Abstract
Situations where normal autografts cannot be used to replace damaged skin often lead to a greater risk of mortality, prolonged hospital stay and increased expenditure for the National Health Service. There is a substantial need for tissue-engineered skin bioconstructs and research is active in this field. Significant progress has been made over the years in the development and clinical use of bioengineered components of the various skin layers. Off-the-shelf availability of such constructs, or production of sufficient quantities of biological materials to aid rapid wound closure, are often the only means to help patients with major skin loss. The aim of this review is to describe those materials already commercially available for clinical use as well as to give a short insight to those under development. It seeks to provide skin scientists/tissue engineers with the information required to not only develop in vitro models of skin, but to move closer to achieving the ultimate goal of an off-the-shelf, complete full-thickness skin replacement.
Keywords: bioengineered skin, skin substitute, dermal substitute, biomaterials, tissue engineering, wound healing
1. Wounds
The loss of skin can occur for many reasons, including genetic disorders (bullous conditions), acute trauma, chronic wounds or even surgical interventions. One of the most common reasons for major skin loss is thermal trauma, where substantial areas of skin can be damaged, often without the possibility of skin regeneration. Burns and scalds sometimes can result in rapid, extensive, deep wounds which cannot be successfully treated with common techniques, and can lead to death.
Wounds can be divided into epidermal, superficial partial-thickness, deep partial-thickness and full-thickness with increasing depth of the injury. Treatment approaches differ accordingly (Papini 2004).
Epidermal injuries, typical of sunburns, light scalds or grazing, are characterized by erythema and minor pain. Such injuries do not require specific surgical treatment as only the epidermis is affected and this regenerates rapidly without scarring, as no extracellular matrix (ECM) deposition occurs to contribute towards the scar tissue.
Superficial partial-thickness wounds affect the epidermis and superficial parts of the dermis, with epidermal blistering and severe pain accompanying this type of injury, especially in the case of thermal trauma. Such wounds heal by epithelialization from the margins of the wound, where basal keratinocytes change into a proliferating migratory cell type and cover the damaged area. Cells migrate either from the wound edge, hair follicle or from sweat gland remnants that lie in the deeper dermis, which has been preserved in this depth of injury (Miller et al. 1998; Papini 2004). Each hair follicle and sweat gland is lined with epithelial cells capable of contributing to epithelial regeneration across the wounded surface. In addition, the hair follicles of human skin contain a reserve of stem cells, located in the bulge region of the follicle, which are capable of self-renewal (Blanpain et al. 2004; Tumbar et al. 2004; Tumbar 2006).
Deep partial-thickness injuries involve greater dermal damage that results in fewer skin appendages remaining and therefore they take longer to heal. Scarring is more pronounced in this depth of injury as fibroplasia is more intensive when compared with superficial partial-thickness wounds.
Full-thickness injuries are characterized by the complete destruction of epithelial-regenerative elements. This type of injury heals by contraction, with epithelialization from only the edge of the wound, leading to cosmetic and functional defects. All full-thickness skin wounds which are more than 1 cm in diameter require skin grafting as they cannot epithelialize on their own and may lead to extensive scarring, resulting in limitations in joint mobility and severe cosmetic deformities (Papini 2004).
In the case of major burn injuries, the currently accepted treatment tactic requires an early excision of a dry scab (eschar) to remove heat-denatured proteins of the skin followed by wound closure (Burke et al. 1976; Papini 2004). This avoids triggering complications such as infection, multiple organ dysfunction syndrome or hypertrophic scar formation. Heat-denatured proteins of the eschar may also cause an uncontrolled inflammatory response and also serve as a good source of nutrients for pathogenic micro-organisms. This is of particular importance in heavily burnt patients as the nature of the injury leads to a temporary suppression of cell-mediated and humoral immunity (Stoilova et al. 2007).
Early permanent wound closure results in minimal or no scarring complications, whereas delayed treatment leads to severe hypertrophic scarring directly proportional to the wound closure delay time. One study of 337 scalded children showed that demarcation time for the wound closure was 21 days, and after this time a much higher incidence of hypertrophic scarring occurred (Cubison et al. 2006). Earlier permanent wound closure is also associated with lower mortality and better functional long-term results (Wolfe et al. 1983).
Wound size plays a major role in the outcome of the injury. Current advances in anaesthesia, ventilation and resuscitation as well as drug and nutrients support of burns patients, new dressings and topical wound-healing agents, as well as technical improvement of specialized burns units allow the successful treatment of extensive burns which would have been considered lethal just half a century ago. According to Bull & Fisher (1954) between 1942 and 1952, the mortality rate of the age group 15–44 years with 60 per cent burns of the total body surface area (TBSA) was 100 per cent. A study undertaken from 1998 to 2003 revealed a reduction in the mortality rate of the same age group with 60 per cent TBSA, to only 41.4 per cent (Chua et al. 2007). Current advances in burns treatment allow the successful treatment of patients with major extensive burns, although treatment of inhalation and deep extensive burns still remains a substantial challenge to the surgeon.
Currently, the clinical ‘gold standard’ in full-thickness injuries treatment is split-thickness autologous skin grafting (Stanton & Billmire 2002; Andreassi et al. 2005; Supp & Boyce 2005). Epidermis with a superficial part of the dermis is harvested with a dermatome from an undamaged skin donor site and applied to the full-thickness wound. Being applied to the wound, capillaries of the split skin graft (SSG) form anastamoses or ‘plug in’ into the existing capillary network to provide nutrients for graft survival; this is referred to as graft ‘take’ (Converse et al. 1975). The donor site heals similarly to the superficial partial-thickness wound by keratinocyte migration from hair follicles, sweat glands and edges of the wound. It heals within a week and can be used for further SSG re-harvesting. Generally, the thicker the SSG is, the less contraction there will be at the site of application but the longer it will take to heal the donor site (Andreassi et al. 2005).
2. The need for tissue-engineered skin substitutes
Patients with 50 per cent TBSA full-thickness wounds have only 50 per cent of undamaged skin left which could be used for split-thickness skin harvesting. Donor sites would add to the total wound size resulting in a wound area covering 100 per cent of the body. An impaired epidermal barrier combined with reduced immunity of heavily burned patients can result in bacterial sepsis which is the main complication in deep extensive burns (Stoilova et al. 2007). Donor sites also heal with some scarring and may be very painful; hence an additional analgesic pharmacological load is required. Moreover, depending on the thickness of the dermis, only three to four split-thickness skin harvests are possible from the same site and re-cropping is delayed by the time necessary for re-epithelialization (Atiyeh & Costagliola 2007).
In the case of a more extensive injury, donor sites are extremely limited and in such cases, meshing techniques can be used where grafted skin is uniformly perforated and stretched to cover greater areas of the wound. Although this method allows greater area coverage and reduces mortality rates, the cosmetic and functional outcomes of such a treatment are inferior when compared with the standard SSG application. This is because of the lack of dermis in the interstices of the stretched meshed skin graft, and slow epithelialization from graft margins across interstices, resulting in a greater graft contraction, delayed healing, scar tissue formation and pronounced ‘crocodile skin’ appearance of the scar. In near-total full-thickness skin injuries even meshing techniques are no help owing to the unavailability of donor sites. In such cases wounds are covered with temporary dressings or cadaver skin to form a mechanical barrier in order to prevent fluid loss and microbial contamination. Only delayed serial autologous split skin grafting can be used to heal injured skin in these cases (Papini 2004). Such wounds are left unhealed for a long time over the course of treatment while awaiting epithelial regeneration, and are prone to severe complications which can result in death.
Alternative life-saving approaches in the treatment of extensive full-thickness wounds, where donor sites for SSG harvesting are not available, include the use of cultured autologous keratinocytes and/or bioengineered skin substitutes. Significant progress has been made recently in the development and clinical use of these products (Horch et al. 2005; Clark et al. 2007; MacNeil 2007; Pham et al. 2007). ‘Off-the-shelf’ availability or the possibility of producing, in a relatively short period of time, sufficient quantities of epithelium capable of permanent wound closure sometimes make these approaches the treatments available in extensive deep injuries.
Because of the great importance and demand for skin-replacement products, there is a long history of material development, and many research groups worldwide have focused on creating biomaterials for skin substitution. Skin substitute biomaterials are commonly referred to by a variety of terms that can lead to confusion. They can be described as bioengineered skin equivalents, tissue-engineered skin, tissue-engineered skin constructs, biological skin substitutes, bioengineered skin substitutes, skin substitute bioconstructs, living skin replacements and, more recently, as bioengineered alternative tissue (Kim et al. 2006). Although these terms differ slightly from each other, and may not truly describe the product, they are considered to be equal and interchangeable by the majority of investigators. For the purpose of this review we shall use these definitions to describe any skin substitute product, produced or modified artificially in any way, including modifications of naturally occurring substances, such as dermis, for the purpose of damaged skin replacement, fully or partially, temporary or permanently, and possessing some similarities with human skin, both anatomical and functional.
All tissue-engineered skin substitute bioconstructs need to comply with three major requirements. They must be safe for the patient, be clinically effective and be convenient in handling and application. Properties of the ‘ideal’ skin substitute for in vivo use have been described elsewhere and recently reviewed by MacNeil (2007). In general, such biomaterials must not be toxic, immunogenic or cause excessive inflammation, and should also have no or low level of transmissible disease risk. The biomaterial for skin reconstruction should be biodegradable, repairable and able to support the reconstruction of normal tissue, with similar physical and mechanical properties to the skin it replaces. It should provide pain relief, prevent fluid and heat loss from the wound surface and protect the wound from infection. It is also of great advantage if the skin substitute bioconstruct is cost-effective, readily available, user-friendly and possesses a long shelf life.
No currently commercially available tissue-engineered skin replacement biomaterials possess all the above-mentioned properties nor can they fully replace the functional and anatomical properties of the native skin. There are, however, a number of bioengineered skin-replacement products which are currently available to clinicians and are used for wound-healing purposes. In general, these tissue replacements only partially address skin functional requirements and surgeons tend to use different products to achieve specific purposes. Shakespeare (2005) outlines four groups of functions which bioengineered skin-replacement products can offer: protection—by establishing a mechanical barrier to micro-organisms and vapour loss; procrastination—following early wound debridement some wound cover is needed until permanent wound closure can be achieved with serial skin grafts or cultured autologous cell applications, especially in extensive burns; promotion—delivery to the wound bed of dermal matrix components, cytokines and growth factors, which can promote and enhance natural host wound-healing responses; provision—of new structures, such as dermal collagen or cultured cells, which are incorporated into the wound and persist during wound healing and/or thereafter.
There are many different classifications of currently available skin-substitute products (Jones et al. 2002; Atiyeh et al. 2005; Horch et al. 2005; Atiyeh & Costagliola 2007; Clark et al. 2007; MacNeil 2007; Patel & Fisher 2008), and they can be summarized as follows.
- Anatomical structure:
- — dermo-epidermal (composite),
- — epidermal,
- — dermal.
- Duration of the cover:
- — permanent,
- — semi-permanent,
- — temporary.
- Type of the biomaterial:
- — biological: autologous, allogeneic, xenogeneic,
- — synthetic: biodegradable, non-biodegradable.
- Skin substitute composition regarding cellular component:
- — cellular,
- — acellular.
- Primary biomaterial loading with cellular component occurs:
- — in vitro,
- — in vivo.
Some of the currently marketed and clinically available tissue-engineered skin-substitute products are reviewed in this paper (tables 1–3) and organized according to anatomical structure classification, which is the most commonly used, but many more are still in the process of investigation (table 4) and are not discussed in detail due either to the unavailability of product information or lack of experimental or clinical results on the materials' performance.
Table 1.
Currently commercially available or marketed dermo-epidermal skin constructs. PEO, polyethylene oxide terephthalate; PBT, polybutylene terephthalate; HAM, hyaluronic acid membrane (microperforated); auto, autologous; allo, allogeneic; xeno, xenogeneic; recomb, recombinant; synth, synthetic.
| brand name/manufacturer | schematic representation | incorporated human cells | primary cellular loading occurs | cell source | scaffold source | scaffold material | duration of the cover |
|---|---|---|---|---|---|---|---|
| allograft (cadaveric) from not for profit skin banks | 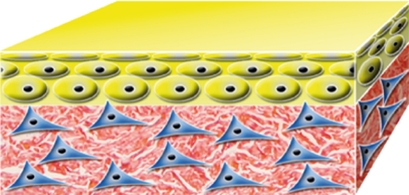 |
native | native | allo | allo | native human skin with dermal and epidermal cells | temporary |
| Karoskin Karocell Tissue Engineering AB, Karolinska University Hospital, Stockholm, Sweden | 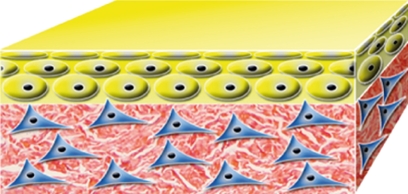 |
native | native | allo | allo | native human cadaver skin with dermal and epidermal cells | temporary |
| Apligraf Organogenesis Inc., Canton, Massachusetts, CA, USA | 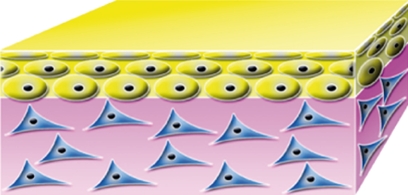 |
cultured keratinocytes and fibroblasts | in vitro | allo | xeno | bovine collagen | temporary |
| OrCel Ortec International, Inc., New York, NY, USA | 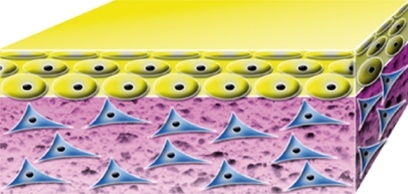 |
cultured keratinocytes and fibroblasts | in vitro | allo | xeno | bovine collagen sponge | temporary |
| PolyActive HC Implants BV, Leiden, The Netherlands | 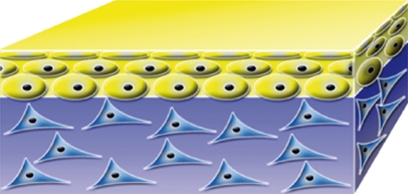 |
cultured keratinocytes and fibroblasts | in vitro | auto | synth | PEO/PBT | temporary |
| TissueTech Autograft System (Laserskin and Hyalograft 3D) Fidia Advanced Biopolymers, Abano Terme, Italy | 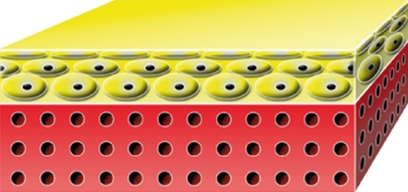 |
cultured keratinocytes and fibroblasts | in vitro | auto | recomb | HAM | permanent |
 |
Table 2.
Currently commercially available or marketed epidermal constructs. HAM, hyaluronic acid membrane (microperforated); auto, autologous; allo, allogeneic; xeno, xenogeneic; recomb, recombinant; synth, synthetic.
| brand name/manufacturer | schematic representation | incorporated human cells | primary cellular loading occurs | cell source | scaffold source | scaffold material | duration of the cover |
|---|---|---|---|---|---|---|---|
| Epicel Genzyme Biosurgery, Cambridge, MA, USA |  |
cultured keratinocytes (confluent cell sheet) | in vitro | auto | — | — | permanent |
| EpiDex Modex Therapeutiques, Lausanne, Switzerland |  |
cultured keratinocytes from outer root sheath of scalp hair follicles (confluent cell sheet) | in vitro | auto | — | — | permanent |
| EPIBASE Laboratoires Genevrier, Sophia-Antipolis, Nice, France |  |
cultured keratinocytes (confluent cell sheet) | in vitro | auto | — | — | permanent |
| MySkin CellTran Ltd, Sheffield, UK |  |
cultured keratinocytes (subconfluent cell sheet) | in vitro | auto | synth | silicone support layer with a specially formulated surface coating | permanent |
| Laserskin or Vivoderm Fidia Advanced Biopolymers, Padua, Italy |  |
cultured keratinocytes (confluent cell sheet) | in vitro | auto | recomb | HAM | permanent |
| Bioseed-S BioTissue Technologies GmbH, Freiburg, Germany |  |
cultured keratinocytes (subconfluent cell suspension) | in vitro | auto | allo | fibrin sealant | permanent |
| CellSpray Clinical Cell Culture (C3), Perth, Australia |  |
non-/cultured keratinocytes (subconfluent cell suspension) | in vitro | auto | — | — | permanent |
Table 3.
Currently commercially available or marketed dermal constructs. PGA, polyglycolic acid (Dexon); PLA, polylactic acid (Vicryl); ECM, extracellular matrix, derived from fibroblasts; HAM, hyaluronic acid membrane (microperforated); HYAFF, a derivative of hyaluronan; GAG, glycosaminoglycan; auto, autologous; allo, allogeneic; xeno, xenogeneic; recomb, recombinant, synth, synthetic.
| brand name/manufacturer | schematic representation | incorporated human cells | primary cellular loading occurs | cell source | scaffold source | scaffold material | duration of the cover |
|---|---|---|---|---|---|---|---|
| AlloDerm LifeCell Corporation, Branchburg, NJ, USA |  |
— | in vivo | — | allo | human acellular lyophilized dermis | permanent |
| Karoderm Karocell Tissue Engineering AB, Karolinska University Hospital, Stockholm, Sweden |  |
— | in vivo | — | allo | human acellular dermis | permanent |
| SureDerm HANS BIOMED Corporation, Seoul, Korea |  |
— | in vivo | — | allo | human acellular lyophilized dermis | permanent |
| GraftJacket Wright Medical Technology, Inc., Arlington, TN, USA |  |
— | in vivo | — | allo | human acellular pre-meshed dermis | permanent |
| Matriderm Dr Suwelack Skin and HealthCare AG, Billerbeck, Germany |  |
— | in vivo | — | xeno | bovine non-cross-linked lyophilized dermis, coated with α-elastin hydrolysate | permanent |
| Permacol Surgical Implant Tissue Science Laboratories plc, Aldershot, UK |  |
— | in vivo | — | xeno | porcine acellular diisocyanite cross-linked dermis | permanent |
| OASIS Wound Matrix Cook Biotech Inc., West Lafayette, IN, USA |  |
— | in vivo | — | xeno | porcine acellular lyophilized small intestine submucosa | permanent |
| EZ Derm Brennen Medical, Inc., MN, USA |  |
— | in vivo | — | xeno | porcine aldehyde cross-linked reconstituted dermal collagen | temporary |
| Integra Dermal Regeneration Template Integra NeuroSciences, Plainsboro, NJ, USA |  |
— | in vivo | — | xeno+synth | polysiloxane, bovine cross-linked tendon collagen, GAG | semi-permanent |
| Terudermis Olympus Terumo Biomaterial Corp., Tokyo, Japan |  |
— | in vivo | — | xeno+synth | silicone, bovine lyophilized cross-linked collagen sponge made of heat-denatured collagen | semi-permanent |
| Pelnac Standard/Pelnac Fortified Gunze Ltd, Medical Materials Center, Kyoto, Japan |  |
— | in vivo | — | xeno+synth | silicone/silicone fortified with silicone gauze TREX, atelocollagen derived from pig tendon | semi-permanent |
| Biobrane/Biobrane-L UDL Laboratories, Inc., Rockford, IL, USA |  |
— | in vivo | — | xeno+synth | silicon film, nylon fabric, porcine collagen | temporary |
| TransCyte (DermagraftTC) Advanced BioHealing, Inc., New York, NY and La Jolla, CA, USA |  |
cultured neonatal fibroblasts | in vitro | allo | xeno+synth | silicon film, nylon mesh, porcine dermal collagen | temporary |
| Dermagraft Advanced BioHealing, Inc., New York, NY and La Jolla, CA, USA |  |
cultured neonatal fibroblasts | in vitro | allo | allo+synth | PGA/PLA, ECM | temporary |
| Hyalomatrix PA Fidia Advanced Biopolymers, Abano Terme, Italy |  |
— | in vivo | — | allo+synth | HYAFF layered on silicone membrane | semi-permanent |
| Hyalograft 3D Fidia Advanced Biopolymers, Abano Terme, Italy |  |
cultured fibroblasts | in vitro | auto | allo | HAM | permanent |
Table 4.
Biomaterials intended for skin substitution which are currently under investigation. PLGA, poly(lactic-co-glycolic acid); FNfds, fibronectin functional domains; auto, autologous; allo, allogeneic; xeno, xenogeneic; recomb, recombinant, synth, synthetic.
| name | manufacturer or investigating group | incorporated human cells | primary cellular loading occurs | cell source | scaffold source | scaffold material | duration of the cover |
|---|---|---|---|---|---|---|---|
| dermo-epidermal constructs | |||||||
| PermaDerm or Cincinnati Shriners Skin Substitute | Cincinnati Shriner's Hospital, Cincinnati, OH, USA | cultured keratinocytes and fibroblasts | in vitro | auto | allo | bovine collagen | permanent |
| AcuDress | DFB Pharmaceuticals, Inc., Fort Worth, TX, USA | cultured keratinocytes | in vitro | auto | allo | fibrin substrate | permanent |
| Allox | DFB Pharmaceuticals, Inc., Fort Worth, TX, USA | sprayed suspension of allogeneic keratinocytes and fibroblasts in fibrin | in vitro | allo | allo | fibrin substrate | temporary |
| epidermal constructs | |||||||
| Karocells | Karocell Tissue Engineering AB, Karolinska University Hospital, Stockholm, Sweden | cultured keratinocytes and fibroblasts | in vitro | auto | — | — | permanent |
| Autoderm | XCELLentis, Gent, Belgium. Merged with Celltran, Sheffield, UK | cultured keratinocytes | in vitro | auto | — | — | permanent |
| TransDerm | XCELLentis, Gent, Belgium. Merged with Celltran, Sheffield, UK | cultured keratinocytes | in vitro | auto | — | — | temporary |
| Lyphoderm | XCELLentis, Gent, Belgium. Merged with Celltran, Sheffield, UK | lyophilized neonatal keratinocytes | in vitro | allo | — | — | temporary |
| Cryoceal | XCELLentis, Gent, Belgium. Merged with Celltran, Sheffield, UK | cryopreserved keratinocytes | in vitro | allo | — | — | temporary |
| dermal constructs | |||||||
| Cyzact (ICX-PRO) chronic wound repair | Intercytex, St John's Innovation Center, Cambridge, UK | cultured dermal fibroblasts | in vitro | allo | allo | fibrin gel | temporary |
| ICX-SKN skin graft replacement | Intercytex, St John's Innovation Center, Cambridge, UK | cultured dermal fibroblasts | in vitro | allo | allo | natural human collagen matrix | temporary |
| polycaprolactone collagen nanofibrous membrane | Nanoscience and Nanotechnology Initiative, Division of Bioengineering, National University of Singapore, Singapore | cultured dermal fibroblasts | in vitro | allo | synth | polycaprolactone-blended collagen electrospun nanofibrous membrane | temporary |
| Tegaderm-nanofibre construct | Nanoscience and Nanotechnology Initiative, Division of Bioengineering, National University of Singapore, Singapore | cultured dermal fibroblasts | in vitro | allo | xeno + synth | poly(ϵ-caprolactone)/gelatin nanofibrous scaffold electrospun on polyurethane dressing | temporary |
| collagen–glycosaminoglycan–chitosan dermal matrix seeded with fibroblasts | INSERM, U553 and Université Paris 7, IUH, Paris, France | cultured dermal fibroblasts | in vitro | allo | xeno | bovine collagen I/ chondroitin-4/6-sulfate/chitosan lyophilized dermal matrix | temporary |
| porcine collagen paste | Blond McIndoe Research Foundation, East Grinstead, UK | — | in vivo | — | xeno | cryomilled porcine acellular diisocyanite cross-linked dermis | permanent |
| human hair keratin-collagen sponge | Department of Histology and Embryology, Southern Medical University, Guangzhou, China | — | in vivo | — | allo | cryomilled porcine acellular diisocyanite cross-linked dermis | permanent |
| hyaluronan-FNfds hydrogel matrix | Department of Biomedical Engineering, SUNY at Stony Brook, New York, USA | — | in vivo | — | allo | hyaluronan coupled with fibronectin functional domains | permanent |
| hybrid nanofibrous PLGA/chitosan membrane | School of Materials Science and Engineering, Tianjin University, Tianjin, China | — | in vivo | — | synth | PLGA/chitosan hybrid electrospun nanofibrous membrane | permanent |
| biodegradable polyurethane microfibres | Department of Materials Science and Engineering, University of Delaware, Newark, DE, USA | — | in vivo | — | synth | biodegradable polyurethane microfibres | permanent |
| silk fibroin and alginate | Department of Veterinary Physiology, College of Veterinary Medicine and School of Agricultural Biotechnology, Seoul National University, Seoul, South Korea | — | in vivo | — | xeno + synth | silk fibroin/alginate-blended sponge | permanent |
| polyvinyl alcohol/chitosan/fibroin-blended sponge | Department of Sericulture and Entomology, National Institute of Agriculture and Technology, Suwon, Korea | — | in vivo | — | xeno + synth | polyvinyl alcohol/chitosan/fibroin-blended sponge | permanent |
| composite nano-titanium oxide–chitosan artificial skin (NTCAS) | Department of Nursing, Cardinal Tien College of Healthcare and Management, Taipei County, Taiwan | — | in vivo | — | allo + recomb | composite nano-titanium oxide–chitosan with gelatin and hyaluronic acid | permanent |
| bacterial cellulose | Vascular Engineering Center, Institution of Surgical Disciplines, Sahlgrenska University Hospital, Göteborg, Sweden | — | in vivo | — | recomb | cellulose nanofibrils synthesized by Acetobacter xylinum | permanent |
| bovine collagen cross-linked with microbial transglutaminase | National Center for Biomedical Engineering Science, National University of Ireland, Galway, Ireland | — | in vivo | — | xeno | freeze-dried bovine collagen scaffold cross-linked with microbial transglutaminase | permanent |
| Collatamp | SYNTACOLL AG, Herisau, Switzerland | — | in vivo | — | xeno | multilayer bovine collagen matrix | permanent |
3. Dermo-epidermal (composite) skin substitutes
Dermo-epidermal or composite skin substitutes aim to mimic the histological structure of normal skin where both epidermal and dermal layers are present. This similarity also provides some functional resemblance to the normal skin. These are not only the most advanced and sophisticated products, when compared with epidermal and dermal substitutes, but also the most expensive tissue-engineered biological constructs for tissue repair (Jones et al. 2002).
Most of these products are based on allogeneic skin cells, incorporated into a dermal scaffold. This approach allows the production of large quantities of uniform batches of the product, with a relative ‘off-the-shelf’ availability. However, these biomaterials act rather like temporary biologically active wound dressings (Supp & Boyce 2005), providing growth factors, cytokines and ECM for host cells while initiating and regulating wound healing. There are reports of host immunogenic tolerance to allogeneic fibroblasts (Coulomb et al. 1998) and their survival in the host up to three weeks (Morimoto et al. 2005). Long-term preservation of allogeneic fibroblasts and their proliferation up to two months in the host without signs of immune rejection have also been reported (Sher et al. 1983; Bell et al. 1984; Eaglstein et al. 1999; Hebda & Dohar 1999; Sandulache et al. 2003; Griffiths et al. 2004). However, porcine studies could not confirm allogeneic fibroblast survival beyond a 7-day time point (Price et al. 2004), nor could some clinical studies when allogeneic fibroblasts were transplanted onto burn wounds (Kolokol'chikova et al. 2001).
Allogeneic keratinocytes provide effective pain relief and accelerate wound healing, but they do not survive longer than a few weeks when applied to the wound because they are rejected by the host (Strande et al. 1997; Clark et al. 2007). It is possible that the expression of the human leucocyte antigen (HLA) is different in fibroblasts and keratinocytes, hence allogeneic fibroblasts are less prone to tissue rejection initiated by the antigen complex. The inability to induce T-cell proliferation by fibroblasts through the cytokine production when HLA class II molecules are involved may indirectly support this observation (Ohyama et al. 2002). In vivo models to investigate acute graft-versus-host disease to study the immunologic tolerance to allogeneic fibroblasts in the host have been suggested (Takakura et al. 1999).
Therefore, in order to produce permanent dermo-epidermal skin substitutes, it appears that either allogeneic or autologous fibroblasts can be used but only autologous keratinocytes can be used to achieve permanent closure of the skin defect.
The current commercially available or marketed dermo-epidermal (composite) skin substitutes are listed in table 1.
3.1. The allograft, Karoskin
Human viable split-thickness cadaveric allograft is used as a temporary measure to cover the wound until it is possible to close it with a permanent skin graft. Cadaveric allograft can be used either fresh or frozen. It incorporates into the deep wound providing pain relief and temporary durable cover during the first few weeks post-injury when the immune response in a patient with extensive burns is pathologically suppressed. When the allograft becomes vascularized, the highly immunogenic epithelial cells trigger the immune response of the host and they are rejected, usually after three to four weeks post-grafting. If the allograft is glycerolized or lyophilized, the cellular component is destroyed and the immunological reaction is diminished; the dermal part of the graft becomes partly incorporated into the wound and serves as a dermal bed for further autologous skin graft applications.
Allografts have been used for decades (Quinby et al. 1981) and remain the standard for comparison of other temporary skin substitutes (Sheridan & Tompkins 1999). Although allografts can be obtained from not-for-profit European skin banks, they can also be purchased as a commercial product, e.g. Karoskin (Karocell Tissue Engineering AB, Karolinska University Hospital, Stockholm, Sweden). However, the use of an allograft is associated with some complexities such as availability of skin banks, denial of application on religious grounds, and its safety for the patient. Rigorous screening for viral diseases and standardized sterilization techniques reduce the risk of infection, but some risk of infective agent transmission still remains.
3.2. Apligraf
Apligraf consists of viable allogeneic neonatal fibroblasts, grown in a bovine type I collagen gel matrix, combined with viable allogeneic neonatal keratinocytes, forming a confluent superficial layer of the construct, thus mimicking the normal structure of human skin. Although this product does not cause immunological rejection, allogeneic cells of the construct do not survive after one to two months in vivo (Eaglstein et al. 1999; Griffiths et al. 2004). Hence, Apligraf, which was marketed initially as an organotypic skin substitute, can only be considered as a temporary bioactive dressing. It is known to deliver ECM components to the wound bed, as well as cytokines and growth factors, such as interferons α and β, PDGF, interleukins 1, 6 and 8 (Eaglstein & Falanga 1998; Ehrenreich & Ruszczak 2006). Nevertheless, reports of Apligraf use in burns treatment are available (Waymack et al. 2000; Hayes, Jr et al. 2001), and some authors consider it to be an alternative to traditional skin grafting in partial-thickness burns. The product cannot be used, however, to deliver a definitive wound closure in full-thickness injuries because of the temporary nature of the grafted allogeneic cells, and therefore it needs co-grafting with an autologous epithelial source. In the study undertaken by Waymack and colleagues (Waymack et al. 2000), Apligraf was combined with autologous meshed SSG, and better cosmetic and functional outcomes were reported when compared with the conventional meshed SSG treatment. The material is licensed only for the treatment of venous leg and diabetic foot ulcers and no results of large clinical trials in burns treatment have yet been reported. Drawbacks include a product shelf life of 5 days, it requires delicate handling and possesses the risk of disease transfer from its allogeneic constituents. Despite these complications it is reported to be the most clinically successful product in its category giving a 25 per cent improvement in ulcer treatment when compared with conventional treatments (Clark et al. 2007). Taking into account its high cost of $28 per cm2, very short shelf life, safety considerations and the temporary nature of cover it is unclear whether this product will find widespread use in burns practice and skin reconstruction in large wounds.
A similar experimentally bioengineered product, based on sterilized human dermis and seeded with autologous keratinocytes and fibroblasts as reported by Hernon et al. (2007), may provide a definitive skin replacement, as autologous cells are not rejected by the host. The limiting factor of slow cellular propagation in this product seemed to be resolved using a low-calcium culture medium that enhances the initial migration and proliferation of keratinocytes (Hernon et al. 2007). However, clinical studies now need to be undertaken.
3.3. OrCell
This tissue-engineered skin construct includes cultured allogeneic fibroblasts and keratinocytes obtained from the same neonatal foreskin. Fibroblasts are seeded into a bovine type I collagen sponge, which has a non-porous collagen-gel coating, on top of which keratinocytes are added to form a confluent layer. The product was licensed in 2001 to treat donor sites in burns and recessive dystrophic epidermolysis bullosa. This bilayered product is reported to produce an array of cytokines and growth factors such as fibroblast growth factor-1, keratinocyte growth factor-1, platelet-derived growth factor, vascular endothelial growth factor and transforming growth factor-α, which are all favourable for host cell migration and wound healing, thus ‘conditioning’ the wound bed for further treatment with skin grafts. This artificial skin substitute product showed reduced scarring, and a shorter healing time was also reported when compared with the acellular bioactive wound dressing Biobrane (Still et al. 2003). Being composed of allogeneic cells, the product performs a temporary role, resorbs in 7–14 days (similar to Apligraf) and no cellular DNA from the product can be found in the wound 14–21 days post-application.
3.4. PolyActive
This bilaminar product is based on autologous cultured keratinocytes and fibroblasts seeded into a PolyActive matrix. This porous matrix consists of a soft polyethylene oxide terephthalate component and a hard polybutylene terephthalate component, which prevents contraction of this polymer (IsoTis NV, Bilthoven, The Netherlands; Xiao et al. 1999; El Ghalbzouri et al. 2004). This polymer is commonly used for bone reconstruction and its use for skin repair is poorly elucidated in the literature. The product uses autologous cells and therefore does not pose the same potential risks as those associated with allogeneic material such as cross-contamination by infective agents or immune rejection, suggesting potential benefits over allogeneic-based bioconstructs. However, the use of autologous cells may limit the product's ‘off-the-shelf’ availability and increase its costs when compared with competitive allogeneic-based products (e.g. Apligraf, OrCell). Perhaps the PolyActive tissue-engineered skin construct may find use as a biologically active dressing in the treatment of partial-thickness wounds and also skin graft donor sites providing growth factors necessary to enhance wound healing. The fact that this product features a non-biodegradable synthetic dermal component precludes its use as a permanent skin substitute.
3.5. TissueTech Autograft System
This system combines two tissue-engineered biomaterials designed by Fidia Advanced Biopolymers (Abano Terme, Italy) and applied consecutively to the wound: dermal replacement construct Hyalograft 3D and epidermal substitute Laserskin (Uccioli 2003). These are based on autologous keratinocytes and fibroblasts, grown on microperforated hyaluronic acid membranes, and described later in this article. According to available publications (Uccioli 2003), this system allowed successful treatment of diabetic foot ulcers as established in randomized clinical trials, where the 70.3 per cent rate of wound closure was achieved in neuroischaemic, ischaemic, neuropathic and post-surgical ulcers, many of which were full-thickness and with the area greater than 5 cm2 in 85 per cent of cases. Recurrence rates were also low (not exceeding 8.2%) when the TissueTech Autograft System was applied. Although this system may allow for definitive wound closure, it is not a ‘true’ bilayered skin substitute where both dermal and epidermal layers are present, as it requires grafting of two products, and may be complicated to use in a clinical setting.
The preceding literature therefore suggests that no commercially available true bilayered ‘skin substitute’ for permanent deep wound closure exists yet.
There are many reports describing combinations of cultured human keratinocytes and fibroblasts with allogeneic or xenogeneic decellularized dermis, but these composites are mainly used for in vitro studies on cell–cell interactions rather than for clinical use (Harrison et al. 2006). There have been attempts to combine commercially available dermal substitutes with either cultured or non-cultured autologous cells in pre-clinical studies (Compton et al. 1998; Boyce et al. 1999; Jones et al. 2003; Wood et al. 2007) with promising results, but no follow-up clinical trial results are available yet. There is only one three-dimensional reconstructed skin substitute which has achieved clinical use and has been found to be very promising—the so-called Cincinnati Shriners Skin Substitute or PermaDerm—which was designed by Boyce and colleagues (Supp & Boyce 2005; Boyce et al. 2006). It is based on the collagen sponge, seeded with autologous fibroblasts and keratinocytes. It therefore delivers permanent wound closure and can be viewed as a ‘true skin substitute’. Although this skin substitute product has won award from the American Burns Association for its clinical performances, the product is not yet commercially available.
Currently available composite skin substitutes use only two cell types—keratinocytes and fibroblasts—therefore they cannot perform all the functions of the skin owing to the lack of innervation, and lack of immune cells, sweat glands and hair follicles. There are sparce reports regarding improvements of these types of skin substitutes where additional cell types, such as endothelial cells, are incorporated for improved functionality of the constructs (Ponec et al. 2004; Tonello et al. 2005), Langerhans cells (Regnier et al. 1998; Dezutter-Dambuyant et al. 2006). Melanocytes, although normally present in fresh keratinocyte cultures and dermo-epidermal constructs (Rehder et al. 2004) were specifically investigated in the work of Hedley and co-workers (Hedley et al. 2002) where the regulatory role for fibroblasts in skin pigmentation was revealed since, when added to in vitro skin constructs, fibroblasts downregulated spontaneous pigmentation. Melanocyte-containing skin constructs are used extensively for in vitro studies of ultraviolet light effects on the skin such as phototoxicity and photoageing (Marrot et al. 1998; Lee et al. 2007).
It was reported recently that murine skin stem cells, found in hair follicle bulges and isolated for in vitro culture, retain their stem cell characteristics and are capable of producing multiple cell types including keratinocytes, hair follicles and functionally active sebaceous glands, when returned to an in vivo situation (Blanpain et al. 2004). There is a significant pool of knowledge generated on human skin stem cells to date (Jahoda 2003; Blanpain et al. 2007; Waters et al. 2007) and this may allow in vivo work to proceed to the same endpoint. If so, this will give a considerable potential to produce histologically similar and fully functional true skin equivalents for the treatment of extensively burnt patients and other acute and chronic skin defects.
4. Epidermal substitutes
As it became possible to cultivate human keratinocytes serially in vitro (Rheinwald & Green 1975) and to rapidly expand the number of patient keratinocytes ex vivo, this technology was rapidly transferred into clinical applications (Gallico III et al. 1984) where it contributed to improve patients' survival rates (Carsin et al. 2000). However, the value of cultured keratinocytes remains disputed and controversial to the present day.
A key step in the designing and production of epidermal substitutes is the isolation of keratinocytes from a donor and the subsequent in vitro culture of these cells, to obtain the necessary number of keratinocytes for therapeutic needs. Differences in approach to the production of epidermal substitutes are dependant on: cell culture techniques (submerged or air–liquid interface models); the stage of cell differentiation and epithelial organization (confluent sheets, subconfluent cell layers and suspensions); the methods of cell delivery to the patient (confluent sheets mounted onto support layer, subconfluent dispersed keratinocytes delivered via aerosol techniques or via microcarrier beads); as well as the use of additional substrates to enhance cell culture and delivery (synthetic and biological; Chester et al. 2004; Atiyeh & Costagliola 2007; MacNeil 2007).
To initiate a culture of autologous cells, a skin biopsy of 2–5 cm2 is usually taken along with initial wound debridement upon the patient's arrival at the clinic. The epidermis is separated from the dermis and single keratinocytes are released from the sheet by exposure to enzymes. These keratinocytes are plated into tissue culture vessels where single cells start to divide to form colonies in the presence of mitotically inactivated mouse fibroblasts and culture medium containing foetal calf serum, and necessary supplements. It is possible to expand keratinocytes in xenogeneic-free conditions where murine fibroblasts and bovine serum are avoided (Notara et al. 2007) but the proliferative lifespan of cells cultured under these conditions is noticeably reduced (Ronfard et al. 2000; Papini et al. 2003). Single colonies of keratinocytes merge together and form stratified epithelial layers which can be enzymatically detached from the culture flasks, mounted onto backing supports (such as paraffin gauze) to maintain basal–apical orientation and the sheet then applied to the wound (Atiyeh & Costagliola 2007).
The quality of such stratified cultured epithelial autografts (CEAs) depends on the clonal cellular composition (Barrandon & Green 1987; Rochat et al. 1994; Papini et al. 2003), which putatively determines graft survival and long-term performance when applied in vivo. Basal keratinocytes, cells which give rise to holoclones (in vitro colonies with the highest proliferative potential), are essential for successful long-term graft survival. Meroclones, consisting of transient amplifying cells, have a variable potential for proliferation and can provide only temporary wound closure if applied in vivo. Committed keratinocytes, or paraclones, form the majority of a normal epithelial cell population but are able to replicate only a few times before differentiation and senescence. Therefore CEA, consisting exclusively of paraclones, cannot serve as a substrate for permanent wound closure. Current culturing techniques allow for holoclone preservation when keratinocytes are cultured in vitro over long periods of time (Papini et al. 2003).
In vitro keratinocyte expansion techniques produce CEA sheets large enough to cover the entire surface of the body in three to four weeks from only a 3 cm2 skin biopsy (Chester et al. 2004). If additional support substances, such as a fibrin matrix, are used to culture keratinocytes, it is possible to further expand the area of CEAs in shorter periods of time. Ronfard and colleagues (Ronfard et al. 2000) obtained 4.1 m2 of graftable epithelium from a 4.5 cm2 skin biopsy cultured for 15 days on a fibrin matrix, compared with 1.4 m2 when cultured on plastic surfaces. Material handling and basement membrane formation were also improved when this technique was employed.
Clinical ‘take’ or integration of such cultured epithelial autografts in a sheet form varies significantly from ‘excellent’ to ‘poor’ (Pandya et al. 1998; Horch et al. 2005; Wood et al. 2006a,b; Atiyeh & Costagliola 2007). This can be partly attributed to the fact that CEAs contain terminally differentiated keratinocytes in which integrin expression, responsible for attachment to the underlaying matrix, is altered (Chester et al. 2004). Among other disadvantages of CEA sheets are: the long culture time; friability of the grafts; and complicated handling and application procedures. There is also a need for precise coordination between the tissue culture facility and the clinic. A major disadvantage of sheet application is the unpredictable clinical outcomes with varied take rates of 15–85% (Williamson et al. 1995; Atiyeh & Costagliola 2007). Poor keratinocyte attachment, resulting in blistering when exposed to minor shearing forces, could be seen months post-grafting in patients treated with confluent CEAs (Gallico III et al. 1984).
It could be postulated that the very nature of the confluent, layered cell culture system is responsible for the unpredictable clinical outcome. As the cell layers build up in the culture vessel, the proliferating basal cells start to be isolated from the nutrients in the cell culture medium. The differentiating, keratinizing cells in the upper layers of the culture are tightly packed together via desmosomal junctions forming a barrier between nutrients and the basal cells. The more cell layers, the easier it is to handle the cell sheet, but the greater the chances of starving the basal cells and therefore the greater the chances of a poor ‘take’ or survival on the wound bed. These shortcomings led to the investigation of the use of subconfluent keratinocytes, which have a greater in vivo proliferative activity, can be harvested much earlier (after 5–7 days in culture) and have a degree of flexibility in the coordination of cell propagation, harvesting and clinical application processes (MacNeil 2007).
Subconfluent keratinocytes can be applied to the wound bed via an aerosol of cell suspension (Navarro et al. 2000). They can be delivered resuspended either in cell culture medium or fibrin glue (Grant 1999; Grant et al. 2002). The fibrin glue improved cell attachment to the wound bed and helped to control bleeding but did not affect keratinocyte take rate or the resulting epithelial cover area (Currie et al. 2003). Subconfluent keratinocyte suspensions contributed to an earlier basement membrane formation with a mature dermal–epidermal junction region when compared with CEA sheets (Andree et al. 2001). As the developed basement membrane is crucial for the strong bonding between the epithelial layer and the underlying tissue this may also explain the poor take levels and long-term results when using CEA sheets (Woodley & Chen 2001).
Another approach to deliver subconfluent keratinocytes to the wound bed is to culture a monolayer of subconfluent keratinocytes on delivery membranes which can be either mechanically peeled off the culture vessel (Ronfard et al. 2000) or can be applied with the cultured cells directly to the wounded site (Hernon et al. 2006). Both techniques obviate the need for enzymatic detachment of cells as enzymatic treatment can alter the structure of anchoring fibrils responsible for the graft attachment to the underlying tissue (Compton et al. 1989; Hernon et al. 2006). Delivery membranes can be made of synthetic materials such as a silicone support membrane with a specially formulated surface coating (MySkin); polyurethane; or based on biological materials such as collagen, fibrin glue, hyaluronic acid or decellularized dermis (Chester et al. 2004). The use of these delivery systems allows for an earlier clinical cell application by reducing the culture, preparation and application times, with the added benefits of convenient material handling as well as the biological properties of some of the delivery membranes which may affect and improve wound healing.
Some commercially produced epidermal substitutes, which have been approved and marketed for clinical use, are listed in table 2.
4.1. Epicel, EPIBASE, EpiDex
These products are manufactured using a patient's own keratinocytes which are grown to confluency within 15 days to form CEA sheets. Epicel and EPIBASE consist of cells derived from a small skin biopsy (Carsin et al. 2000; Vacher 2003), whereas EpiDex is cultured from keratinocytes obtained from the outer root sheath of scalp hair follicles (Tausche et al. 2003).
This is the oldest approach in keratinocyte delivery and shares the previously described disadvantages of long culture time; difficulties in handling and application; variable take rate; poor long-term results; necessity for dermal support; high cost; and a short (24 h) shelf-life (Horch et al. 2005). Despite these difficulties, as well as a declining interest and rising doubt in the usefulness of CEA products, they still remain a valuable life-saving treatment in cases of extensively burned patients (Atiyeh & Costagliola 2007).
4.2. MySkin
This product uses subconfluent autologous living keratinocytes which are grown on a silicone support layer with a specially formulated surface coating (Moustafa et al. 2004). Such an approach allows not only easier handling and application of keratinocyte grafts, but a decreased time for cell culture. Another advantage is that proliferatively active keratinocytes could be delivered to the patient with greater time flexibility (Hernon et al. 2006) that cannot be achieved with confluent cultured epithelial sheet grafts. This product is indicated for the treatment of neuropathic, pressure and diabetic foot ulcers, superficial burns and skin graft donor sites with reported positive clinical outcomes (Zhu et al. 2005; Moustafa et al. 2007). It can also be applied to full-thickness wounds in combination with meshed skin grafts but cannot be used alone for deep-wound treatment and in this way is similar to other epithelial bioengineered constructs.
4.3. Laserskin (Vivoderm)
Laserskin was designed and manufactured by Fidia Advanced Biopolymers (Italy) with rights to manufacture and distribute this product granted to ConvaTec, a division of Bristol-Myers Squibb Company under the Vivoderm trade name. The product consists of autologous keratinocytes cultured on a hyaluronic acid membrane which is laser-microperforated. This allows the keratinocyte migration from a support material down to the wound bed (Ramos-e-Silva & Ribeiro de Castro 2002). Hyaluronic acid is a naturally synthesized polymer of the human skin ECM which is reported to promote fibroblast and keratinocyte migration and proliferation. It is also reported to participate in scarless foetal wound healing (Price et al. 2007). Preliminary studies with Laserskin have shown the promising potential of the product giving good graft take rate, biocompatibility as well as low infection rates in a pre-clinical animal model (Lam et al. 1999; Myers et al. 2007) and small clinical trials (Price et al. 2006).
4.4. Bioseed-S
This product consists of 3–6 × 106 ml−1 cultivated subconfluent autologous keratinocytes resuspended in a fibrin sealant (Tissucol Duo S Immuno, Baxter). To date it has mainly been used to treat therapy-resistant chronic venous leg ulcers (Johnsen et al. 2005) and multinational randomized controlled clinical trials suggest almost 50 per cent increase in wound-healing efficiency when compared with standard treatment (Vanscheidt et al. 2007). No information regarding the use of this material in burns patients is available, although there is potential for its use in this area.
An animal study with analogous material, where autologous keratinocytes were resuspended in autologous fibrin sealant, applied to full-thickness wounds, revealed the usefulness of such application methods resulting in a good epithelialization (Grant et al. 2002). The fibrin did not improve the take rate of keratinocytes when compared with sprayed keratinocytes without fibrin glue, although improved handling, cell attachment, haemostasis and wound healing were noted.
4.5. CellSpray
CellSpray products, provided by Clinical Cell Culture company (C3, Perth, Australia), use either cultured or non-cultured autologous keratinocytes. This technology is based on the possibility of harvesting subconfluent keratinocytes in their most active proliferating state followed by their application to the wound bed by spraying. This allows further in vivo proliferation to confluency (wound closure), and cell differentiation to form a recognizable epithelial structure (Navarro et al. 2000; Chester et al. 2004). Such an approach results in a reduced cell culture time with earlier wound coverage by viable activated proliferating keratinocytes (Atiyeh & Costagliola 2007). Although this method allows a more convenient way of delivering keratinocytes to the wound bed at earlier stages post-wounding, such an application is limited to partial-thickness and graft donor site wounds. Full-thickness wounds still require a dermal element to achieve functional permanent skin restoration (Wood et al. 2006a,b).
The listed epidermal substitute products provide permanent wound closure. They are effective in treating chronic ulcers and improving the quality of life of these patients, although their efficiency and long-term outcomes for burns treatment are still questioned by many. It is also widely appreciated that combination with some sort of dermal substitute is needed in order to achieve effective full-thickness wound healing.
5. Dermal substitutes
Wound bed preparation and the resultant recipient surface are very important for an effective graft take. It is reported that cultured epithelial autografts would take in only 15 per cent of cases when grafted onto chronic granulation tissue, in 28–47% of cases if grafted onto early granulation tissue or a freshly debrided wound, but would have a 45–75% chance of integration when applied to the wound with a dermal or neodermal bed (Orgill et al. 1998). Other clinical trials also outline the importance of dermal pregraftment for the successful take of cultured autologous keratinocytes (Travia et al. 2003). In vivo studies have also shown the importance of a dermal bed for successful full-thickness wound epithelialization by sprayed keratinocytes (Wood et al. 2007).
The majority of products for dermal substitutions are acellular, based either on allogeneic, xenogeneic or synthetic materials (Anthony et al. 2006). It is much easier to manufacture these and to obtain a licence for clinical application when compared with cell-containing bilayered skin substitute constructs. The ability to produce large batches associated with rigorous quality control and reduced costs has resulted in the array of products which have found their way to the clinic, where some of them have been widely adopted. Currently commercially available or marketed dermal bioengineered constructs are listed in table 3.
5.1. AlloDerm, Karoderm, SureDerm, GraftJacket
AlloDerm, Karoderm, SureDerm and GraftJacket all represent human accellular dermal matrix products.
AlloDerm is a freeze-dried human accellular dermal matrix, with preserved basement membrane, acting similarly to cadaver allograft, readily incorporates into the wound without rejection and does not cause immunogenic response owing to the lack of a cellular component. Initially intended as a dermal replacement material, it showed uncertain rates of vascularization (Shakespeare 2005) and has therefore recently gained more popularity for abdominal wall hernia reconstruction (Espinosa-de-los-Monteros et al. 2007; Patton et al. 2007; Lipman et al. 2008), subcutaneous mastectomy (Ashikari et al. 2008), rhinoplasty and temporomandibular joint reconstruction (Khariwala et al. 2007), periodontal surgery (Zigdon & Horwitz 2006), and rectovaginal, rectourethral or tracheoesophageal fistulae reconstruction (Shelton & Welton 2006; Lesser et al. 2008; Su et al. 2008), where immediate revascularization is of less importance. It is still used for acute thermal injury treatment with very promising results. It is being used in one-stage operating procedures in combination with extra-thin split-thickness skin grafts (Tsai et al. 1999; Callcut et al. 2006), but longer follow-up and more clinical trials are needed. It is reported to produce acceptable functional and cosmetic results but, being a human-derived biomaterial, it is associated with potential safety and ethical issues and avoided on moral grounds by some clinicians and patients.
GraftJacket is a similar product, 0.4–0.8 mm thick, pre-meshed for the convenience of application, often used for tendon (Valentin et al. 2006; Furukawa et al. 2007) and low extremity wounds repair (Brigido 2006). Successful treatment of superficial and deep wounds has been reported (Kim et al. 2006) but information regarding thermal injuries treatment is limited because of the novelty of this biomaterial.
SureDerm is produced by HansBiomed (Korea) and uses human allogeneic acellular lyophilized dermis (Kim et al. 2003). It is indicated for the replacement or repair of damaged soft tissue including hypertrophic scar revision and burns wounds. The material can be stored up to 2 years, requires 10 min rehydration before application, is permanently incorporated into the wound bed and provides a dermal bed for subsequent skin grafting.
5.2. Permacol Surgical Implant, Matriderm
These decellularized dermal products are similar to AlloDerm but of animal origin. This reduces risks associated with transferable human viral diseases, such as HIV and HepB. The wide availability of raw materials makes these easier and cheaper to produce.
Permacol Surgical Implant is a decellularized porcine dermal layer containing collagen and elastin fibres. Material is cross-linked with diisocyanate by a patented technology. It is used mainly for abdominal wall hernia reconstruction (Parker et al. 2006), especially when microbial contamination is present (Catena et al. 2007). Its use for dermal reconstruction is limited owing to the slow biointegration and vascularization (MacLeod et al. 2004, 2005).
Matriderm is of bovine origin, consists of 1 mm-thick structurally intact native collagen matrix coated with α-elastin hydrolysate from the ligament, freeze-dried and non-cross-linked. In small clinical trials to treat full-thickness burns it has shown promising results when applied simultaneously with split-thickness skin grafts in a single-stage operative procedure (van Zuijlen et al. 2000; Haslik et al. 2007; Ryssel et al. 2008).
5.3. OASIS Wound Matrix
OASIS Wound Matrix is produced from porcine small-intestine submucosa and intended for wound closure stimulation in acute, chronic and burns wounds. It is freeze-dried and decellularized to prevent immunological responses. Positive results have been obtained in randomized prospective controlled multicentre trials for chronic leg ulcer treatment where faster healing time and less ulcer recurrence was achieved (Mostow et al. 2005). OASIS Wound Matrix has also been shown to support in vitro epidermal differentiation and basement membrane formation (Lindberg & Badylak 2001). It was also evaluated in vivo as a wound dressing in rodent full-thickness wounds where it contributed towards contraction minimization and had no effect on epithelialization (Prevel et al. 1995). No results of clinical trials regarding its use in full-thickness wound management have been published yet.
5.4. EZ Derm
EZ Derm is a reconstituted collagen of porcine origin which is cross-linked with aldehyde to increase its tensile strength. The product does not incorporate into the wound and has to be removed (Bello et al. 2001). It is therefore marketed as a bioactive wound dressing. Comparison of this dressing with petrolatum non-adherent gauze dressing for partial-thickness burns care revealed no differences in bacterial colonization rate, healing time, pain relief and frequency of dressing change procedures (Healy & Boorman 1989).
5.5. Integra Dermal Regeneration Template, Terudermis, Pelnac Standard Type/Pelnac Fortified With Mesh Type
Integra Dermal Regeneration Template, initially designed by Yannas & Burke (1980), consists of a porous dermal component made of bovine type I collagen and shark chondroitin-6-sulphate glycosaminoglycan which is bonded to a silicone pseudo-epidermis (Yannas & Burke 1980). The dermal component of the bioconstruct becomes populated with host cells, including fibroblasts, which contribute towards neodermis formation while the material's scaffold degrades and the pseudo-epidermal component protects wounds from vapour loss and bacterial contamination. When Integra vascularization and neodermis formation are complete, usually within 15–20 days, the silicone layer is peeled off and the wound can be closed permanently with an SSG. This material was successfully clinically tested in managing burn wounds in 1981 (Burke et al. 1981) and since then has become widely adopted for full-thickness burns treatment (Heimbach et al. 1988, 2003; Heitland et al. 2004) becoming clinically a ‘gold standard’ dermal substitute biomaterial. It is also used for chronic ulcer treatment (Silverstein 2006) and full-thickness non-thermal skin wound management (Violas et al. 2005). Advantages of the product include its long shelf life, simple handling, low risks of immunogenic response and disease transmission, good cosmetic outcomes with reduced rates of contraction and scarring (Anthony et al. 2006; Kim et al. 2006). Meticulous surgical preparation of the wound bed is required to guarantee a good take of Integra. It cannot be used on infected wounds, it requires a relatively long time of 10–14 days for vascularization and also requires a second operative procedure to achieve permanent wound closure with an SSG. In attempts to achieve a single-stage surgical procedure, the product has been seeded with disaggregated cultured (Jones et al. 2003) or non-cultured (Wood et al. 2007) autologous keratinocytes, using in vivo experimental models. Results were promising, but further clinical follow-up is required.
Terudermis consists of a layer of lyophilized collagen sponge reconstituted from a mixture of fibrous and heat-denatured bovine collagen which is cross-linked by dehydrothermal treatment. The collagen layer is bonded to the silicone membrane which controls bacterial contamination and vapour loss during engraftment, similar to Integra. The material is designed for deep burns treatment, where bone, muscle or ligament exposure is present (Choi et al. 1999). It is also reported to be useful for skin flap donor site regeneration (Lee et al. 2005), post-traumatic deformity corrections (Yurugi et al. 2002) and in otological surgery (Bessho et al. 1998). Terudermis, when loaded with cultured fibroblasts, endothelial cells, platelet-derived growth factor and then applied to rodent in vivo models, showed not only angiogenesis enhancement but also the potential to use the material simultaneously with an SSG for a one-step operative procedure (Soejima et al. 2006).
Pelnac Standard Type/Fortified With Mesh Type are produced by Gunze Ltd, Medical Materials Center, Kyoto, Japan, and consist of superficial silicone film layer and porcine collagen sponge layer (made of atelocollagen derived from pig tendon, about 3 mm thick with pores 60–110 µm in diameter). Pelnac Fortified With Mesh Type has additional non-adhesive silicone gauze (TREX), which is inserted into silicone film to provide additional reinforcement of tensile strength of the material. Pelnac is refreeze-dried after cross-linking, stored in dry condition and can be stored at room temperature for 3 years after production. This product is indicated for third grade burn injuries, traumatic skin defects, skin defects after excision of tumours or nervus, and donor sites of skin flaps. Pelnac was reported to deliver good to excellent long-term results (the mean 6 years 10 months) in 90 per cent of cases when used in combination with extra-thin split-thickness skin grafts to treat full-thickness skin defects (tumour, naevus, scar or skin ulcer removal), deep burns and to eliminate hypertrophic skin-graft donor-site scarring (Suzuki et al. 2000). It also was found to be easy, safe and useful in lower extremity reconstruction after necrotic skin lesions and necrotizing fasciitis owing to Streptococcus, methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa (Akita et al. 2006). In another study of 55 patients (Nam et al. 2006), Pelnac was found to be useful for reconstruction of acute burns, burn scar contractures and soft tissue defects. Rapid neodermis formation was noted with Pelnac before it was grafted with an SSG (14 ± 1.8 days), and it was superior to Integra (17.5 ± 1.4) and Terudermis (18.5 ± 2.2); however, two-stage operative procedure may potentially limit the widespread use of the product.
5.6. Biobrane, Biobrane-L, TransCyte
These temporary dressings consist of a pseudo-epidermal semipermeable silicon film bonded to nylon fabric (trifilament for Biobrane and monofilament for Biobrane-L for reduced adhesiveness to the wound) with incorporated porcine collagen. TransCyte (earlier name, Dermagraft-TC) additionally has viable cultured neonatal allogeneic fibroblasts incorporated into the scaffold. Materials are indicated for partial-thickness burn wounds and donor sites treatment or as a dressing over meshed autografts (Demling 1995; Barret et al. 2000; Pape & Byrne 2000). As reviewed by Whitaker and colleagues (Whitaker et al. 2008), Biobrane-L is particularly useful for the management of partial-thickness burns in children; however it can also be used as a temporary dressing for the management of toxic epidermal necrolysis, paraneoplastic pemphigus, after skin-graft harvesting and dermabrasion, and in chronic wounds. These bioengineered constructs provide matrix proteins, growth factors and cytokines necessary for wound healing improvement; they are effective for vapour loss control, pain relief and are reported to reduce healing time when compared with conventional dressings (Lal et al. 2000; Lukish et al. 2001). Being non-degradable and synthetic, the materials must be removed after 7–14 days and are intended for a temporary wound cover until permanent closure with either autologous skin graft or cultured epithelial cells are achieved.
5.7. Dermagraft
This cryopreserved material is composed of polyglactin mesh seeded with living cultured neonatal fibroblasts from foreskin tissue. The scaffold degrades by hydrolysis in 20–30 days while fibroblasts produce growth factors and ECM components (vitronectin, tenascin, collagens and glycosaminoglycans), helping to reconstitute a dermal layer (Kim et al. 2006). The material is licensed and mainly used for chronic diabetic foot ulcers (Marston et al. 2003) and venous ulcers (Omar et al. 2004) with positive effect over conventional treatment, facilitating fibrovascular ingrowths and re-epithelialization by keratinocyte migration from the wound margin. This material can also be used for burns treatment when combined with skin grafts; however, clinical trials did not reveal significant differences between the application of meshed skin graft and meshed skin graft combined with Dermagraft (Hansbrough et al. 1992), and its performances were found to be similar to allograft (Pham et al. 2007). The disadvantages include a necessity for multiple applications, higher cost and safety issues owing to allogeneic cells incorporated into this bioconstruct (Kim et al. 2006).
5.8. Hyalomatrix PA, Hyalograft 3D
Both products are based on hyaluronic acid derivates. Hyaluronic acid is one of the main polysaccharide components of dermal ECM and promotes migration and proliferation of skin fibroblasts and keratinocytes (Price et al. 2007). The most common sources of the hyaluronic acid are rooster combs extraction, and recombinant production using Streptococcus bacterium (Manna et al. 1999; Price et al. 2007). Esterification of hyaluronic acid with benzyl alcohol is used to obtain HYAFF—an ester of hyaluronic acid used in Hyalomatrix PA production. Hyalomatrix PA has a temporary silicone layer which acts like an epidermis, while the dermal component of the construct incorporates into the wound so preparing it for the subsequent skin grafting. Hyalograft 3D has no pseudo-epidermal layer but the product's effects are strengthened by the cultured autologous fibroblasts that provide the healing wound with growth factors and cytokines. It also lays down ECM components ‘conditioning’ the wound for split skin grafting. This material is reported to improve in vitro epithelial organization and dermal–epidermal junction maturation in organotypic skin bioconstructs (Stark et al. 2004, 2006). Clinically, Hyalograft 3D is primarily used for feet ulcer treatment in combination with Laserskin autologous epidermal bioconstructs (Caravaggi et al. 2003). Such combinations of hyaluronic acid-based products incorporating autologous fibroblasts and keratinocytes did not show any improvements in plantar diabetic feet ulcer treatment when compared with standard treatment (Caravaggi et al. 2003). Contrarily, successful treatment of severe scleroderma cutaneous ulcers employing this technique has been reported (Giuggioli et al. 2003). Similar material combinations for deep burns treatment have also revealed advantages of Hyalograft 3D grafting, which enhanced keratinocyte take, and reduced hypertrophy and wound contracture rates when compared with exclusive application of keratinocyte cultures (Travia et al. 2003). Hyalograft 3D also contributes towards rapid basement membrane formation (Stark et al. 2004). Hyalomatrix PA has been investigated with favourable outcomes in a porcine preclinical model of full-thickness wounds (Myers et al. 2007), and it has also been used clinically for the treatment of deep partial-thickness burns. The material served as a temporary dressing to stimulate wound regeneration after dermabrasion and was reported to be a good and feasible approach for such wound treatments (Gravante et al. 2007). It is also reported to give favourable outcomes in deep paediatric burns treatment (Tamisani 2004). Both products are also appealing from the safety point of view—they do not contain any animal or allogeneic human-derived components.
6. Potential biomaterials for skin substitution
Besides the above listed biomaterials, the majority of which are based on collagen as the most studied, traditional and convenient component of ECM known for its biocompatibility and bioconductivity and therefore used for bioengineering of skin substitutes (Cen et al. 2008), there is a variety of different skin and dermal substitute constructs that are currently under investigation. Some of them are still in the process of in vitro investigation; however, some have entered stage II–III clinical trials and could possibly be on the market shortly available for patients and health practitioners. Novel potential skin substitute biomaterials and scaffolds include human hair keratin-collagen sponge (Chen et al. 2006), hyaluronan coupled with fibronectin functional domains (Ghosh et al. 2006), poly(lactic-co-glycolic acid)/chitosan hybrid nanofibrous membrane (Duan et al. 2007), biodegradable polyurethane microfibres (Rockwood et al. 2007), polycaprolactone (PCL) collagen nanofibrous membrane (Venugopal et al. 2006), silk fibroin and alginate (Roh et al. 2006), polyvinyl alcohol/chitosan/fibroin blended sponge (Yeo et al. 2000), Tegaderm-nanofibre construct (Chong et al. 2007), bacterial cellulose (Helenius et al. 2006), ICX-SKN skin graft replacement (Boyd et al. 2007), porcine collagen paste (Shevchenko et al. 2008), bovine collagen cross-linked with microbial transglutaminase (Garcia et al. 2008), collagen–glycosaminoglycan–chitosan dermal matrix seeded with fibroblasts (Kellouche et al. 2007), composite nano-titanium oxide–chitosan artificial skin (Peng et al. 2008), keratinocytes and fibroblasts grown on Collatamp, deacetylated chitin or plant cellulose transfer membranes (Johnen et al. 2008) and many others (table 4). Some of these experimental biomaterials, like PermaDerm, have produced promising clinical results (Boyce et al. 1999, 2006) and have a potential to be licensed and marketed for clinical use. However, with such variety of available biomaterials for skin substitution a differentiated evaluative approach should be employed, especially by medical practitioners. Although some biomaterials like Suprathel are described as skin substitutes (Schwarze et al. 2008), the material's performances clearly state it can only be considered as a temporary bioabsorbable synthetic wound dressing.
7. Biomechanical characteristics of skin substitutes
In comparison with the literature addressing clinical behaviour, and support of cell growth in tissue engineering skin substitutes, surprisingly few reports consider the mechanical properties of such skin substitutes. In our view, a number of problems need to be addressed before an objective and coherent approach to both design and testing of the mechanical properties of skin substitutes can be considered.
How closely should skin substitutes' properties mimic those of natural skin at the time of clinical application? Other properties such as ease of handling by the surgeon, deformability to follow anatomical contours or resistance to tearing may be more important immediately upon application.
After a period of cell invasion and remodelling, mechanical properties closer to those of natural skin would probably be more appropriate. If so, those variables affecting changes in mechanical properties during cell invasion and remodelling need to be identified.
The most appropriate mechanical properties predicting skin substitute behaviour need to be identified. For instance, should we be measuring elasticity, stiffness, viscosity, viscoelasticity, failure, brittleness, etc.? Also under what kind of loading should these variables be measured: static tension, compression, creep, dynamic oscillatory testing, etc.?
More pragmatically, what mode of measurement of these properties should be employed? Does simple extension or compression to obtain a Young's modulus in a moving beam instrument tell us enough, or do we need to resort to rheological methods such as cone–plate or plate–plate oscillatory or creep testing?
If we are to compare the mechanical properties of skin substitutes with those of native skin, how should the latter be measured? Should we use values produced in vivo or ex vivo, should we measure full- or partial-thickness skin samples, and if an in vivo measurement is attempted, how do we know how much underlying tissue contributes to the values obtained?
Many reports in the literature present data obtained from in vivo tests on the human forearm. The question remains as to how representative these data are of skin at sites elsewhere in the body.
7.1. The basic engineering principles
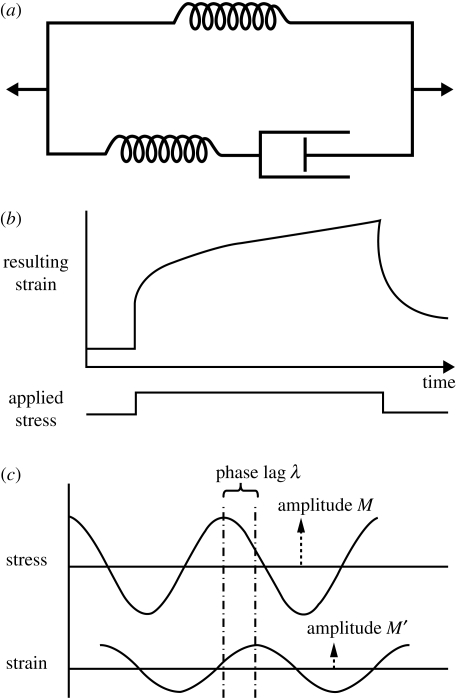
Both skin and skin substitutes such as cross-linked collagen gels display viscoelastic (VE) properties (Edwards & Marks 1995; Sheu et al. 2001). Viscoelastic behaviour implies that energy used in deforming a material is partly stored (elasticity) and partly dissipated (viscosity). In skin, the elastic components are chiefly proteins such as collagen and elastin, while the viscous components would be water, and highly hydrated macromolecules such as glycosaminoglycans (Edwards & Marks 1995). Such materials generally show time-dependent behaviour, and thus should be tested dynamically. Modelling of such material is traditionally done by combinations of springs and dashpots (dampers), with the springs modelling elastic components and the dashpots the viscous (time-dependent) components. So, for instance, a spring and a dashpot in series (Maxwell element) will model a VE material which shows permanent ‘set’ when extended, while a spring and a dashpot in parallel (Voight element) displays ‘creep’ behaviour. A combination of Maxwell and Voight elements (Standard Linear Solid Model (SLSM)) is usually considered to be a better description of the behaviour of skin and skin substitutes (figure 1a).
Figure 1.
The basic engineering principles used to characterize skin biomechanics. (a) The Linear Standard Solid Model; (b) a typical creep curve; (c) the basis of oscillatory testing, where G′ and G″ can be derived from M/M′, the amplitude ratio, and λ, the phase lag.
The literature has several reports of static analysis of both skin and skin substitutes, where a stress is applied to a sample, and the resulting strain measured, yielding the Young's modulus. However, the SLSM suggests that the result will vary, depending upon the rate at which the stress is applied. This is why such testing should be done dynamically, so that the time-dependent aspect can be captured. Two major approaches have been employed. Firstly, an instantaneous stress is applied to the sample, and the strain recorded over time. This can be extended by then instantaneously removing the stress and recording the relaxation over time also. This gives a so-called creep curve (e.g. figure 1b) or an equivalent depending upon the geometry of the testing system used.
Secondly, the material can be subjected to a sinusoidally oscillating stress of small amplitude, and the resulting strain measured both for amplitude and phase lag of the output strain when compared with the input stress. A perfectly elastic material would show zero phase lag, a perfectly viscous material would display 90° phase lag, and a VE material somewhere in between (figure 1c). The characteristic moduli derived from this kind of testing are the storage modulus (a measure of dynamic elasticity) G′ and the loss modulus (a measure of dynamic viscosity) G″. Both of these approaches have been used from time to time to study both skin and skin substitutes.
7.2. Biomechanical properties of human skin
While a full review of the recent advances in the measurement of the mechanical properties of skin is beyond the scope of this review, a summary of some relevant observations may prove useful.
This subject has been usefully reviewed well over a decade ago by Edwards & Marks (1995). These authors emphasize the distinction between measurements made in vivo, which may be confounded by contributions from deeper tissues, and those made in vitro, where the tests can be performed only a limited number of times on each sample. They also note that skin is anisotropic, particularly around joints, and thus measurements along one axis might be quite different from those made along an orthogonal axis. Indeed, Khatyr et al. (2004) have shown that not only is this anisotropy a dominant property, but that it varies from one person to another. As long ago as the 1970s, Thacker et al. (1977) observed the importance of skin biomechanics in plastic surgery, stating that directions of tensions in skin must be properly aligned to ensure that subsequent scarring is narrow and inconspicuous. Edwards & Marks (1995) also reinforce the principle outlined above, in that measurement of skin mechanical properties must take account of the time element, and if a simple stress–strain curve, or hysteresis loop, is attempted, it must be done very slowly so that time-dependent elements have time to relax during stretching, compression or shear (e.g. in figure 1a, the dashpot has time to completely open, so that properties of the springs only are measured, such as in an estimation of Young's modulus). Perhaps, even more importantly, a test of ultimate strength, i.e. the measurement of breaking strain, can also be time-dependent. Based largely on the work of Vogel (1987a,b) these authors quote values for tensile strength of skin (as measured by uniaxial loading in vitro) as having a mean of 21 N mm−2 in a child, declining to a mean of 17 N mm−2 in an elderly person. The ultimate modulus of elasticity has a mean of 70 N mm−2 in a child, declining to 60 N mm−2 in the elderly. Furthermore, the mean ultimate strain at rupture is quoted as 75 per cent at birth declining to 60 per cent in the elderly. However, all of these values display wide ranges over the population studied.
In vivo static testing includes uniaxial extensometry, where tabs are glued to the skin and pulled apart, the resulting stress or strain being measured and modifications to allow biaxial or torsional testing to be achieved. Testing of skin hardness has been attempted by measuring the deformation caused by a stylus pressed into the skin (so-called indentometry). However, for the reasons outlined above, a dynamic approach will yield more informative data, and a wide variety of methodologies exist in the literature. Such techniques include transforming the static tests into an oscillatory mode, but also by the measurement of shear wave propagation, and suction cup techniques, often with the use of ultrasound scanning to identify movements in underlying tissues. More recently, considerable progress has been made in the modelling of the viscoelastic properties of skin; for instance a model using only four parameters has been developed by Khatyr et al. (2004) which describes the behaviour of skin well, and sets of important material parameters which should be considered in modelling skin have been identified by Kvistedal & Nielsen (2009). Of particular interest when considering the interface of biomaterials with native skin are the findings of Holt et al. (2008) while measuring the viscoelasticity of skin under low shear and low frequency conditions. They found that the epidermis appeared to contribute rigidity and elasticity, while the dermis was far more viscoelastic in nature, suggesting that the skin is mechanically a two-phase system. This finding could pose serious problems when designing skin substitutes which interface with a wound bed and healing tissue.
7.3. Biomechanical properties of skin substitutes
Investigations into the biomechanical characteristics of skin substitutes have concerned themselves chiefly with either elucidating the properties of the fresh matrix, or attempting to follow changes in matrix properties as they become populated with cells, and start to degrade. Many of the matrices investigated have been of the collagen–glycosaminoglycan type, because these are commonly used clinically, but some others are presently under investigation.
An interesting approach has been adopted by Harley et al. (2007) in modelling such a matrix. They adopt a cellular solids description, treating the matrix as a foam, consisting of a network of interconnected struts. Under compression, this shows three phases of collapse: (i) a linear elastic region as the struts bend; (ii) a collapse bending region as the struts buckle; and (iii) a densification region as the pores have collapsed and the bulk properties of the material predominate. The experimental results, both in terms of the properties of individual struts tested by atomic force microscopy and the properties of the whole material in compression and tension, agree rather well with this model. This modelling has importance when the ingrowth of cells into the material is considered as fibroblasts, for instance, are known to collapse such struts, and the mechanical properties of the material at this scale are believed to affect cellular population of the matrix. Furthermore, this model suggested that the mechanical properties of the matrix are independent of pore size, but that post-production covalent cross-linking increased stiffness dramatically.
In terms of the effects of such cross-linking, Powell & Boyce (2006) have cross-linked collagen–glycosaminoglycan matrices with increasing amounts of the water-soluble carbodiimide 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). The stiffness of the matrix increases to a point where brittleness renders the matrix mechanically unsuitable for tissue replacement, but when tested for the growth of human fibroblasts and keratinocytes, cytotoxicity was observed at higher EDC concentrations, an optimum between increased mechanical stability and reduced cytotoxicity existing at 5 mM EDC. An alternative approach has been adopted by Garcia et al. (2008) by cross-linking the matrix to improve its mechanical stability using an enzymic approach with microbial transglutaminase. The introduction of the glutamyl-lysine cross-links into the collagen matrix modified its properties such that wound contraction in an in vivo model was significantly reduced. This approach to mechanical modification also avoids potential cytotoxicity contributed by chemical cross-linkers.
The mechanical behaviour of these collagen matrices, but measured on the nanoscale, has been investigated by Chaudhry et al. (2009). Using a nanoindentation approach, they were able to discriminate between surface and bulk properties, and reported values for the storage modulus of 0.71 GPa, and for the loss modulus of 0.40 GPa. Constantinides et al. (2008) report a value for the elastic modulus of porcine skin of 222 kPa also using a nanoindentation approach, but this was derived from a creep compliance approach. Nevertheless, this does suggest at least some discrepancy between collagen matrix and skin in terms of mechanical properties at the nanometre scale. This is important as cell attachment is likely to be influenced more at dimensions similar to those of focal adhesions than bulk dimensions, and cells are known to respond to the mechanical stiffness of their substrate (Discher et al. 2005). A direct comparison of ultimate tensile strength, stress–strain behaviour, stress relaxation and creep parameters between native excised human skin and four tissue engineering scaffolds was attempted by Zhang et al. (2007). Unfortunately, the scaffolds used are not well characterized in the report, but are two cross-linked dermal matrices from human and pig, and two non-cross-linked matrices from pig and goat. The parameters for the non-cross-linked matrices approximated those of the natural excised skin, while the gluteraldehyde-fixed matrices gave results significantly higher than the natural skin.
In an effort to improve the mechanical properties of skin substitutes at the bulk level, several workers have attempted to introduce synthetic polymers into natural polymer matrices such as collagen. Interestingly, Powell & Boyce (2009) have added increasing amounts of PCL to an electrospun collagen scaffold, and have shown that increasing PCL ratios increase the stiffness of the material, making it easier to handle for the surgeon. However, after growth of human keratinocytes and fibroblasts into the scaffolds, the mechanical properties were either not significantly different, or even worse than those of the collagen alone. This suggests that investigation of the mechanical properties of tissue-engineering matrices both before and during the invasion of cellular components is critical in the design of artificial skin.
The estimation of changes in mechanical properties of skin substitutes after population by cells has been the subject of a number of recent studies. For instance, Saddiq et al. (2009) have measured the ultimate tensile strength and stiffness of four collagen gel matrices before and after the growth of either 3T3 mouse fibroblasts or primary human fibroblasts into the matrix. The matrices used were collagen; collagen–glycosaminoglycan (GAG); collagen cross-linked with carbodiimide and putrescine, and collagen–GAG cross-linked with carbodiimide and putrescence. While the addition of GAGs and cross-linkers increased both the ultimate tensile strength and the stiffness initially, after 6 days of fibroblast growth (of both types) into the matrices, these values dropped significantly. Perhaps, even more surprisingly, the values to which these parameters fell appeared independent of the original gel treatment, with cross-linking offering no protection from matrix degradation.
A number of workers have adopted a different approach for obtaining a mechanically acceptable skin substitute matrix, which is to create a cell-derived matrix directly from cell culture, rather than developing a collagen, or similar, matrix separately. For instance, Ahlfors & Billiar (2007) have developed a rapid method for producing a fibroblast-derived matrix that promotes further organized growth of cells. Mechanical testing of the final product using an inflation method similar to that of Billiar et al. (2005), in terms of ultimate tensile strength showed it to be superior (313 kPa) to collagen and fibrin gels, but still not as strong as native skin (713 kPa).
Recently, a novel approach to measuring skin substitute mechanical properties in vivo has been reported by Kim et al. (2008) using ultrasound elasticity measurements. This report describes the use with a tissue phantom, but suggests this method as a useful approach to non-invasive measurement of the properties of skin substitutes as they become populated with cells during the wound-healing process.
8. Future perspectives
Currently available tissue-engineered products for skin substitution, including dermal and epidermal constructs, although not perfect, occupy a specific niche within a complex approach to treat full-thickness extensive burns, improving patients' survival rates and their quality of life after injury (MacNeil 2007). Such products target only limited specific roles in the wound-healing process. Predominantly, they serve as temporary biologically active dressings, donators of cytokines and structural molecules necessary for wound healing while the patient's own skin regenerates to be used for serial autografting. Products based on autologous cultured keratinocytes and fibroblasts are more likely to contribute to actual skin substitution and results of clinical trials are encouraging (Boyce et al. 2006); however, no one will agree that these products at the current level of sophistication can fully replace damaged skin (MacNeil 2007; Metcalfe & Ferguson 2007b).
There are many challenges faced by bioengineers supplying live cell products that should also be taken into account. There are long, complicated and expensive cultivation procedures, specific (and expensive) transport and storage conditions, a limited shelf-life, the friable nature of cell-containing biomaterials, especially for products based on live cells, a need for precise coordination between the tissue culture facility and the clinic if autologous cells are used just to name a few. All the above reasons as well as an unattractive cost-effectiveness of cell-based biomaterials which are only partially effective at fulfilling skin functions make it also very difficult for any cell-based skin substitute product to reach the clinic.
Currently available products for permanent skin substitution can only partially replace the protective barrier function of skin, but other functions, including touch and temperature sensation, excretion, perspiration, thermoregulation, protection from ultraviolet rays, synthetic function, not to mention the aesthetic function, are not restored by the existing skin tissue-engineered products (MacNeil 2007; Metcalfe & Ferguson 2007b). The simultaneous combination of different skin cell types including keratinocytes, melanocytes, fibroblasts and endothelial cells derived from postnatal skin is aiming to create a functional skin replacement (Regnier et al. 1998; Hedley et al. 2002; Ponec et al. 2004; Tonello et al. 2005; Dezutter-Dambuyant et al. 2006). Attempts are being made to restore skin appendages, such as hair follicles and sebaceous glands to maximally functionalize skin-replacement bioconstructs (Blanpain et al. 2004). Bone marrow-derived cells have also been looked at as a potential cell source for skin-substitute products (Fioretti et al. 2008; Yoshikawa et al. 2008). Another approach to even further functionalize artificial scaffolds for skin substitution is the addition of signalling molecules for the regulation of cell–cell and cell–matrix interactions either to accelerate biointegration (Tsuji-Saso et al. 2007; Wilcke et al. 2007; Garcia et al. 2008; Ma 2008) or to adjust it according to the phases of the wound-healing process. Such ‘intelligent’ biomimetic hybrid materials are termed ‘smart’ with the aim of producing a more natural skin restoration (Rosso et al. 2005; Metcalfe & Ferguson 2007b).
No matter how complicated a strategy is adopted to create a skin replacement product that is based on postnatal cellular material, it is unlikely to succeed. Such products use mechanisms of tissue repair rather than tissue regeneration (Metcalfe & Ferguson 2007a). Normal wound-healing processes, required to repair wounded sites, have evolved over thousands of years of human evolution to provide an efficient way to cope with skin injuries, most of which resulted from bites and mechanical trauma. These wounds were contaminated with saliva, blood, dirt and micro-organisms. There was no pharmacological means of decontamination, and therefore natural non-specific and specific immunities evolved. Fibrin clots with a provisional fibrin matrix helped to stop bleeding; an acute inflammatory response dealt with bacteria and any wound contamination with non-biological materials; and a rapid expansion of fibrovascular granulation tissue allowed filling-in and closing-up of any wound defects within a relatively short period of time in order to preserve the individual life and the continuation of the given species (Clark 1993; Li et al. 2007).
Skin wounds and the clinical strategies to treat them have significantly changed over the past century: sharp or laser aseptic surgical dissection of the damaged tissues; meticulous wound bed preparation (Panuncialman & Falanga 2007); pharmacological medications to avoid wound bacterial colonization (Khan & Naqvi 2006; White et al. 2006; Landis 2008); and biomaterials to replace damaged tissue (Jones et al. 2002; Horch et al. 2005; Ehrenreich & Ruszczak 2006; Clark et al. 2007) significantly obviated the need for the natural protective cascades involved in wound healing. Apart from function restoration, accepted by our ancestors as appropriate results of wound healing, issues of cosmesis, improved functionality and quality of life are now of great importance during skin-restoration treatment. The inflammatory response, the production of cytokines to promote fibrovascular tissue proliferation, is considered a normal process to cope with wounds to deliver their ‘repair’. However, in the light of current advances in medical science, these are now more likely to hinder optimal tissue ‘regeneration’ as they nearly always result in scarring (Martin & Leibovich 2005). Any bioengineered product based on ‘natural’ mechanisms of wound healing will result in scarring as well as limited functionality, rather than giving fully functional skin regeneration (Metcalfe & Ferguson 2007a).
True regeneration in vertebrates is seen in Xenopus and axolotl where the entire limb is restored (Goss & Holt 1992; Gardiner & Bryant 1996). Examples of tissue regeneration in humans consist of the regeneration of the digit tip in a child (Illingworth 1974); regeneration of the liver (Fausto 2000); and teeth regeneration (Chai & Slavkin 2003). Human skin does not regenerate postnatally. However, during the early antenatal period, when injured, all skin layers and appendages are regenerated. Foetal wound healing does not result in scar formation after injury (Lorenz & Adzick 1993; Yannas 2005). Understanding the mechanisms by which foetal wounds heal could result in a real breakthrough in adult wound healing with the possibility of real skin regeneration rather than defective and inferior scar-like skin repair (Ferguson & O'Kane 2004).
Scars are the outcomes of any postnatal healing of wounds caused by burns, trauma, surgical intervention, or indeed anywhere tissue damage occurs, including integration of tissue-engineered skin-substitute products. Uncontrolled scarring may result in defective aesthetics and the possible loss of function where excessive tissue production and contraction occurs. Prevention of scarring is therefore the second major problem to be addressed after the restoration of the damaged skin. Insights into foetal wound healing and understanding that scarless healing is partly attributed to a decreased inflammatory response (Martin & Leibovich 2005), reduced fibrin clot formation and platelet degranulation has already allowed production of therapeutic measures directed at scar-free wound healing (Ferguson & O'Kane 2004; Metcalfe & Ferguson 2007b). Growth factors from the TGF-β family have been closely studied in relation to scar-free healing. It was established that during foetal wound healing TGF-β1 and -β2 isoforms are low or absent, whereas the concentration of TGF-β3 isoform is significantly higher. During adult wound healing, the amount of TGF-β3 is insignificant, but amounts of TGF-β1 and -β2 are elevated by platelet degranulation and synthesis by cells of monocytic lineage during the inflammatory phase of wound healing (Ferguson & O'Kane 2004). Manipulation with adult wound healing by immunogenic blocking of TGF-β1 and -β2 isoforms and exogenous addition of the TGF-β3 isoform to the wound in rodent, pig and human studies resulted in significantly reduced scarring (Shah et al. 1995; O'Kane & Ferguson 1997; Ferguson & O'Kane 2004). Interestingly, neutralization of all three TGF-β isoforms did not result in reduced scarring confirming complexity of the molecular interplay and alternative pathways during wound healing. Not only particular signalling molecules, but their concentrations, time-dependent release cascades and pathways of their interaction with cells and ECM need to be fully exposed and understood to use this knowledge in creating clinically safe and efficient products to control scarring in postnatal organisms.
Another approach where both true skin regeneration and avoidance of scarring may be successfully achieved is the use of either embryonic or adult stem cells. While embryonic stem cell research is delayed by ethical and political debate, the number of investigations in the use of adult stem cells is constantly growing, including skin research. It is well established that the hair follicle outer root sheath contains pluripotent epithelial stem cells capable of self-renewal (Blanpain et al. 2004; Tumbar et al. 2004; Tumbar 2006). Although many in vitro and in vivo assays exist to label, isolate and analyse skin stem cells, it is still impossible to identify a stem cell within an intact human skin tissue without ambiguity (Tumbar 2006). Nevertheless, pluripotent epithelial stem cells are isolated from hair follicles and grown into epithelial sheets to be used clinically for epithelial restoration (Limat et al. 2003; Tausche et al. 2003). Although positive results regarding wound epithelialization were reported, no restoration of hair follicles or sweat glands was seen; such stem cells exhibited stable self-renewal potential, but their plasticity was limited (Lemoli et al. 2005). Varying microenvironmental stimuli may affect stem cell plasticity, and result in acquiring stem cell functions by more differentiated cells and limiting stem capacity by pushing stem cells into a more differentiated state (Lemoli et al. 2005). Hence, unsuitable biochemical and mechanical conditions in a wound may limit plasticity and proliferative activity of implanted stem cells. Multipotent adult stem cells isolated from rodent dermis have been shown to transdifferentiate into cells of several embryonic lineages such as adipocytes, smooth muscle cells, glial and neuron cells retaining their regenerative capacity (Toma et al. 2001). Studies have shown that these cells can be subcultivated as distinct diverse cell types for at least 1 year without the loss of regenerative capacity (Toma et al. 2001). Novel therapeutical approaches in wound healing could well be established if such precursors can be identified within a human dermis and isolated. Another study on murine skin stem cells of hair follicle bulges, isolated for in vitro culture, revealed their ability to retain stem cell capacity and plasticity by producing multiple cell types like keratinocytes, hair follicles and functionally active sebaceous glands, when engrafted in vivo (Blanpain et al. 2004, 2007). Successful replication of these experiments in humans would hold out promise to produce fully functional true skin equivalents.
9. Conclusions
It can be seen from this overview of tissue-engineered skin-substitute products that there is no ideal composite skin substitute for permanent wound closure currently commercially available and all the epidermal- and dermal-bioengineered products require either multiple-stage operating procedures or autologous skin grafting to achieve a definitive wound epithelialization.
Rapid progress in tissue engineering and different approaches to design a skin substitute biomaterial, including the use of stem cells, may give us hope that such a product will be developed in the near future. Currently, adult stem cell research is still in its infancy but provides potential candidates for tissue-engineering approaches to regenerate skin for the treatment of extensively burned patients and other acute and chronic skin defects. Great scientific, public and commercial interests may lead to significant progress in this field in the coming years.
References
- Ahlfors J. E., Billiar K. L. 2007. Biomechanical and biochemical characteristics of a human fibroblast-produced and remodeled matrix. Biomaterials 28, 2183–2191. ( 10.1016/j.biomaterials.2006.12.030) [DOI] [PubMed] [Google Scholar]
- Akita S., Tanaka K., Hirano A. 2006. Lower extremity reconstruction after necrotising fasciitis and necrotic skin lesions using a porcine-derived skin substitute. J. Plast. Reconstr. Aesthet. Surg. 59, 759–763. ( 10.1016/j.bjps.2005.11.021) [DOI] [PubMed] [Google Scholar]
- Andreassi A., Bilenchi R., Biagioli M., D'Aniello C. 2005. Classification and pathophysiology of skin grafts. Clin. Dermatol. 23, 332–337. ( 10.1016/j.clindermatol.2004.07.024) [DOI] [PubMed] [Google Scholar]
- Andree C., Reimer C., Page C. P., Slama J., Stark B. G., Eriksson E. 2001. Basement membrane formation during wound healing is dependent on epidermal transplants. Plast. Reconstr. Surg. 107, 97–104. ( 10.1097/00006534-200101000-00015) [DOI] [PubMed] [Google Scholar]
- Anthony E. T., Syed M., Myers S., Moir G., Navsaria H. 2006. The development of novel dermal matrices for cutaneous wound repair. Drug Discov. Today: Therapeut. Strat. 3, 81–86. ( 10.1016/j.ddstr.2006.03.001) [DOI] [Google Scholar]
- Ashikari R. H., Ashikari A. Y., Kelemen P. R., Salzberg C. A. 2008. Subcutaneous mastectomy and immediate reconstruction for prevention of breast cancer for high-risk patients. Breast Cancer 15, 185–191. ( 10.1007/s12282-008-0059-7) [DOI] [PubMed] [Google Scholar]
- Atiyeh B. S., Costagliola M. 2007. Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns 33, 405–413. ( 10.1016/j.burns.2006.11.002) [DOI] [PubMed] [Google Scholar]
- Atiyeh B. S., Hayek S. N., Gunn S. W. 2005. New technologies for burn wound closure and healing—review of the literature. Burns 31, 944–956. ( 10.1016/j.burns.2005.08.023) [DOI] [PubMed] [Google Scholar]
- Barrandon Y., Green H. 1987. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl Acad. Sci. USA 84, 2302–2306. ( 10.1073/pnas.84.8.2302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barret J. P., Dziewulski P., Ramzy P. I., Wolf S. E., Desai M. H., Herndon D. N. 2000. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast. Reconstr. Surg. 105, 62–65. [DOI] [PubMed] [Google Scholar]
- Bell E., Sher S., Hull B. 1984. The living skin-equivalent as a structural and immunological model in skin grafting. Scan. Electron Microsc., 1957–1962. [PubMed] [Google Scholar]
- Bello Y. M., Falabella A. F., Eaglstein W. H. 2001. Tissue-engineered skin. Current status in wound healing. Am. J. Clin. Dermatol. 2, 305–313. ( 10.2165/00128071-200102050-00005) [DOI] [PubMed] [Google Scholar]
- Bessho K., Murakami K., Iizuka T. 1998. The use of a new bilayer artificial dermis for vestibular extension. Br. J. Oral Maxillofac. Surg. 36, 457–459. ( 10.1016/S0266-4356(98)90463-6) [DOI] [PubMed] [Google Scholar]
- Billiar K. L., Throm A. M., Frey M. T. 2005. Biaxial failure properties of planar living tissue equivalents. J. Biomed. Mater. Res. A 73, 182–191. ( 10.1002/jbm.a.30282) [DOI] [PubMed] [Google Scholar]
- Blanpain C., Lowry W. E., Geoghegan A., Polak L., Fuchs E. 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648. ( 10.1016/j.cell.2004.08.012) [DOI] [PubMed] [Google Scholar]
- Blanpain C., Horsley V., Fuchs E. 2007. Epithelial stem cells: turning over new leaves. Cell 128, 445–458. ( 10.1016/j.cell.2007.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce S. T., Kagan R. J., Meyer N. A., Yakuboff K. P., Warden G. D. 1999. Cultured skin substitutes combined with Integra Artificial Skin to replace native skin autograft and allograft for the closure of excised full-thickness burns. The 1999 clinical research award. J. Burn Care Rehab. 20, 453–461. ( 10.1097/00004630-199920060-00006) [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Kagan R. J., Greenhalgh D. G., Warner P., Yakuboff K. P., Palmieri T., Warden G. D. 2006. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J. Trauma 60, 821–829. ( 10.1097/01.ta.0000196802.91829.cc) [DOI] [PubMed] [Google Scholar]
- Boyd M., Flasza M., Johnson P. A., Roberts J. S., Kemp P. 2007. Integration and persistence of an investigational human living skin equivalent (ICX-SKN) in human surgical wounds. Regen. Med. 2, 363–370. ( 10.2217/17460751.2.4.363) [DOI] [PubMed] [Google Scholar]
- Brigido S. A. 2006. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. Int. Wound J. 3, 181–187. ( 10.1111/j.1742-481X.2006.00209.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull J. P., Fisher A. J. 1954. A study of mortality in a burns unit: a revised estimate. Ann. Surg. 139, 269–274. ( 10.1097/00000658-195403000-00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F., Quinby W. C., Jr, Bondoc C. C. 1976. Primary excision and prompt grafting as routine therapy for the treatment of thermal burns in children. Surg. Clin. North Am. 56, 477–494. [DOI] [PubMed] [Google Scholar]
- Burke J. F., Yannas I. V., Quinby W. C., Jr, Bondoc C. C., Jung W. K. 1981. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 194, 413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callcut R. A., Schurr M. J., Sloan M., Faucher L. D. 2006. Clinical experience with Alloderm: a one-staged composite dermal/epidermal replacement utilizing processed cadaver dermis and thin autografts. Burns 32, 583–588. ( 10.1016/j.burns.2005.12.002) [DOI] [PubMed] [Google Scholar]
- Caravaggi C., et al. 2003. HYAFF 11-based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: a prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 26, 2853–2859. ( 10.2337/diacare.26.10.2853) [DOI] [PubMed] [Google Scholar]
- Carsin H., Ainaud P., Le Bever H., Rives J., Lakhel A., Stephanazzi J., Lambert F., Perrot J. 2000. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns 26, 379–387. ( 10.1016/S0305-4179(99)00143-6) [DOI] [PubMed] [Google Scholar]
- Catena F., Ansaloni L., Gazzotti F., Gagliardi S., Di Saverio S., D'Alessandro L., Pinna A. D. 2007. Use of porcine dermal collagen graft (Permacol) for hernia repair in contaminated fields. Hernia 11, 57–60. ( 10.1007/s10029-006-0171-6) [DOI] [PubMed] [Google Scholar]
- Cen L., Liu W., Cui L., Zhang W., Cao Y. 2008. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr. Res. 63, 492–496. ( 10.1203/PDR.0b013e31816c5bc3) [DOI] [PubMed] [Google Scholar]
- Chai Y., Slavkin H. C. 2003. Prospects for tooth regeneration in the 21st century: a perspective. Microsc. Res. Tech. 60, 469–479. ( 10.1002/jemt.10287) [DOI] [PubMed] [Google Scholar]
- Chaudhry B., Ashton H., Muhamed A., Yost M., Bull S., Frankel D. 2009. Nanoscale viscoelastic properties of an aligned collagen scaffold. J. Mater. Sci. Mater. Med. 20, 257–263. ( 10.1007/s10856-008-3574-3) [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Dong W. R., Xiao Y. Q., Zhao B. L., Hu G. D., An L. B. 2006. Preparation and bioactivity of human hair keratin-collagen sponge, a new type of dermal analogue. Nan Fang Yi Ke Da Xue Xue Bao 26, 131–138. [PubMed] [Google Scholar]
- Chester D. L., Balderson D. S., Papini R. P. 2004. A review of keratinocyte delivery to the wound bed. J. Burn Care Rehab. 25, 266–275. [DOI] [PubMed] [Google Scholar]
- Choi M. H., Yi S. B., Hwang J. W., Yang W. S., Lee K. K. 1999. Treatment of bone and tendon-exposed wounds using Terudermis. J. Kor. Soc. Plast. Reconstr. Surg. 26, 491–497. [Google Scholar]
- Chong E. J., Phan T. T., Lim I. J., Zhang Y. Z., Bay B. H., Ramakrishna S., Lim C. T. 2007. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 3, 321–330. ( 10.1016/j.actbio.2007.01.002) [DOI] [PubMed] [Google Scholar]
- Chua A., Song C., Chai A., Kong S., Tan K. C. 2007. Use of skin allograft and its donation rate in singapore: an 11-year retrospective review for burns treatment. Transplant. Proc. 39, 1314–1316. ( 10.1016/j.transproceed.2006.11.028) [DOI] [PubMed] [Google Scholar]
- Clark R. A. 1993. Biology of dermal wound repair. Dermatol. Clin. 11, 647–666. [PubMed] [Google Scholar]
- Clark R. A., Ghosh K., Tonnesen M. G. 2007. Tissue engineering for cutaneous wounds. J. Invest. Dermatol. 127, 1018–1029. ( 10.1038/sj.jid.5700715) [DOI] [PubMed] [Google Scholar]
- Compton C. C., Gill J. M., Bradford D. A., Regauer S., Gallico G. G., O'Connor N. E. 1989. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab. Invest. 60, 600–612. [PubMed] [Google Scholar]
- Compton C. C., Butler C. E., Yannas I. V., Warland G., Orgill D. P. 1998. Organized skin structure is regenerated in vivo from collagen-GAG matrices seeded with autologous keratinocytes. J. Invest. Dermatol. 110, 908–916. ( 10.1046/j.1523-1747.1998.00200.x) [DOI] [PubMed] [Google Scholar]
- Constantinides G., Kalcioglu Z. I., McFarland M., Smith J. F., Van Vliet K. J. 2008. Probing mechanical properties of fully hydrated gels and biological tissues. J. Biomech. 41, 3285–3289. ( 10.1016/j.jbiomech.2008.08.015) [DOI] [PubMed] [Google Scholar]
- Converse J. M., Smahel J., Ballantyne D. L., Jr, Harper A. D. 1975. Inosculation of vessels of skin graft and host bed: a fortuitous encounter. Br. J. Plast. Surg. 28, 274–282. ( 10.1016/0007-1226(75)90031-4) [DOI] [PubMed] [Google Scholar]
- Coulomb B., Friteau L., Baruch J., Guilbaud J., Chretien-Marquet B., Glicenstein J., Lebreton-Decoster C., Bell E., Dubertret L. 1998. Advantage of the presence of living dermal fibroblasts within in vitro reconstructed skin for grafting in humans. Plast. Reconstr. Surg. 101, 1891–1903. [DOI] [PubMed] [Google Scholar]
- Cubison T. C., Pape S. A., Parkhouse N. 2006. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns 32, 992–999. ( 10.1016/j.burns.2006.02.007) [DOI] [PubMed] [Google Scholar]
- Currie L. J., Martin R., Sharpe J. R., James S. E. 2003. A comparison of keratinocyte cell sprays with and without fibrin glue. Burns 29, 677–685. ( 10.1016/S0305-4179(03)00155-4) [DOI] [PubMed] [Google Scholar]
- Demling R. H. 1995. Use of Biobrane in management of scalds. J. Burn Care Rehab. 16, 329–330. ( 10.1097/00004630-199505000-00021) [DOI] [PubMed] [Google Scholar]
- Dezutter-Dambuyant C., et al. 2006. Evolutive skin reconstructions: from the dermal collagen-glycosaminoglycan-chitosane substrate to an immunocompetent reconstructed skin. Biomed. Mater. Eng 16, S85–S94. [PubMed] [Google Scholar]
- Discher D. E., Janmey P., Wang Y. L. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143. ( 10.1126/science.1116995) [DOI] [PubMed] [Google Scholar]
- Duan B., Wu L., Yuan X., Hu Z., Li X., Zhang Y., Yao K., Wang M. 2007. Hybrid nanofibrous membranes of PLGA/chitosan fabricated via an electrospinning array. J. Biomed. Mater. Res. A 83A, 868–878. ( 10.1002/jbm.a.31408) [DOI] [PubMed] [Google Scholar]
- Eaglstein W. H., Falanga V. 1998. Tissue engineering for skin: an update. J. Am. Acad. Dermatol. 39, 1007–1010. ( 10.1016/S0190-9622(98)70278-6) [DOI] [PubMed] [Google Scholar]
- Eaglstein W. H., et al. 1999. Acute excisional wounds treated with a tissue-engineered skin (Apligraf). Dermatol. Surg. 25, 195–201. ( 10.1046/j.1524-4725.1999.08186.x) [DOI] [PubMed] [Google Scholar]
- Edwards C., Marks R. 1995. Evaluation of biomechanical properties of human skin. Clin. Dermatol. 13, 375–380. ( 10.1016/0738-081X(95)00078-T) [DOI] [PubMed] [Google Scholar]
- Ehrenreich M., Ruszczak Z. 2006. Update on tissue-engineered biological dressings. Tissue Eng. 12, 2407–2424. ( 10.1089/ten.2006.12.2407) [DOI] [PubMed] [Google Scholar]
- El Ghalbzouri A., Lamme E. N., van Blitterswijk C., Koopman J., Ponec M. 2004. The use of PEGT/PBT as a dermal scaffold for skin tissue engineering. Biomaterials 25, 2987–2996. ( 10.1016/j.biomaterials.2003.09.098) [DOI] [PubMed] [Google Scholar]
- Espinosa-de-los-Monteros A., de la Torre J. I., Marrero I., Andrades P., Davis M. R., Vasconez L. O. 2007. Utilization of human cadaveric acellular dermis for abdominal hernia reconstruction. Ann. Plast. Surg. 58, 264–267. ( 10.1097/01.sap.0000254410.91132.a8) [DOI] [PubMed] [Google Scholar]
- Fausto N. 2000. Liver regeneration. J. Hepatol. 32, 19–31. ( 10.1016/S0168-8278(00)80412-2) [DOI] [PubMed] [Google Scholar]
- Ferguson M. W., O'Kane S. 2004. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Phil. Trans. R. Soc. Lond. B 359, 839–850. ( 10.1098/rstb.2004.1475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioretti F., Lebreton-Decoster C., Gueniche F., Yousfi M., Humbert P., Godeau G., Senni K., Desmouliere A., Coulomb B. 2008. Human bone marrow-derived cells: an attractive source to populate dermal substitutes. Wound Repair Regen. 16, 87–94. ( 10.1111/j.1524-475X.2007.00304.x) [DOI] [PubMed] [Google Scholar]
- Furukawa K., Pichora J., Steinmann S., Faber K. J., Johnson J. A., King G. J. 2007. Efficacy of interference screw and double-docking methods using palmaris longus and GraftJacket for medial collateral ligament reconstruction of the elbow. J. Shoulder Elbow Surg. 16, 449–453. ( 10.1016/j.jse.2006.09.020) [DOI] [PubMed] [Google Scholar]
- Gallico G. G., III, O'Connor N. E., Compton C. C., Kehinde O., Green H. 1984. Permanent coverage of large burn wounds with autologous cultured human epithelium. N. Engl. J. Med. 311, 448–451. [DOI] [PubMed] [Google Scholar]
- Garcia Y., Wilkins B., Collighan R. J., Griffin M., Pandit A. 2008. Towards development of a dermal rudiment for enhanced wound healing response. Biomaterials 29, 857–868. ( 10.1016/j.biomaterials.2007.10.053) [DOI] [PubMed] [Google Scholar]
- Gardiner D. M., Bryant S. V. 1996. Molecular mechanisms in the control of limb regeneration: the role of homeobox genes. Int. J. Dev. Biol. 40, 797–805. [PubMed] [Google Scholar]
- Ghosh K., Ren X. D., Shu X. Z., Prestwich G. D., Clark R. A. 2006. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 12, 601–613. ( 10.1089/ten.2006.12.601) [DOI] [PubMed] [Google Scholar]
- Giuggioli D., Sebastiani M., Cazzato M., Piaggesi A., Abatangelo G., Ferri C. 2003. Autologous skin grafting in the treatment of severe scleroderma cutaneous ulcers: a case report. Rheumatology (Oxford) 42, 694–696. ( 10.1093/rheumatology/keg106) [DOI] [PubMed] [Google Scholar]
- Goss R. J., Holt R. 1992. Epimorphic vs. tissue regeneration in Xenopus forelimbs. J. Exp. Zool. 261, 451–457. ( 10.1002/jez.1402610412) [DOI] [PubMed] [Google Scholar]
- Grant I. 1999. An investigation of novel methods of cultured keratinocyte delivery in a pig model. MD dissertation, University of Oxford, UK. [Google Scholar]
- Grant I., Warwick K., Marshall J., Green C., Martin R. 2002. The co-application of sprayed cultured autologous keratinocytes and autologous fibrin sealant in a porcine wound model. Br. J. Plast. Surg. 55, 219–227. ( 10.1054/bjps.2002.3810) [DOI] [PubMed] [Google Scholar]
- Gravante G., Delogu D., Giordan N., Morano G., Montone A., Esposito G. 2007. The use of hyalomatrix PA in the treatment of deep partial-thickness burns. J. Burn Care Res. 28, 269–274. [DOI] [PubMed] [Google Scholar]
- Griffiths M., Ojeh N., Livingstone R., Price R., Navsaria H. 2004. Survival of Apligraf in acute human wounds. Tissue Eng. 10, 1180–1195. ( 10.1089/ten.2004.10.1180) [DOI] [PubMed] [Google Scholar]
- Hansbrough J. F., Dore C., Hansbrough W. B. 1992. Clinical trials of a living dermal tissue replacement placed beneath meshed, split-thickness skin grafts on excised burn wounds. J. Burn Care Rehab. 13, 519–529. [DOI] [PubMed] [Google Scholar]
- Harley B. A., Leung J. H., Silva E. C., Gibson L. J. 2007. Mechanical characterization of collagen-glycosaminoglycan scaffolds. Acta Biomater. 3, 463–474. ( 10.1016/j.actbio.2006.12.009) [DOI] [PubMed] [Google Scholar]
- Harrison C. A., Heaton M. J., Layton C. M., MacNeil S. 2006. Use of an in vitro model of tissue-engineered human skin to study keratinocyte attachment and migration in the process of reepithelialization. Wound Repair Regen. 14, 203–209. ( 10.1111/j.1743-6109.2006.00111.x) [DOI] [PubMed] [Google Scholar]
- Haslik W., Kamolz L. P., Nathschlager G., Andel H., Meissl G., Frey M. 2007. First experiences with the collagen-elastin matrix Matriderm as a dermal substitute in severe burn injuries of the hand. Burns 33, 364–368. ( 10.1016/j.burns.2006.07.021) [DOI] [PubMed] [Google Scholar]
- Hayes D. W., Jr, Webb G. E., Mandracchia V. J., John K. J. 2001. Full-thickness burn of the foot: successful treatment with Apligraf. A case report. Clin. Podiatr. Med. Surg. 18, 179–188. [PubMed] [Google Scholar]
- Healy C. M., Boorman J. G. 1989. Comparison of E-Z Derm and Jelonet dressings for partial skin thickness burns. Burns Incl. Therm. Inj. 15, 52–54. [DOI] [PubMed] [Google Scholar]
- Hebda P. A., Dohar J. E. 1999. Transplanted fetal fibroblasts: survival and distribution over time in normal adult dermis compared with autogenic, allogenic, and xenogenic adult fibroblasts. Otolaryngol. Head Neck Surg. 121, 245–251. ( 10.1016/S0194-5998(99)70179-8) [DOI] [PubMed] [Google Scholar]
- Hedley S. J., Layton C., Heaton M., Chakrabarty K. H., Dawson R. A., Gawkrodger D. J., MacNeil S. 2002. Fibroblasts play a regulatory role in the control of pigmentation in reconstructed human skin from skin types I and II. Pigment Cell Res. 15, 49–56. ( 10.1034/j.1600-0749.2002.00067.x) [DOI] [PubMed] [Google Scholar]
- Heimbach D., et al. 1988. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann. Surg. 208, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbach D. M., et al. 2003. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J. Burn Care Rehab. 24, 42–48. ( 10.1097/00004630-200301000-00009) [DOI] [PubMed] [Google Scholar]
- Heitland A., Piatkowski A., Noah E. M., Pallua N. 2004. Update on the use of collagen/glycosaminoglycate skin substitute—six years of experiences with artificial skin in 15 German burn centers. Burns 30, 471–475. ( 10.1016/j.burns.2004.01.010) [DOI] [PubMed] [Google Scholar]
- Helenius G., Backdahl H., Bodin A., Nannmark U., Gatenholm P., Risberg B. 2006. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. A 76, 431–438. ( 10.1002/jbm.a.30570) [DOI] [PubMed] [Google Scholar]
- Hernon C. A., Dawson R. A., Freedlander E., Short R., Haddow D. B., Brotherston M., MacNeil S. 2006. Clinical experience using cultured epithelial autografts leads to an alternative methodology for transferring skin cells from the laboratory to the patient. Regen. Med. 1, 809–821. ( 10.2217/17460751.1.6.809) [DOI] [PubMed] [Google Scholar]
- Hernon C. A., Harrison C. A., Thornton D. J., MacNeil S. 2007. Enhancement of keratinocyte performance in the production of tissue-engineered skin using a low-calcium medium. Wound Repair Regen. 15, 718–726. ( 10.1111/j.1524-475X.2007.00275.x) [DOI] [PubMed] [Google Scholar]
- Holt B., Tripathi A., Morgan J. 2008. Viscoelastic response of human skin to low magnitude physiologically relevant shear. J. Biomech. 41, 2689–2695. ( 10.1016/j.jbiomech.2008.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch R. E., Kopp J., Kneser U., Beier J., Bach A. D. 2005. Tissue engineering of cultured skin substitutes. J. Cell Mol. Med. 9, 592–608. ( 10.1111/j.1582-4934.2005.tb00491.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth C. M. 1974. Trapped fingers and amputated finger tips in children. J. Pediatr. Surg. 9, 853–858. ( 10.1016/S0022-3468(74)80220-4) [DOI] [PubMed] [Google Scholar]
- Jahoda C. A. 2003. Cell movement in the hair follicle dermis—more than a two-way street? J. Invest. Dermatol. 121, ix–xi. ( 10.1111/j.1523-1747.2003.12585.x) [DOI] [PubMed] [Google Scholar]
- Johnen C., et al. 2008. Culture of subconfluent human fibroblasts and keratinocytes using biodegradable transfer membranes. Burns 34, 655–663. ( 10.1016/j.burns.2007.08.023) [DOI] [PubMed] [Google Scholar]
- Johnsen S., et al. 2005. Treatment of therapy-refractive ulcera cruris of various origins with autologous keratinocytes in fibrin sealant. Vasa 34, 25–29. ( 10.1024/0301-1526.34.1.25) [DOI] [PubMed] [Google Scholar]
- Jones I., Currie L., Martin R. 2002. A guide to biological skin substitutes. Br. J. Plast. Surg. 55, 185–193. ( 10.1054/bjps.2002.3800) [DOI] [PubMed] [Google Scholar]
- Jones I., James S. E., Rubin P., Martin R. 2003. Upward migration of cultured autologous keratinocytes in Integra artificial skin: a preliminary report. Wound Repair Regen. 11, 132–138. ( 10.1046/j.1524-475X.2003.11209.x) [DOI] [PubMed] [Google Scholar]
- Kellouche S., et al. 2007. Tissue engineering for full-thickness burns: a dermal substitute from bench to bedside. Biochem. Biophys. Res. Commun. 363, 472–478. ( 10.1016/j.bbrc.2007.08.155) [DOI] [PubMed] [Google Scholar]
- Khan M. N., Naqvi A. H. 2006. Antiseptics, iodine, povidone iodine and traumatic wound cleansing. J. Tissue Viability 16, 6–10. [DOI] [PubMed] [Google Scholar]
- Khariwala S. S., Chan J., Blackwell K. E., Alam D. S. 2007. Temporomandibular joint reconstruction using a vascularized bone graft with Alloderm. J. Reconstr. Microsurg. 23, 25–30. ( 10.1055/s-2006-958698) [DOI] [PubMed] [Google Scholar]
- Khatyr F., Imberdis C., Vescovo P., Varchon D., Lagarde J. M. 2004. Model of the viscoelastic behaviour of skin in vivo and study of anisotropy. Skin Res. Technol. 10, 96–103. ( 10.1111/j.1600-0846.2004.00057.x) [DOI] [PubMed] [Google Scholar]
- Kim H. T., Ahn S. T., Park J. G. 2003. Absorption rates of various-thickness human acellular dermal grafts (SureDerm(R)). J. Korean Soc. Plast. Reconstr. Surg. 30, 224–230. [Google Scholar]
- Kim K., Jeong C. G., Hollister S. J. 2008. Non-invasive monitoring of tissue scaffold degradation using ultrasound elasticity imaging. Acta Biomater. 4, 783–790. ( 10.1016/j.actbio.2008.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. J., Dybowski K. S., Steinberg J. S. 2006. Feature: a closer look at bioengineered alternative tissues. Podiatry Today 19, 38–55. [Google Scholar]
- Kolokol'chikova E. G., Budkevich L. I., Bobrovnikov A. E., Badikova A. K., Tumanov V. P. 2001. Morphological changes in burn wounds after transplantation of allogenic fibroblasts. Bull. Exp. Biol. Med. 131, 89–93. ( 10.1023/A:1017503301550) [DOI] [PubMed] [Google Scholar]
- Kvistedal Y. A., Nielsen P. M. 2009. Estimating material parameters of human skin in vivo. Biomech. Model. Mechanobiol. 8, 1–8. ( 10.1007/s10237-007-0112-z) [DOI] [PubMed] [Google Scholar]
- Lal S., Barrow R. E., Wolf S. E., Chinkes D. L., Hart D. W., Heggers J. P., Herndon D. N. 2000. Biobrane improves wound healing in burned children without increased risk of infection. Shock 14, 314–318. ( 10.1097/00024382-200014030-00013) [DOI] [PubMed] [Google Scholar]
- Lam P. K., Chan E. S., To E. W., Lau C. H., Yen S. C., King W. W. 1999. Development and evaluation of a new composite Laserskin graft. J. Trauma 47, 918–922. ( 10.1097/00005373-199911000-00017) [DOI] [PubMed] [Google Scholar]
- Landis S. J. 2008. Chronic wound infection and antimicrobial use. Adv. Skin Wound Care 21, 531–540. ( 10.1097/01.ASW.0000323578.87700.a5) [DOI] [PubMed] [Google Scholar]
- Lee J. W., Jang Y. C., Oh S. J. 2005. Use of the artificial dermis for free radial forearm flap donor site. Ann. Plast. Surg. 55, 500–502. ( 10.1097/01.sap.0000183789.00146.c6) [DOI] [PubMed] [Google Scholar]
- Lee J. H., Kim J. E., Kim B. J., Cho K. H. 2007. In vitro phototoxicity test using artificial skin with melanocytes. Photodermatol. Photoimmunol. Photomed. 23, 73–80. ( 10.1111/j.1600-0781.2007.00279.x) [DOI] [PubMed] [Google Scholar]
- Lemoli R. M., et al. 2005. Stem cell plasticity: time for a reappraisal? Haematologica 90, 360–381. [PubMed] [Google Scholar]
- Lesser T., Aboseif S., Abbas M. A. 2008. Combined endorectal advancement flap with Alloderm graft repair of radiation and cryoablation-induced rectourethral fistula. Am. Surg. 74, 341–345. [PubMed] [Google Scholar]
- Li J., Chen J., Kirsner R. 2007. Pathophysiology of acute wound healing. Clin. Dermatol. 25, 9–18. ( 10.1016/j.clindermatol.2006.09.007) [DOI] [PubMed] [Google Scholar]
- Limat A., French L. E., Blal L., Saurat J. H., Hunziker T., Salomon D. 2003. Organotypic cultures of autologous hair follicle keratinocytes for the treatment of recurrent leg ulcers. J. Am. Acad. Dermatol. 48, 207–214. ( 10.1067/mjd.2003.69) [DOI] [PubMed] [Google Scholar]
- Lindberg K., Badylak S. F. 2001. Porcine small intestinal submucosa (SIS): a bioscaffold supporting in vitro primary human epidermal cell differentiation and synthesis of basement membrane proteins. Burns 27, 254–266. ( 10.1016/S0305-4179(00)00113-3) [DOI] [PubMed] [Google Scholar]
- Lipman J., Medalie D., Rosen M. J. 2008. Staged repair of massive incisional hernias with loss of abdominal domain: a novel approach. Am. J. Surg. 195, 84–88. ( 10.1016/j.amjsurg.2007.02.017) [DOI] [PubMed] [Google Scholar]
- Lorenz H. P., Adzick N. S. 1993. Scarless skin wound repair in the fetus. West J. Med. 159, 350–355. [PMC free article] [PubMed] [Google Scholar]
- Lukish J. R., et al. 2001. The use of a bioactive skin substitute decreases length of stay for pediatric burn patients. J. Pediatr. Surg. 36, 1118–1121. ( 10.1053/jpsu.2001.25678) [DOI] [PubMed] [Google Scholar]
- Ma P. X. 2008. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 60, 184–198. ( 10.1016/j.addr.2007.08.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod T. M., Sarathchandra P., Williams G., Sanders R., Green C. J. 2004. The diamond CO2 laser as a method of improving the vascularisation of a permanent collagen implant. Burns 30, 704–712. ( 10.1016/j.burns.2004.03.008) [DOI] [PubMed] [Google Scholar]
- MacLeod T. M., Williams G., Sanders R., Green C. J. 2005. Histological evaluation of Permacol as a subcutaneous implant over a 20-week period in the rat model. Br. J. Plast. Surg. 58, 518–532. ( 10.1016/j.bjps.2004.12.012) [DOI] [PubMed] [Google Scholar]
- MacNeil S. 2007. Progress and opportunities for tissue-engineered skin. Nature 445, 874–880. ( 10.1038/nature05664) [DOI] [PubMed] [Google Scholar]
- Manna F., Dentini M., Desideri P., De Pita O., Mortilla E., Maras B. 1999. Comparative chemical evaluation of two commercially available derivatives of hyaluronic acid (hylaform from rooster combs and restylane from streptococcus) used for soft tissue augmentation. J. Eur. Acad. Dermatol. Venereol. 13, 183–192. [PubMed] [Google Scholar]
- Marrot L., Belaidi J. P., Chaubo C., Meunier J. R., Perez P., Agapakis-Causse C. 1998. An in vitro strategy to evaluate the phototoxicity of solar UV at the molecular and cellular level: application to photoprotection assessment. Eur. J. Dermatol. 8, 403–412. [PubMed] [Google Scholar]
- Marston W. A., Hanft J., Norwood P., Pollak R. 2003. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26, 1701–1705. ( 10.2337/diacare.26.6.1701) [DOI] [PubMed] [Google Scholar]
- Martin P., Leibovich S. J. 2005. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 15, 599–607. ( 10.1016/j.tcb.2005.09.002) [DOI] [PubMed] [Google Scholar]
- Metcalfe A. D., Ferguson M. W. 2007a. Bioengineering skin using mechanisms of regeneration and repair. Biomaterials 28, 5100–5113. ( 10.1016/j.biomaterials.2007.07.031) [DOI] [PubMed] [Google Scholar]
- Metcalfe A. D., Ferguson M. W. 2007b. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 4, 413–437. ( 10.1098/rsif.2006.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. J., Burke E. M., Rader M. D., Coulombe P. A., Lavker R. M. 1998. Re-epithelialization of porcine skin by the sweat apparatus. J. Invest. Dermatol. 110, 13–19. ( 10.1046/j.1523-1747.1998.00087.x) [DOI] [PubMed] [Google Scholar]
- Morimoto N., Saso Y., Tomihata K., Taira T., Takahashi Y., Ohta M., Suzuki S. 2005. Viability and function of autologous and allogeneic fibroblasts seeded in dermal substitutes after implantation. J. Surg. Res. 125, 56–67. ( 10.1016/j.jss.2004.11.012) [DOI] [PubMed] [Google Scholar]
- Mostow E. N., Haraway G. D., Dalsing M., Hodde J. P., King D. 2005. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J. Vasc. Surg. 41, 837–843. ( 10.1016/j.jvs.2005.01.042) [DOI] [PubMed] [Google Scholar]
- Moustafa M., et al. 2004. A new autologous keratinocyte dressing treatment for non-healing diabetic neuropathic foot ulcers. Diabet. Med. 21, 786–789. ( 10.1111/j.1464-5491.2004.01166.x) [DOI] [PubMed] [Google Scholar]
- Moustafa M., et al. 2007. Randomized, controlled, single-blind study on use of autologous keratinocytes on a transfer dressing to treat nonhealing diabetic ulcers. Regen. Med. 2, 887–902. ( 10.2217/17460751.2.6.887) [DOI] [PubMed] [Google Scholar]
- Myers S. R., Partha V. N., Soranzo C., Price R. D., Navsaria H. A. 2007. Hyalomatrix: a temporary epidermal barrier, hyaluronan delivery, and neodermis induction system for keratinocyte stem cell therapy. Tissue Eng. 13, 2733–2741. ( 10.1089/ten.2007.0109) [DOI] [PubMed] [Google Scholar]
- Nam Y. O., Lee J. W., Koh J. H., Seo D. K., Oh S. J., Jang Y. C. 2006. Burn management and reconstruction using artificial dermis Pelnac. J. Kor. Burn Soc. 9, 115–120. [Google Scholar]
- Navarro F. A., Stoner M. L., Park C. S., Huertas J. C., Lee H. B., Wood F. M., Orgill D. P. 2000. Sprayed keratinocyte suspensions accelerate epidermal coverage in a porcine microwound model. J. Burn Care Rehab. 21, 513–518. ( 10.1097/00004630-200021060-00007) [DOI] [PubMed] [Google Scholar]
- Notara M., Bullett N. A., Deshpande P., Haddow D. B., MacNeil S., Daniels J. T. 2007. Plasma polymer coated surfaces for serum-free culture of limbal epithelium for ocular surface disease. J. Mater. Sci. Mater. Med. 18, 329–338. ( 10.1007/s10856-006-0697-2) [DOI] [PubMed] [Google Scholar]
- O'Kane S., Ferguson M. W. 1997. Transforming growth factor βs and wound healing. Int. J. Biochem. Cell Biol. 29, 63–78. ( 10.1016/S1357-2725(96)00120-3) [DOI] [PubMed] [Google Scholar]
- Ohyama H., Nishimura F., Meguro M., Takashiba S., Murayama Y., Matsushita S. 2002. Counter-antigen presentation: fibroblasts produce cytokines by signalling through HLA class II molecules without inducing T-cell proliferation. Cytokine 17, 175–181. ( 10.1006/cyto.2001.0976) [DOI] [PubMed] [Google Scholar]
- Omar A. A., Mavor A. I., Jones A. M., Homer-Vanniasinkam S. 2004. Treatment of venous leg ulcers with Dermagraft. Eur. J. Vasc. Endovasc. Surg. 27, 666–672. ( 10.1016/j.ejvs.2004.03.001) [DOI] [PubMed] [Google Scholar]
- Orgill D. P., Butler C., Regan J. F., Barlow M. S., Yannas I. V., Compton C. C. 1998. Vascularized collagen-glycosaminoglycan matrix provides a dermal substrate and improves take of cultured epithelial autografts. Plast. Reconstr. Surg. 102, 423–429. [DOI] [PubMed] [Google Scholar]
- Pandya A. N., Woodward B., Parkhouse N. 1998. The use of cultured autologous keratinocytes with Integra in the resurfacing of acute burns. Plast. Reconstr. Surg. 102, 825–828. [PubMed] [Google Scholar]
- Panuncialman J., Falanga V. 2007. The science of wound bed preparation. Clin. Plast. Surg. 34, 621–632. ( 10.1016/j.cps.2007.07.003) [DOI] [PubMed] [Google Scholar]
- Pape S. A., Byrne P. O. 2000. Safety and efficacy of TransCyte for the treatment of partial-thickness burns. J. Burn Care Rehab. 21, 390. [PubMed] [Google Scholar]
- Papini R. 2004. Management of burn injuries of various depths. Br. Med. J. 329, 158–160. ( 10.1136/bmj.329.7458.158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini S., Cecchetti D., Campani D., Fitzgerald W., Grivel J. C., Chen S., Margolis L., Revoltella R. P. 2003. Isolation and clonal analysis of human epidermal keratinocyte stem cells in long-term culture. Stem Cells 21, 481–494. ( 10.1634/stemcells.21-4-481) [DOI] [PubMed] [Google Scholar]
- Parker D. M., Armstrong P. J., Frizzi J. D., North J. H., Jr 2006. Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr. Surg. 63, 255–258. ( 10.1016/j.cursur.2006.05.003) [DOI] [PubMed] [Google Scholar]
- Patel M., Fisher J. P. 2008. Biomaterial scaffolds in pediatric tissue engineering. Pediatr. Res. 63, 497–501. ( 10.1203/01.PDR.0b013e318165eb3e) [DOI] [PubMed] [Google Scholar]
- Patton J. H., Jr, Berry S., Kralovich K. A. 2007. Use of human acellular dermal matrix in complex and contaminated abdominal wall reconstructions. Am. J. Surg. 193, 360–363. [DOI] [PubMed] [Google Scholar]
- Peng C. C., Yang M. H., Chiu W. T., Chiu C. H., Yang C. S., Chen Y. W., Chen K. C., Peng R. Y. 2008. Composite nano-titanium oxide-chitosan artificial skin exhibits strong wound-healing effect—an approach with anti-inflammatory and bactericidal kinetics. Macromol. Biosci. 8, 316–327. ( 10.1002/mabi.200700188) [DOI] [PubMed] [Google Scholar]
- Pham C., Greenwood J., Cleland H., Woodruff P., Maddern G. 2007. Bioengineered skin substitutes for the management of burns: a systematic review. Burns 33, 946–957. ( 10.1016/j.burns.2007.03.020) [DOI] [PubMed] [Google Scholar]
- Ponec M., El Ghalbzouri A., Dijkman R., Kempenaar J., van der Pluijm G., Koolwijk P. 2004. Endothelial network formed with human dermal microvascular endothelial cells in autologous multicellular skin substitutes. Angiogenesis 7, 295–305. ( 10.1007/s10456-004-6315-3) [DOI] [PubMed] [Google Scholar]
- Powell H. M., Boyce S. T. 2006. EDC cross-linking improves skin substitute strength and stability. Biomaterials 27, 5821–5827. ( 10.1016/j.biomaterials.2006.07.030) [DOI] [PubMed] [Google Scholar]
- Powell H. M., Boyce S. T. 2009. Engineered human skin fabricated using electrospun collagen-PCL blends: morphogenesis and mechanical properties. Tissue Eng. A 15, 2177–2187. ( 10.1089/ten.tea.2008.0473) [DOI] [PubMed] [Google Scholar]
- Prevel C. D., Eppley B. L., Summerlin D. J., Sidner R., Jackson J. R., McCarty M., Badylak S. F. 1995. Small intestinal submucosa: utilization as a wound dressing in full-thickness rodent wounds. Ann. Plast. Surg. 35, 381–388. [PubMed] [Google Scholar]
- Price R. D., Das-Gupta V., Harris P. A., Leigh I. M., Navsaria H. A. 2004. The role of allogenic fibroblasts in an acute wound healing model. Plast. Reconstr. Surg. 113, 1719–1729. ( 10.1097/01.PRS.0000117367.86893.CE) [DOI] [PubMed] [Google Scholar]
- Price R. D., Das-Gupta V., Leigh I. M., Navsaria H. A. 2006. A comparison of tissue-engineered hyaluronic acid dermal matrices in a human wound model. Tissue Eng. 12, 2985–2995. ( 10.1089/ten.2006.12.2985) [DOI] [PubMed] [Google Scholar]
- Price R. D., Berry M. G., Navsaria H. A. 2007. Hyaluronic acid: the scientific and clinical evidence. J. Plast. Reconstr. Aesthet. Surg. 60, 1110–1119. ( 10.1016/j.bjps.2007.03.005) [DOI] [PubMed] [Google Scholar]
- Quinby W. C., Jr, Burke J. F., Bondoc C. C. 1981. Primary excision and immediate wound closure. Intensive Care Med. 7, 71–76. ( 10.1007/BF01687263) [DOI] [PubMed] [Google Scholar]
- Ramos-e-Silva M., Ribeiro de Castro M. C. 2002. New dressings, including tissue-engineered living skin. Clin. Dermatol. 20, 715–723. ( 10.1016/S0738-081X(02)00298-5) [DOI] [PubMed] [Google Scholar]
- Regnier M., Patwardhan A., Scheynius A., Schmidt R. 1998. Reconstructed human epidermis composed of keratinocytes, melanocytes and Langerhans cells. Med. Biol. Eng. Comput. 36, 821–824. ( 10.1007/BF02518889) [DOI] [PubMed] [Google Scholar]
- Rehder J., Souto L. R., Issa C. M., Puzzi M. B. 2004. Model of human epidermis reconstructed in vitro with keratinocytes and melanocytes on dead de-epidermized human dermis. Sao Paulo Med. J. 122, 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. 1975. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343. ( 10.1016/S0092-8674(75)80001-8) [DOI] [PubMed] [Google Scholar]
- Rochat A., Kobayashi K., Barrandon Y. 1994. Location of stem cells of human hair follicles by clonal analysis. Cell 76, 1063–1073. ( 10.1016/0092-8674(94)90383-2) [DOI] [PubMed] [Google Scholar]
- Rockwood D. N., Woodhouse K. A., Fromstein J. D., Chase D. B., Rabolt J. F. 2007. Characterization of biodegradable polyurethane microfibers for tissue engineering. J. Biomater. Sci. Polym. Ed. 18, 743–758. ( 10.1163/156856207781034115) [DOI] [PubMed] [Google Scholar]
- Roh D. H., et al. 2006. Wound healing effect of silk fibroin/alginate-blended sponge in full thickness skin defect of rat. J. Mater. Sci. Mater. Med. 17, 547–552. ( 10.1007/s10856-006-8938-y) [DOI] [PubMed] [Google Scholar]
- Ronfard V., Rives J. M., Neveux Y., Carsin H., Barrandon Y. 2000. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation 70, 1588–1598. ( 10.1097/00007890-200012150-00009) [DOI] [PubMed] [Google Scholar]
- Rosso F., Marino G., Giordano A., Barbarisi M., Parmeggiani D., Barbarisi A. 2005. Smart materials as scaffolds for tissue engineering. J. Cell Physiol. 203, 465–470. ( 10.1002/jcp.20270) [DOI] [PubMed] [Google Scholar]
- Ryssel H., Gazyakan E., Germann G., Ohlbauer M. 2008. The use of MatriDerm® in early excision and simultaneous autologous skin grafting in burns—a pilot study. Burns 34, 93–97. ( 10.1016/j.burns.2007.01.018) [DOI] [PubMed] [Google Scholar]
- Saddiq Z. A., Barbenel J. C., Grant M. H. 2009. The mechanical strength of collagen gels containing glycosaminoglycans and populated with fibroblasts. J. Biomed. Mater. Res. A 89, 697–706. ( 10.1002/jbm.a.32007) [DOI] [PubMed] [Google Scholar]
- Sandulache V. C., Zhou Z., Sherman A., Dohar J. E., Hebda P. A. 2003. Impact of transplanted fibroblasts on rabbit skin wounds. Arch. Otolaryngol. Head Neck Surg. 129, 345–350. [DOI] [PubMed] [Google Scholar]
- Schwarze H., Kuntscher M., Uhlig C., Hierlemann H., Prantl L., Ottomann C., Hartmann B. 2008. Suprathel, a new skin substitute, in the management of partial-thickness burn wounds: results of a clinical study. Ann. Plast. Surg. 60, 181–185. ( 10.1097/SAP.0b013e318056bbf6) [DOI] [PubMed] [Google Scholar]
- Shah M., Foreman D. M., Ferguson M. W. 1995. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 108, 985–1002. [DOI] [PubMed] [Google Scholar]
- Shakespeare P. G. 2005. The role of skin substitutes in the treatment of burn injuries. Clin. Dermatol. 23, 413–418. ( 10.1016/j.clindermatol.2004.07.015) [DOI] [PubMed] [Google Scholar]
- Shelton A. A., Welton M. L. 2006. Transperineal repair of persistent rectovaginal fistulas using an acellular cadaveric dermal graft (AlloDerm). Dis. Colon Rectum 49, 1454–1457. ( 10.1007/s10350-006-0619-x) [DOI] [PubMed] [Google Scholar]
- Sher S. E., Hull B. E., Rosen S., Church D., Friedman L., Bell E. 1983. Acceptance of allogeneic fibroblasts in skin equivalent transplants. Transplantation 36, 552–557. [DOI] [PubMed] [Google Scholar]
- Sheridan R. L., Tompkins R. G. 1999. Skin substitutes in burns. Burns 25, 97–103. ( 10.1016/S0305-4179(98)00176-4) [DOI] [PubMed] [Google Scholar]
- Sheu M. T., Huang J. C., Yeh G. C., Ho H. O. 2001. Characterization of collagen gel solutions and collagen matrices for cell culture. Biomaterials 22, 1713–1719. ( 10.1016/S0142-9612(00)00315-X) [DOI] [PubMed] [Google Scholar]
- Shevchenko R. V., Sibbons P. D., Sharpe J. R., James S. E. 2008. Use of a novel porcine collagen paste as a dermal substitute in full-thickness wounds Wound Repair Regen. 16, 198–207. ( 10.1111/j.1524-475X.2008.00360.x) [DOI] [PubMed] [Google Scholar]
- Silverstein G. 2006. Dermal regeneration template in the surgical management of diabetic foot ulcers: a series of five cases. J. Foot Ankle Surg. 45, 28–33. ( 10.1053/j.jfas.2005.10.005) [DOI] [PubMed] [Google Scholar]
- Soejima K., Chen X., Nozaki M., Hori K., Sakurai H., Takeuchi M. 2006. Novel application method of artificial dermis: one-step grafting procedure of artificial dermis and skin, rat experimental study. Burns 32, 312–318. ( 10.1016/j.burns.2005.10.013) [DOI] [PubMed] [Google Scholar]
- Stanton R. A., Billmire D. A. 2002. Skin resurfacing for the burned patient. Clin. Plast. Surg. 29, 29–51. ( 10.1016/S0094-1298(03)00085-3) [DOI] [PubMed] [Google Scholar]
- Stark H. J., Willhauck M. J., Mirancea N., Boehnke K., Nord I., Breitkreutz D., Pavesio A., Boukamp P., Fusenig N. E. 2004. Authentic fibroblast matrix in dermal equivalents normalises epidermal histogenesis and dermoepidermal junction in organotypic co-culture. Eur. J. Cell Biol. 83, 631–645. ( 10.1078/0171-9335-00435) [DOI] [PubMed] [Google Scholar]
- Stark H. J., Boehnke K., Mirancea N., Willhauck M. J., Pavesio A., Fusenig N. E., Boukamp P. 2006. Epidermal homeostasis in long-term scaffold-enforced skin equivalents. J. Invest. Dermatol. Symp. Proc. 11, 93–105. ( 10.1038/sj.jidsymp.5650015) [DOI] [PubMed] [Google Scholar]
- Still J., Glat P., Silverstein P., Griswold J., Mozingo D. 2003. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns 29, 837–841. ( 10.1016/S0305-4179(03)00164-5) [DOI] [PubMed] [Google Scholar]
- Stoilova Y. D., Haidushkal I. A., Murdjeval M. A., Traikov I. Z., Popova T. A., Kevorkyan A. K. 2007. Immunological and microbiological investigations of patients with burn injuries. Folia Med. (Plovdiv.) 49, 49–58. [PubMed] [Google Scholar]
- Strande L. F., Foley S. T., Doolin E. J., Hewitt C. W. 1997. In vitro bioartificial skin culture model of tissue rejection and inflammatory/immune mechanisms. Transplant. Proc. 29, 2118–2119. ( 10.1016/S0041-1345(97)00256-X) [DOI] [PubMed] [Google Scholar]
- Su J. W., Mason D. P., Murthy S. C., Rice T. W. 2008. Closure of a large tracheoesophageal fistula using AlloDerm. J. Thorac. Cardiovasc. Surg. 135, 706–707. [DOI] [PubMed] [Google Scholar]
- Supp D. M., Boyce S. T. 2005. Engineered skin substitutes: practices and potentials. Clin. Dermatol. 23, 403–412. ( 10.1016/j.clindermatol.2004.07.023) [DOI] [PubMed] [Google Scholar]
- Suzuki S., Kawai K., Ashoori F., Morimoto N., Nishimura Y., Ikada Y. 2000. Long-term follow-up study of artificial dermis composed of outer silicone layer and inner collagen sponge. Br. J. Plast. Surg. 53, 659–666. ( 10.1054/bjps.2000.3426) [DOI] [PubMed] [Google Scholar]
- Takakura I., et al. 1999. An in vivo model of human skin acute graft-versus-host disease: transplantation of cultured human epidermal cells and dermal fibroblasts with human lymphocytes into SCID mice. Exp. Hematol. 27, 1815–1821. ( 10.1016/S0301-472X(99)00111-3) [DOI] [PubMed] [Google Scholar]
- Tamisani A. M. 2004. The use of hyalomatrix in deep paediatric burns. Ann. Burns Fire Disast. XVII. [Google Scholar]
- Tausche A. K., et al. 2003. An autologous epidermal equivalent tissue-engineered from follicular outer root sheath keratinocytes is as effective as split-thickness skin autograft in recalcitrant vascular leg ulcers. Wound Repair Regen. 11, 248–252. ( 10.1046/j.1524-475X.2003.11403.x) [DOI] [PubMed] [Google Scholar]
- Thacker J. G., Stalnecker M. C., Allaire P. E., Edgerton M. T., Rodeheaver G. T., Edlich R. F. 1977. Practical applications of skin biomechanics. Clin. Plast. Surg. 4, 167–171. [PubMed] [Google Scholar]
- Toma J. G., Akhavan M., Fernandes K. J., Barnabe-Heider F., Sadikot A., Kaplan D. R., Miller F. D. 2001. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 3, 778–784. ( 10.1038/ncb0901-778) [DOI] [PubMed] [Google Scholar]
- Tonello C., Vindigni V., Zavan B., Abatangelo S., Abatangelo G., Brun P., Cortivo R. 2005. In vitro reconstruction of an endothelialized skin substitute provided with a microcapillary network using biopolymer scaffolds. FASEB J. 19, 1546–1548. [DOI] [PubMed] [Google Scholar]
- Travia G., Palmisano P. A., Cervelli V., Esposito G., Casciani C. U. 2003. The use of fibroblast and keratinocyte cultures in burns treatment. Ann. Burns Fire Disast. XVI. [Google Scholar]
- Tsai C. C., Lin S. D., Lai C. S., Lin T. M. 1999. The use of composite acellular allodermis-ultrathin autograft on joint area in major burn patients—one year follow-up. Kaohsiung. J. Med. Sci. 15, 651–658. [PubMed] [Google Scholar]
- Tsuji-Saso Y., Kawazoe T., Morimoto N., Tabata Y., Taira T., Tomihata K., Utani A., Suzuki S. 2007. Incorporation of basic fibroblast growth factor into preconfluent cultured skin substitute to accelerate neovascularisation and skin reconstruction after transplantation. Scand. J. Plast. Reconstr. Surg. Hand Surg. 41, 228–235. [DOI] [PubMed] [Google Scholar]
- Tumbar T. 2006. Epithelial skin stem cells. Methods Enzymol. 419, 73–99. [DOI] [PubMed] [Google Scholar]
- Tumbar T., Guasch G., Greco V., Blanpain C., Lowry W. E., Rendl M., Fuchs E. 2004. Defining the epithelial stem cell niche in skin. Science 303, 359–363. ( 10.1126/science.1092436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccioli L. 2003. A clinical investigation on the characteristics and outcomes of treating chronic lower extremity wounds using the tissuetech autograft system. Int. J. Low Extrem. Wounds 2, 140–151. ( 10.1177/1534734603258480) [DOI] [PubMed] [Google Scholar]
- Vacher D. 2003. Autologous epidermal sheets production for skin cellular therapy. Ann. Pharm. Fr. 61, 203–206. [PubMed] [Google Scholar]
- Valentin J. E., Badylak J. S., McCabe G. P., Badylak S. F. 2006. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J. Bone Joint Surg. Am. 88, 2673–2686. ( 10.2106/JBJS.E.01008) [DOI] [PubMed] [Google Scholar]
- van Zuijlen P. P., van Trier A. J., Vloemans J. F., Groenevelt F., Kreis R. W., Middelkoop E. 2000. Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a one-stage grafting model. Plast. Reconstr. Surg. 106, 615–623. ( 10.1097/00006534-200009030-00014) [DOI] [PubMed] [Google Scholar]
- Vanscheidt W., et al. 2007. Treatment of recalcitrant venous leg ulcers with autologous keratinocytes in fibrin sealant: a multinational randomized controlled clinical trial. Wound Repair Regen. 15, 308–315. ( 10.1111/j.1524-475X.2007.00231.x) [DOI] [PubMed] [Google Scholar]
- Venugopal J. R., Zhang Y., Ramakrishna S. 2006. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif. Organs 30, 440–446. ( 10.1111/j.1525-1594.2006.00239.x) [DOI] [PubMed] [Google Scholar]
- Violas P., Abid A., Darodes P., Galinier P., de Gauzy J. S., Cahuzac J. P. 2005. Integra artificial skin in the management of severe tissue defects, including bone exposure, in injured children. J. Pediatr. Orthop. B 14, 381–384. [DOI] [PubMed] [Google Scholar]
- Vogel H. G. 1987a. Age dependence of mechanical and biochemical properties of human skin. Part I. Stress–strain experiments, skin thickness and biochemical analysis. Bioeng. Skin 3, 67–91. [Google Scholar]
- Vogel H. G. 1987b. Age dependence of mechanical and biochemical properties of human skin. Part II. Hysteresis, relaxation, creep and repeated strain experiments. Bioeng. Skin 3, 141–176. [Google Scholar]
- Waters J. M., Richardson G. D., Jahoda C. A. 2007. Hair follicle stem cells. Semin. Cell Dev. Biol. 18, 245–254. ( 10.1016/j.semcdb.2007.02.003) [DOI] [PubMed] [Google Scholar]
- Waymack P., Duff R. G., Sabolinski M. 2000. The effect of a tissue engineered bilayered living skin analog, over meshed split-thickness autografts on the healing of excised burn wounds. The Apligraf Burn Study Group. Burns 26, 609–619. ( 10.1016/S0305-4179(00)00017-6) [DOI] [PubMed] [Google Scholar]
- Whitaker I. S., Prowse S., Potokar T. S. 2008. A critical evaluation of the use of Biobrane as a biologic skin substitute: a versatile tool for the plastic and reconstructive surgeon. Ann. Plast. Surg. 60, 333–337. ( 10.1097/SAP.0b013e31806bf446) [DOI] [PubMed] [Google Scholar]
- White R. J., Cutting K., Kingsley A. 2006. Topical antimicrobials in the control of wound bioburden. Ostomy. Wound Manage. 52, 26–58. [PubMed] [Google Scholar]
- Wilcke I., Lohmeyer J. A., Liu S., Condurache A., Kruger S., Mailander P., Machens H. G. 2007. VEGF(165) and bFGF protein-based therapy in a slow release system to improve angiogenesis in a bioartificial dermal substitute in vitro and in vivo. Langenbecks Arch. Surg. 392, 305–314. ( 10.1007/s00423-007-0194-1) [DOI] [PubMed] [Google Scholar]
- Williamson J. S., Snelling C. F., Clugston P., Macdonald I. B., Germann E. 1995. Cultured epithelial autograft: five years of clinical experience with twenty-eight patients. J. Trauma 39, 309–319. ( 10.1097/00005373-199508000-00020) [DOI] [PubMed] [Google Scholar]
- Wolfe R. A., Roi L. D., Flora J. D., Feller I., Cornell R. G. 1983. Mortality differences and speed of wound closure among specialized burn care facilities. JAMA 250, 763–766. ( 10.1001/jama.250.6.763) [DOI] [PubMed] [Google Scholar]
- Wood F. M., Kolybaba M. L., Allen P. 2006a. The use of cultured epithelial autograft in the treatment of major burn injuries: a critical review of the literature. Burns 32, 395–401. ( 10.1016/j.burns.2006.01.008) [DOI] [PubMed] [Google Scholar]
- Wood F. M., Kolybaba M. L., Allen P. 2006b. The use of cultured epithelial autograft in the treatment of major burn wounds: eleven years of clinical experience. Burns 32, 538–544. ( 10.1016/j.burns.2006.02.025) [DOI] [PubMed] [Google Scholar]
- Wood F. M., Stoner M. L., Fowler B. V., Fear M. W. 2007. The use of a non-cultured autologous cell suspension and Integra dermal regeneration template to repair full-thickness skin wounds in a porcine model: a one-step process. Burns 33, 693–700. ( 10.1016/j.burns.2006.10.388) [DOI] [PubMed] [Google Scholar]
- Woodley D. T., Chen M. 2001. The basement membrane zone. In The biology of the skin (eds Frenkel R. K., Woodley D. T.), pp. 133–152. London, UK: The Parthenon Publishing Group. [Google Scholar]
- Xiao Y. L., Riesle J., Van Blitterswijk C. A. 1999. Static and dynamic fibroblast seeding and cultivation in porous PEO/PBT scaffolds. J. Mater. Sci. Mater. Med. 10, 773–777. ( 10.1023/A:1008946832443) [DOI] [PubMed] [Google Scholar]
- Yannas I. V. 2005. Similarities and differences between induced organ regeneration in adults and early foetal regeneration. J. R. Soc. Interface 2, 403–417. ( 10.1098/rsif.2005.0062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannas I. V., Burke J. F. 1980. Design of an artificial skin. I. Basic design principles. J. Biomed. Mater. Res. 14, 65–81. ( 10.1002/jbm.820140108) [DOI] [PubMed] [Google Scholar]
- Yeo J. H., Lee K. G., Kim H. C., Oh H. Y. L., Kim A. J., Kim S. Y. 2000. The effects of Pva/chitosan/fibroin (PCF)-blended spongy sheets on wound healing in rats. Biol. Pharm. Bull. 23, 1220–1223. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Mitsuno H., Nonaka I., Sen Y., Kawanishi K., Inada Y., Takakura Y., Okuchi K., Nonomura A. 2008. Wound therapy by marrow mesenchymal cell transplantation. Plast. Reconstr. Surg. 121, 860–877. ( 10.1097/01.prs.0000299922.96006.24) [DOI] [PubMed] [Google Scholar]
- Yurugi S., Hatoko M., Kuwahara M., Tanaka A., Iioka H., Niitsuma K. 2002. Usefulness and limitations of artificial dermis implantation for posttraumatic deformity. Aesthetic Plast. Surg. 26, 360–364. ( 10.1007/s00266-002-2048-0) [DOI] [PubMed] [Google Scholar]
- Zhang G. A., Ning F. G., Zhao N. M. 2007. Biomechanical properties of four dermal substitutes. Chin. Med. J. (Engl.) 120, 1454–1455. [PubMed] [Google Scholar]
- Zhu N., et al. 2005. Treatment of burns and chronic wounds using a new cell transfer dressing for delivery of autologous keratinocytes. Eur. J. Plast. Surg. 28, 319–330. ( 10.1007/s00238-005-0777-4) [DOI] [Google Scholar]
- Zigdon H., Horwitz J. 2006. Using acellular dermal matrix (ADM) allograft in periodontal surgery—a literature review and case reports. Refuat. Hapeh. Vehashinayim. 24, 19–29, 92. [PubMed] [Google Scholar]