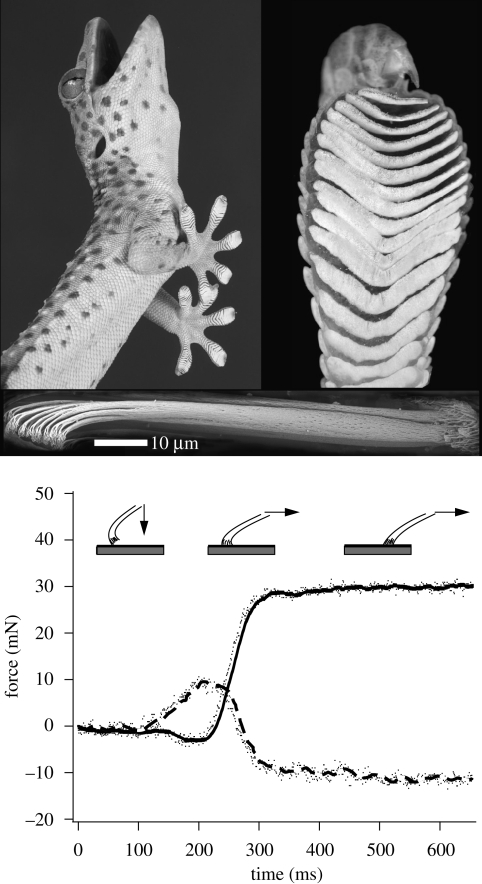
Figure 1.
The tokay gecko (approx. 30 cm in length) possesses adhesive pads on the undersides of its toes (approx. 1 cm in length). Arrays of branched setae (approx. 110 µm in length) achieve intimate contact with surfaces to engage intermolecular van der Waals forces. Setae are adhesive only under the application of a shear force. Setae are first applied vertically to a surface and then sheared parallel to the surface. The setal shafts load elastically until the elastic component is saturated, at which point the setae slide across the substrate while remaining in adhesion. As the adhesive begins to slide (300 ms), friction and adhesion forces remain constant and do not exhibit any notable decrease, uncharacteristic of solid contact mechanics. The solid line represents friction and the dashed line adhesion. Note that for normal force, adhesion is negative while compression is positive.
1.2. Rate dependence of contact forces
Friction is one of the most well-studied natural processes in the history of science (Bhushan 2002), yet only recently, with the inventions of the surface force apparatus (see Israelachvili (1992) for a review) and the atomic force microscope, have researchers begun to probe tribological processes at the nanoscale (Krim 1996). Relating macroscopic friction to the underlying nanoscale processes is challenging because of the complex dynamics of millions of single-asperity contacts between rubbing surfaces (Robbins & Smith 1996; Porto et al. 2000; Ringlein & Robbins 2004; Israelachvili 2005; Luan & Robbins 2005). Gecko adhesion has attracted considerable attention in surface science because it is a macroscopic system in which the contact mechanics are a consequence of well-defined nanoscopic geometry (Autumn et al. 2000; Huber et al. 2005a; Tian et al. 2006; Zhao et al. 2008).
Solid friction between multi-contact interfaces usually abides by the rate–state friction model (Rice & Ruina 1983; Baumberger et al. 1999) where contact time increases contact area (contact aging; Sills et al. 2007), and thus kinetic forces are typically smaller than static forces. However, theory suggests that branched gecko setae reduce the stress concentrations typically associated with fracture (Hui et al. 2004), and in fact setae attach well in both the static and kinetic regimes (Autumn et al. 2000, 2006a; Zhao et al. 2008). Synthetic setae may resist stress-induced wear through compliance and load-sharing (Bhushan et al. 2008). Stable and repeatable adhesion while sliding over distances thousands of times greater than the length of a single spatular bond (200 nm) implies the presence of non-trivial kinetic contact mechanics in the gecko adhesive.
We investigated friction and adhesion dynamics in isolated tokay gecko setal arrays by measuring contact forces over five orders of magnitude of shear velocity, and proposed a model based on a population of nanoscopic stick–slip events, explaining how wear resistance and viscous-like kinetic forces can occur at the macroscale in a dry, hard material. To test our model, we explored the dynamic response of a novel GSA.
2. Methods
2.1. Samples and measurement
Setal arrays were gathered from live non-moulting tokay geckos (Gekko gecko) and mounted on scanning electron microscope stubs using Loctite 410 (Henkel Co., CT) as described previously (Autumn et al. 2002; Gravish et al. 2007). Prior to testing, the samples were inspected to ensure no wicking of glue occurred in the setal shafts.
The setal array test platform, RoboToe, has been described previously (Autumn et al. 2006a; Gravish et al. 2007). Mounted setal array samples were held in a specimen chuck attached to a Kistler 9328A three-axis piezoelectric force sensor (Kistler, Winterthur, Switzerland). The force sensor and sample were mounted atop a Newport RP Reliance breadboard table (Newport, Irvine, CA). New pre-cleaned slides (Erie Scientific, Portsmouth, NH) were cleaned in a bath of 2 M NaOH (Ted Pella, Redding, CA) for 15 min followed by a triple series of ultrapure deionized water rinses and Kimwipe dry (Kimberly-Clark, Neenah, WI) to ensure that the substrate was clean. Freshly cleaned slides were mounted on a two-axis nanopositioning system comprising two Aerotech ANT-50L (Aerotech, Pittsburgh, PA) linear actuators with maximum travel of 50 mm and velocities from 10 nm s−1 to 250 mm s−1.
The resolution of the force sensor was 2.5 mN and the actuator resolution was 10 nm. The stiffness of the measurement system was approximately 320 N mm−1 (Autumn et al. 2006c), which is more than 30 times greater than the 1–10 N mm−1 average shear stiffness of setal arrays (Autumn et al. 2000; Gravish et al. 2007), and thus, considering the force sensor and setae in series, the elastic contribution from the measurement system is negligible. We aligned the sample and substrate surfaces to be coplanar using two Newport goniometers as well as a linear slope correction in software.
2.2. Rate dependence and wear resistance in gecko setae
We measured friction and adhesion forces at 12 different drag velocities over the velocity range of 500 nm s−1 to 158 mm s−1. Due to temporal constraints on the length of individual tests, we had to run slower tests over smaller displacements and faster tests over larger displacements similar to the methods of Vorvolakos & Chaudhury (2003), Tambe & Bhushan (2005) and Tao & Bhushan (2007). We chose five drag distances (from 1 µm to 10 mm), each an order of magnitude larger than the last to test for velocity dependence. In all, 24 individual velocity and distance combinations were tested five times, totaling 120 tests per sample. In total, 25 setal arrays were subjected to 120 shear tests for a total of 3000 trials. However, as discussed in §3, 14 arrays survived the battery of tests undamaged, leaving 1680 of the 3000 trials in the analysis.
Approximately 70 µm tall setal arrays (Autumn et al. 2006c) were preloaded to a normal displacement of 35 µm. Normal displacement was measured from the point at which setal array and slide made first contact. Once preloaded, each test began with a 50 µm engagement drag at a constant velocity of 100 µm s−1. Immediately following the engagement drag, the slide accelerated at 1500 mm s−2 to the predetermined velocity and dragged over the specified distance until completed. After completion of the velocity drag, arrays were unloaded at 45° until out of contact with the glass slide. Loading and unloading motions were performed at 500 µm s−1. Perpendicular load–pull tests were conducted at the beginning and end of each test set to calculate array stiffness to identify (and discard) any arrays damaged during the experiments (Autumn et al. 2006c). To assess the wear resistance of setae, we subjected two samples to a battery of 30 000 load–drag–pull tests at a velocity of 10 mm s−1 over a distance of 10 mm per trial, totalling a net displacement of 300 m. We recorded friction, adhesion and sample stiffness in the normal direction every 1000 trials.
2.3. Fabrication of GSA
Since gecko spatulae are smaller than the wavelength of visible light, the details of the sliding mechanism for natural gecko setal arrays occur at a scale that is not accessible optically. To test the fibrillar stick–slip model, we explored the dynamic response of a GSA with dimensions greater than those of the natural gecko material. Because contact mechanics are controlled by a balance between (surface-dependent) adhesive energy and (volume-dependent) elastic energy, increasing the size of the system while maintaining a similar contact geometry requires a decrease in the elastic modulus of the material. Our GSA comprised wedge-like fibrils 45 µm in width and 155 µm in height (approx. 3.5 : 1 aspect ratio; Santos 2009; Parness et al. in press), so we chose a polydimethylsiloxane elastomer (PDMS, Sylgard-170; E = 1.75 MPa) in proxy of hard keratin. We employed a lithographic process with two exposure angles to create moulds for the GSA out of a negative tone photoresist, SU-8 (Microchem.com). We cast the Sylgard elastomer in the SU-8 mould under vacuum, spun it down to the desired backing layer thickness of 155 µm, then heat cured it and pulled it out of the mould by hand.
2.4. Rate dependence and wear resistance in GSA
Taking into account the GSA's larger contact geometry, we conducted similar frictional–adhesion velocity and durability tests on the synthetic structure over an increased drag distance range from 100 µm to 30 mm and velocities from 5 µm s−1 to 158 mm s−1. We recorded kinetic friction and adhesion forces at 13 drag distance/velocity combinations of nine trials each, totaling 117 tests. We subjected the synthetic adhesive to the same durability test as that performed on setal arrays: 30 000 load–drag–pull tests at a velocity of 10 mm s−1 over a distance of 10 mm per trial, totalling a net displacement of 300 m. We recorded friction, adhesion and sample stiffness in the normal direction every 1000 trials. Lastly, we recorded high-speed videos of the real-time contact area of the synthetic adhesive using frustrated total internal reflection (FTIR). Drags at speeds of 0.05, 0.1, 0.5, 1, and 10 mm s−1 were recorded at 250 fps. The adhesive's dynamic contact area was isolated in the image and digitized, with each pixel yielding a time-intensity waveform. We then performed a fast Fourier transform (FFT) analysis on the waveform, and averaged the FFT magnitude for each point to determine an average frequency response of the adhesive at a given speed.
Force and motion data were sampled at 1 kHz and recorded with software. A custom LabVIEW (National Instruments, Austin, TX) program controlled test parameters and data acquisition. Piezoelectric sensor drift was compensated for by fitting the zero-force variation before and after testing with a cubic spline in LabVIEW. Steady-state drag forces were calculated by averaging the forces between 25 and 75 per cent of the duration of the drag. We scaled the measured stiffness of setal arrays by area, A, and height (approx. 70 µm) to calculate the effective elastic modulus, 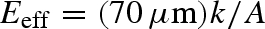 , where k is the compressive stiffness of the setal array, as described in Autumn et al. (2006c). All results are mean ± standard error.
, where k is the compressive stiffness of the setal array, as described in Autumn et al. (2006c). All results are mean ± standard error.
3. Results
3.1. Rate dependence and wear resistance in gecko setae
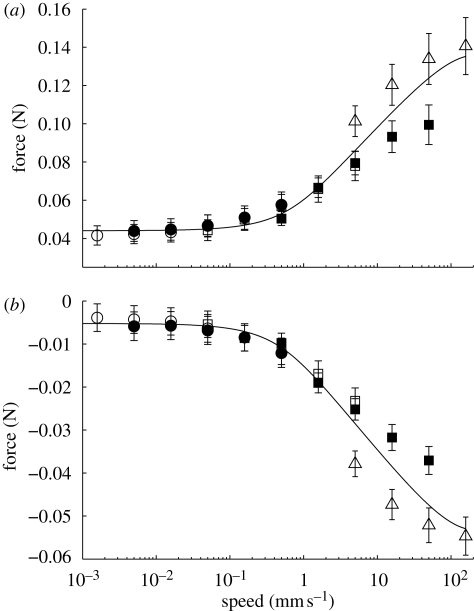
Friction and adhesion forces were rate dependent (figure 2). Sliding arrays in their adhesive direction resulted in monotonically increasing magnitude of friction–adhesion with speed. We measured an average 3.3× increase in friction and 10.3× increase in adhesion over the almost six orders of magnitude velocity test range (500 nm s−1–158 mm s−1). As setae transitioned from the static to the kinetic regime, frictional–adhesion forces remained consistent and did not decrease once the array began to slide, as would be predicted for typical dry, hard materials (figure 1).
Figure 2.
Steady-state contact forces increased in magnitude as a function of drag speed over drag distances of 10−3 mm (open circles), 10−2 mm (filled circles), 10−1 mm (open squares), 100 mm (filled squares), and 101 mm (open triangles). (a) Friction forces increased 3.3× over the velocity range. (b) Adhesion forces increased 10.3× over the test velocity range. Line fits are Fshear = (0.018 N)(1 + v/(1.11 × 103 mm s−1))−1 × sinh−1(v/(0.972 mm s−1)) + 0.044 N (R2 = 0.99) and Fnormal =−(0.009 N)(1 + v/(1.11 × 103 mm s−1))−1 sinh−1(v/(0.751 mm s−1)) − 0.009 N (R2 = 0.99).
Setal arrays were highly wear resistant. Over a battery of 30 000 load–drag–pulls of 10 mm displacement each, totaling 300 m of sliding, we measured a 25 per cent increase in adhesion and only a 5 per cent decrease in friction. Frictional–adhesion forces decreased initially, followed by a linear increase in contact forces (electronic supplementary material). We observed a 9 per cent decrease in the setal array effective elastic modulus over the 30 000 tests. During the velocity tests, we observed a larger 13 per cent decrease in average effective elastic modulus of 163 ± 19.2 kPa prior to testing, and 143 ± 14.8 kPa after testing (electronic supplementary material). The decrease in stiffness over repeated tests may have been due to an increase in alignment of the setae under repeated loading.
In total, we tested 25 setal array samples and rejected nine samples that completely or partially fractured from the SEM stub during testing. Five of the nine rejected samples tore from their mount at the highest shear speed of 158 mm s−1, consistent with our observations that contact force was maximized at higher velocity. We rejected an additional two samples due to glue contamination from the mounting procedure, leaving 14 samples in our analysis.
3.2. Rate dependence and wear resistance in GSA
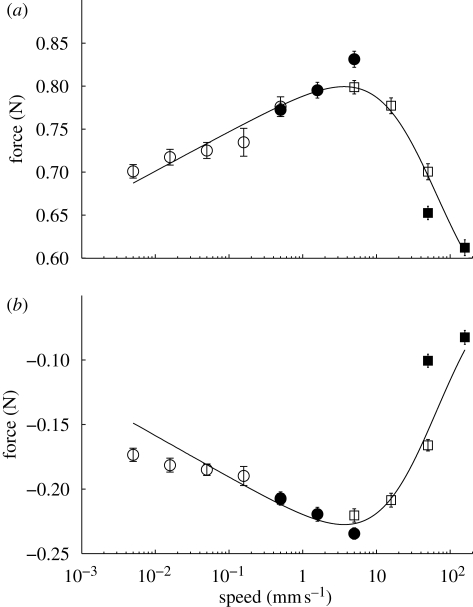
Friction and adhesion in the GSA were also velocity dependent. We observed logarithmically increasing frictional–adhesion forces with speed, which reached a maximum at 5 mm s−1 and steadily declined at higher speeds (figure 3). During shearing of the GSA, we observed uncorrelated stick–slip of the microscopic fibrils, while at the macroscopic scale, the adhesive slid smoothly. Durability measurements of the synthetic material exhibited only 7.2 per cent decrease in friction and 9.6 per cent decrease in adhesion over 30 000 tests totaling 300 m of sliding.
Figure 3.
Steady-state contact forces of the GSA as a function of drag speed over drag distances of 10−1 mm (open circles), 100 mm (filled circles), 101 mm (open squares) and 3× 101 mm (filled squares). (a) Friction forces increased with speed until approximately 10 mm s−1 at which point friction decreased with speed. (b) Adhesion force followed the friction force. Line fits are Fshear = (0.020 N)(1 + v/(52.4 mm s−1))−1sinh−1(v/(1.5 × 10−6 mm s−1)) + 0.511 N (R2 = 0.96) and Fnormal = (−0.014 N)(1 + v/(52.4 mm s−1))−1 sinh−1(v/(1.5 × 10−6 mm s−1)) − 0.025 N (R2 = 0.91).
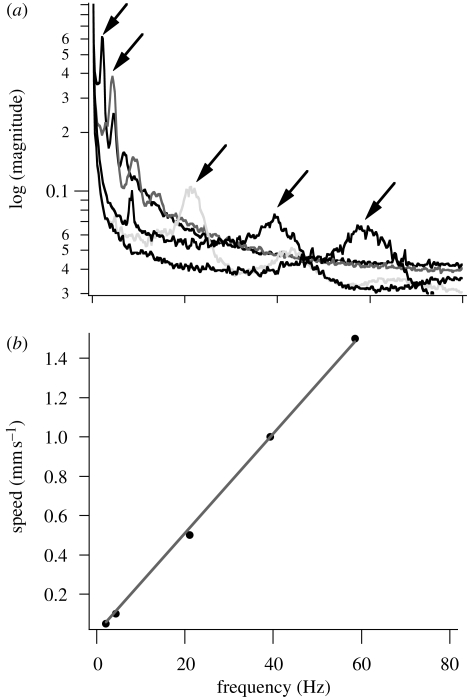
Using a high-speed video camera and FTIR, we observed stick–slip occurring in the frictionally adhering GSA. At drag speeds of 0.05, 0.50, 1 and 10 mm s−1, we observed noticeable uncorrelated stick–slip events at the interface that could be observed by the naked eye. Performing an FFT analysis on the contact area determined by FTIR, imaged at 250 fps, we observed a dominant oscillation frequency at each speed corresponding to individual fibril stick–slip at the surface (figure 4). The stick–slip frequency varied linearly with speed (f = (43.881 mm−1)v) and the slope of this fit suggests a stick–slip distance of λ = 25.3 µm, which we were able to confirm through microscopy.
Figure 4.
Stick–slip frequency analysis of the GSA. (a) The averaged FFT magnitude from high-speed video shows dominant stick–slip frequencies that increase in proportion to velocity. The 0.05, 0.10, 0.50, 1.00 and 1.50 mm s−1 FFT traces are highlighted with arrows from left to right in increasing order. (b) The dominant stick–slip frequencies (arrows) varied linearly with speed as f = (25.3 µm)v. Using equation (4.4), the fit predicts a stick–slip displacement of λ = 25.3 µm.
4. Discussion
When a gecko's toes slide, it does not detach from the surface, even if the surface is vertical or inverted (Huber et al. 2007; Jusufi et al. 2008), suggesting that gecko setae maintain adhesion and friction during sliding. Ordinarily, dry, hard materials slip more easily as they slide more rapidly, in part due to wear caused by rubbing (Bhushan 2002). Instead, we discovered that gecko setae became stickier as they slid more rapidly (figure 2), and resisted wear over a net displacement of 300 m. We observed a threefold increase in friction and 10-fold increase in adhesion forces over shear velocities from 500 nm s−1 to 158 mm s−1. The logarithmic increase in contact force in gecko setae is similar in form to that observed in polymer friction experiments (Vorvolakos & Chaudhury 2003; Vajpayee et al. 2009), raising the possibility that there is a common underlying mechanism despite the obvious differences in structure between gecko setal arrays and flat polymer surfaces.
Macroscale stick–slip is typically an unwanted behaviour that results in wear, chatter and unstable motion in sliding systems (Rabinowicz 1995; Bhushan 2002); however, nanoscale stick–slip dynamics are notably different (Gnecco et al. 2000; Richetti et al. 2001; Riedo et al. 2003; Tambe & Bhushan 2005), and it has been proposed that smooth macroscopic sliding may result from uncorrelated nanoscale stick–slip (Braun et al. 2005; Persson 1995). Nanoscale stick–slip models are ubiquitous in the mechanics of sliding solids (Rice & Ruina 1983; Baumberger et al. 1999; Gnecco et al. 2000; Richetti et al. 2001; Riedo et al. 2003; Tambe & Bhushan 2005; Bureau et al. 2006), elastomers (Chernyak & Leonov 1986; Schallamach 1963; Ghatak et al. 2000; Vorvolakos & Chaudhury 2003) and boundary lubricated systems (He & Robbins 2001; Richetti et al. 2001; Tao & Bhushan 2007). We propose that wear resistance, and stable frictional adhesion forces during sliding, may emerge from the stochastic stick–slip of a population of individual fibrils with high resonant frequencies.
4.1. A stick–slip model of kinetic friction and adhesion
In a fibrillar adhesive, contact stress should be evenly distributed among the fibrils (Hui et al. 2004). Therefore, considering the high modulus of keratin (Peattie et al. 2007), it is reasonable to assume that during sliding, setal shafts remain in tension and are effectively rigid (Gravish et al. 2007), while the spatular shafts undergo uncorrelated stick–slip. To model stick–slip of a spatular shaft (fibril), we assume that during sliding, fibrils cycle through a stick–slip process governed by two characteristic times: tstick, the average lifetime of a fibril–substrate bond, and τ0, the characteristic time for a fibril to reattach to the substrate after bond rupture. Assuming fibrils act as vertical or curved cantilever beams (Jagota & Bennison 2002; Sitti & Fearing 2003; Hui et al. 2004; Autumn et al. 2006c; Gravish et al. 2007; Chen et al. 2008; figure 5), we estimate the characteristic reattachment time to be given by 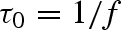 , where f is the fibre's resonant frequency. Assuming a purely elastic system, a cylindrical fibre of material density ρ, radius R, area A = πR2, length l, Young's modulus E and bending inertia
, where f is the fibre's resonant frequency. Assuming a purely elastic system, a cylindrical fibre of material density ρ, radius R, area A = πR2, length l, Young's modulus E and bending inertia 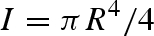 ,
,
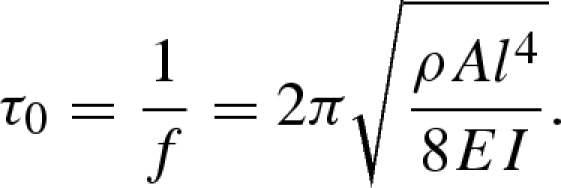 |
4.1 |
Figure 5.
Fibrillar geometry of (a) natural gecko setae and (b) GSA.
The slip time (equal to τ0) is independent of sliding velocity (v), whereas the stick time (tstick) is a function of v and a characteristic yield displacement (λ) at which the adhesive bond is broken. Thus, the average lifetime of a fibril bond is given by
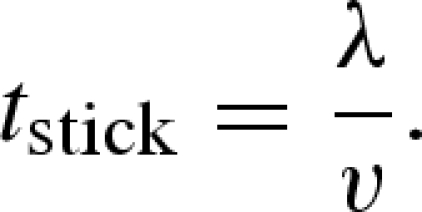 |
4.2 |
Our assumption here is that the fibril–surface contact will break when the macroscopic array has moved by a distance λ. The microscopic details are unknown at this point but could involve a combination of stretching of the spatula (Yamaguchi et al. 2009) and rate-dependent plastic yielding of the actual contact as suggested in the state and rate model (Baumberger et al. 1999).
To analyse the stick–slip response of fibrillar adhesives, we employ a dimensionless Deborah number (Reiner 1964; Huilgol 1975; Israelachvili & Berman 1995), which is ordinarily defined in rheology as the ratio of the characteristic relaxation time of the system to the observation time. For stick–slip systems, however, we can define the Deborah number as
| 4.3 |
the ratio of the sliding velocity (v) to the critical relaxation velocity V*, the ratio of the slip length λ to the characteristic time τ0 (Richetti et al. 2001; Filippov et al. 2004):
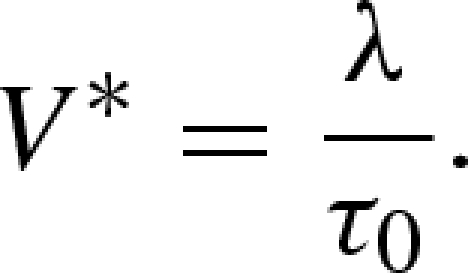 |
4.4 |
The critical relaxation velocity defines an inflection point in the adhesive's sliding behaviour; above V*, individual fibrils spend more time reattaching to the substrate than they do sticking to it. Consider N0 fibrils undergoing stick–slip: the number of fibrils attached to the substrate simultaneously is given by (Schallamach 1963)
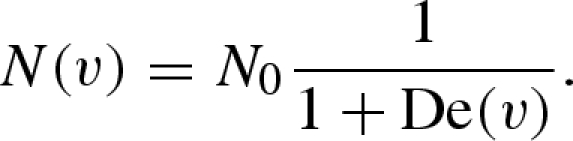 |
4.5 |
When the system is driven at a velocity lower than V*, then De < 1, and fibrils are predominantly stuck to the substrate with reattachment occurring rapidly relative to the speed of the substrate. At low v ≪ V*, N(v) is near N0. When the system is driven at a velocity faster than V*, then De > 1, and the reattachment time becomes slow compared with the motion of the substrate. At v ≫ V*, the response of the system becomes consistent with the decline in force with velocity expected in friction between solids in contact (Rice & Ruina 1983; Baumberger et al. 1999). In this case, force declines with velocity because fewer fibrils can attach simultaneously as velocity increases.
We can now estimate the model predictions for gecko spatulae. In tokay geckos, flat spatula pads (200 × 300 × 5 nm pads) are attached to approximately 800 nm long × 50 nm diameter shafts (Ruibal & Ernst 1965; Russell 1975; Williams & Peterson 1982; Tian et al. 2006). Keratin modulus is E = 1.5 GPa (Peattie et al. 2007) and we assume a density of ρ = 1000 kg m−3. Thus, for fibrils of l = 800 nm, R = 25 nm, bending inertia is I = 3.07 × 10−31 m4, and equation (4.1) yields a characteristic time of τ0 = 0.09 µs for relaxation of the fibril during slip and a natural frequency of 10.8 MHz. Adhering spatulae are between 100 and 1000 nm apart and single spatula measurements typically observe vertical detachment within 100–1000 nm displacement (Huber et al. 2005a; Sun et al. 2005). Therefore, we use λ = 100 nm as a conservative lower bound of the slip length of the spatula, yielding a predicted critical velocity V* = 100 nm/0.09 µs = 1.08 m s−1 (equation (4.4)). This suggests that De ≪ 1 over our entire experimental velocity range (500 nm s−1–158 mm s−1; equation (4.3)) because V* is at least 1 m s−1 in gecko setae.
4.2. Rate dependence of detachment force
The last, but important, point that remains to be discussed is the detachment condition for the spatulae. If the detachment force was independent of tstick, then for De < 1, the time-averaged force could only be constant with increasing velocity, which is inconsistent with the experimental data. This suggests that the force to detach a spatula f(v) is not independent of tstick, but increases as tstick decreases, i.e. as the strain rate of the spatulae increases, as discussed by Yamaguchi et al. (2009).
The velocity dependence of the gecko adhesive and GSA was logarithmic above a threshold speed (figures 2 and 3). Logarithmic force–velocity models are abundant in the mechanics of atomic stick–slip (Bennewitz et al. 1999; Gnecco et al. 2000; Richetti et al. 2001; Riedo et al. 2003; Tambe & Bhushan 2005; Tao & Bhushan 2007), yielding in polymers (Mulliken 2004; Ward & Sweeney 2004; Bureau et al. 2006; Mulliken & Boyce 2006), elastomer friction (Schallamach 1963; Vorvolakos & Chaudhury 2003; Vajpayee et al. 2009) and boundary lubricated surfaces (He & Robbins 2001; Richetti et al. 2001; Tao & Bhushan 2007). To model our data, we apply a velocity-dependent function that is constant at low velocity, and logarithmic at higher velocity
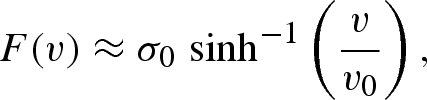 |
4.6 |
where v is the macroscopic relative motion between the setal array and the substrate and v0 is an adjustable parameter setting the velocity where F is proportional to σ0.
We can now model the rate dependence of the total force of a fibrillar array (Farray) by combining equation (4.5) (the rate dependence of number of contacts) and equation (4.6) (the rate dependence of the force of each contact):
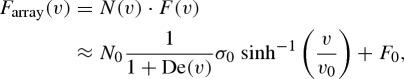 |
4.7 |
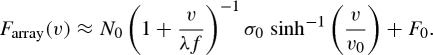 |
4.8 |
It is worthwhile to note that rate-dependent stick–slip models of polymer yielding (Krausz & Eyring 1975; Ward & Sweeney 2004), elastomer friction (Schallamach 1963; Vorvolakos & Chaudhury 2003), boundary lubricated friction (He & Robbins 2001; Richetti et al. 2001; Tao & Bhushan 2007), atomic stick–slip (Bennewitz et al. 1999; Gnecco et al. 2001; Riedo et al. 2003; Tambe & Bhushan 2005) and even earthquakes (Huisman & Fasolino 2005) all suggest that smooth macroscopic kinetic friction is the result of uncorrelated stick–slip of all the discrete microscale contact elements sliding along the surface, precisely what we observed in the GSA and what we predicted for gecko setae.
It may be the case that gecko spatulae are subject to rate-dependent yielding during stick–slip, as in unlubricated friction of polymer glasses (Creton et al. 1999; Bureau et al. 2006). As discussed earlier, spatulae are not elastomers, but rather are made of β-keratin, a stiff material (Rizzo et al. 2006; Peattie et al. 2007) analogous to a polymer glass. Sliding of polymer glasses on a smooth surface occurs as microscopic contact points move by shearing of highly localized nanoscopic layers that are deformed beyond the yield stress of the polymer. Fracture of spatular contacts in mixed mode (shear and tension) may then involve local plasticity at the interface between the spatula and the surface. An order of magnitude estimate for the plausibility of this mechanism comes from measurements of individual setae. A single seta can sustain 200 µN in shear (Autumn et al. 2000). Assuming approximately 500 spatulae per seta, the shear force is 0.4 µN per spatula, which is supported by an area of roughly 0.01 µm2. The average shear stress to be transferred to the surface by each spatula is then of the order 40 µN µm−2 or 40 MPa, of the order of magnitude of the yield stress of a molecular solid such as β-keratin. Further microscopic investigations will elucidate this issue.
4.3. Rate dependence and wear resistance in GSA
Shearing the GSA resulted in smooth friction and adhesion forces during macroscopic sliding while stick–slip events at the fibrillar level were visible at the microscale (see video in the electronic supplementary material). While some GSA fibrils slid steadily, stick–slip was the dominant mechanism, accounting for the remarkable durability of the GSA, which maintained over 90 per cent of its adhesion over a total sliding distance of 300 m. The stick–slip model predicts that the frequency of stick events (f) should vary linearly with the drag velocity as v = λf. As predicted (equation (4.2)), stick–slip frequency varied linearly with velocity (figure 4; v = (25.3 µm)f; R2 = 0.99) and from the slope of this fit and equation (4.2), we estimate that the 155 µm tall GSA structures have a characteristic stick–slip distance of λ = 25.3 µm, which was similar to the displacements observed visually (23 µm). This confirms that smooth, macroscopic friction–adhesion can result from uncorrelated stick–slip events at the fibrillar level.
It is clear that significant differences exist between the sliding motion of natural gecko setae and that of the GSA (compare the electronic supplementary material of this study with the electronic supplementary material of Gravish et al. (2007; http://rsif.royalsocietypublishing.org/content/5/20/339/suppl/DC1)). This may be due in part to the difference in dissipative mechanisms resisting detachment (viscoelastic for the Sylgard elastomer and plastic for the keratin), so we must be cautious about the direct applicability of the GSA results to the gecko system.
We determined V* for the GSA experimentally (figure 3) and then compared it with a prediction based on the resonant frequency of a PDMS cantilever. To predict V* in the GSA, we estimated the natural frequency of a tapered beam (Volterra & Zachmanoglou 1965) as
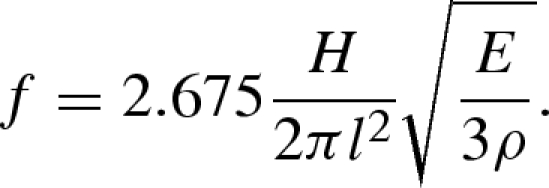 |
4.9 |
For our structures (see §2), E = 1.75 MPa, ρ = 1370 kg m−3, H = 45 µm and l = 155 µm, yielding a natural frequency of 15.8 kHz and a characteristic time τ0 = 63 µs. We observed a slip distance of λ = 23 µm. Therefore, the predicted critical velocity (equation (4.4)) is V* = 364 mm s−1, roughly two orders of magnitude larger than the observed velocity at which forces were maximal (figure 3). The prediction (equation (4.9)) does not account for the elastomeric backing layer that the GSA array is mounted upon, nor any damping in the material. Moreover, estimating f from equation (4.9) assumes that fibres return to their unloaded state without resistance. In contrast, we observed that following detachment, fibres maintained frictional contact with the surface while relaxing to a freestanding position (electronic supplementary material). Our calculation (equation 4.9) represents an upper bound to the GSA's critical velocity and the difference in our measured V* suggests that friction and surface dynamics play at least as important a role as the free resonant response.
4.4. Why geckos do not fall when they slip
Selection for dynamic locomotion in unpredictable environments may have driven the evolution of adhesive nanostructures in geckos. Slip resistance in biological attachment mechanisms has evolved through the use of fluids (Denny 1980; Federle et al. 2006) or compliant materials (Gorb 1998; Federle et al. 2004; Santos et al. 2005). Instead, geckos evolved branched setal arrays made of a high modulus material (1.5 GPa; Peattie et al. 2007) with millions of redundant high-aspect ratio contact points, each with a very high resonant frequency (10 MHz), resulting in a novel dry adhesive that responds to sliding by becoming stickier. This is why geckos do not fall when their toes slide. If the rate of sliding is lower than the critical velocity V* (approx. 1 m s−1), then De < 1 and the majority of spatulae undergoing stick–slip will be sticking to the substrate simultaneously. This design also promotes wear resistance since sliding at the macroscale can occur while individual contacts remain static. Dynamic friction alone could be the functional role for non-adhesive setae in phylogenetically basal geckos such as Aeluroscalabotes felinus (Peattie & Full 2007), which represent a puzzling intermediate evolutionary step between the nano-spiny epidermis of gecko ancestors, and highly adhesive branched setae of derived geckos.
4.5. Design of failure-resistant GSAs
Our results and model suggest design principles for the development of GSAs. Approaching the performance of the gecko will require a very fine hierarchical structure of fibrils made of a hard material, which is difficult to manufacture. Yet, recent results showing strong static attachment and shear adhesion (Lee et al. 2008; Qu et al. 2008; Schubert et al. 2008; Vajpayee et al. 2009) are promising.
The principle of uncorrelated stick–slip motion can be applied to softer and larger structures, as demonstrated in our GSA. As the fibril size increases, the elastic energy of deformation of the fibril will become more important relative to adhesive forces. Therefore, it becomes necessary to use a softer material such as a rubber for the fibrils. A viscoelastic rubber would seem a good choice for maximizing pull-off force because of the high dissipation at each crack tip, as contacts start to peel. However, maximization of pull-off is not ideal for dynamic applications such as climbing robots (Autumn et al. 2005; Kim et al. 2007), where for safety, it is desirable to maintain, or even increase, the aggregate shear and adhesive forces when sliding occurs. The good sliding performance of the GSA (figure 3) was a consequence of using a PDMS elastomer with relatively low damping. In contrast, GSAs fabricated from a more dissipative urethane elastomer had high adhesion statically, but performance declined rapidly at the onset of sliding (Santos et al. 2007; Santos 2009).
5. Conclusions
The gecko adhesive is a rich and complex tribological system. Recent theoretical attempts to reconcile molecular dynamics and empirical tribology results have proposed that macroscopically smooth sliding can be the result of a population of stick–slip events at the atomic scale (Braun et al. 2005; Huisman & Fasolino 2005). Our observations support this model and suggest that the dynamics of gecko setae and GSAs may be of interest to scientists studying macro- to nanoscale phenomena as diverse as earthquakes (Rice 1980; Carlson et al. 1994; Heslot et al. 1994) and atomic friction (Bennewitz et al. 1999; Gnecco et al. 2001; Riedo et al. 2003; Tambe & Bhushan 2005).
Acknowledgements
We thank Jacob Israelachvili, Anne Peattie and three anonymous reviewers for helpful comments. Work performed at the Stanford Nanofabrication Facility of NNIN was supported by the National Science Foundation under grant ECS-9731293. This work was also supported in part by DARPA-Zman, NSF Center of Integrated Nanomechanical Systems and CIS New Users Grant Program. Work performed at Lewis & Clark College was supported by NSF-NIRT 0304730 and DCI/NGIA HM1582-05-2022 grants to K.A.
References
- Alibardi L. 2003. Ultrastructural autoradiographic and immunocytochemical analysis of setae formation and keratinization in the digital pads of the gecko Hemidactylus turcicus (Gekkonidae, Reptilia). Tissue Cell 35, 288–296. ( 10.1016/S0040-8166(03)00050-8) [DOI] [PubMed] [Google Scholar]
- Aristotle 350 BCE 1918. Historia animalium (transl. by Thompson D. W.). Oxford, UK: The Clarendon Press. [Google Scholar]
- Arzt E., Gorb S., Spolenak R. 2003. From micro to nano contacts in biological attachment devices. Proc. Natl Acad. Sci USA 100, 10 603–10 606. ( 10.1073/pnas.1534701100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autumn K. 2006. Properties, principles, and parameters of the gecko adhesive system. In Biological adhesives (eds Smith A., Callow J.), pp. 225–255. Berlin, Germany: Springer Verlag. [Google Scholar]
- Autumn K., Hansen W. 2006. Ultrahydrophobicity indicates a nonadhesive default state in gecko setae. J. Comp. Physiol. A 192, 1205–1212. ( 10.1007/s00359-006-0149-y) [DOI] [PubMed] [Google Scholar]
- Autumn K., Liang Y. A., Hsieh S. T., Zesch W., Chan W.-P., Kenny W. T., Fearing R., Full R. J. 2000. Adhesive force of a single gecko foot-hair. Nature 405, 681–685. ( 10.1038/35015073) [DOI] [PubMed] [Google Scholar]
- Autumn K., et al. 2002. Evidence for van der Waals adhesion in gecko setae. Proc. Natl Acad. Sci. USA 99, 12 252–12 256. ( 10.1073/pnas.192252799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autumn K., et al. 2005. Robotics in scansorial environments. Proc. SPIE 5804, 291–302. ( 10.1117/12.606157) [DOI] [Google Scholar]
- Autumn K., Dittmore A., Santos D., Spenko M., Cutkosky M. 2006a. Frictional adhesion: a new angle on gecko attachment. J. Exp. Biol. 209, 3569–3579. ( 10.1242/jeb.02486) [DOI] [PubMed] [Google Scholar]
- Autumn K., Hsieh S. T., Dudek D. M., Chen J., Chitaphan C., Full R. J. 2006b. Dynamics of geckos running vertically. J. Exp. Biol. 209, 260–272. ( 10.1242/jeb.01980) [DOI] [PubMed] [Google Scholar]
- Autumn K., Majidi C., Groff R., Dittmore A., Fearing R. 2006c. Effective elastic modulus of isolated gecko setal arrays. J. Exp. Biol. 209, 3558–3568. ( 10.1242/jeb.02469) [DOI] [PubMed] [Google Scholar]
- Autumn K., Gravish N., Wilkinson M., Santos D., Spenko M., Cutkosky M. 2007. Frictional adhesion of natural and synthetic gecko setal arrays. In Proc. 30th Annu. Meeting of the Adhesion Society, pp. 58–60. [Google Scholar]
- Baumberger T., Berthoud P., Caroli C. 1999. Physical analysis of the state- and rate-dependent friction law. II. Dynamic friction. Phys. Rev. B 60, 3928–3939. ( 10.1103/PhysRevB.60.3928) [DOI] [Google Scholar]
- Bennewitz R., Gyalog T., Guggisberg M., Bammerlin M., Meyer E., Guntherodt H. J. 1999. Atomic-scale stick-slip processes on Cu(111). Phys. Rev. B 60, R11 301–R11 304. ( 10.1103/PhysRevB.60.R11301) [DOI] [Google Scholar]
- Bhushan B. 2002. Introduction to tribology. New York, NY: John Wiley & Sons. [Google Scholar]
- Bhushan B., Galasso B., Bignardi C., Nguyen C., Dai L., Qu L. 2008. Adhesion, friction and wear on the nanoscale of MWNT tips and SWNT and MWNT arrays. Nanotechnology 19, 125 702 ( 10.1088/0957-4484/19/12/125702) [DOI] [PubMed] [Google Scholar]
- Braun O. M., Peyrard M., Bortolani V., Franchini A., Vanossi A. 2005. Transition from smooth sliding to stick-slip motion in a single frictional contact. Phys. Rev. E 72, 056116 ( 10.1103/PhysRevE.72.056116) [DOI] [PubMed] [Google Scholar]
- Bullock J. M. R., Drechsler P., Federle W. 2008. Comparison of smooth and hairy attachment pads in insects: friction, adhesion and mechanisms for direction-dependence. J. Exp. Biol. 211, 3333–3343. ( 10.1242/jeb.020941) [DOI] [PubMed] [Google Scholar]
- Bureau L., Caroli C., Baumberger T. 2006. Frictional dissipation and interfacial glass transition of polymeric solids. Phys. Rev. Lett. 97, 225 501 ( 10.1103/PhysRevLett.97.225501) [DOI] [PubMed] [Google Scholar]
- Carlson J. M., Langer J. S., Shaw B. E. 1994. Dynamics of earthquake faults. Rev. Mod. Phys. 66, 657–670. ( 10.1103/RevModPhys.66.657) [DOI] [Google Scholar]
- Chen B., Wu P. D., Gao H. 2008. Hierarchical modelling of attachment and detachment mechanisms of gecko toe adhesion. Proc. R. Soc. A 464, 1639–1652. ( 10.1098/rspa.2007.0350) [DOI] [Google Scholar]
- Chen B., Wu P. D., Gao H. 2009. Pre-tension generates strongly reversible adhesion of a spatula pad on substrate. J. R. Soc. Interface 6, 529–537. ( 10.1098/rsif.2008.0322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak Y. B., Leonov A. I. 1986. On the theory of the adhesive friction of elastomers. Wear 108, 105–138. ( 10.1016/0043-1648(86)90092-X) [DOI] [Google Scholar]
- Creton C. 2003. Pressure-sensitive adhesives: an introductory course. MRS Bull. 28, 434–439. [Google Scholar]
- Creton C., Halary J. L., Monnerie L. 1999. Plasticity of polystyrene-poly(2,6,dimethyl,1,4,phenylene oxide) blends. Polymer 40, 199–206. ( 10.1016/S0032-3861(98)00206-7) [DOI] [Google Scholar]
- Denny M. 1980. The role of gastropod pedal mucus in locomotion. Nature 285, 160–161. ( 10.1038/285160a0) [DOI] [Google Scholar]
- Eimuller T., Guttmann P., Gorb S. N. 2008. Terminal contact elements of insect attachment devices studied by transmission X-ray microscopy. J. Exp. Biol. 211, 1958–1963. ( 10.1242/jeb.014308) [DOI] [PubMed] [Google Scholar]
- Federle W., Baumgartner W., Holldobler B. 2004. Biomechanics of ant adhesive pads: frictional forces are rate- and temperature-dependent. J. Exp. Biol. 207, 67–74. ( 10.1242/jeb.00716) [DOI] [PubMed] [Google Scholar]
- Federle W., Barnes W. J. P., Baumgartner W., Drechsler P., Smith J. M. 2006. Wet but not slippery: boundary friction in tree frog adhesive toe pads. J. R. Soc. Interface 3, 689–697. ( 10.1098/rsif.2006.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov A. E., Klafter J., Urbakh M. 2004. Friction through dynamical formation and rupture of molecular bonds. Phys. Rev. Lett. 92, 135 503 ( 10.1103/PhysRevLett.92.135503) [DOI] [PubMed] [Google Scholar]
- Ghatak A., Vorvolakos K., She H. Q., Malotky D. L., Chaudhury M. K. 2000. Interfacial rate processes in adhesion and friction. J. Phys. Chem. B 104, 4018–4030. ( 10.1021/jp9942973) [DOI] [Google Scholar]
- Gnecco E., Bennewitz R., Gyalog T., Loppacher C., Bammerlin M., Meyer E., Guntherodt H. J. 2000. Velocity dependence of atomic friction. Phys. Rev. Lett. 84, 1172–1175. ( 10.1103/PhysRevLett.84.1172) [DOI] [PubMed] [Google Scholar]
- Gnecco E., Bennewitz R., Gyalog T., Meyer E. 2001. Friction experiments on the nanometre scale. J. Phys. Condens. Matter 13, R619–R642. ( 10.1088/0953-8984/13/31/202) [DOI] [Google Scholar]
- Gorb S. N. 1998. The design of the fly adhesive pad: distal tenent setae are adapted to the delivery of an adhesive secretion. Proc. R. Soc. Lond. B 265, 747–752. ( 10.1098/rspb.1998.0356) [DOI] [Google Scholar]
- Gravish N., Wilkinson M., Autumn K. 2007. Frictional and elastic energy in gecko adhesive detachment. J. R. Soc. Interface 5, 339–348. ( 10.1098/rsif.2007.1077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen W., Autumn K. 2005. Evidence for self-cleaning in gecko setae. Proc. Natl Acad. Sci. USA 102, 385–389. ( 10.1073/pnas.0408304102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G., Robbins M. O. 2001. Simulations of the kinetic friction due to adsorbed surface layers. Tribology Lett. 10, 7–14. ( 10.1023/A:1009030413641) [DOI] [Google Scholar]
- Heslot F., Baumberger T., Perrin B., Caroli B., Caroli C. 1994. Creep, stick-slip, and dry-friction dynamics—experiments and a heuristic model. Phys. Rev. E 49, 4973–4988. ( 10.1103/PhysRevE.49.4973) [DOI] [PubMed] [Google Scholar]
- Huber G., Gorb S., Spolenak R., Arzt E. 2005a. Resolving the nanoscale adhesion of individual gecko spatulae by atomic force microscopy. Biol. Lett. 1, 2–4. ( 10.1098/rsbl.2004.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber G., Mantz H., Spolenak R., Mecke K., Jacobs K., Gorb S., Arzt E. 2005b. Evidence for capillarity contributions to gecko adhesion from single spatula nanomechanical measurements. Proc. Natl Acad. Sci. USA 102, 16 293–16 296. ( 10.1073/pnas.0506328102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber G., Gorb S., Hosoda N., Spolenak R., Arzt E. 2007. Influence of surface roughness on gecko adhesion. Acta Biomater. 3, 607–610. ( 10.1016/j.actbio.2007.01.007) [DOI] [PubMed] [Google Scholar]
- Hui C. Y., Glassmaker N. J., Tang T., Jagota A. 2004. Design of biomimetic fibrillar interfaces: 2. Mechanics of enhanced adhesion. J. R. Soc. Interface 1, 35–48. ( 10.1098/rsif.2004.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. Y., Shen L., Jagota A., Autumn K. 2006. Mechanics of anti-fouling or self-cleaning in gecko setae. In Proc. 29th Annu. Meeting of the Adhesion Society. [Google Scholar]
- Huilgol R. R. 1975. On the concept of the Deborah number. Trans. Soc. Rheology 19, 297–306. ( 10.1122/1.549372) [DOI] [Google Scholar]
- Huisman B. A. H., Fasolino A. 2005. Transition to strictly solitary motion in the Burridge–Knopoff model of multicontact friction. Phys. Rev. E 72, 016 107 ( 10.1103/PhysRevE.72.016107) [DOI] [PubMed] [Google Scholar]
- Israelachvili J. 1992. Intermolecular and surface forces. New York, NY: Academic Press. [Google Scholar]
- Israelachvili J. N. 2005. Engineering—skimming the surface. Nature 435, 893–895. ( 10.1038/435893a) [DOI] [PubMed] [Google Scholar]
- Israelachvili J., Berman A. 1995. Irreversibility, energy-dissipation, and time effects in intermolecular and surface interactions. Isr. J. Chem. 35, 85–91. [Google Scholar]
- Jagota A., Bennison S. 2002. Mechanics of adhesion through a fibrillar microstructure. Int. Comp. Biol. 42, 1140–1145. ( 10.1093/icb/42.6.1140) [DOI] [PubMed] [Google Scholar]
- Jusufi A., Goldman D., Revzen S., Full R. 2008. Active tails enhance arboreal acrobatics in geckos. Proc. Natl Acad. Sci. USA 105, 4215–4219. ( 10.1073/pnas.0711944105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Spenko M., Trujillo S., Heyneman B., Mattoli V., Cutkosky M. R. 2007. Whole body adhesion: hierarchical, directional and distributed control of adhesive forces for a climbing robot. In IEEE Int. Conf. on Robotics and Automation, Rome, Italy, pp. 1268–1273. [Google Scholar]
- Krausz A. S., Eyring H. 1975. Deformation kinetics. New York, NY: John Wiley & Sons. [Google Scholar]
- Krim J. 1996. Friction at the atomic scale. Sci. Am. 275, 74–80. [Google Scholar]
- Lamb T., Bauer A. M. 2006. Footprints in the sand: independent reduction of subdigital lamellae in the Namib–Kalahari burrowing geckos. Proc. R. Soc. B 273, 855–864. ( 10.1098/rspb.2005.3390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Fearing R. S. 2008. Contact self-cleaning of synthetic gecko adhesive from polymer microfibers. Langmuir 24, 10 587–10 591. ( 10.1021/la8021485) [DOI] [PubMed] [Google Scholar]
- Lee J., Majidi C., Schubert B., Fearing R. S. 2008. Sliding induced adhesion of stiff polymer microfiber arrays: 1. Macroscale bahaviour. J. R. Soc. Interface 5, 835–844. ( 10.1098/rsif.2007.1308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan B. Q., Robbins M. O. 2005. The breakdown of continuum models for mechanical contacts. Nature 435, 929–932. ( 10.1038/nature03700) [DOI] [PubMed] [Google Scholar]
- Maderson P. F. A. 1964. Keratinized epidermal derivatives as an aid to climbing in gekkonid lizards. Nature 203, 780–781. ( 10.1038/203780a0) [DOI] [Google Scholar]
- Mulliken A. 2004. Low to high strain rate deformation of amorphous polymers: experiments and modeling. MS thesis, Department of Mechanical Engineering, MIT. [Google Scholar]
- Mulliken A., Boyce M. 2006. Mechanics of the rate-dependent elastic–plastic deformation of glassy polymers from low to high strain rates. Int. J. Solids Struct. 43, 1331–1356. ( 10.1016/j.ijsolstr.2005.04.016) [DOI] [Google Scholar]
- Parness A., Soto D., Esparza N., Gravish N., Wilkinson M., Autumn K., Cutkosky M. In press. A microfabricated wedge-shaped adhesive array displaying gecko-like dynamic adhesion, directionality and long lifetime. J. R. Soc. Interface. ( 10.1098/rsif.2009.0048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie A. M., Full R. J. 2007. Phylogenetic analysis of the scaling of wet and dry biological fibrillar adhesives. Proc. Natl Acad. Sci. USA 104, 18 595–18 600. ( 10.1073/pnas.0707591104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie A. M., Majidi C., Corder A., Full R. J. 2007. Ancestrally high elastic modulus of gecko setal β-keratin. J. R. Soc. Interface 4, 1071–1076. ( 10.1098/rsif.2007.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B. N. J. 1995. Theory of friction—stress domains, relaxation, and creep. Phys. Rev. B 51, 13 568–13 585. ( 10.1103/PhysRevB.51.13568) [DOI] [PubMed] [Google Scholar]
- Persson B. N. J. 2003. On the mechanism of adhesion in biological systems. J. Chem. Phys. 118, 7614–7621. ( 10.1063/1.1562192) [DOI] [Google Scholar]
- Pianka E. R., Sweet S. S. 2005. Integrative biology of sticky feet in geckos. BioEssays 27, 647–652. ( 10.1002/bies.20237) [DOI] [PubMed] [Google Scholar]
- Pocius A. V. 2002. Adhesion and adhesives technology: an introduction, 2nd edn. Munich, Germany: Hanser Verlag. [Google Scholar]
- Porto M., Zaloj V., Urbakh M., Klafter J. 2000. Macroscopic versus microscopic description of friction. Tribology Lett. 9, 45–54. ( 10.1023/A:1018800309732) [DOI] [Google Scholar]
- Qu L. T., Dai L. M., Stone M., Xia Z. H., Wang Z. L. 2008. Carbon nanotube arrays with strong shear binding-on and easy normal lifting-off. Science 322, 238–242. ( 10.1126/science.1159503) [DOI] [PubMed] [Google Scholar]
- Rabinowicz E. 1995. Friction and wear of materials. New York, NY: Wiley-Interscience. [Google Scholar]
- Reiner M. 1964. The Deborah number. Phys. Today 17, 62 ( 10.1063/1.3051374) [DOI] [Google Scholar]
- Rice J. R. 1980. The mechanics of earthquake rupture. In Physics of the earth's interior (eds Dziewonski A. M., Boschi E.), pp. 555–649. Amsterdam, The Netherlands: Italian Physical Society and North-Holland. [Google Scholar]
- Rice J. R., Ruina A. L. 1983. Stability of steady frictional slipping. J. Appl. Mech. Trans. ASME 50, 343–349. [Google Scholar]
- Richetti P., Drummond C., Israelachvili J., In M., Zana R. 2001. Inverted stick-slip friction. Europhys. Lett. 55, 653–659. ( 10.1209/epl/i2001-00109-0) [DOI] [Google Scholar]
- Riedo E., Gnecco E., Bennewitz R., Meyer E., Brune H. 2003. Interaction potential and hopping dynamics governing sliding friction. Phys. Rev. Lett. 91, 084 502 ( 10.1103/PhysRevLett.91.084502) [DOI] [PubMed] [Google Scholar]
- Ringlein J., Robbins M. O. 2004. Understanding and illustrating the atomic origins of friction. Am. J. Phys. 72, 884–891. ( 10.1119/1.1715107) [DOI] [Google Scholar]
- Rizzo N., Gardner K., Walls D., Keiper-Hrynko N., Ganzke T., Hallahan D. 2006. Characterization of the structure and composition of gecko adhesive setae. J. R. Soc. Interface 3, 441–451. ( 10.1098/rsif.2005.0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins M. O., Smith E. D. 1996. Connecting molecular-scale and macroscopic tribology. Langmuir 12, 4543–4547. ( 10.1021/la9505576) [DOI] [Google Scholar]
- Ruibal R., Ernst V. 1965. The structure of the digital setae of lizards. J. Morphol. 117, 271–294. ( 10.1002/jmor.1051170302) [DOI] [PubMed] [Google Scholar]
- Russell A. P. 1975. A contribution to the functional morphology of the foot of the tokay, Gekko gecko (Reptilia, Gekkonidae). J. Zool. Lond. 176, 437–476. [Google Scholar]
- Santos D. O. 2009. Contact modeling and directional adhesion for climbing robots. PhD thesis, Stanford University. [Google Scholar]
- Santos R., Gorb S., Jamar V., Flammang P. 2005. Adhesion of echinoderm tube feet to rough surfaces. J. Exp. Biol. 208, 2555–2567. ( 10.1242/jeb.01683) [DOI] [PubMed] [Google Scholar]
- Santos D., Spenko M., Parness A., Kim S., Cutkosky M. 2007. Directional adhesion for climbing: theoretical and practical considerations. J. Adh. Sci. Technol. 21, 1317–1341. ( 10.1163/156856107782328399) [DOI] [Google Scholar]
- Schallamach A. 1963. A theory of dynamic rubber friction. Wear 6, 375–382. ( 10.1016/0043-1648(63)90206-0) [DOI] [Google Scholar]
- Schubert B., Lee J., Majidi C., Fearing R. S. 2008. Sliding induced adhesion of stiff polymer microfiber arrays: 2. Microscale behaviour. J. R. Soc. Interface 5, 845–853. ( 10.1098/rsif.2007.1309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S., Ge L., Ci L., Ajayan P. M., Dhinojwala A. 2008. Gecko-inspired carbon nanotube-based self-cleaning adhesives. Nano Lett. 8, 822–825. ( 10.1021/nl0727765) [DOI] [PubMed] [Google Scholar]
- Sills S., Vorvolakos K., Chaudhury M. K., Overney R. M. 2007. Molecular origins of elastomeric friction. In Fundamentals of friction and wear at the nanoscale (eds Gnecco E., Meyer E.). New York, NY: Springer. [Google Scholar]
- Sitti M., Fearing R. S. 2003. Synthetic gecko foot-hair micro/nano structures as dry adhesives. J. Adh. Sci. Technol. 17, 1055–1073. ( 10.1163/156856103322113788) [DOI] [Google Scholar]
- Sun W., Neuzil P., Kustandi T. S., Oh S., Samper V. D. 2005. The nature of the gecko lizard adhesive force. Biophys. J. 89, L14–L17. ( 10.1529/biophysj.105.065268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambe N. S., Bhushan B. 2005. Friction model for the velocity dependence of nanoscale friction. Nanotechnology 16, 2309–2324. ( 10.1088/0957-4484/16/10/054) [DOI] [PubMed] [Google Scholar]
- Tao Z., Bhushan B. 2007. Velocity dependence and rest time effect on nanoscale friction of ultrathin films at high sliding velocities. J. Vac. Sci. Technol. A 25, 1267–1274. ( 10.1116/1.2435381) [DOI] [Google Scholar]
- Tian Y., Pesika N., Zeng H., Rosenberg K., Zhao B., McGuiggan P., Autumn K., Israelachvili J. 2006. Adhesion and friction in gecko toe attachment and detachment. Proc. Natl Acad. Sci. USA 103, 19 320–19 325. ( 10.1073/pnas.0608841103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajpayee S., Long R., Shen L. L., Jagota A., Hui C. Y. 2009. Effect of rate on adhesion and static friction of a film-terminated fibrillar interface. Langmuir 25, 2765–2771. ( 10.1021/la8033885) [DOI] [PubMed] [Google Scholar]
- Volterra E., Zachmanoglou E. 1965. Dynamics of vibrations. Columbus, OH: Charles E. Merrill Books. [Google Scholar]
- Vorvolakos K., Chaudhury M. K. 2003. The effects of molecular weight and temperature on the kinetic friction of silicone rubbers. Langmuir 19, 6778–6787. ( 10.1021/la027061q) [DOI] [Google Scholar]
- Ward I. M., Sweeney J. 2004. An introduction to the mechanical properties of solid polymers. Chichester, UK: John Wiley & Sons. [Google Scholar]
- Williams E. E., Peterson J. A. 1982. Convergent and alternative designs in the digital adhesive pads of scincid lizards. Science 215, 1509–1511. ( 10.1126/science.215.4539.1509) [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Gravish N., Autumn K., Creton C. 2009. Microscopic modelling of the dynamics of frictional adhesion in the gecko attachment system. J. Phys. Chem. 113, 3622–3628. ( 10.1021/jp8067415) [DOI] [PubMed] [Google Scholar]
- Zhao B. X., Pesika N., Rosenberg K., Tian Y., Zeng H. B., McGuiggan P., Autumn K., Israelachvili J. 2008. Adhesion and friction force coupling of gecko setal arrays: implications for structured adhesive surfaces. Langmuir 24, 1517–1524. ( 10.1021/la702126k) [DOI] [PubMed] [Google Scholar]