Abstract
The pre-sacral vertebrae of most sauropod dinosaurs were surrounded by interconnected, air-filled diverticula, penetrating into the bones and creating an intricate internal cavity system within the vertebrae. Computational finite-element models of two sauropod cervical vertebrae now demonstrate the mechanical reason for vertebral pneumaticity. The analyses show that the structure of the cervical vertebrae leads to an even distribution of all occurring stress fields along the vertebrae, concentrated mainly on their external surface and the vertebral laminae. The regions between vertebral laminae and the interior part of the vertebral body including thin bony struts and septa are mostly unloaded and pneumatic structures are positioned in these regions of minimal stress. The morphology of sauropod cervical vertebrae was influenced by strongly segmented axial neck muscles, which require only small attachment areas on each vertebra, and pneumatic epithelia that are able to resorb bone that is not mechanically loaded. The interaction of these soft tissues with the bony tissue of the vertebrae produced lightweight, air-filled vertebrae in which most stresses were borne by the external cortical bone. Cervical pneumaticity was therefore an important prerequisite for neck enlargement in sauropods. Thus, we expect that vertebral pneumaticity in other parts of the body to have a similar role in enabling gigantism.
Keywords: sauropoda, vertebral pneumaticity, finite-element analysis, cervical vertebrae, gigantism
1. Introduction
Extensive vertebral pneumaticity is one of the most striking anatomical features of sauropod dinosaurs (Britt 1993; Wedel 2003a,b; Wedel 2005; O'Connor 2006; Sereno et al. 2007), the largest terrestrial vertebrates of all time. Among extant vertebrates, only birds possess similar extensive postcranial pneumaticity, with pneumatic diverticula invading large parts of the postcranial skeleton (Müller 1908; Duncker 1971; O'Connor 2004). Osteological comparisons with extant birds have yielded convincing evidence for skeletal pneumaticity in pterosaurs (Claessens et al. 2009), theropod dinosaurs and sauropod dinosaurs, although pneumatic structures may have been present in basal non-ornithodiran archosaurs too (Gower 2001). Many hypotheses exist about the biological roles of vertebral pneumaticity in sauropods but these focus largely on their importance in weight-saving and respiration (Wedel 2003b, 2005, 2007; Perry & Sander 2004). Using anatomical comparisons with birds, the pneumatic diverticula of sauropod cervicals were reconstructed. This revealed that in most sauropods the positions of the pneumatic diverticula are similar, although there is considerable variation in the size of the diverticula and the number of subdivisions within them (Wedel 2005; Schwarz & Fritsch 2006; Schwarz et al. 2007). Computed-tomographic (CT) images expose the distribution and geometry of the pneumatic cavity system within the cervical vertebrae, which ranges in different sauropod taxa from having a few large camerae to many small camellae or a honeycomb-like cavity system (Wedel et al. 2000). CT sections allow a quantification of the amount of pneumatic weight reduction in the vertebra, which in an adult neosauropod is around 50–60%, but could range up to 79 per cent in the largest neosauropods like Sauroposeidon (see electronic supplementary material and Wedel 2005).
It has been postulated that pneumatic structures form in unstressed areas of the bone (Bremer 1940; Witmer 1997) and that pneumatic bones would have a mechanical advantage in allowing the bone material to be concentrated far away from the centre of rotation (Hunter 1774; Currey & Alexander 1985). The mechanical basis of pneumatization in sauropod cervical vertebrae up to 1.3 m or more in length has not been studied previously using biomechanical approaches (Lovelace et al. 2007). Therefore, we applied a finite-element analysis (FEA) to investigate the mechanical configuration of a pneumatic mid-cervical vertebra from an undetermined diplodocid and the third cervical vertebra of Brachiosaurus (see electronic supplementary material and figure 1). We used three loading scenarios that we consider to have occurred habitually in all sauropod necks: (i) an extension of the neck by a quick elevation of 4 g, (ii) lateral movement of the neck with an lateral acceleration of 2 g and (iii) lowering of the neck by gravity allowing for cervical ribs to overlap in Brachiosaurus as well as and contact between the cervical ribs in the case of Diplodocus.
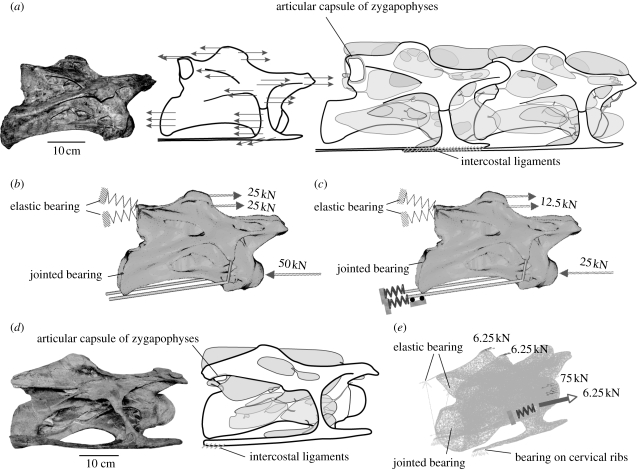
Figure 1.
Used soft-tissue insertions (after Schwarz et al. 2007; Schwarz & Frey 2008) and simplified models for FE analysis. (a) Fourth cervical vertebra of Brachiosaurus brancai (MB.R.2080.25), line drawing with vectors of assumed axial cervical muscles indicated in their insertion sites, and reconstruction of fourth and fifth cervical vertebrae of B. brancai with pneumatic diverticula and articular soft tissues; right lateral view. (b) Model for the fourth cervical vertebra of B. brancai used in the FEA with applied forces during extension. (c) Same model with applied forces during ventral flexion and suspension on the cervical ribs. (d) Mid-cervical vertebra of Diplodocus sp. (SMA L25-3) in right lateral view and with reconstructed pneumatic diverticula and soft tissues used for FEA in the load case of lateral flexion. (e) Model for the mid-cervical vertebra of Diplodocus used in the FEA with applied forces during lateral flexion.
2. Results of finite-element analysis
(a). Caveats
The results of the FEA (see also electronic supplementary material) are expected to realistically display the distribution and direction of the stresses across the studied vertebrae. However, the absolute values obtained from the analyses are imprecise and not significant, because:
the mass and weight of the neck cannot be determined precisely,
the geometry of the models are approximate since it was necessary to simplify the dataset and because cross-linking with linear tetrahedrons yields imprecise results,
the material properties and bone mineral fraction of dinosaur bone are not known precisely and anisotropy of the bone was not considered. Both factors influence stress and strain distribution within the bone.
the insertation points of tendons and muscles in the model were approximated. In some areas, forces were only applied at a single point resulting in extreme localized stress.
(b). Extension of the neck
The main and the comparative stresses are similar. High stresses occur mainly uniaxially, affecting only the longitudinal axis as compression along the massive bar-like connection between the cranial and caudal articular surface of the vertebra (figure 2 and electronic supplementary material). Multiaxial stresses, affecting longitudinal, transverse and vertical axes, and tensional stresses occur around the zygapophyses (figure 2). Comparative loads at the contact between costal capitulum and parapophysis and the caudal part of the cervical ribs (preserved in our model in Diplodocus only) display high stress dependence on the relationship between the stiffness of the rib and the zygapophyseal ligaments (figure 3). The occurrence of a well-rounded transitional area (figure 3, see arrow) indicates a region normally exposed to such elevated stresses. The tendency of the vertebrae for lateral buckling and torque between the segments is counteracted by the zygapophyseal articulations, which contribute to the even stress distribution across the vertebrae.
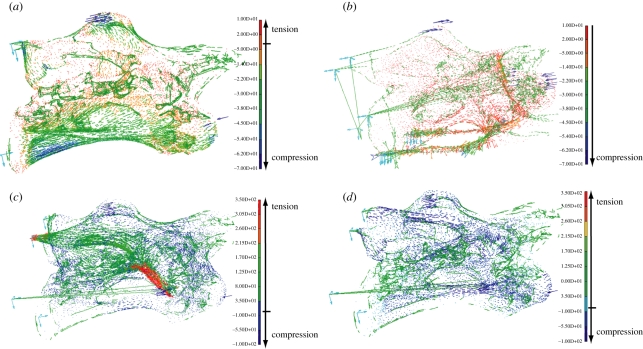
Figure 2.
Distribution of main (=real) stress in the models for cervical vertebrae of Brachiosaurus and Diplodocus, displayed as vector plots in N mm−2 (note that minimum, negative stress values represent compression, but maximum, positive stress values represent tension). (a) Brachiosaurus vertebra during extension: high stress occurs mainly uniaxially (affecting only the longitudinal axis) and as compression along the vertebral laminae as shown by the contours of the vertebra. (b) Diplodocus vertebra during lateral flexion, showing main compressive stress (filtered by displaying only minimum mathematical stress values), concentrated mainly on vertebral body and to a smaller extent on neural arch. The compressive stress occurring is at least one order of magnitude higher than the tensional stress, which is not displayed here. (c) Brachiosaurus vertebra during lateral flexion, showing tensional stress by focusing on mathematically maximum values. A maximum of tensionsal stress occurs around the diapophysis and zygapophyseal articular capsule of the flexed body side. (d) Same Brachiosaurus cervical vertebra during lateral flexion, showing compressive stress by focusing on mathematically minimum values, which are regularly distributed along the external side of the vertebra.
Figure 3.
Von Mises comparative stresses shows a situation where all stress acting on the object in all three dimensions (=multiaxial) is combined and simplified in an uniaxial stress situation displayed in N mm−2. Diplodocus cervical vertebra during extension: (a) high comparative stress occurs around the parapophysis, fused costal capitulum, and cervical rib (arrow marks well-rounded transitional area described in the text), (b) cross section reveals nearly unloaded interior of the vertebra. Brachiosaurus cervical vertebra, (c) extended side during lateral flexion: comparative stress concentration is visible in green and yellow colours on vertebral laminae, (d) cross section during lateral flexion reveals nearly stress-free interior of the vertebra.
(c). Lateral movement of the neck
High forces act on the neck during lateral movement because of the combination of gravity, compression and tension. Thus, both vertebrae examined are exposed to higher compressional and tensional stresses than during neck extension, with compression being generally an order of magnitude higher. The distribution of main stresses exposes high tensional stresses in the dorsal part of the vertebra, i.e. in the region of the zygapophyses and the diapophysis. High compressional loads occur at the vertebral body between the condyle and cotyla. Von Mises comparative stresses show that on the extended side of the vertebrae, the spino-pre-zygapophyseal and spino-post-zygapophyseal lamina, the postzygodiapophyseal lamina, posterior centro-diapophyseal lamina and the posterior centroparapophyseal lamina are loaded (figure 3). On its flexed side, the pre- and post-zygodiapophyseal lamina and the posterior centrodiapophyseal lamina are loaded. On the flexed side of the vertebra of Brachiosaurus, preserved without cervical rib, the main tensional stresses are highest in the region of the diapophysis (see electronic supplementary material). In contrast, the presence of fused cervical ribs like in Diplodocus results in stress at the diapophysis, rib tuberculum and the caudally adjacent part of the cervical rib corpus. On the extended side, the diapophysis is only slightly loaded in Brachiosaurus and nearly unloaded in Diplodocus, with stresses being concentrated to the cervical rib and adjacent parapophysis.
(d). Supposition of the neck vertebrae on the cervical ribs
There are two possibilities for soft-tissue reconstructions in cervical ribs of sauropods and both use osteological correlates on the ribs. In the first scenario, the cervical ribs are connected by intercostal ligament fibres (figure 1a,c) like in extant Crocodylia (Frey 1988; Schwarz et al. 2007). In the second scenario, the long and thin cervical ribs of sauropods represent ossified tendons of hypaxial neck muscles like in extant Aves (Wedel & Sanders 2002). Consequently, they are not connected by intercostal ligament fibres. For the FEA, the cervical ribs of sauropods were first modelled as ossified tendons of hypaxial neck muscles without interconnection. In this case, an even and mainly compressional stress distribution occurred, and the cervical ribs had no support effect at all. The neck was supported exclusively by dorsal ligaments and muscles. In the second analysis, cervical ribs were connected by intercostal fibres. This arrangement also formed an even and mainly compressional stress distribution in both vertebrae but the units of ribs interconnected by elastic ligament fibres reduced the forces at the intervertebral articulation. The amount of force reduction depends on the spring stiffness, c, but assuming a similar spring stiffness for the dorsal and ventral ligaments, this reached up to 40 per cent (cu/co=1) (figure 4).
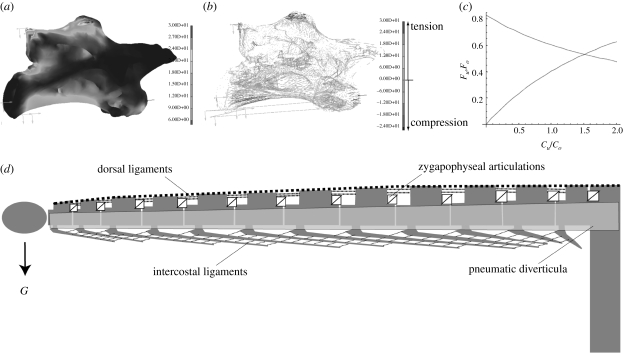
Figure 4.
Flexion of the cervical vertebra of Brachiosaurus and suspension on the ribs results in a regular, mainly compressional stress distribution. (a) Von Mises comparative stresses, (b) main stress displayed as vector plots in N mm−2, (c) diagram of necessary forces (F) and spring stiffness (C) in relation with FEA: force reduction is dependent on the assumed spring stiffness; (d) biomechanical model for a generalized sauropod neck with main bracing structures, representing a segmented cantilever loaded by its own weight (G). The intercostal ligaments represent only one reconstruction possibility, the alternative to which would be ossified tendons of axial neck muscles.
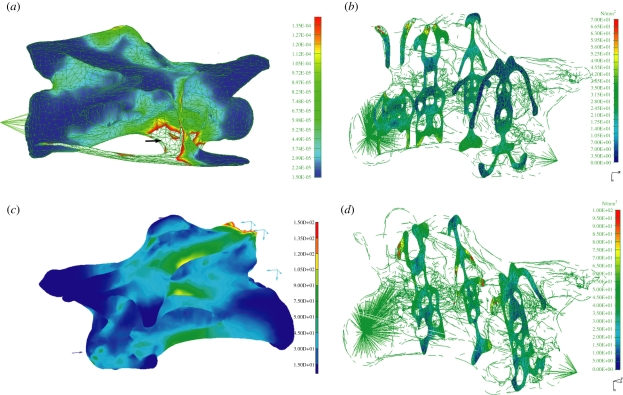
(e). Cross sections and the pneumatic cavity system
Comparative stresses are distributed evenly around the vertebrae and mainly on the bone cortex. Peak stresses occur only at points where the tendons and muscles are inserting because the insertion areas used were small resulting in extreme localized stresses. The interior of both vertebrae is nearly stress free. Almost no stresses occur around the cavities and in their bony walls (figure 3).
3. Discussion
The FEA reveals an even distribution of all occurring stress fields along the vertebrae and no peak stresses occur. The main stress is mostly compressional and positioned within the vertebral body, whereas tensional stress is an order of magnitude lower and restricted to the neural arch (figures 2 and 3). Both sauropod vertebrae investigated appear to be optimized for compressional loads, which are distributed along vertebral laminae, leaving the regions between laminae and the interior part of the vertebral body unloaded (figure 3). Pneumatic cavities are positioned in regions of minimal stress as indicated by the almost unloaded pneumatic cavity walls (figure 3). This demonstrates that the position of the pneumatic spaces is consistent with the distribution of main stresses within the vertebrae. The absence of higher loads within the vertebral body and neural arch allows thin and even perforate internal and median bony septa within the vertebrae (see Wedel et al. (2000) and Schwarz & Fritsch (2006) for CT images).
The pneumatic epithelium that forms the diverticula is, by its close association with osteoclasts, capable of extensive resorption of bone material (Witmer 1997). Additionally, the reconstructed localized insertion of strongly segmented axial cervical muscles in sauropod dinosaurs (Wedel & Sanders 2002; Schwarz et al. 2007) would keep the required attachment areas on the bone surface small, allowing the formation of large pneumatic fossae and foramina penetrating the bone surface. Wilson (1999) mentions that vertebral laminae and narrow bone struts control the distribution of stress. Our analysis confirms this role as we demonstrate that the existence of pneumatic epithelium in the post-cranium and small-scale insertion areas of segmented axial muscles permit the extensive pneumatization of sauropod cervical vertebrae and makes them, in accordance with Wolff's law (Wolff 1892; Witzel & Preuschoft 2005), true light-weight constructions. Explanations for a similar mechanism for the cranial pneumatic sinuses of archosaurs were given by Witmer (1997). He argued that the sinuses can only expand as much as the biomechanical loading regime of the skull allows while remaining stable. The results of the FEA give no explanation for the different sizes and geometries of pneumatic diverticula, i.e. large camerae versus small camellae, which might be due to the variation in pneumatization, caused by small differences in loading between vertebrae. In the examples, the presence of camellae in Brachiosaurus seems to result in a slightly higher amount of bone reduction combined with a reduction in weight (see also electronic supplementary material). However, the size of pneumatic cavities in sauropods might also depend on physiological and/or ontogenetic factors. The FEA also gives no hint about the posture of the neck in these sauropods (e.g. Christian 2002; Dzemski & Christian 2007; Sander et al. 2009; Seymour 2009a,b; Stevens & Parrish 1999; Taylor et al. 2009), although the absence of peak stresses in the vertebrae indicates that the test scenarios would have been possible for these sauropods at least occasionally. The horizontal neck position tested here represents a case in which higher stresses act on the vertebrae compared to a more vertical neck position (Taylor et al. 2009). This is because bending moments occurring in a horizontal neck are much weaker than in a vertical neck.
The results of the FEA were incorporated into a biomechanical model of the sauropod neck that represents a segmented cantilever loaded by its own weight (figure 4). The distribution of main stresses in the FEA vertebra models is consistent with the proposed loading of such a cantilever: the ventral part (the vertebral body) is loaded by compression, whereas the dorsal part (the neural arch) is subject to tension. Bracing structures that prevent sagging of the neck are the dorsal ligament apparatus (Martin et al. 1998; Tsuihiji 2004) and the strongly segmented axial neck musculature (Wedel & Sanders 2002; Schwarz et al. 2007). During all tested FEA load cases, the zygapophyseal articulations between the neck vertebrae form short V-shaped levers acting as dove-tail guidance, stabilizing the neck against torsion and lateral tilting of the neck vertebrae against each other. The dove-tail guidance mechanism of the zygapophyses is especially important during lateral movements of the neck, where high forces are exerted on the neck vertebrae by the axial muscles. Finally, assuming that the cervical ribs of Brachiosaurus (spanning over one to three vertebrae) and of the chosen Diplodocus specimen (overlapping with their cranial and caudal tips) were interconnected by intercostal ligament fibres like in extant crocodylians, the FEA results demonstrate that the cervical ribs would form an additional ventral support of the neck as mentioned (Martin 1987; Martin et al. 1998) (figure 4). However, assuming an alternative, bird-like soft-tissue reconstruction with an absence of intercostal ligament fibres, the ribs did not produce a support effect when represented as compressional elements. In the case of a bird-like configuration of the hypaxial neck muscles in sauropods, the ossified tendons (=cervical ribs) might also be loaded by tension during active ventral movement of the neck, which would result in a different loading scenario than those tested here. In any case, neck support by cervical ribs is absent in most diplodocid dinosaurs, such as Apatosaurus and Diplodocus carnegii, which possess short cervical ribs.
Owing to the connection of adjacent pneumatic diverticula in the neck by ducts and their close proximity to each other, the diverticula units could, even at pressures less than 1 kPa, have behaved like shock absorbers, buffering oscillations and torque of the long neck during walking. The shock absorbing effect would have allowed reduction of respective control muscles and thus provided further weight reduction. The occurrence of intravertebral pneumaticity in sauropods opened the evolutionary pathway for their specific lightweight construction and, in combination with the specific support systems, resulted in freely carried, extremely long necks (Wedel 2003b). The consequence of increasing vertebral pneumaticity is a step-by-step replacement of a heavy tendinomuscular system by a true lightweight construction without any loss of overall stability. The pattern of this structural change follows strict mechanical rules as is seen in the results of the FEA (Wolff 1892; Witzel & Preuschoft 2005). The variation of neck lengths and flexibilities in sauropods by the variation of bracing mechanisms resulted in the ecological separation of feeding ranges in different, often contemporary sauropod taxa (Upchurch & Barrett 2000). The significant effect of vertebral pneumaticity on weight reduction and the shock absorber effect of the pneumatic diverticula were certainly important factors in the dorsal vertebral column. These were major prerequisites for the evolution of light but massive bodies and paved the way for gigantism among sauropods. Thus, the mechanical design of sauropod vertebrae in combination with the presence of pneumatic epithelium is another important factor for the achievement of extreme body sizes in sauropods. This should therefore be added alongside physiological and ecological reasons for sauropod gigantism which have been recently discussed (Sander & Clauss 2008; McNab 2009).
Acknowledgements
We thank D. Unwin (formerly Museum für Naturkunde Berlin, Germany; now University of Leicester) and H.-J. Siber (Sauriermuseum Aathal, Switzerland) for access to the specimens studied with CT, H.-G. Bongartz and G. Fritsch for carrying out the CT data, R. Luchsinger for help with questions of industrially used pneumatic systems, U. Witzel for discussion and valuable hints on the interpretation of the FEA results, O. Wings for critical comments and J. Liston and M. Pittman for linguistic help. We are grateful for the helpful suggestions and critical comments of three referees that helped improving this work significantly. D.S.W. gratefully acknowledges the financial support of the Swiss National Science Foundation (SNF) through contract nos. 200021-101494/1 and 200020-109131/1.
References
- Bremer J. L.1940The pneumatization of the humerus in the common fowl and the associated activity of theelin. Anat. Rec. 77, 197–211 (doi:10.1002/ar.1090770209) [Google Scholar]
- Britt B. B.1993. Pneumatic postcranial bones in dinosaurs and other archosaurs. PhD thesis, University of Calgary, Alberta [Google Scholar]
- Christian A.2002Neck posture and overall body design in sauropods. Mitteilungen aus dem Museum für Naturkunde Berlin, Geowissenschaftliche Reihe 5, 271–281 [Google Scholar]
- Claessens L. P. A. M., O'Connor M. P., Unwin D. M.2009Respiratory evolution facilitated the origin of pterosaur flight and aerial gigantism. PLoS ONE 4, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currey J. D., Alexander R. M.1985The thickness of the walls of tubular bones. J. Zool. Lond. 206, 453–468 [Google Scholar]
- Duncker R.1971The lung air sac system of birds. Adv. Anat. Embryol. Cell Biol. 45, 1–171 [Google Scholar]
- Dzemski G., Christian A.2007Flexibility along the neck of the ostrich (Struthio camelus) and consequences for the reconstruction of dinosaurs with extreme neck lengths. J. Morphol. 268, 701–714 (doi:10.1002/jmor.10542) [DOI] [PubMed] [Google Scholar]
- Frey E.1988Anatomie des Körperstammes von Alligator mississippiensis Daudin. Stuttgarter Beiträge zur Naturkunde A 24, 1–106 [Google Scholar]
- Gower D. J.2001Possible postcranial pneumaticty in the last common ancestor of birds and crocodilians: evidence from Erythrosuchus and other Mesozoic archosaurs. Naturwissenschaften 88, 119–122 (doi:10.1007/s001140100206) [DOI] [PubMed] [Google Scholar]
- Hunter J.1774An account of certain receptacles of air, in birds, which communicate with the lungs, and are lodged both among the fleshy parts and in the hollow bones of those animals. Phil. Trans. 64, 205–213 [Google Scholar]
- Lovelace D., Hartman S., Wahl W.2007Morphology of a specimen of Supersaurus (Dinosauria, Sauropoda) from the Morrison Formation of Wyoming, and a re-evaluation of diplodocid phylogeny. Arquivos do Museu Nacional, Rio de Janeiro 65, 527–544 [Google Scholar]
- Martin J.1987Mobility and feeding of Cetiosaurus (Saurischia: Sauropoda) - why the long neck? In Fourth Symp. on Mesozoic Terrestrial Ecosystems, Short Papers, vol. 3 (eds Currie P. J., Koster E. H.), pp. 154–159 Drumheller, Alberta: Occasional Papers of the Tyrell Museum for Palaeontology [Google Scholar]
- Martin J., Martin-Rolland V., Frey E.1998Not cranes or masts, but beams: The biomechanics of sauropod necks. Oryctos 1, 113–120 [Google Scholar]
- McNab B. K.2009Resources and energetics determined dinosaur maximal size. Proc. Natl Acad. Sci. USA 106, 12 184–12 188 (doi:10.1073/pnas.0904000106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B.1908The air sacs of the pigeon. Smithsonian Misc. Collect. 50, 365–414 [Google Scholar]
- O'Connor M. P.2004Pulmonary pneumaticity in the postcranial skeleton of extant aves: a case study examining Anseriformes. J. Morphol. 261, 141–161 (doi:10.1002/jmor.10190) [DOI] [PubMed] [Google Scholar]
- O'Connor M. P.2006Postcranial pneumaticity: An evaluation of soft-tissue influences on the postcranial skeleton and the reconstruction of pulmonary anatomy in archosaurs. J. Morphol. 267, 1199–1226 (doi:10.1002/jmor.10470) [DOI] [PubMed] [Google Scholar]
- Perry S. F., Sander P. M.2004Reconstruction of the evolution of the respiratory apparatus in tetrapods. Respir. Physiol. Neurobiol. 144, 125–139 (doi:10.1016/j.resp.2004.06.018) [DOI] [PubMed] [Google Scholar]
- Sander P. M., Clauss M.2008Sauropod gigantism. Science 322, 200–201 (doi:10.1126/science.1160904) [DOI] [PubMed] [Google Scholar]
- Sander P. M., Christian A., Gee C. T.2009Response to ‘Sauropods kept their head down’. Science 323, 1671–167219325098 [Google Scholar]
- Schwarz D., Fritsch G.2006Pneumatic structures in the cervical vertebrae of the Late Jurassic (Kimmerigian-Tithonian) Tendaguru sauropods Brachiosaurus brancai and Dicraeosaurus. Eclogae Geol. Helv. 99, 65–78 (doi:10.1007/s00015-006-1177-x) [Google Scholar]
- Schwarz D., Frey E.2008Is there an option for a pneumatic stabilization of sauropod necks? An experimental and anatomical approach. Palaeontol. Electron. 11, 17A:26p [Google Scholar]
- Schwarz D., Frey E., Meyer C. A.2007Pneumaticity and soft-tissue reconstructions in the neck of diplodocid and dicraeosaurid sauropods. Acta Palaeontol. Polon. 52, 167–188 [Google Scholar]
- Sereno P. C., Wilson J. A., Witmer L. M., Whitlock J. A., Maga A., Ide O., Rowe T.2007Structural extremes in a cretaceous dinosaur. PLoS ONE 2, e1230 (doi:10.1371/journal.pone.0001230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour R. S.2009aRaising the sauropod neck: it costs more to get less. Biol. Lett. 5, 317–319 (doi:10.1098/rsbl.2009.0096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour R. S.2009bSauropods kept their heads down. Science 323, 1671–1672 (doi:10.1126/science.323.5922.1671) [DOI] [PubMed] [Google Scholar]
- Stevens K. A., Parrish M. J.1999The posture and feeding habits of two Jurassic sauropod dinosaurs. Science 284, 798–800 (doi:10.1126/science.284.5415.798) [DOI] [PubMed] [Google Scholar]
- Taylor M. P., Wedel M. J., Naish D.2009Head and neck posture in sauropod dinosaurs inferred from extant animals. Acta Palaeontol. Polon. 54, 213–220 [Google Scholar]
- Tsuihiji T.2004The ligament system in the neck of Rhea americana and ist implications for the bifurcated neural spines of sauropod dinosaurs. J. Verteb. Paleontol. 24, 165–172 (doi:10.1671/A1129-12) [Google Scholar]
- Upchurch P., Barrett P. M.2000The evolution of sauropod feeding mechanisms. In Evolution of herbivory in terrestrial vertebrates (ed. Sues H.-D.), pp. 79–121 Cambridge, UK: Cambridge University Press [Google Scholar]
- Wedel M. J.2003aThe evolution of vertebral pneumaticity in sauropod dinosaurs. J. Verteb. Paleontol. 23, 344–357 (doi:10.1671/0272-4634(2003)023[0344:TEOVPI]2.0.CO;2) [Google Scholar]
- Wedel M. J.2003bVertebral pneumaticity, air sacs, and the physiology of sauropod dinosaurs. Paleobiology 29, 243–255 (doi:10.1666/0094-8373(2003)029<0243:VPASAT>2.0.CO;2) [Google Scholar]
- Wedel M. J.2005Postcranial skeletal pneumaticity in sauropods and its implications for mass estimates. In The sauropods: evolution and paleobiology (eds Curry Rogers K. A., Wilson J. A.), pp. 201–228 Berkeley, CA: University of California Press [Google Scholar]
- Wedel M. J.2007. Postcranial pneumaticity in dinosaurs and the origin of the avian lung. PhD thesis, University of California, Berkeley [Google Scholar]
- Wedel M. J., Sanders R. K.2002Osteological correlates of cervical musculature in Aves and Sauropoda (Dinosauria: Saurischia), with comments on the cervical ribs of Apatosaurus. PaleoBios 22, 1–6 [Google Scholar]
- Wedel M. J., Cifelli R. I., Sanders R. K.2000Osteology, paleobiology, and relationships of the sauropod dinosaur Sauroposeidon. Acta Palaeontol. Polon. 45, 343–388 [Google Scholar]
- Wilson J. A.1999A nomenclature for vertebral laminae in sauropods and other saurischian dinosaurs. J. Verteb. Paleontol. 19, 639–653 [Google Scholar]
- Witmer L. M.1997The evolution of the antorbital cavity in archosaurs: a study in soft-tissue reconstruction in the fossil record with analysis of the function of pneumaticity. J. Vert. Paleontol., Mem. 3, 1–73 (doi:10.2307/3889342) [Google Scholar]
- Witzel U., Preuschoft H.2005Finite-element model construction for the virtual synthesis of the skulls in vertebrates: case study of Diplodocus. Anat. Rec. A 283, 391–401 (doi:10.1002/ar.a.20174) [DOI] [PubMed] [Google Scholar]
- Wolff J.1892Das Gesetz der Transformation der Knochen Berlin, Germany: Hirschwald [Google Scholar]