Abstract
Quiescence, or a sleep-like state, is a common and important feature of the daily lives of animals from both invertebrate and vertebrate taxa, suggesting that sleep appeared early in animal evolution. Recently, Drosophila melanogaster has been shown to be a relevant and powerful model for the genetic analysis of sleep behaviour. The sleep architecture of D. melanogaster is sexually dimorphic, with females sleeping much less than males during day-time, presumably because reproductive success requires greater foraging activity by the female as well as the search for egg-laying sites. However, this loss of sleep and increase in locomotor activity will heighten the risk for the female from environmental and predator hazards. In this study, we show that virgin females can minimize this risk by behaving like males, with an extended afternoon ‘siesta’. Copulation results in the female losing 70 per cent of day-time sleep and becoming more active. This behaviour lasts for at least 8 days after copulation and is abolished if the mating males lack sex peptide (SP), normally present in the seminal fluid. Our results suggest that SP is the molecular switch that promotes wakefulness in the post-mated female, a change of behaviour compatible with increased foraging and egg-laying activity. The stress resulting from SP-dependent sleep deprivation might be an important contribution to the toxic side-effects of male accessory gland products that are known to reduce lifespan in post-mated females.
Keywords: Drosophila, sleep, sex peptide, foraging, egg-laying
1. Introduction
Sleep is a sustained and reversible quiescent state in which animals display reduced motor activity and are less responsive to external stimuli (Bonnet 2005; Dinges et al. 2005). Sleep is important for normal wakeful behaviour, such as alertness, memory consolidation, cognitive performance and appetite, and sleep duration is linked to cardiovascular disease and obesity, as well as lifespan (Taheri et al. 2004; Bonnet 2005; Dinges et al. 2005; Hamet & Tremblay 2006). Sleep is often considered as having a restorative metabolic function after intense activity during wakeful periods, but this does not explain the large diversity in sleep quotas among species. Other theories, which are not mutually exclusive, have also been proposed for the need to sleep (Zepelin et al. 2005). These include the need for synaptic homeostasis for neuronal plasticity (Lesku et al. 2006, 2008; Capellini et al. 2008) and energy conservation by enforcing a sustained period of rest and low metabolic rate, which in turn reduce levels of damaging reactive oxygen species (Siegel 2005). Several comparative studies using mammalian sleep and ecological data have highlighted the relationship between sleep architecture and ecological variables, especially foraging strategies and predatory danger (Allison & Cicchetti 1976; Lesku et al. 2006, 2008; Capellini et al. 2008). Animals that spend a large proportion of the day asleep in a stable and benign environment reduce the risks from exposure to hostile features of environments such as predation, but miss opportunities to mate and forage for food. In contrast, animals that spend less time sleeping are more likely to perceive and adapt to changes in their surroundings. Therefore, although the biological functions of sleep are not fully understood, it is increasingly being recognized that there is a critical trade-off between the benefits and costs of sleep and wakefulness. Differences in sleep quotas among species might, therefore, reflect specific ecological contexts.
Quiescent sleep-like states also occur in invertebrate species, suggesting that sleep is an ancient behaviour with evolutionarily conserved biological functions (Siegel 2008). Most of our knowledge of sleep and its regulation in invertebrates has come from studying the fruitfly, Drosophila melanogaster, a model organism that displays many of the defining properties of mammalian sleep (preferred resting body posture and increased arousal threshold, homeostatic response to sleep deprivation, robust circadian control of the sleep–activity cycle and age-related changes to sleep architecture; Hendricks 2003; Shaw 2003; Koh et al. 2006). Similarities also extend to the effect of pharmacological agents (e.g. caffeine, cyclohexyladenosine and hydroxyzine) on sleep and the involvement of signalling molecules such as GABA, biogenic amines and neuropeptides in the regulation of sleep (Andretic et al. 2005; Kume et al. 2005; Yuan et al. 2006; Agosto et al. 2008; Liu et al. 2008). In adult D. melanogaster, kept in a 24 h light–dark cycle under standard laboratory conditions, there are two periods of sleep in a day separated by peaks of locomotor activity coinciding with lights-on (dawn) and lights-off (dusk; Hendricks et al. 2000; Shaw et al. 2000). The duration and intensity of the sleep–wake periods is determined by photoperiod and temperature (Majercak et al. 1999). This regulation relies on the endogenous circadian clock and appears to be an important adaptation allowing D. melanogaster to colonize temperate climates where daylight hours and temperature can vary greatly with the seasons (Majercak et al. 1999; Low et al. 2008). Thus, D. melanogaster responds to an increase in environmental temperature by reducing locomotor activity and increasing sleep levels during the midday ‘siesta’ period and moving the peak of evening locomotor activity further into night-time. This mechanism can reduce foraging activity during the long hot daylight hours of the summer months, thus lowering the risk of desiccation (Chen et al. 2007).
The sleep–wake cycle of D. melanogaster is seen in both males and females, but there is a large sex difference between the amounts of sleep experienced during daylight hours, with females sleeping for only 40 per cent of the time that male flies sleep (Huber et al. 2004; Andretic & Shaw 2005). This sexual dimorphic sleep has been observed in several strains of D. melanogaster and probably reflects the greater need of females in a natural environment to stay awake to forage for food and to select sites for egg laying, while males can spend more time conserving energy and avoiding physical and predatory dangers by remaining still. The greater locomotor activity observed in females during midday and afternoon might expose the fly not only to predation but also to thermal stress and water loss, a particular problem for small insects with a high surface area/volume ratio. Therefore, female D. melanogaster has to balance these possible life-threatening eventualities with the need to acquire nutrients for egg production, and to seek and select ovipositing sites. We might therefore expect that virgin females with no immediate need either to increase calorie intake or to oviposit will behave differently from mated females by having a male-like quiescent midday period after the peak morning activity, and that a more high-risk foraging and egg-laying behaviour will be adopted only after mating.
In this study we show that copulation results in a loss of day-time sleep and a switch to a more active ambulatory state compatible with foraging, feeding and ovipositing behaviours typical of post-mated females. This switch in behaviour requires the male sex peptide (SP), a product of the male accessory gland.
2. Material and methods
(a). Fly strains
Oregon R flies were from an established stock maintained in our laboratory for over 20 years. The Canton-S and Dahomey wild-type strains were provided by S. T. Sweeney, University of York, UK, and T. Chapman, University of East Anglia, UK. SP null mutants (SP0) and control wild-type flies (SP+) were generated as described previously (Liu & Kubli 2003) using mutant stocks, originating from the laboratory of E. Kubli (provided by B. J. Dickson, Research Institute of Molecular Pathology, Vienna). tud1 bw1/CyO flies were obtained from the Bloomington Stock Center. Male survivors from tud1/tud1 females are sterile with testes that lack germ cells.
(b). Fly culture
Flies were cultured on oatmeal/molasses/yeast/agar medium at 25°C in 12 : 12 h light : dark cycle and were sexed at the pupal stage on the basis of presence/absence of male sex combs.
(c). Sleep behaviour
All experiments were conducted at 25°C in a 12 : 12 h light : dark cycle. Unless otherwise stated, female flies were mated with males by placing 10 virgin females with 10 virgin males in vials (8 × 2 cm) containing oatmeal/molasses/agar diet for 3 days from eclosion. Virgin females were kept in groups of 20 for the same length of time under identical conditions. Flies were anaesthetised using CO2 and placed in glass tubes (65 mm long, 5 mm diameter) plugged at one end with 2 per cent agar containing 5 per cent sucrose and at the other with a ball of cotton wool. Tubes were placed in activity monitors (DAM2, Trikinetics Inc., Waltham, MA, USA) that use an infrared beam to detect movement as the fly walks along the glass tube. The number of beam breaks occurring in either 5 min or 30 min time-bins was recorded for individual flies and the data analysed using Microsoft Excel. Flies were allowed to recover from CO2 anaesthesia for 12 h before locomotor data were stored. A sleep period was defined as a 5 min time-bin with no locomotor activity and sleep latency was the time in minutes between lights-off and the first recorded night-time sleep period (Andretic & Shaw 2005). For determining the role of SP, single pairs of Oregon R females and either mutant (SP0) or wild-type (SP+) males were observed in vials with standard food until mating occurred. After mating, females were separated from the males and placed in activity tubes for monitoring activity–sleep behaviour. Activity and sleep data were statistically analysed using the Student's t-test.
3. Results
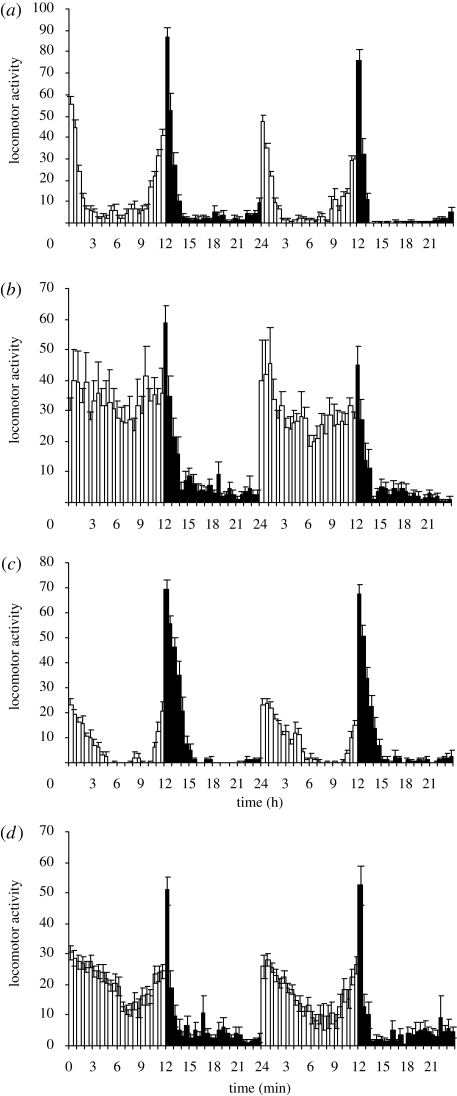
To test our hypothesis that virgin and post-mated females differ in their sleep–wake architecture, we compared the rhythmic walking activity of age-matched virgin female, mated female and male flies of the Oregon R strain in a 12 : 12 h light : dark cycle. Comparison of mated females with males revealed the sexual dimorphic locomotor behaviour that has been reported previously (figure 1; Shaw et al. 2000). Male flies have peaks of walking activity at the transition between lights-on and lights-off, with troughs of activity at night and during daylight hours. Mated females also have very low activity during night-time, but differ markedly from males in the sustained and robust activity evident during day time. However, this difference in walking activity in the afternoon period is not seen with virgin female flies, whose activity profile is more similar to that of males. The mating-induced increase in ambulation in post-mated females during the ‘siesta’ period was also observed when wild-type females were mated with males derived from tud1/tud1 females that lack sperm (figure 1d), establishing that sperm was not critical for switching the behaviour of virgin females. However, there was a decline in total day-time locomotor activity from 504 beam breaks on day 1 to 396 on day 2 of the recordings (p = 0.01, Student's t-test), suggesting that sperm might help to sustain the post-mating response.
Figure 1.
Sexually dimorphic locomotor rhythms of Oregon R flies ((a) virgin males; (b) female mated to wild-type male; (c) virgin females; (d) females mated to spermless males) entrained in a 12 : 12 h light : dark cycle. Locomotor activities are expressed as number of beam breaks in 30 min (mean ± s.e.m., n = 22). White and black bars indicate day-time and night-time, respectively. Time is expressed as zeitgeber time with 0 h being the time of lights-on and 12 h being the time of lights-off.
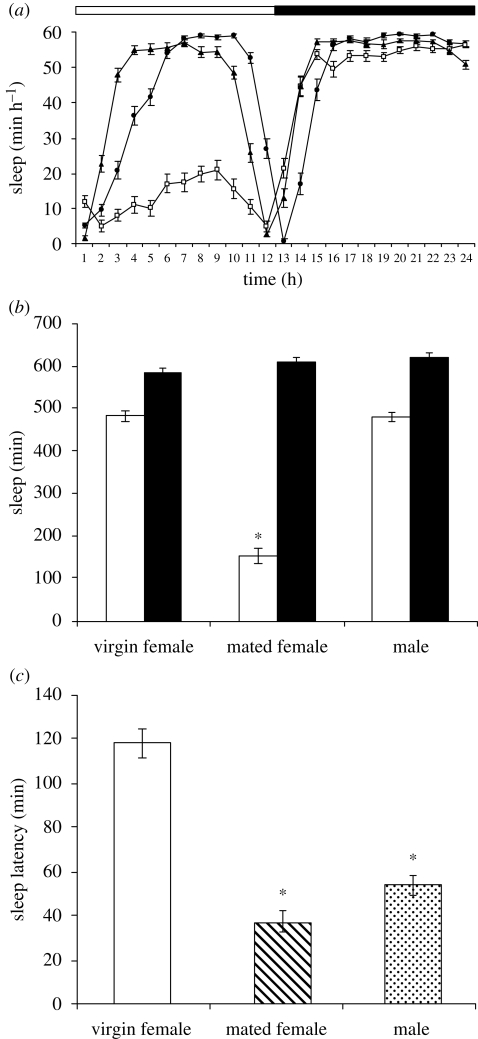
A sleep-like state was quantified from the data generated by the activity monitors with one sleep unit defined as a 5 min time-bin with no registered beam breaks (Andretic & Shaw 2005). This analysis shows that mated females experience 70 per cent less sleep during daylight hours compared to both virgin female and male flies, whereas total night-time sleep is hardly affected by the mating status of the females (figure 2a,b). The sleep profiles also showed that virgin females take longer to initiate sleep after lights-off. This evening sleep latency, measured by recording the first sleep bout of the evening, was threefold longer for virgins compared with mated females (figure 2c) and is indicative of an increase in sleep-drive in response to the mating-induced reduction in day-time sleep. Similar results were obtained when the same studies were repeated with two additional wild-type D. melanogaster strains (Canton-S and Dahomey; electronic supplementary material, fig. S1), showing that these differences between virgin and mated female flies are not restricted to the Oregon R strain.
Figure 2.
Mating results in loss of day-time sleep (a,b) and a reduction in sleep onset latency (c) in female Oregon R flies (filled circle, virgin female; open square, mated female; filled triangle, male). White and black bars in (a) and (b) indicate day-time and night-time, respectively. Sleep is expressed as minutes of sleep/hour (mean ± s.e.m., n = 22). *p < 0.0001, statistical significance of the difference in (i) day-time sleep between females mated to wild-type Oregon R males and virgin Oregon R males and females, (ii) sleep latency between virgin females and either females mated to wild-type Oregon R males or virgin Oregon R males (Student's t-test). A sleep period was defined as a 5 min period with no locomotor activity and sleep latency was the time in minutes between lights-off and the first recorded night-time sleep period. Time is expressed as zeitgeber time.
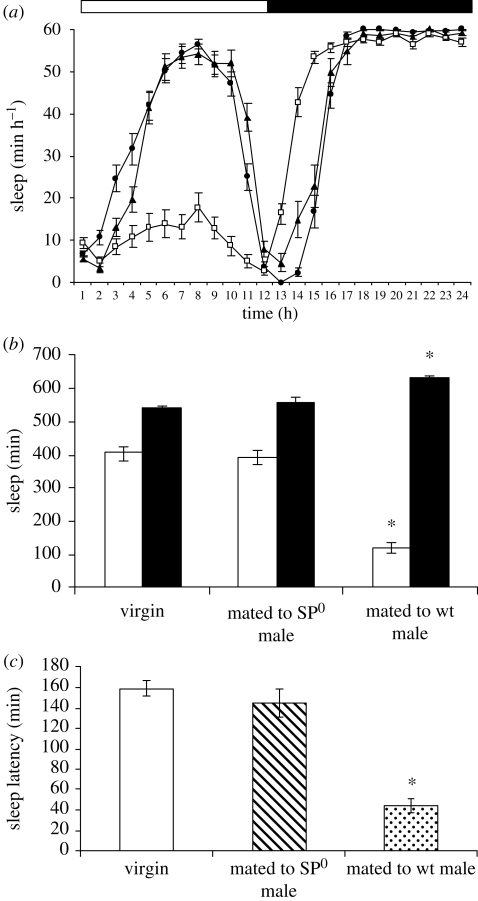
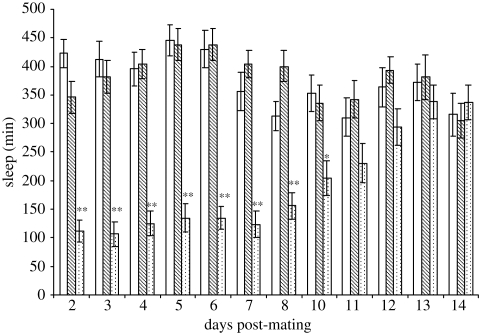
It is well established that molecules present in the seminal fluid of male D. melanogaster elicit profound changes in the physiology and behaviour of mated females (Chapman & Davies 2004). SP is the best known of these signalling molecules and is responsible for triggering several post-mating responses (Kubli 2003). We considered the possibility that promotion of day-time activity is yet another component of the SP signalling repertoire. SP null (SP0) males carrying a targeted deletion of the SP gene were used to inseminate wild-type females with sperm and seminal fluid minus SP. These females experienced the same amount of midday sleep as virgins, whereas copulation with control males (SP+) of the same genetic background resulted in a 70 per cent reduction of day-time sleep (figure 3a,b). The reduction in sleep onset latency that accompanies the sleep deprivation in post-mated females was also abolished when females were mated with SP0 males (figure 3c). These results show that SP is a wake-promoting molecule that alters the day-time behaviour of females from male-like laziness to a more active state necessary for feeding and ovipositing activities. The loss of sleep resulting from a single mating with wild-type (SP+) males was sustained for up to 10 days after copulation, after which day-time sleep approached levels seen in both virgin females and those mated with SP0 males (figure 4). Thus, wake promotion and increased walking activity is a long-term post-mating response to a single copulation.
Figure 3.
Mating-induced changes in sleep behaviours of female Oregon R flies are dependent upon male SP. Sleep and sleep latency are defined as in legend to figure 2 (filled circle, virgin; filled triangle, mated to SP0 male; open square, mated to wild-type male). Unlike wild-type males (wt, SP+), males lacking SP (SP0) do not elicit a change in total day-time sleep (a,b) and do not reduce sleep-onset latency (c) of post-mated female flies. White and black bars in (a) and (b) indicate day-time and night-time, respectively. Values are means ± s.e.m., n = 20–23. *p < 0.0002, statistical significance of the differences in sleep and sleep latency between females mated to wild-type males (SP+) and either virgin females or females mated to SP0 males (Student's t-test). Time is expressed as zeitgeber time.
Figure 4.
SP-dependent loss of total day-time sleep in female (Oregon R) flies persists for 10 days before returning to ‘virgin’ levels by day 14. Data for day 9 were omitted because flies were transferred to fresh food on this day. Values of total day-time sleep are means ± s.e.m., n = 20–23. **p < 0.00 001, *p < 0.05, statistical significances of the differences in daylight sleep between virgin females and females mated to wild-type SP+ males (Student's t-test; open bar, virgin; hatched bar, mated to SP0 male; dotted bar, mated to wt male).
4. Discussion
In this study, we have shown that the sexually dimorphic nature of the sleep–wake behaviour of adult D. melanogaster is dependent on the mating status of the female. Male flies display a marked bimodal sleep pattern, with day-time and night-time sleep periods separated by intense periods of locomotor activity coinciding with dawn and dusk. Mated females maintain a similar level of night-time sleep as males, but are much more active during the day time. However, this wakefulness is greatly reduced in virgin females, who display a male-like tendency for robust day-time sleep. We propose that this behaviour of virgin females is advantageous by permitting courtship, mating and adequate foraging, and at the same time minimizes risks posed by hazardous environmental conditions, such as high temperatures of the midday and afternoon. After mating, females switch from a low-risk behaviour to a more adventurous lifestyle that involves increased day-time wakefulness and locomotor activity, which is necessary to find food to satisfy the greater nutritional demands imposed by the very high rate of egg production, and for seeking suitable egg-laying sites. Locomotor activity and quiescence were analysed using commercially available equipment (Trikinetics DAM2) that has the advantage of being able to monitor a large number of individual flies over several days and is the standard method used in Drosophila sleep studies (Andretic & Shaw 2005). It is recognized, however, that such a system is limited to recording walking and cannot provide a full description of adult behaviour.
The observed changes in female behaviour did not require transfer of sperm for initiation, but were totally dependent upon SP, a peptide made by the male accessory gland and secreted into the seminal fluid. SP is one of many seminal fluid proteins/peptides synthesized by the male accessory glands and transferred into the female reproductive tract during copulation (Chen et al. 1988; Kubli 2003). These accessory gland products have diverse biological roles (e.g. structural components of temporary mating plugs, immune defense, facilitating sperm transfer, storage and viability, metabolic enzymes, regulating ovulation, and altering female behaviour) that together maximize an individual male's chances of paternity (Chapman & Davies 2004; Wolfner 2007). SP has a central role in this strategy by eliciting male-rejection behaviour in post-mated females, who, after a single copulation, avoid further mating for around one week by extruding the ovipositor and closing the vaginal pore (Chen et al. 1988; Kubli 1992). SP also elicits an increase in the rate of ovulation and the production of antimicrobial peptides in the post-mated female (Kubli 2003; Peng et al. 2005b). The rejection behaviour and increase in egg production elicited by SP is mediated by a small number of sensory neurons in the female reproductive tract that express the SP receptor (Yapici et al. 2008; Hasemeyer et al. 2009; Yang et al. 2009). SP is a 36-amino-acid peptide with a tryptophan-rich N-terminal region by which it attaches to the external surface of sperm tails, a mechanism that carries SP with sperm to the sperm storage organs of the female (Liu & Kubli 2003; Peng et al. 2005a). Biologically active SP is slowly released from this attachment by proteolysis, providing sufficient peptide in the female reproductive tract to prolong the response of the female for as long as one week after copulation (Peng et al. 2005a). In our study we have shown that the loss of day-time sleep in the post-mated female lasts for between 8 and 10 days after a single copulation before returning to virgin female levels. This long-term response is therefore probably sustained by the processing of soluble SP from sperm stored in the female.
Recently, it has been shown that SP stimulates an increase in feeding rate, presumably to satisfy the reproductive demand for nutrients in post-mated females, who can lay as many as 100 eggs per day (Carvalho et al. 2006). This appears to be an indirect effect of SP signalling since the elevated feeding depends on the ability to produce eggs (Barnes et al. 2008). Our study suggests that SP changes feeding-related behaviours not only by elevating appetite, but also by stimulating foraging behaviour by modulating day-time sleep–wake activity. Unlike the SP-induced increase in the rate of food ingestion, the change in female locomotor behaviour after mating is not linked to the ability to lay eggs, since SP elicited these effects in adult females fed solely with sucrose, which inhibits egg production (Ashburner et al. 2005). SP is responsible, at least in part, for a reduction in lifespan in multiple mated females (Wigby & Chapman 2005). This cost to the mated female might result from the SP-induced increase in food consumption since calorific restriction increases lifespan in D. melanogaster (Carvalho et al. 2006). However, a recent study concluded that over-eating does not have a direct effect on longevity and suggests that other, at present unknown, mechanisms are responsible (Barnes et al. 2008). It is known that adult female D. melanogaster begin to die after 60 hours of sleep deprivation and over-expression of stress response proteins can protect against this lethality (Shaw et al. 2002). Therefore it is possible that the SP-induced loss of sleep and the accompanying stress directly contribute to the reduction in lifespan of mated females.
Acknowledgements
We thank Sean Sweeney, Tracy Chapman and Barry Dickson for providing fly stocks and Carol Sowden and Alan Reynolds for technical assistance.
References
- Agosto J., Choi J. C., Parisky K. M., Stilwell G., Rosbash M., Griffith L. C.2008Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat. Neurosci. 11, 354–359 (doi:10.1038/nn2046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T., Cicchetti D. V.1976Sleep in mammals: ecological and constitutional correlates. Science 194, 732–734 (doi:10.1126/science.982039) [DOI] [PubMed] [Google Scholar]
- Andretic R., Shaw P. J.2005Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 393, 759–772 [DOI] [PubMed] [Google Scholar]
- Andretic R., van Swinderen B., Greenspan R. J.2005Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 15, 1165–1175 (doi:10.1016/j.cub.2005.05.025) [DOI] [PubMed] [Google Scholar]
- Ashburner M., Golic K. G., Hawley R. S.2005Drosophila: a laboratory handbook New York, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Barnes A. I., Wigby S., Boone J. M., Partridge L., Chapman T.2008Feeding, fecundity and lifespan in female Drosophila melanogaster. Proc. R. Soc. B 275, 1675–1683 (doi:10.1098/rspb.2008.0139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet M. H.2005Acute sleep deprivation. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 51–66 Philadelphia, PA: Elsevier Saunders [Google Scholar]
- Capellini I., Barton R. A., McNamara P., Preston B. T., Nunn C. L.2008Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution 62, 1764–1776 (doi:10.1111/j.1558-5646.2008.00392.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho G. B., Kapahi P., Anderson D. J., Benzer S.2006Allocrine modulation of feeding behaviour by the sex peptide of Drosophila. Curr. Biol. 16, 692–696 (doi:10.1016/j.cub.2006.02.064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T., Davies S. J.2004Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides 25, 1477–1490 (doi:10.1016/j.peptides.2003.10.023) [DOI] [PubMed] [Google Scholar]
- Chen P. S., Stumm-Zollinger E., Aigaki T., Balmer J., Bienz M., Bohlen P.1988A male accessory gland peptide that regulates reproductive behaviour of female D. melanogaster. Cell 54, 291–298 (doi:10.1016/0092-8674(88)90192-4) [DOI] [PubMed] [Google Scholar]
- Chen W. F., Low K. H., Lim C., Edery I.2007Thermosensitive splicing of a clock gene and seasonal adaptation. Cold Spring Harb. Symp. Quant. Biol. 72, 599–606 (doi:10.1101/sqb.2007.72.021) [DOI] [PubMed] [Google Scholar]
- Dinges D. F., Rogers N. L., Baynard M. D.2005Chronic sleep deprivation. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 67–76 Philadelphia, PA: Elsevier Saunders [Google Scholar]
- Hamet P., Tremblay J.2006Genetics of the sleep–wake cycle and its disorders. Metabolism 55, S7–S12 (doi:10.1016/j.metabol.2006.07.006) [DOI] [PubMed] [Google Scholar]
- Hasemeyer M., Yapici N., Heberlein U., Dickson B. J.2009Sensory neurons in the Drosophila genital tract regulate female reproductive behaviour. Neuron 61, 511–518 (doi:10.1016/j.neuron.2009.01.009) [DOI] [PubMed] [Google Scholar]
- Hendricks J. C.2003Invited review: sleeping flies don't lie: the use of Drosophila melanogaster to study sleep and circadian rhythms. J. Appl. Physiol. 94, 1660–1672 [DOI] [PubMed] [Google Scholar]
- Hendricks J. C., Finn S. M., Panckeri K. A., Chavkin J., Williams J. A., Sehgal A., Pack A. I.2000Rest in Drosophila is a sleep-like state. Neuron 25, 129–138 (doi:10.1016/S0896-6273(00)80877-6) [DOI] [PubMed] [Google Scholar]
- Huber R., Hill S. L., Holladay C., Biesiadecki M., Tononi G., Cirelli C.2004Sleep homeostasis in Drosophila melanogaster. Sleep 27, 628–639 [DOI] [PubMed] [Google Scholar]
- Koh K., Evans J. M., Hendricks J. C., Sehgal A.2006A Drosophila model for age-associated changes in sleep: wake cycles. Proc. Natl Acad. Sci. USA 103, 13 843–13 847 (doi:10.1073/pnas.0605903103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E.1992The sex-peptide. Bioessays 14, 779–784 (doi:10.1002/bies.950141111) [DOI] [PubMed] [Google Scholar]
- Kubli E.2003Sex-peptides: seminal peptides of the Drosophila male. Cell Mol. Life Sci. 60, 1689–1704 (doi:10.1007/s00018-003-3052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K., Kume S., Park S. K., Hirsh J., Jackson F. R.2005Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25, 7377–7384 (doi:10.1523/JNEUROSCI.2048-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesku J. A., Roth T. C., 2nd, Amlaner C. J., Lima S. L.2006A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. Am. Nat. 168, 441–453 (doi:10.1086/506973) [DOI] [PubMed] [Google Scholar]
- Lesku J. A., Roth T. C., Rattenborg N. C., Amlaner C. J., Lima S. L.2008Phylogenetics and the correlates of mammalian sleep: a reappraisal. Sleep Med. Rev. 12, 229–244 (doi:10.1016/j.smrv.2007.10.003) [DOI] [PubMed] [Google Scholar]
- Liu H., Kubli E.2003Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 100, 9929–9933 (doi:10.1073/pnas.1631700100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Guo F., Lu B., Guo A.2008Amnesiac regulates sleep onset and maintenance in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 372, 798–803 (doi:10.1016/j.bbrc.2008.05.119) [DOI] [PubMed] [Google Scholar]
- Low K. H., Lim C., Ko H. W., Edery I.2008Natural variation in the splice site strength of a clock gene and species-specific thermal adaptation. Neuron 60, 1054–1067 (doi:10.1016/j.neuron.2008.10.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majercak J., Sidote D., Hardin P. E., Edery I.1999How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron 24, 219–230 (doi:10.1016/S0896-6273(00)80834-X) [DOI] [PubMed] [Google Scholar]
- Peng J., Chen S., Busser S., Liu H., Honegger T., Kubli E.2005aGradual release of sperm bound sex-peptide controls female postmating behaviour in Drosophila. Curr. Biol. 15, 207–213 (doi:10.1016/j.cub.2005.01.034) [DOI] [PubMed] [Google Scholar]
- Peng J., Zipperlen P., Kubli E.2005bDrosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr. Biol. 15, 1690–1694 (doi:10.1016/j.cub.2005.08.048) [DOI] [PubMed] [Google Scholar]
- Shaw P.2003Awakening to the behavioural analysis of sleep in Drosophila. J. Biol. Rhythms 18, 4–11 (doi:10.1177/0748730402239672) [DOI] [PubMed] [Google Scholar]
- Shaw P. J., Cirelli C., Greenspan R. J., Tononi G.2000Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 (doi:10.1126/science.287.5459.1834) [DOI] [PubMed] [Google Scholar]
- Shaw P. J., Tononi G., Greenspan R. J., Robinson D. F.2002Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature 417, 287–291 (doi:10.1038/417287a) [DOI] [PubMed] [Google Scholar]
- Siegel J. M.2005Clues to the functions of mammalian sleep. Nature 437, 1264–1271 (doi:10.1038/nature04285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. M.2008Do all animals sleep? Trends Neurosci. 31, 208–213 (doi:10.1016/j.tins.2008.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S., Lin L., Austin D., Young T., Mignot E.2004Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 1, e62 (doi:10.1371/journal.pmed.0010062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigby S., Chapman T.2005Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15, 316–321 (doi:10.1016/j.cub.2005.01.051) [DOI] [PubMed] [Google Scholar]
- Wolfner M. F.2007‘S.P.E.R.M.’ (seminal proteins (are) essential reproductive modulators): the view from Drosophila. Soc. Reprod. Fertil. Suppl. 65, 183–199 [PubMed] [Google Scholar]
- Yang C. H., Rumpf S., Xiang Y., Gordon M. D., Song W., Jan L. Y., Jan Y. N.2009Control of the postmating behavioural switch in Drosophila females by internal sensory neurons. Neuron 61, 519–526 (doi:10.1016/j.neuron.2008.12.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N., Kim Y. J., Ribeiro C., Dickson B. J.2008A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451, 33–37 (doi:10.1038/nature06483) [DOI] [PubMed] [Google Scholar]
- Yuan Q., Joiner W. J., Sehgal A.2006A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr. Biol 16, 1051–1062 (doi:10.1016/j.cub.2006.04.032) [DOI] [PubMed] [Google Scholar]
- Zepelin H., Siegel J. M., Tobler I.2005Mammalian sleep. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 91–100 Philadelphia, PA: Elsevier Saunders [Google Scholar]