Abstract
Animals frequently use metabolites produced by symbiotic bacteria as agents against pathogens and parasites. Secretions from the preen gland of birds are used for this purpose, although its chemicals apparently are produced by the birds themselves. European hoopoes Upupa epops and green woodhoopoes Phoeniculus purpureus harbour symbiotic bacteria in the uropygial gland that might be partly responsible for the chemical composition of secretions. Here we investigate the antimicrobial activity of the volatile fraction of chemicals in hoopoe preen secretions, and, by means of experimental antibiotic injections, test whether symbiotic bacteria living within the uropygial gland are responsible for their production. Hoopoes produce two different kinds of secretions that differ drastically in their chemical composition. While the malodorous dark secretions produced by nestlings included a complex mix of volatiles, these chemicals did not appear in white secretions produced by non-nesting birds. All volatiles detected showed strong antibacterial activity, and a mixture of the chemicals at the concentrations measured in nestling glands inhibited the growth of all bacterial strains assayed. We found support for the hypothesized role of bacteria in the production of such antimicrobial chemicals because experimental clearance of bacteria from glands of nestlings with antibiotics resulted in secretions without most of the volatiles detected in control individuals. Thus, the presence of symbiotic bacteria in the uropygial gland provides hoopoes with potent antimicrobials for topical use.
Keywords: antimicrobial substances, chemical defence, coevolution, symbiotic bacteria, Upupa epops, uropygial gland
1. Introduction
The biosynthetic ability of animals is limited, so they frequently use chemical substances obtained from other organisms to increase their fitness. For instance, some barriers against parasites and pathogens depend on substances that animals ingest in order to mount an adequate immune response. This is the case with carotenoids from plants or prey (Møller et al. 2000; Blount et al. 2003), or with vitamins derived from the metabolism of gut bacteria (Hooper & Gordon 2001; Dillon & Dillon 2004). Moreover, antimicrobials, anti-helmintic products or insecticides produced by plants, fungi or bacteria are frequently directly used either in the diet or topically (see review in Lozano 1998).
Antimicrobial substances that animals use by spreading them onto their body surface include resins (Gompper & Hoylman 1993) or fruit juices (Baker 1996) produced by plants, whole plants (i.e. leaves, Clark & Mason 1985) or even animals (e.g. anting behaviour, Longino 1984; Ehrlich et al. 1986), as well as mineral substances such as iron oxides (Arlettaz et al. 2002). Animals may also use chemicals they produce, such as those from external glands, for external application. Birds, for instance, synthesize a secretion in their uropygial gland which is spread over the skin and feathers, and among other purposes, is used to fight against external pathogens (Bandyopadhyay & Bhattacharyya 1996; Shawkey et al. 2003; Reneerkens et al. 2008).
The uropygial secretion has a complex chemical composition that varies, among species and seasonally (e.g. Reneerkens et al. 2002), but usually includes mono- or diester waxes of fatty acids and alcohols, sterols and hydrocarbons (Jacob & Ziswiler 1982). All these chemicals are considered to be secreted directly by uropygial cells after processing substrates available in the bird's diet. However, European hoopoes (Upupa epops, Upupidae) and green woodhoopoes (Phoeniculus purpureus, Phoeniculidae) produce preen secretions with an unusual brown colour and pungent odour, which have been interpreted as reducing the risk of predation (Cramp 1998; Ligon 2001; du Plessis et al. unpublished cited in Burger et al. 2004). In addition, the uropygial secretion of European hoopoes inhibits growth of the feather-degrading bacterium Bacillus licheniformis (Soler et al. 2008), and consequently, the particular secretion of these species may have both anti-predator and antimicrobial functions.
Another peculiarity of uropygial gland secretions of both woodhoopoes (Law-Brown & Meyers 2003) and hoopoes (Martín-Platero et al. 2006; Soler et al. 2008) is that they harbour symbiotic Enterococcus bacteria, although this only occurs during the breeding season in the European hoopoe (Soler et al. 2008; Martín-Vivaldi et al. 2009). Interestingly, in experimental studies with both woodhoopoes and hoopoes in which the symbiotic bacteria were eliminated by means of antibiotics, the special characteristics (i.e. colour and odours) of secretions changed (Law-Brown 2001 cited in Law-Brown & Meyers 2003; Martín-Vivaldi et al. 2009), suggesting a role of bacteria in mediating these particular secretions. Enterococci, as most bacteria, are able to produce antimicrobial chemicals and, consequently, we hypothesized that the antimicrobial power of hoopoe uropygial secretions may be mediated by the presence of bacteria in the uropygial gland. The use of bacterial metabolites with defensive roles by animals, plants or even eucariotic unicellular organisms is common (Piel 2004; Schmidt 2008), but, among animals, it is mainly restricted to invertebrates (reviewed in Piel 2004). Therefore, the system formed by the hoopoe and its symbionts is one of the few cases described where vertebrates may use defensive products derived from bacteria. We have shown that Enterococcus living in the uropygial gland of hoopoes produce in vitro at least two different bacteriocins, active against a variety of bacteria strains (Martín-Platero et al. 2006). Symbiotic bacteria, however, may even be responsible for some other antimicrobial chemicals of the secretion.
The chemical composition of woodhoopoe secretions, but not that of European hoopoes, has already been studied. Burger et al. (2004) found in woodhoopoes, a complex mix of volatile compounds usually not present in other species. For instance, they considered that some of these chemicals (indole, benzaldehyde, together with short-chain acids or dimethyl disulphide) are responsible for the particular pungently unpleasant smell of the secretion of this species (Burger et al. 2004). The presence of these chemicals also suggests a role for symbionts in the composition of the secretion, given that some of these chemical products such as indole are known to be metabolites of bacteria (Leitão & Rios 2000).
The hypothetical role of bacteria in explaining the production of these potential antimicrobial and antipredator substances by Upupiformes has, to our knowledge, never been tested, and such a test is the main purpose of this article. Here we (i) test the antimicrobial properties of the volatiles found in dark secretions of European hoopoes, and (ii) compare the composition of uropygial secretions of antibiotic-treated birds and two control groups. If bacteria were responsible for the production of chemicals with antimicrobial activity in the dark secretion of hoopoes, we would expect an effect of the antibiotic treatment on the chemical composition of secretions, particularly for chemicals with antagonistic properties.
2. Material and methods
(a). General methods
The field study was performed during the breeding season in 2007 in Hoya de Guadix (37°18′ N, 38°11′ W), southern Spain, where hoopoes breed in nest-boxes installed in trees and on buildings (for a more detailed description of the study area see Martín-Vivaldi et al. 1999).
Nest-boxes were visited twice per week from mid-February to the end of July to record laying dates, clutch size and hatching dates. Incubation usually starts with the first or second egg, followed by complete hatching asynchrony in which eggs hatch every 24 h or at even greater intervals (Cramp 1998). This generates a marked size hierarchy within the brood that can be used to deduce hatching order (Martín-Vivaldi et al. 2006). For recognition, nestlings were individually painted with permanent markers on their tarsus every 2 days until they were ringed with numbered metal rings.
(b). Sampling of secretions
The birds used for chemical analyses of preen secretions were sampled in the laboratory early in the morning. The secretions were sampled from birds in the laboratory and, afterwards, they were released back into the nest-box in which we captured them (nestlings), or in the surroundings (adults) a few hours after having been caught by hand (nestlings) or with mist-nets (adults). Samples were taken using a 10 µl micropipette, with the tip gently introduced through the opening of the papilla after the circlet and surrounding skin was softly washed with a cotton swab soaked in ethanol to reduce the risk of contamination of the secretion with external bacteria. The amount of secretion obtained was estimated by the number of times that the pipette was filled with a particular volume. A maximum of 15 µl per bird was used for the extraction in 100 µl of dichloromethane. For individuals with less than 15 µl available, the entire secretion obtained was used after the volume was estimated with the micropipette. From the quantification of volatiles in each extraction, we estimated the concentration of each compound per microlitre of the original secretions.
The antibiotic experiment was performed with seven broods from which we analysed the secretions of one randomly selected nestling per treatment. In one brood, only two nestlings survived until the end of the experiment (the antibiotic and the saline-water treated ones). This brood was not included in the global analysis, but was used for post hoc paired comparisons among treatments (the results were similar when excluding this brood), and, therefore, sample size differs among paired comparisons.
(c). Chemical analysis
The volatile fraction of constituents in hoopoe uropigial secretion was analysed by gas-chromatography and mass-spectrometry (GC-MS) (appendix S1, electronic supplementary material). Extracts were injected immediately after sampling of glands of birds in the laboratory to avoid possible alteration of the composition during storage or transport.
The volatile organic compounds found in the chromatograms were identified comparing spectra and retention times with the list of compounds previously identified in the descriptive analysis of the secretions of a set of 11 hoopoe nestlings from 11 broods (for a detailed description, see appendix S1 in the electronic supplementary material).
(d). Antibiotic experiment
In order to determine whether there is an association between the presence of volatile compounds found in hoopoe secretions and the presence of symbiotic bacteria, we performed an experiment in which we compared the chemical composition of secretions of antibiotic treated and control nestlings. The microscopic study of the dark secretion produced by hoopoe nestlings and females during their stay within the nest showed that it harbours bacteria at a very high density (Soler et al. 2008; Martín-Vivaldi et al. 2009). These symbiotic bacteria are concentrated in the cavity of the papilla, where the secretion is stored by the bird until used, while they are not found within the tissues surrounding this cavity (Soler et al. 2008) nor in the tubules of the secretory lobes (M. Martin-Vivaldi 2006, unpublished data). Therefore, the papilla of the gland is the place where the antibiotic acts to kill symbiotic bacteria.
Nestlings of the antibiotic group were injected with 0.04 ml of amoxyciline diluted in saline water (100 mg ml−1, Clamoxyl GlaxoSmithKline, South Africa) in their uropygial gland. Half of this volume (0.02 ml) was injected through the wall of each of the two secretor lobes, directly into the secretory tissues. In all cases, a small part of the antibiotic solution poured out of the papilla, which ensured that the papilla was full of antibiotic. A second group of nestlings (control) was injected with 0.04 ml of saline solution. A third group (uninjected) was handled in the same way, but was not injected with any solution. Hatchlings in each nest were ranked according to their body mass and randomly assigned to the three experimental groups.
Nestling glands start to have visible lobes and to produce secretion after the fourth day of life (Cramp 1998). We started the experiment when the oldest nestling in the nest was 11 days old and all nestlings were injected daily for 6 days. The evening of the second day after the last injection, nestlings were transported to the laboratory in an empty nest-box, which allowed us to perform chemical analyses the next day early in the morning. We always left at least one nestling in the nest of origin, either on its own or from a different brood to avoid nest desertion by parents. After sampling of the secretion, and until their return to the original nest some (i.e. 3–5) hours later, experimental nestlings were hand-fed with crickets.
In a previous experiment with antibiotics, we showed that injection of amoxyciline in nestling glands drastically reduced the prevalence of Enterococcus bacteria (Martín-Vivaldi et al. 2009). Thus, to maximize the available volume of secretion for the chemical analyses, we did not check the effectiveness of the antibiotic injection in killing bacteria in experimental nestlings, but, given previous results, we assumed a similar effect. Such effect, however, does not mean complete disappearance of bacteria from glands. The antibiotic treatment in our previous study did not completely eliminate bacteria and there was still some bacterial growth in 21 per cent of nestlings. Nevertheless, there was a highly significant reduction in bacterial load of antibiotic treated nestlings in comparison with their control nest-mates (comparison of the number of colonies grown per µl of secretion in specific medium for Enterococci, Wilcoxon matched pair test using averages per treatment within nests, z = 2.76, p = 0.0058, n = 12; antibiotic group: median (min–max) = 0 (0–26); control group: median (min–max) = 179 (0–1600)). Such reduction in bacterial load was enough to cause very evident changes in the colour of secretions (Martín-Vivaldi et al. 2009), which should be the result of changes in its chemical composition.
(e). Antimicrobial properties of chemical volatiles
We tested the antibacterial activity of pure commercial standards of the identified compounds against 15 bacterial strains (including Gram-positive and Gram-negative ones) widely used as indicator strains in studies of antagonistic ability of bacteriocin producers. Afterwards, we estimated the minimum inhibitory concentration of each compound against a selection of five Gram-positive and two Gram-negative pathogenic strains and compared it with the concentration found in the secretions of nestling hoopoes. Finally, we tested the antimicrobial activity of experimental chemical mixtures reflecting (median of concentration values) the volatile fraction of the secretion composition of antibiotic-treated and uninjected birds against a battery of 19 indicator strains.
The determination of the minimum inhibitory concentration of each compound and differences in the activity of the volatile fractions of control and experimental groups were carried out on micro-plates (Corning 96-Well Plates, Sigma-Aldrich, Germany). Briefly, each well of the micro-plate containing 200 µl of growth medium inoculated with approximately 5 × 106 cfu ml−1 of the indicator strain was added to the amount of each compound needed to obtain the desired concentration in the final volume of the well. Plates were incubated at 37°C overnight and checked for growth of bacteria which was confirmed by the turbidity of the medium.
3. Results
(a). Chemical volatiles in hoopoe secretions
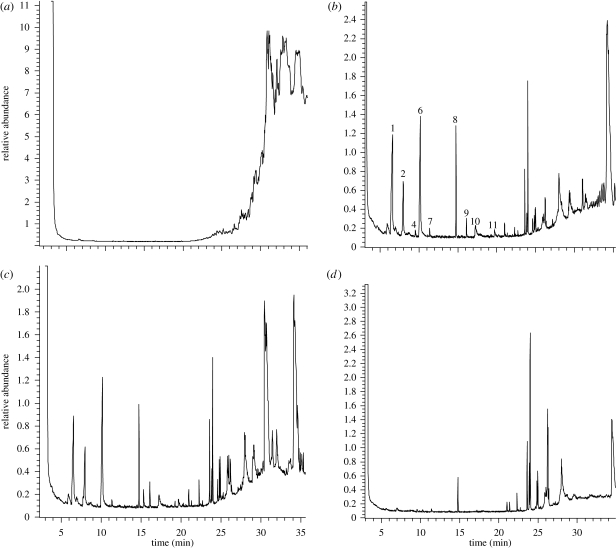
A typical ion chromatogram obtained from a GC-MS analysis of dark uropygial secretions of hoopoe nestlings is shown in figure 1b. The profile shows several peaks at short elution times (below 21 min), corresponding to volatile compounds.
Figure 1.
Chromatograms of hoopoe preen secretions. (a) The white preen secretion of a non-breeding adult, (b–d) dark secretions of three nestling hoopoes of the same brood after a 6 day treatment with (b) only manipulation, without injection, (c) saline water injected within uropygial gland lobes and (d) amoxyciline injected within uropygial gland lobes. Peaks are numbered according to the list of compounds detected in the descriptive analysis of brown secretions (appendix S1, electronic supplementary material), and their identity is shown in table 1.
The chromatogram profile of white uropygial secretions corresponding to non-nesting adult birds is quite different (figure 1a). Profiles of all seven adult non-breeding individuals monitored differed from those of dark secretions of nestlings, as seen in figure 1a,b. Most peaks detected in the brown secretion corresponded to volatile chemicals; however, they were absent in white secretions.
(b). Antibiotic experiment
The experimental antibiotic injections in the uropygial gland during nestling development produced drastic changes in the composition of hoopoe nestling uropygial gland secretions. The profile of the chromatograms of antibiotic-treated nestlings lacked most peaks of the most volatile compounds present in both kinds of control birds (figure 1). We found statistically significant differences among treatments in the concentration of seven out of 10 chemicals found in control secretions (table 1). Butanoic acid, 2-methyl butanoic acid, 4-methyl pentanoic acid, unidentified compound, indole, 3-phenyl propanoic acid and 4-chloro indole were significantly less concentrated in antibiotic treated birds than in either uninjected or control-injected birds (table 1). In two out of the remaining three volatiles (phenol and phenyl acetaldehyde), the concentrations were also lower for the antibiotic group, although not significantly so and only benzaldehyde was unaffected by the clearance of bacteria from nestling glands (see concentrations in appendix S2, electronic supplementary material).
Table 1.
Comparison of the concentration of chemical volatiles in the secretions of antibiotic-treated (AB), saline water-treated (SA) and uninjected (NAT) nestlings of the same brood. (The paired comparisons for phenol (in parentheses) were analysed with the sign test owing to the high rate of zeros in the two groups. Compounds are numbered in elution order from descriptive analyses (appendix S1, electronic supplementary material).)
| Friedman ANOVA n = 6, d.f. = 2 |
Wilcoxon matched pairs test |
||||
|---|---|---|---|---|---|
| χ2 | p-value | AB-SA, p-value (n = 7) | AB-NAT, p-value (n = 6) | SA-NAT, p-value (n = 6) | |
| (1) butanoic acid | 9.33 | 0.009 | 0.018 | 0.028 | 0.345 |
| (2) 2-methyl butanoic acid | 7.91 | 0.019 | 0.018 | 0.043 | 0.753 |
| (4) benzaldehyde | 4.33 | 0.115 | 0.310 | 0.345 | 0.345 |
| (5) phenol | 3.00 | 0.223 | (0.480) | (0.480) | (0.480) |
| (6) 4-methyl pentanoic acid | 8.44 | 0.015 | 0.028 | 0.028 | 0.345 |
| (7) phenyl acetaldehyde | 2.33 | 0.311 | 0.128 | 0.116 | 0.916 |
| (8) unidentified | 7.64 | 0.022 | 0.018 | 0.046 | 0.144 |
| (9) indole | 9.00 | 0.011 | 0.018 | 0.028 | 0.600 |
| (10) 3-phenyl propanoic acid | 8.09 | 0.018 | 0.018 | 0.043 | 0.917 |
| (11) 4-chloro indole | 7.91 | 0.019 | 0.028 | 0.028 | 0.917 |
(c). Antimicrobial activity
All volatiles found in hoopoe dark secretions inhibited growth of some of the bacterial strains assayed when using pure commercial standards (appendix S3, electronic supplementary material), but only five of them were found in hoopoe secretions in concentrations sufficiently high to reach the minimum inhibitory concentration necessary for inhibiting growth of between one and five indicator strains (table 2). However, when testing the antimicrobial effects of the volatiles in combination (i.e. median concentrations of the compounds), the mix of volatiles simulating the natural combination found in uninjected nestlings was effective in inhibiting the growth of all 19 bacterial strains assayed (table 2). Interestingly, the mix of volatile chemicals at the concentrations found in hoopoe nestlings, whose uropygial glands were treated with antibiotics during nestling development (with experimentally reduced loads of symbiotic bacteria), was not effective against any bacterial indicator strain (comparison of the number of strains inhibited between control and experimental mixtures, Fisher's exact test p < 0.0001; table 2).
Table 2.
Antimicrobial power of the natural concentrations of volatiles found in hoopoe dark secretions. (The table shows the minimum inhibitory concentration (mg 100 µl−1) of the different volatile chemicals found in hoopoe uropygial secretions for seven bacteria indicator strains, the mean concentration of the same compounds in nestling hoopoe secretions (from descriptive analyses in appendix S1, electronic supplementary material), and the results of an experiment testing for differences in the inhibitory power of a mix of volatiles at the median concentration found in control uninjected nestlings (MIX 1), and at the median concentration found in experimental nestlings whose uropygial bacterial community was cleared with antibiotics (MIX 2). Underlining indicates that the concentration of the compound in hoopoe secretions is equal or larger than the minimum inhibitory concentration and therefore would be sufficient to inhibit the indicator strain. In some cases the indicator strains were resistant to all concentrations assayed with this method, which is indicated by R. For the experiment with mixes, the growth inhibition of the indicator strains is showed by a plus symbol, while a negative symbol means bacterial growth. Strains marked with 1 were from the collection of the laboratory of Microbiology at the University of Granada, those marked with 2 are from the Spanish Type Culture Collection (CECT) and those marked with 3 are referred to in Yagi & Clewell (1980).
| minimum inhibitory concentration for individual compounds |
MIX 1 control nestlings | MIX 2 exp. nestlings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| But ac | 2-MeBut ac | benzal | phenol | 4-Me-pent ac | Phe-acetal | indole | 3-phe-prop ac | 4-Cl indole | |||
| Gram positive | |||||||||||
| Listeria monocytogenes 40322 | 1.0 | 0.3 | 0.5 | 1.2 | 0.3 | 0.001 | 0.2 | R | R | + | − |
| L. monocytogenes 10342 | — | — | — | — | — | — | — | — | — | + | − |
| Listeria innocua 40302 | — | — | — | — | — | — | — | — | — | + | − |
| B. licheniformis D131 | 0.01 | 0.3 | 0.3 | 0.6 | 0.3 | 0.001 | 0.2 | 0.1 | 2.0 | + | − |
| B. cereus LW11 | — | — | — | — | — | — | — | — | — | + | − |
| E. faecalis S-471 | 1.0 | 0.3 | 0.5 | 1.2 | 0.3 | 0.001 | 0.2 | R | R | + | − |
| E. faecalis A48/321 | — | — | — | — | — | — | — | — | — | + | − |
| Enterococcus faecium 341 | 1.0 | 0.3 | 0.5 | 0.6 | 0.3 | 0.1 | 0.2 | R | R | + | − |
| E. faecium 32/81 | — | — | — | — | — | — | — | — | — | + | − |
| Staphylococcus aureus 2402 | 1.0 | 0.3 | 0.5 | 0.6 | 0.5 | 0.001 | 0.3 | 0.2 | 2.0 | + | − |
| Staphylococcus carnosus 44912 | — | — | — | — | — | — | — | — | — | + | − |
| L. lactis LM23013 | — | — | — | — | — | — | — | — | — | + | − |
| Micrococcus sp.1 | — | — | — | — | — | — | — | — | — | + | − |
| Gram negative | |||||||||||
| Escherichia coli 7742 | 0.5 | 0.1 | 0.5 | 0.6 | 0.3 | 0.1 | 0.3 | R | 2.0 | + | − |
| E. coli U91 | — | — | — | — | — | — | — | — | — | + | − |
| Salmonella choleraesius 4432 | 0.5 | 0.5 | 0.3 | 0.6 | 1.0 | 0.001 | 0.3 | 0.4 | 2.0 | + | − |
| Salmonella enterica 1022 | — | — | — | — | — | — | — | — | — | + | − |
| Proteus sp.1 | — | — | — | — | — | — | — | — | — | + | − |
| Klebsiella sp.1 | — | — | — | — | — | — | — | — | — | + | − |
| mean concentration in glands | 0.5 | 0.1 | <0.01 | 0.2 | 0.3 | 0.01 | <0.01 | 0.4 | 0.02 | ||
4. Discussion
Our main results were that: (i) there is a different chemical composition of dark and white secretions of uropygial glands of hoopoes, with abundant volatile compounds in dark, but not in white secretions; (ii) these volatiles show antimicrobial activity against a wide set of bacterial strains; and (iii) most volatiles disappear from secretions of individuals experimentally injected with antibiotic during development, suggesting a central role of bacteria in the presence of these compounds.
We have shown evidence of a relationship between presence of bacteria in uropygial glands (experimental treatment) and the presence of antimicrobial volatile substances. In a previous experiment with antibiotics, we found that body condition, growth rate and immune response to a novel antigen were not affected by antibiotic injections in uropygial glands of nestlings (Martín-Vivaldi et al. 2009); therefore, the differences found in chemical composition of secretions were not caused by an indirect effect of the antibiotic on general condition or health of birds. Interestingly, we found that the mixture of volatile compounds in individuals with bacteria in their uropygial gland, but not in that of individuals with experimentally induced bacterial clearance, demonstrated antagonistic capacity against all bacterial strains tested, which include the feather-degrading bacilli B. licheniformis (Burtt & Ichida 1999) and Bacillus cereus (E. H. Burtt Jr 2009, personal communication), and several potentially pathogenic bacteria. These compounds were not of a peptide nature, and, therefore, our results suggest that not only bacteriocins (Martín-Platero et al. 2006), but also a variety of chemical defensive products of the uropygial secretion are of bacterial origin. The antimicrobial effects of these symbiont-produced chemicals would even be higher when in combination with other compounds of the secretion such as antimicrobial peptides (bacteriocins) owing to synergistic effects (e.g. Ananou et al. 2007). Therefore, the antagonistic activity shown in our experiments by the combination of chemicals would underestimate the real antimicrobial power of uropygial secretions.
We have used a wide-spectrum antibiotic during six consecutive days of the growth period that probably killed any strain of bacteria living in hoopoe glands. Despite the fact that the most common cultivable hoopoe gland symbionts are Enterococcus faecalis, other Enterococcus species are frequently isolated (Soler et al. 2008). Furthermore, given that most bacterial strains are not cultivable with standard methods (Pace 1997), we cannot ignore the possibility that other non-enterococcal species may live within hoopoe glands and that they produce some of the detected chemicals that are not common for enterococci. For example, 4-methyl pentanoic and 3-phenyl propanoic acids are considered typical metabolites of Clostridia (Arellano et al. 2000), which may also be present in hoopoe glands. In any case, and independently of whether detected volatiles were from one or several bacterial groups, our experiment demonstrates a link between the presence of symbiotic bacteria and detection of volatile chemicals with antimicrobial properties in the uropygial secretion of hoopoes.
These results further support a symbiotic association between hoopoes and bacteria living in their uropygial gland that is maintained by the benefits that bacteria provide to the birds in their chemical defence against feather-degrading bacteria and pathogens. This interpretation is further supported by previous findings of enhanced antimicrobial activity of brown secretions and bacteriocin production by the strains isolated from hoopoe glands (Martín-Platero et al. 2006; Soler et al. 2008).
(a). Seasonal and interspecific differences in chemical composition of uropygial gland secretions
We have found that the chemical composition of uropygial secretions of European hoopoes changed drastically between the breeding and the non-breeding seasons. Chromatogram profiles of white secretions produced by both sexes outside the breeding season were similar to those shown in most bird species, mainly including waxes (Jacob & Ziswiler 1982), but completely lacking volatile chemicals. By contrast, dark secretions produced by breeding females and nestlings include a variety of volatiles only found in secretions of the closely related woodhoopoes (Phoeniculidae, Burger et al. 2004; Feduccia 1975). These components are probably responsible for the unpleasant smell of the very special uropygial secretion of both species. Contrary to the exaggerated seasonal changes, not only in colour and odours (Martín-Vivaldi et al. 2009), but also in chemical profile detected in European hoopoes, woodhoopoe secretion does not appear to vary during the annual cycle (Burger et al. 2004). Previous studies have described seasonal changes in the chemical composition of secretions in several groups of birds such as ducks (e.g. Kolattukudy et al. 1987), shorebirds (Piersma et al. 1999; Reneerkens et al. 2002, 2005, 2008) or passerines (Bhattacharyya & Chowdhury 1995; Haribal et al. 2005; Soini et al. 2007), which are considered adaptations to variation in selection pressures from parasites or predators among seasons. In most cases, however, the changes affect the kind of waxes present in the secretions but not the presence of volatile substances, except in the dark-eyed junco Junco hyemalis, for which more volatile compounds such as organic acids and alcohols are incorporated into the secretions in the breeding season (Soini et al. 2007). These studies, however, have not found phenolic or indolic compounds and the short-chain organic acids present in hoopoe secretions during the stay within the nest, which suggests that the presence of bacteria is at least partially responsible for the drastic change in composition found in European hoopoe uropygial secretions.
As in European hoopoes, some volatile chemical compounds in the woodhoopoe secretion could be a secondary product of symbiotic bacteria living in their uropygial gland. This suggestion is based on the effect of experimental injections of antibiotics on secretion colour (Law-Brown 2001, cited in Law-Brown & Meyers 2003). The cultivable bacteria in both species are Enterococcus sp., but the new species E. phoeniculicola found in woodhoopoes (Law-Brown & Meyers 2003) was not detected in samples of European hoopoes (Soler et al. 2008). Therefore, it is possible that interspecific differences in volatile chemicals of secretions can be explained by different bacterial communities living within their glands.
We conclude that the composition of uropygial gland secretions of European hoopoes is a result of the combined action of the birds and symbiotic bacteria living within their uropygial glands that provide nestlings and breeding females with a variety of antimicrobial chemicals for their fight against feather-degrading bacteria and pathogens.
Acknowledgements
Consejería de Medio Ambiente of Junta de Andalucía provided permits required to perform the present research according to Spanish regulations.
Pilar López and José Martín provided useful advice on sampling, extraction and analytical methods and performed a preliminary analysis that helped in the planning of the study. Rafael Núñez-Gómez at the Scientific Instrumentation Service, Estación Experimetal Zaidín (CSIC), helped in GC-MS analyses. Edward H. Burtt Jr, Juan Moreno, Anders Møller and an anonymous referee revised a previous draft of the manuscript and suggested changes that improved its quality. Funds were provided by Ministerio de Ciencia y Tecnología (projects CGL2005-06975/BOSFEDER and CGL2007-61251/BOSFEDER) and Junta de Andalucía (projects P06-RNM-02177, JTR/EB RNM345, RNM 339, RNM 340).
References
- Ananou S., Maqueda M., Martinez-Bueno M., Galvez A., Valdivia E.2007Bactericidal synergism through enterocin AS-48 and chemical preservatives against Staphylococcus aureus. Lett. Appl. Microbiol. 45, 19–23 (doi:10.1111/j.1472-765X.2007.02155.x) [DOI] [PubMed] [Google Scholar]
- Arellano M., Jomard P., El Kaddouri S., Roques C., Nepveu F., Couderc F.2000Routine analysis of short-chain fatty acids for anaerobic bacteria identification using capillary electrophoresis and indirect ultraviolet detection. J. Chromatogr. B 741, 89–100 [DOI] [PubMed] [Google Scholar]
- Arlettaz R., Christe P., Surai P. F., Møller A. P.2002Deliberate rusty staining of plumage in the bearded vulture: does function precede art? Anim. Behav. 64, F1–F3 (doi:10.1006/anbe.2002.3097) [Google Scholar]
- Baker M.1996Fur rubbing: use of medicinal plants by capuchin monkeys (Cebus capucinus). Am. J. Primatol. 38, 263–270 (doi:10.1002/(SICI)1098-2345(1996)38:3%3C263::AID-AJP5%3E3.0.CO;2-X) [Google Scholar]
- Bandyopadhyay A., Bhattacharyya S. P.1996Influence of fowl uropygial gland and its secretory lipid components on growth of skin surface bacteria of fowl. Indian J. Exp. Biol. 34, 48–52 [PubMed] [Google Scholar]
- Bhattacharyya S. P., Chowdhury S. R.1995Seasonal-variation in the secretory lipids of the uropygial gland of a subtropical wild passerine bird, Pycnonotus-cafer (l) in relation to the testicular cycle. Biol. Rhythm Res. 26, 79–87 (doi:10.1080/09291019509360326) [Google Scholar]
- Blount J. D., Metcalfe N. B., Birkhead T. R., Surai P. F.2003Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300, 125–127 (doi:10.1126/science.1082142) [DOI] [PubMed] [Google Scholar]
- Burger B. V., Reiter B., Borzyk O., Du Plessis M. A.2004Avian exocrine secretions. I. Chemical characterization of the volatile fraction of the uropygial secretion of the green woodhoopoe, Phoeniculus purpureus. J. Chem. Ecol. 30, 1603–1611 (doi:10.1023/B:JOEC.0000042071.65335.f3) [DOI] [PubMed] [Google Scholar]
- Burtt E. H. J., Ichida J. M.1999Occurrence of feather-degrading bacilli in the plumage of birds. Auk 116, 364–372 [Google Scholar]
- Clark L., Mason J. R.1985Use of nest material as insecticidal and anti-pathogenic agents by the European starling. Oecologia 67, 169–176 (doi:10.1007/BF00384280) [DOI] [PubMed] [Google Scholar]
- Cramp S.1998The complete birds of the Western Palearctic on CD-ROM Oxford, UK: Software Optimedia, Oxford University Press [Google Scholar]
- Dillon R. J., Dillon V. M.2004The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 49, 71–92 (doi:10.1146/annurev.ento.49.061802.123416) [DOI] [PubMed] [Google Scholar]
- Ehrlich P. R., Dobkin D. S., Wheye D.1986The adaptive significance of anting. Auk 103, 835 [Google Scholar]
- Feduccia A.1975The bony stapes in the Upupidae and Phoeniculidae: evidence for common ancestry. Wilson Bull. 87, 416–417 [Google Scholar]
- Gompper M. E., Hoylman A. M.1993Grooming with Trattinnickia resin: possible pharmaceutical plant use by coatis in Panama. J. Trop. Ecol. 9, 533–540 (doi:10.1017/S0266467400007616) [Google Scholar]
- Haribal M., Dhondt A. A., Rosane D., Rodriguez E.2005Chemistry of preen gland secretions of passerines: different pathways to same goal? why? Chemoecology 15, 251–260 (doi:10.1007/s00049-005-0318-4) [Google Scholar]
- Hooper L. V., Gordon J. I.2001Commensal host-bacterial relationships in the gut. Science 292, 1115–1118 (doi:10.1126/science.1058709) [DOI] [PubMed] [Google Scholar]
- Jacob J., Ziswiler V.1982The uropygial gland. Avian biology, pp. 199–324 London, UK: Academic Press [Google Scholar]
- Kolattukudy P. E., Bohnet S., Rogers L.1987Diesters of 3-hydroxy fatty-acids produced by the uropygial glands of female mallards uniquely during the mating season. J. Lipid Res. 28, 582–588 [PubMed] [Google Scholar]
- Law-Brown J.2001. Chemical defence in the red-billed woodhoopoe Phoeniculus purpureus. MSc thesis, University of Cape Town [Google Scholar]
- Law-Brown J., Meyers P. R.2003Enterococcus phoeniculicola sp nov., a novel member of the enterococci isolated from the uropygial gland of the red-billed woodhoopoe, Phoeniculus purpureus. Int. J. Syst. Evol. Micr. 53, 683–685 (doi:10.1099/ijs.0.02334-0) [DOI] [PubMed] [Google Scholar]
- Leitão M. F. F., Rios D. P. A.2000Microbiological and chemical changes in freshwater prawn (Macrobrachium rosembergii) stored under refrigeration. Braz. J. Microbiol. 31, 178–183 [Google Scholar]
- Ligon J. D.2001Family Phoeniculidae (woodhoopoes). In Handbook of the birds of the world (eds del Hoyo J., Elliot A., Sargatal J.), pp. 412–434 Barcelona, Spain: Lynx Edicions [Google Scholar]
- Longino J. T.1984True anting by the capuchin (Cebus capucinus). Primates 25, 243–245 (doi:10.1007/BF02382396) [Google Scholar]
- Lozano G. A.1998Parasitic stress and self-medication in wild animals. Adv. Stud. Behav. 27, 291–317 (doi:10.1016/S0065-3454(08)60367-8) [Google Scholar]
- Martín-Platero A. M., Valdivia E., Ruiz-Rodríguez M., Soler J. J., Martín-Vivaldi M., Maqueda M., Martínez-Bueno M.2006Characterization of antimicrobial substances produced by Enterococcus faecalis MRR 10–3, isolated from the uropygial gland of the hoopoe (Upupa epops). Appl. Environ. Microbiol. 72, 4245–4249 (doi:10.1128/AEM.02940-05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Vivaldi M., Palomino J. J., Soler M., Soler J. J.1999Determinants of reproductive success in the hoopoe (Upupa epops), a hole-nesting non-passerine bird with asynchronous hatching. Bird Study 46, 205–216 (doi:10.1080/00063659909461132) [Google Scholar]
- Martín-Vivaldi M., Ruiz-Rodríguez M., Méndez M., Soler J. J.2006Relative importance of factors affecting nestling immune response differs between junior and senior nestlings within broods of hoopoes Upupa epops. J. Avian Biol. 37, 467–476 (doi:10.1111/j.0908-8857.2006.03660.x) [Google Scholar]
- Martín-Vivaldi M., Ruiz-Rodriguez M., Soler J. J., Peralta-Sánchez J. M., Méndez M., Valdivia E., Martín-Platero A. M., Martínez-Bueno M.2009Seasonal, sexual and developmental differences in hoopoe preen gland morphology and secretions. Evidence for a role of bacteria. J. Avian Biol. 40, 191–205 (doi:10.1111/j.1600-048X.2009.04393.x) [Google Scholar]
- Møller A. P., Biard C., Blount J. D., Houston D. C., Ninni P., Saino N., Surai P. F.2000Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult. Biol. Rev. 11, 137–159 [Google Scholar]
- Pace N. R.1997A molecular view of microbial diversity and the biosphere. Science 276, 734–740 (doi:10.1126/science.276.5313.734) [DOI] [PubMed] [Google Scholar]
- Piel J.2004Metabolites from symbiotic bacteria. Nat. Prod. Rep. 21, 519–538 (doi:10.1039/b310175b) [DOI] [PubMed] [Google Scholar]
- Piersma T., Dekker M., Damsté J. S. S.1999An avian equivalent of make-up? Ecol. Lett. 2, 201–203 (doi:10.1046/j.1461-0248.1999.00078.x) [Google Scholar]
- Reneerkens J., Piersma T., Damsté J. S. S.2002Sandpipers (Scolopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why? Proc. R. Soc. Lond. B 269, 2135–2139 (doi:10.1098/rspb.2002.2132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneerkens J., Piersma T., Damsté J. S. S.2005Switch to diester preen waxes may reduce avian nest predation by mammalian predators using olfactory cues. J. Exp. Biol. 208, 4199–4202 (doi:10.1242/jeb.01872) [DOI] [PubMed] [Google Scholar]
- Reneerkens J., Versteegh M. A., Schneider A. M., Piersma T., Burtt E. H., Jr2008Seasonally changing preen wax composition: red knots’ (Calidris canutus) flexible defense against feather-degrading bacteria? Auk 125, 285–290 (doi:10.1525/auk.2008.06217) [Google Scholar]
- Schmidt E. W.2008Trading molecules and tracking targets in symbiotic interactions. Nat. Chem. Biol. 4, 466–473 (doi:10.1038/nchembio.101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey M. D., Pillai S. R., Hill G. E.2003Chemical warfare? Effects of uropygial oil on feather-degrading bacteria. J. Avian Biol. 34, 345–349 (doi:10.1111/j.0908-8857.2003.03193.x) [Google Scholar]
- Soini H. A., Schrock S. E., Bruce K. E., Wiesler D., Ketterson E. D., Novotny M. V.2007Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J. Chem. Ecol. 33, 183–198 (doi:10.1007/s10886-006-9210-0) [DOI] [PubMed] [Google Scholar]
- Soler J. J., Martín-Vivaldi M., Ruiz-Rodríguez M., Valdivia E., Martín-Platero A. M., Martínez-Bueno M., Peralta-Sánchez J. M., Méndez M.2008Symbiotic association between hoopoes and antibiotic-producing bacteria that live in their uropygial gland. Funct. Ecol. 22, 864–871 (doi:10.1111/j.1365-2435.2008.01448.x) [Google Scholar]
- Yagi Y., Clewell D. B.1980Recombination-deficient mutant of Streptococcus faecalis. J. Bacteriol. 143, 966–970 [DOI] [PMC free article] [PubMed] [Google Scholar]