Abstract
A conventional five-step chemo-mechanical cycle of the myosin–actin ATPase reaction, which implies myosin detachment from actin upon release of hydrolysis products (ADP and phosphate, Pi) and binding of a new ATP molecule, is able to fit the [Pi] dependence of the force and number of myosin motors during isometric contraction of skeletal muscle. However, this scheme is not able to explain why the isometric ATPase rate of fast skeletal muscle is decreased by an increase in [Pi] much less than the number of motors. The question can be solved assuming the presence of a branch in the cycle: in isometric contraction, when the force generation process by the myosin motor is biased at the start of the working stroke, the motor can detach at an early stage of the ATPase cycle, with Pi still bound to its catalytic site, and then rapidly release the hydrolysis products and bind another ATP. In this way, the model predicts that in fast skeletal muscle the energetic cost of isometric contraction increases with [Pi]. The large dissociation constant of the product release in the branched pathway allows the isometric myosin–actin reaction to fit the equilibrium constant of the ATPase.
Keywords: chemo-mechanical cycle in muscle, myosin–actin ATPase, skinned fibre mechanochemistry, kinetic model of myosin motor
1. Introduction
(a). The myosin working stroke in isometric contraction
During muscle contraction, the globular head of the myosin molecule (M) extending from the thick filament cyclically attaches to the actin site (A) on the thin filament and undergoes a structural working stroke accounted for by the energy released by the hydrolysis of one ATP molecule (Huxley 1969; Huxley & Simmons 1971; Lymn & Taylor 1971). According to the crystallographic model (Dominguez et al. 1998; Geeves & Holmes 2005) the working stroke consists of a 70° tilting of the light chain domain of the myosin head (the lever arm) about a fulcrum in the catalytic domain firmly attached to actin, corresponding to an axial movement (D) of 10 nm between the catalytic domain and the attachment of the lever arm to the myosin filament. A similar amount of filament sliding has been found in single muscle fibres when the force of the half-sarcomere is suddenly reduced to synchronize the working stroke in the actin attached myosin motors (Huxley & Simmons 1971; Piazzesi et al. 2002; Reconditi et al. 2004). The biochemical step associated with the working stroke is the release of the hydrolysis product orthophosphate (Pi), while the release of ADP follows the execution of the working stroke (Bagshaw & Trentham 1974; Sleep & Hutton 1980; Ferenczi et al. 1984; Hibberd et al. 1985).
The probability of the completion of the ATPase cycle is reduced when the myosin motors act under high load, with respect to low load, as proven by the reduction of the rate of energy liberation (Fenn 1923; Hill 1938) and ATP hydrolysis (Kushmerick & Davies 1969). Recently, it has been shown that the average strain (s) in the myosin motors in isometric contraction is one order of magnitude smaller than the size of the working stroke D (Decostre et al. 2005; Piazzesi et al. 2007). The energy required to strain the motor elastic element during an isometric working stroke is ½ εD2, where ε is the stiffness of the myosin cross-bridge. In a single fibre of frog skeletal muscle, ε is approximately 3.2 pN nm−1 (Decostre et al. 2005; Piazzesi et al. 2007). In this case, for a single working stroke of 10 nm, the energy would be 160 zJ, twice that released by the hydrolysis of one molecule of ATP (Pate & Cooke 1989a) and approximately 40 kbΘ (where kb is the Boltzmann constant and Θ the absolute temperature). The corresponding equilibrium constant of the reaction would be too low to explain the isometric force on the basis of motors that have undergone the whole working stroke transition. Note that the argument remains valid also assuming that the stiffness of the myosin motor is as low as approximately 1.7 pN nm−1, the value found in skinned fibres from rabbit psoas (Linari et al. 2007; Lewalle et al. 2008), as also in this case the energy for the working stroke transition in the isometric condition would be greater than 20 kbΘ. A possible solution to the problem is suggested by recent X-ray experiments (Reconditi et al. 2004; Piazzesi et al. 2007), demonstrating that the 10 nm working stroke occurs only at low load, while, for loads greater than or equal to 0.5 T0, the working stroke is approximately 6 nm. These results can be explained assuming that the working stroke is made up of a series of steps and the detachment probability increases sharply for motor movements above 6 nm. The average strain per motor in isometric contraction is approximately 1.7 nm at 5°C and is accounted for by the first of a series of steps of approximately 2.8 nm between motor states that have the same stiffness (Decostre et al. 2005). The energy for the first transition is (½·3.2·2.82=) 12.5 zJ, that is approximately 3kbΘ, a value that is compatible with the possibility that the transition occurs by thermal fluctuation.
(b). The effect of Pi on mechanics and energetics of isometric contraction
In Ca2+-activated skinned muscle fibres, addition of Pi has been found to increase the rate of force generation following photolysis of caged ATP starting from rigor (Hibberd et al. 1985). Accordingly, the rate constant of development of isometric force following a period of unloaded shortening (rF) and the rate constant of the force transient elicited by a jump in [Pi] (rPi) are faster at higher [Pi] (Millar & Homsher 1990; Dantzig et al. 1992; Walker et al. 1992; Regnier et al. 1995; Tesi et al. 2000, 2002; Caremani et al. 2008). At any [Pi] above 3 mM, rPi is larger than rF and both rate constants show saturation at large [Pi]. The steady isometric force (T0) developed by a muscle fibre is decreased by increase in [Pi] (Brandt et al. 1982; Hibberd et al. 1985; Pate & Cooke 1985; Kawai et al. 1987; Cooke et al. 1988; Pate & Cooke 1989b; Millar & Homsher 1990; Fortune et al. 1991; Kawai & Halvorson 1991; Dantzig et al. 1992; Martyn & Gordon 1992). Also the ATPase rate of isometrically contracting fibres is reduced by Pi, but less than in proportion to the reduction of force (Bowater & Sleep 1988; Potma & Stienen 1996). These findings were explained by assuming that the effect of Pi depends on the strain of the myosin motor (Pate & Cooke 1989a) as it is the case if Pi release is associated with the transition to higher force-generating states of the myosin motor. However, this idea is contradicted by the recent finding that the Pi-dependent reduction in force is explained by a proportional decrease in the number of myosin motors without any effect of Pi on the force per motor (Caremani et al. 2008). This finding makes existing conventional models unable to explain the reduced effect of Pi on the ATPase rate (Woledge et al. 2009).
2. Results and discussion
The models described here are intended to explain the kinetics of the chemo-mechanical coupling in isometric conditions; therefore, the myosin motors experience only a relatively narrow range of strains and a detailed description of the dependency of the rate constants controlling state transitions on the axial position of the motors is not necessary. The rate constants for forward and backward transitions and the corresponding equilibrium constants are defined as kx, k−x and Kx, respectively. Time-dependent distributions of myosin motors among the various states following Pi jump or unloaded shortening were calculated by solving a system of linear differential equations as reported in the electronic supplementary material.
(a). Predictions of a conventional cycle
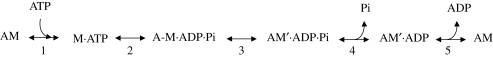
The effects of Pi on the isometric force under steady-state and transient conditions can be explained by a five-step reaction scheme (scheme 1) for the myosin–actin ATPase cycle similar to that described in the 1990s (Fortune et al. 1991; Kawai & Halvorson 1991; Dantzig et al. 1992).
Scheme 1.
Conventional chemo-mechanical cycle.
ATP binds to the actomyosin (AM) complex (step 1), promotes rapid dissociation of myosin from actin (incorporated in step 1) and then is hydrolysed (step 2). The myosin with the hydrolysis products is in rapid equilibrium with the weakly bound A-M·ADP·Pi state. Actin attachment by the closure of the actin binding cleft of the myosin head (Geeves & Holmes 2005) forms the strongly bound, stiffness generating, AM′·ADP·Pi state that, without significant delay undergoes an interdomain structural change leading to the strained conformation responsible for isometric force (step 3). Thus, step 3 is the combination of two processes, formation of strongly bound cross-bridges and force generation, with the kinetics determined by the much slower attachment process. This definition of step 3 is in agreement with a fast kinetics for force generation (Huxley & Simmons 1971) and is supported by the recent evidence that force rises in proportion to number of myosin motors during isometric force development (Caremani et al. 2008). Pi is released in a rapid reaction (step 4, Dantzig et al. 1992), without any further contribution to force. Thus both the AM′·ADP·Pi and the AM′·ADP states are a mixture of different force-generating states of cross-bridges in rapid equilibrium and the sum of the occupancies of these two states constitutes the fraction (f) of actin attached motors. Therefore each of the two states contributes equally to the fibre force and stiffness, in agreement with the finding that an increase in Pi decreases the ensemble force and the number of motors by the same amount (Caremani et al. 2008). The subsequent ADP-release step (step 5) occurs at a rate that, in the isometric contraction is low (Nyitrai & Geeves 2004; Sleep et al. 2005; West et al. 2005). The cycle is repeated as long as ATP is available and the fibre is activated.
In the scheme, the development of isometric force is rate limited by both the ATP hydrolysis step (step 2) and the attachment of cross-bridges (step 3), while the steady-state flux through the whole cycle (the ATPase activity) is limited by the rate of the ADP release (step 5). The constraint is removed, according to the view that the rate of ADP release becomes very rapid following the stroke-dependent conformational change (Nyitrai & Geeves 2004; Geeves & Holmes 2005), to obtain the state distribution during the period of unloaded shortening that precedes the development of isometric force. During this period, the rate constant for ADP release (k5) is set to 1000 s−1.
The reference experimental data are from Caremani et al. (2008), for the development of isometric force and from Dantzig et al. (1992), for the force transient following a Pi jump.
(i). Rate constants
Unless differently specified, the values of the rate constants of the transitions (listed in table 1) have been chosen in agreement with those reported in previous kinetic studies on rabbit psoas actomyosin both in solution and in skinned fibres, as specified in the electronic supplementary material. Rapid equilibrium reactions that follow a kinetically relevant step are incorporated in the step itself.
Table 1.
Rate constants for the forward (kx) and the backward (k−x) transitions and corresponding equilibrium constants (Kx) in schemes 1 and 2. x represents the step number, as reported in brackets in the first row. For the second-order rate constants, the apparent rate constants are calculated assuming [MgATP] = 5 mM (step 1), [ADP] = 20 µM (step 5) and [Pi] according to the experimental conditions (step 4). The unloaded shortening condition is simulated by rising k5 from 8 to 1000 s−1.
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
|---|---|---|---|---|---|---|---|
| scheme 1 | |||||||
| kx | 106 M−1 s−1 | 18 s−1 | 21 s−1 | 500 s−1 | 8 s−1 | ||
| k−x | 0.1 s−1 | 15 s−1 | 100 s−1 | 40 × 103 M−1 s−1 | 105 M−1 s−1 | ||
| Kx | 107 M−1 | 1.2 | 0.21 | 12.5 × 10−3 M | 8 × 10−5 M | ||
| scheme 2 | |||||||
| kx | 106 M−1 s−1 | 14 s−1 | 21 s−1 | 500 s−1 | 8 s−1 | 24 s−1 | |
| k−x | 0.1 s−1 | 9 s−1 | 90 s−1 | 33 × 103 M−1 s−1 | 105 M−1 s−1 | 8 × 10−3 | |
| Kx | 107 M−1 | 1.56 | 0.23 | 15.2 × 10−3 M | 8 × 10−5 M | 3 × 103 | 5 × 102 M |
(ii). Equilibrium constant of ATP hydrolysis
The product of the equilibrium constants of all steps reported in table 1 gives an equilibrium constant for ATP hydrolysis (KATP) in isometric contraction of 2.5 M, which is smaller than that reported in literature (2–5 × 105 M, Guynn & Weech 1973) by a factor of approximately 105. This corresponds to a difference in the associated energy of approximately 50 zJ, consistent with the energy of the working stroke (White & Taylor 1976). This difference is accounted for by the difference in the combined equilibrium constant of steps 3 and 4 (isomerization and Pi release steps) between unloaded conditions and isometric conditions, which explains why steps 3 and 4 are reversible in the isometric contraction and no net work is done.
(iii). Simulation of the effect of Pi on the isometric force during steady state and transient conditions
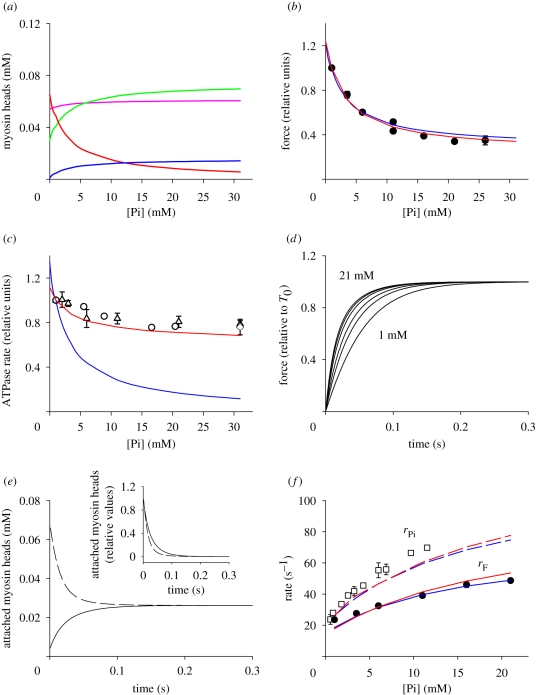
The effect of [Pi] on the occupancy of the various states of the reaction scheme (except the AM state that is not significantly populated at the physiological [ATP]) during steady isometric contraction are plotted in figure 1a. With a concentration of myosin heads in skeletal muscle of 0.15 mM (He et al. 1997; Sun et al. 2001), the concentration of motors bearing stiffness and force in isometric contraction in control solution (no added Pi, corresponding to [Pi] = 1 mM) is approximately 0.052 mM, which gives a fractional number of (0.052 mM/0.15 mM=) 0.34, in agreement with that fraction of motors estimated from mechanical measurements under the same conditions (Zhao & Kawai 1991; Linari et al. 2007; Piazzesi et al. 2007). The number of motors (and thus the force of the motor ensemble in each half-sarcomere) decreases with increases in [Pi] (figure 1b, blue line), in agreement with the experimental data up to a minimum of 35 per cent of the value in control solution (circles, from Caremani et al. 2008; similar data for the force–Pi relation are reported by Bowater & Sleep 1988; Millar & Homsher 1990; Potma et al. 1995; Potma & Stienen 1996).
Figure 1.
Responses of the simulation. (a) Effect of [Pi] on the occupancy of the cross-bridge states (as defined by the colours) during isometric contraction, calculated with scheme 1. The attached AM state is not significantly populated and is omitted. Pink line, M·ATP; green line, A-M·ADP·Pi; blue line, AM′·ADP·Pi; red line, AM′·ADP. (b) Pi-dependence of isometric force (relative to the force in control solution, 1 mM Pi). Observed relation, filled circles (data from figs 1f and 6a of Caremani et al. 2008). Simulated relations: blue line (scheme 1) and red line (scheme 2). (c) Pi-dependence of ATPase rate: observed relation, open symbols (circles, Bowater & Sleep 1988; triangles, Potma et al. 1995; diamonds, Potma & Stienen 1996). Simulated relations: blue line (scheme 1) and red line (scheme 2). Values relative to those in control solution. (d) Time course (calculated with scheme 1) of the number of attached cross-bridges (the sum of the occupancy of AM′·ADP·Pi and AM′·ADP) following the end of the unloaded shortening in control solution ([Pi], 1 mM) and at different [Pi] (3.5, 6, 11, 16 and 21 mM). Values on the ordinate are normalized for the steady isometric value at the respective [Pi]. (e) Superposition of the time courses (calculated with scheme 1) of the number of attached cross-bridges following either a step of [Pi] from 0 to 11 mM (dashed line) or a period of unloaded shortening in 11 mM Pi (continuous line). For facilitating the comparison, in the inset, the data are plotted after making them relative to their respective maximum change and applying the operation (1 − y) to data (y) for the rise in number of cross-bridges following unloaded shortening. (f) Pi dependences of rF and rPi. rF relation: observed (filled circles) from fig. 6c of Caremani et al. (2008); simulated: scheme 1 (blue continuous line) and scheme 2 (red continuous line). rPi relation: observed (open squares) from fig. 6 of Dantzig et al. 1992; simulated: scheme 1 (blue-dashed line) and scheme 2 (red-dashed line). The simulated rPi relations lie slightly below the observed relation probably because of the different procedure to estimate the rate constant of the force transient. While in the experimental records of Dantzig et al. (1992), the initial lag does not contribute to the estimate of the rate constant, in our simulation the lag is incorporated in the estimate.
To simulate the rate of isometric force development following unloaded shortening, the starting distribution of myosin states induced by unloaded shortening is obtained by increasing k5 to 1000 s−1, and 15 ms after the jump in k5 (a time similar to that of unloaded shortening in Caremani et al. 2008) the motor states with significant occupancy are only M·ATP and AM·ADP·Pi. The rise in the number of actin-attached motors when k5 is re-assigned the isometric value is shown in figure 1d (data relative to the steady isometric value at the respective [Pi]). The rate constant of force rise (rF), estimated by the reciprocal of the time from the force at time 0–63% of the steady isometric force (see Caremani et al. 2008), increases with [Pi] (blue continuous line in figure 1f) in agreement with the experimental results (filled circles from fig. 6C of Caremani et al. 2008). The same set of rate constants was used to reproduce the force transient elicited by a Pi jump. The simulated time course of the number of attached cross-bridges in response to a Pi jump from 0 to 11 mM Pi (figure 1e, dashed line), is faster than that following unloaded shortening in the presence of 11 mM Pi (continuous line). The calculated relation between the rate of the force transient elicited by a Pi jump (rPi) and [Pi] (blue dashed line in figure 1f) reproduces quite satisfactorily the observed relation (open squares from fig. 6 of Dantzig et al. 1992) and both lie above the rF–Pi relations (filled circles and continuous line) as a consequence of the fact that the weight of each process in approaching the new distribution is determined by the position of the perturbation in the cycle (see also Caremani et al. 2008).
(iv). Simulation of the effect of Pi on the isometric ATPase rate
In control solution, the calculated ATPase rate is 0.38 mM s−1, in agreement with the values reported for fast skeletal muscle (Potma & Stienen 1996). With scheme 1, the increase in [Pi] reduces the ATPase rate (blue line in figure 1c) in proportion to the reduction of the occupancy of the AM′·ADP state (red line in figure 1a). Thus, the Pi-dependent decrease in the ATPase rate is larger than that of isometric force (blue line in figure 1b), as the force results from the occupancy of both the decreasing AM′·ADP state and the increasing AM′·ADP·Pi state (blue line in figure 1a). In contrast to the simulated relation, the observed relation (open symbols in figure 1c) obtained from several studies at comparable temperature (11–15°C, Bowater & Sleep 1988; Potma et al. 1995; Potma & Stienen 1996) shows that the ATPase rate is reduced less than the isometric force (and the fraction of attached cross-bridges): an increase of [Pi] to 15–20 mM produces a reduction in the isometric ATPase of only approximately 20 per cent. This demonstrates that the conventional reaction scheme is not adequate to explain the energetics of the isometric contraction.
(b). Unconventional cycle with the release of hydrolysis products after motor detachment
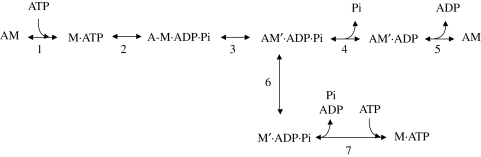
The contradiction can be solved by allowing a substantial ATPase activity to occur also at high [Pi]. This is provided in scheme 2 by the branched pathway that allows the AM′·ADP·Pi force-generating state to detach before the release of hydrolysis products (step 6).
Scheme 2.
Unconventional chemo-mechanical cycle.
The detached M′·ADP·Pi state derives from a strongly bound state that has undergone the conformational changes that generates the stiff and strained conformation responsible for force, and therefore it is structurally and kinetically different from the M·ADP·Pi state in rapid equilibrium with the weakly bound A-M·ADP·Pi state. We hypothesize that the M′·ADP·Pi state, undergoes a rapid sequence of events consisting of the completion of both the structural change normally associated to the execution of the 10 nm working stroke in the attached myosin head and the ATPase cycle by an almost irreversible release of Pi and ADP and binding of a new ATP (step 7). Rapid release of the hydrolysis products from myosin in the absence of actin has not been observed in solution (White et al. 1997). However, in fibres, the release occurs from a myosin state that, as a consequence of the reaction with actin, has assumed a strained conformation that responds to detachment with the immediate opening of the nucleotide binding pocket and product release. In support of this view, recent X-ray experiments on intact frog muscle fibres suggest that the rate of events that terminate the AM interaction (ADP release, ATP binding and the ensuing detachment of myosin from actin) is controlled specifically by the conformation of the myosin head (Piazzesi et al. 2007). Since it is not possible to investigate the hypothesized state in solution, there is no direct conflict between our hypothesis and solution studies.
(i). Rate constants
Unless otherwise specified, the values of the rate constants of the transitions (listed in table 1) are the same as those selected for the simulation with scheme 1. Details for the selection of rate constants of the steps 6 and 7 are given in the electronic supplementary material. The combined equilibrium constant of steps 6 and 7 is 1.5 × 106 M, so that the equilibrium constant of the unconventional cycle (K2·K3·K6·K7) is 5.3 × 105 M. Thus, in contrast to the conventional cycle, the unconventional cycle accounts for the equilibrium constant of ATP hydrolysis also in isometric contraction. As a corollary, it must be noted that the unconventional cycle provides a straightforward explanation for the finding that in isometric contraction the free energy of the ATP hydrolysis is mostly released as heat.
(ii). Simulation of the effect of Pi on the isometric force and on the ATPase rate
With scheme 2, the effects of [Pi] on the occupancy of the relevant states of the reaction scheme (figure 2a) are substantially similar to those in scheme 1 (figure 1a), because the new M′·ADP·Pi state is only a transient intermediate and its occupancy is relatively low even at the highest [Pi].
Figure 2.
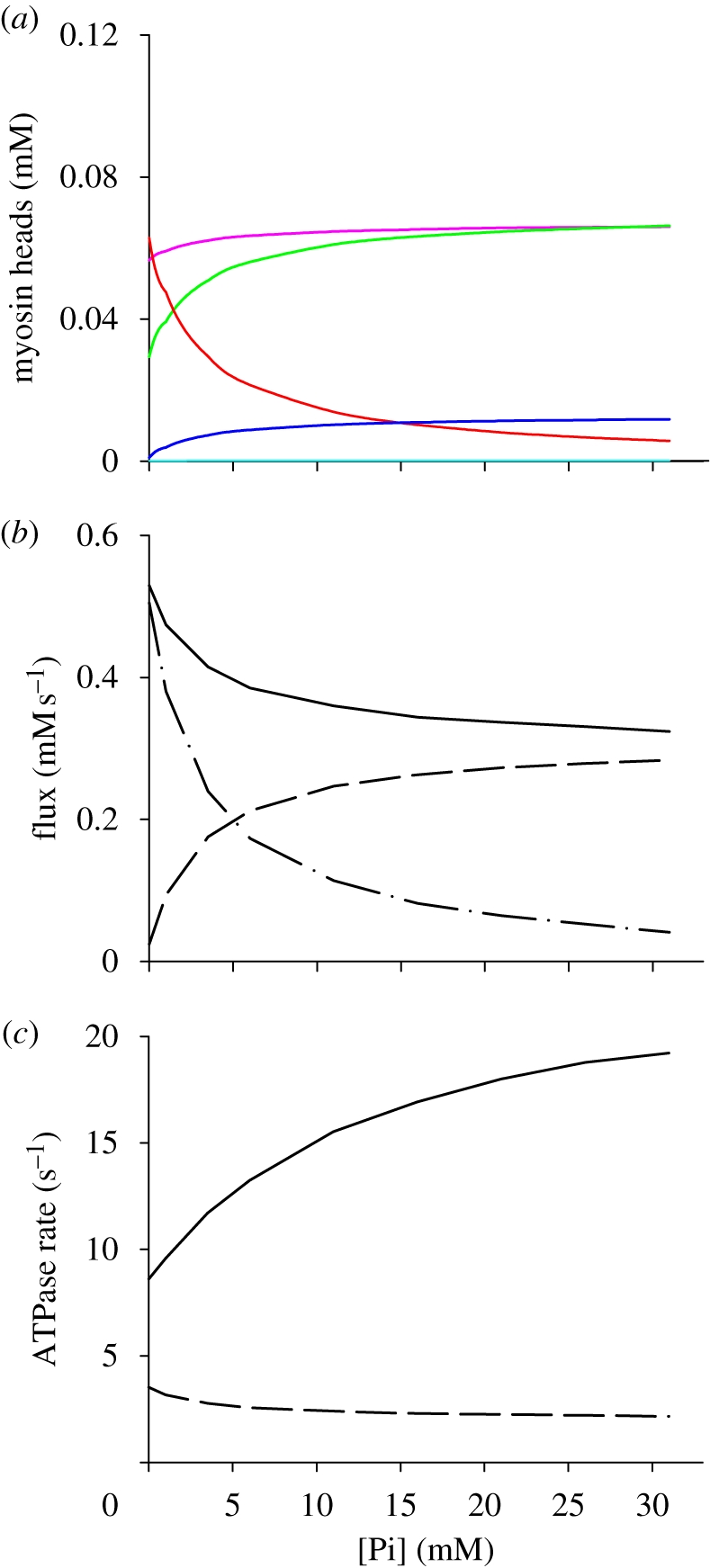
Responses of the simulation with scheme 2. (a) Effect of [Pi] on the occupancy of the cross-bridge states (as defined by the colours: pink line, M·ATP; green line, A-M·ADP·Pi; dark blue line, AM′·ADP·Pi; red line, AM′·ADP; light blue line, M′·ADP·Pi) during isometric contraction. (b) Pi dependence of fluxes through the conventional pathway (dot-dashed line) and the branched pathway (dashed line) and simulated ATPase rate (continuous line, given by the sum of the two fluxes). (c) Pi-dependence of the ATPase rate per myosin head (dashed line) and per attached myosin head (continuous line) calculated as described in the text.
As shown in figure 2b, in control solution the ATPase rate (continuous line) is 0.47 mM s−1, similar to that observed (Potma & Stienen 1996) and is 80 per cent accounted for by the flux through the conventional path (dot-dashed line) and 20 per cent by the flux through the branched path (dashed line). An increase in [Pi] alters the fluxes through the two pathways by mass action in opposite ways: the flux through the conventional pathway is reduced, while that through the branched pathway is increased. At 25 mM Pi, the ATPase rate has dropped only to 0.33 mM s−1 and is 82 per cent accounted for by the flux through the branched pathway. The reduced effect of [Pi] on the ATPase is in agreement with the data reported by all the studies on fast skeletal muscle (figure 1c, red line). At the same time, the model maintains the ability to fit the effects of Pi on the steady isometric force (figure 1b, red line), on the rate of force development rF (figure 1f, red continuous line) and on the rate of the force transient following a Pi jump, rPi (red dashed line).
(c). Kinetics, energetics and structural dynamics of the myosin motor and their Pi dependence
With a concentration of myosin heads in skeletal muscle of 0.15 mM (He et al. 1997; Sun et al. 2001) and using the relation of ATPase rate versus Pi (red line in figure 1c), the Pi dependence of the ATPase rate per myosin head can be calculated (figure 2c, dashed line): in control solution the ATPase rate per myosin head is (0.47 mM s−1/0.15 mM=) 3.1 s−1 and drops with increases in [Pi] up to a minimum of (0.33 mM s−1/0.15 mM=) 2.2 s−1 at 25 mM Pi. In isometric contraction in control solution, the fraction of cross-bridges attached, f, is approximately 0.3 (Linari et al. 2007) and drops with Pi, attaining at 25 mM Pi a minimum of approximately 0.4 the control value (approx. 0.12, Caremani et al. 2008). Using the value of f at any [Pi] as the normalization factor for the ATPase rate, we can calculate the Pi dependence of the ATPase rate per attached cross-bridge (figure 2c, continuous line). In the control solution, each cross-bridge splits ATP at approximately 9 s−1; increasing [Pi] the rate of ATP splitting increases up to approximately 19 s−1 at 25 mM Pi, as a consequence of the presence of the branched pathway. Thus, this pathway explains why the metabolic cost of the isometric force increases with the increase in [Pi].
If filamentary sliding and thus the execution of the myosin working stroke are prevented by the isometric condition, the process that leads to the release of Pi upon attachment to actin can be reversed by the increase in [Pi], because all steps in the process are in dynamic equilibrium (Geeves & Holmes 2005). The assumption in scheme 2 that in the isometric contraction the myosin cross-bridge can detach from actin when it is in the strongly bound force generating AM′·ADP·Pi state implies a qualitatively different mechanism, as in this case the myosin cross-bridge has gone through the liberation of part of the free energy of ATP hydrolysis during the actin attachment period. Therefore, after detachment, this myosin cross-bridge is committed for completing the biochemical cycle with release of the hydrolysis products and rebinding of a new ATP. To consider the likelihood of such a different pathway for the myosin–actin ATPase reaction, it must be taken into account that, in a complex molecule such as the myosin head, the path between the two structural states representing the beginning and the end of the working stroke may be different in relation to conditions (such as [Pi] or strain) that alter the free energy landscape between the two states.
In structural terms, scheme 2 implies that the myosin cross-bridge can detach from actin at a stage of the working stroke that has progressed only by the extent necessary to account for the isometric force. The possibility that the myosin–actin interaction implies different sizes of the working stroke finds support in the idea, promoted by the recent evidence that the stiffness of the myosin cross-bridge is quite high (1.7–3.2 pN nm−1, Decostre et al. 2005; Linari et al. 2007; Lewalle et al. 2008), that the working stroke is made by a series of structural steps and that only the first steps are allowed at high load (Reconditi et al. 2004; Decostre et al. 2005; Piazzesi et al. 2007).
Schemes of the cross-bridge cycle with a branched pathway were previously hypothesized (Kawai et al. 1987; Smith & Mijailovich 2008; Woledge et al. 2009); however, none of them is adequate to account for the different Pi dependence of the number of cross-bridges and ATPase rate in isometric contraction. On the other hand, the idea that the myosin can detach from actin at different stages of the working stroke, allowing rapid termination of the biochemical and structural steps of the actomyosin interaction at high load, probably applies also at physiological [Pi], and thus after the Pi release. Under this hypothesis, which provides the general mechanism by which the equilibrium constant of ATP hydrolysis is accounted for at high loads, the dot-dashed line in figure 2b would represent the flux of the cross-bridges through cycles that imply detachment from actin following the Pi release.
(d). Comparative kinetic and energetical aspects
In fibres from slow skeletal muscle, the ATPase rate during isometric contraction is one order of magnitude smaller than in fibres from fast skeletal muscle (Potma et al. 1995); moreover, in contrast with fast fibres, in slow fibres the increase in [Pi] reduces the isometric force and the ATPase rate in proportion (Potma et al. 1995). In terms of our kinetic model, we can predict that these features of slow fibres can be explained by suppression of the branched pathway and adequate decrease of the rate constant of the ATP hydrolysis. Consequently, the metabolic cost of the isometric force should be lower in slow fibres compared with fast fibres, as proven by the datum that the maintenance heat (the heat liberated during the isometric contraction) is lower in slow fibres than in fast fibres (Woledge et al. 1985; Barclay et al. 1993).
The most important mechanical and energetic difference in the two fibre types is the power output during steady shortening, which is much higher in fast fibres (Woledge et al. 1985; Barclay et al. 1993; Linari et al. 2004). A kinetic mechanical model of the myosin cross-bridge in muscle was able to explain these differences (Piazzesi & Lombardi 1995a,b). The higher power of fast fibres could be predicted by that model by increasing the probability of cross-bridges to detach at an intermediate stage of the working stroke and reattach more rapidly than cross-bridges detached at the end of the working stroke. The conclusion of this work that, in fast fibres, the myosin cross-bridge can detach via the branched pathway with the hydrolysis products still bound to its catalytic site is not per se sufficient to fit the predictions of the mechanical model of Piazzesi & Lombardi (1995a), because the unconventional cycle still implies that the apparent rate constant of attachment is moderate, as it includes both the hydrolysis step and the attachment/force-generation step. In any case, following this work, one can anticipate that a model able to fit the kinetics and energetics of steady shortening should consider in detail not only the strain dependency of the rate constants of transitions between attached states, but also several other features such as the conformational and biochemical states for detachment in the unconventional cycles and the steric role played at the level of a single myosin–actin interaction by the dimeric structure of myosin (e.g. Huxley & Tideswell, 1997) and by the array arrangement of myosin motors in each thick filament.
Acknowledgements
The authors thank Alessandro Cuccoli for his suggestions for the analytical solution of the model, Malcolm Irving and Gabriella Piazzesi for helpful discussion and Mario Dolfi for software assistance. This research was supported by the National Institutes of Health (grant R01 AR049033), by the Ministero dell'Università e della Ricerca (MIUR-COFIN 2006), by the Istituto di Tecnologie Biomediche (ITB-CNR) and by the Ministero del Lavoro, della Salute e delle Politiche Sociali (grant no. RF-MUL-2007-666195).
References
- Bagshaw C. R., Trentham D. R.1974The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem. J. 141, 331–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay C. J., Constable J. K., Gibbs C. L.1993Energetics of fast- and slow-twitch muscles of the mouse. J. Physiol. 472, 61–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowater R., Sleep J.1988Demembranated muscle fibers catalyze a more rapid exchange between phosphate and adenosine triphosphate than actomyosin subfragment 1. Biochemistry 27, 5314–5323 (doi:10.1021/bi00414a055) [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Cox R. N., Kawai M., Robinson T.1982Regulation of tension in skinned muscle fibers. Effect of cross-bridge kinetics on apparent Ca2+ sensitivity. J. Gen. Physiol. 79, 997–1016 (doi:10.1085/jgp.79.6.997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caremani M., Dantzig J. A., Goldman Y. E., Lombardi V., Linari M.2008The effect of inorganic phosphate on the force and number of myosin cross-bridges during the isometric contraction of permeabilized muscle fibers from rabbit psoas. Biophys. J. 95, 5798–5808 (doi:10.1529/biophysj.108.130435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E.1988The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J. Physiol. 395, 77–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig J. A., Goldman Y. E., Millar N. C., Lacktis J., Homsher E.1992Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J. Physiol. 451, 247–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decostre V., Bianco P., Lombardi V., Piazzesi G.2005Effect of temperature on the working stroke of muscle myosin. Proc. Natl Acad. Sci. USA 102, 13927–13932 (doi:10.1073/pnas.0506795102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R., Freyzon Y., Trybus K. M., Cohen C.1998Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualization of the pre-power stroke state. Cell 94, 559–571 (doi:10.1016/S0092-8674(00)81598-6) [DOI] [PubMed] [Google Scholar]
- Fenn W. O.1923A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J. Physiol. 58, 175–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Trentham D. R.1984The kinetics of magnesium adenosine triphosphate cleavage in skinned muscle fibres of the rabbit. J. Physiol. 352, 575–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune N. S., Geeves M. A., Ranatunga K. W.1991Tension responses to rapid pressure release in glycerinated rabbit muscle fibers. Proc. Natl Acad. Sci. USA 88, 7323–7327 (doi:10.1073/pnas.88.16.7323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves M. A., Holmes K. C.2005The molecular mechanism of muscle contraction. Adv. Protein Chem. 71, 161–193 (doi:10.1016/S0065-3233(04)71005-0) [DOI] [PubMed] [Google Scholar]
- Guynn R. W., Weech R. L.1973The equilibrium constants of the adenosine triphosphate hydrolysis and the adenosine triphosphate-citrate lyase reactions. J. Biol. Chem. 248, 6966–6972 [PubMed] [Google Scholar]
- He Z. H., Chillingworth R. K., Brune M., Corrie J. E., Trentham D. R., Webb M. R., Ferenczi M. A.1997ATPase kinetics on activation of rabbit and frog permeabilized isometric muscle fibres: a real time phosphate assay. J. Physiol. 501, 125–148 (doi:10.1111/j.1469-7793.1997.125bo.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E.1985Phosphate release and force generation in skeletal muscle fibers. Science 228, 1317–1319 (doi:10.1126/science.3159090) [DOI] [PubMed] [Google Scholar]
- Hill A. V.1938The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. B 126, 136–195 (doi:10.1098/rspb.1938.0050) [Google Scholar]
- Huxley H. E.1969The mechanism of muscular contraction. Science 164, 1356–1365 (doi:10.1126/science.164.3886.1356) [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M.1971Proposed mechanism of force generation in striated muscle. Nature 233, 533–538 (doi:10.1038/233533a0) [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Tideswell S.1997Rapid regeneration of the power stroke in contracting muscle by attachment of second myosin head. J. Muscle Res. Cell Motil. 18, 111–114 (doi:10.1023/A:1018641218961) [DOI] [PubMed] [Google Scholar]
- Kawai M., Halvorson H. R.1991Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas muscle. Biophys. J. 59, 329–342 (doi:10.1016/S0006-3495(91)82227-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Guth K., Winnikes K., Haist C., Ruegg J. C.1987The effect of inorganic phosphate on the ATP hydrolysis rate and the tension transients in chemically skinned rabbit psoas fibers. Pflugers Arch. 408, 1–9 (doi:10.1007/BF00581833) [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E.1969The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Proc. R. Soc. Lond. B 174, 315–353 (doi:10.1098/rspb.1969.0096) [DOI] [PubMed] [Google Scholar]
- Lewalle A., Steffen W., Stevenson O., Ouyang Z., Sleep J.2008Single molecule measurements of the stiffness of the rigor myosin head. Biophys. J. 94, 2160–2169 (doi:10.1529/biophysj.107.119396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M., Bottinelli R., Pellegrino M. A., Reconditi M., Reggiani C., Lombardi V.2004The mechanism of the force response to stretch in human skinned muscle fibres with different myosin isoforms. J. Physiol. 554, 335–352 (doi:10.1113/jphysiol.2003.051748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M., Caremani M., Piperio C., Brandt P., Lombardi V.2007Stiffness and fraction of myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas. Biophys. J. 92, 2476–2490 (doi:10.1529/biophysj.106.099549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W.1971Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10, 4617–4624 (doi:10.1021/bi00801a004) [DOI] [PubMed] [Google Scholar]
- Martyn D. A., Gordon A. M.1992Force and stiffness in glycerinated rabbit psoas fibers. Effects of calcium and elevated phosphate. J. Gen. Physiol. 99, 795–816 (doi:10.1085/jgp.99.5.795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar N. C., Homsher E.1990The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J. Biol. Chem. 265, 20234–20240 [PubMed] [Google Scholar]
- Nyitrai M., Geeves M. A.2004Adenosine diphosphate and strain sensitivity in myosin motors. Phil. Trans. R. Soc. Lond. B 359, 1867–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E., Cooke R.1985The inhibition of muscle contraction by adenosine 5′ (beta, gamma-imido) triphosphate and by pyrophosphate. Biophys. J. 47, 773–780 (doi:10.1016/S0006-3495(85)83980-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E., Cooke R.1989aA model of crossbridge action: the effects of ATP, ADP and Pi. J. Muscle Res. Cell Motil. 10, 181–196 (doi:10.1007/BF01739809) [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R.1989bAddition of phosphate to active muscle fibers probes actomyosin states within the powerstroke. Pflügers Arch. 414, 73–81 (doi:10.1007/BF00585629) [DOI] [PubMed] [Google Scholar]
- Piazzesi G., Lombardi V.1995aA cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys. J. 68, 1966–1979 (doi:10.1016/S0006-3495(95)80374-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G., Lombardi V.1995bA model of cross-bridge action able to explain mechanical and energetic properties of muscle. Biophys. J. 68, 356s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G., Reconditi M., Linari M., Lucii L., Sun Y.-B., Narayanan T., Boesecke P., Lombardi V., Irving M.2002Mechanism of force generation by myosin heads in skeletal muscle. Nature 415, 659–662 (doi:10.1038/415659a) [DOI] [PubMed] [Google Scholar]
- Piazzesi G., et al. 2007Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131, 784–795 (doi:10.1016/j.cell.2007.09.045) [DOI] [PubMed] [Google Scholar]
- Potma E. J., Stienen G. J.1996Increase in ATP consumption during shortening in skinned fibres from rabbit psoas muscle: effects of inorganic phosphate. J. Physiol. 496, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potma E. J., van Grass I. A., Stienen G. J.1995Influence of inorganic phosphate and pH on ATP utilization in fast and slow skeletal muscle fibers. Biophys. J. 69, 2580–2589 (doi:10.1016/S0006-3495(95)80129-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reconditi M., et al. 2004The myosin motor in muscle generates a smaller and slower working stroke at higher load. Nature 428, 578–581 (doi:10.1038/nature02380) [DOI] [PubMed] [Google Scholar]
- Regnier M., Morris C., Homsher E.1995Regulation of the cross-bridge transition from a weakly to strongly bound state in skinned rabbit muscle fibers. Am. J. Physiol. 269, C1532–C1539 [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Hutton R. L.1980Exchange between inorganic phosphate and adenosine 5′-triphosphate in the medium by actomyosin subfragment 1. Biochemistry 19, 1276–1283 (doi:10.1021/bi00548a002) [DOI] [PubMed] [Google Scholar]
- Sleep J., Irving M., Burton K.2005The ATP hydrolysis and phosphate release steps control the time course of force development in rabbit skeletal muscle. J. Physiol. 563, 671–687 (doi:10.1113/jphysiol.2004.078873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. A., Mijailovich S. M.2008Toward a unified theory of muscle contraction. II: predictions with the mean-field approximation. Ann. Biomed. Eng. 26, 1353–1371 (doi:10.1007/s10439-008-9573-1) [DOI] [PubMed] [Google Scholar]
- Sun Y.-B., Hilber K., Irving M.2001Effect of active shortening on the rate of ATP utilisation by rabbit psoas muscle fibres. J. Physiol. 531, 781–791 (doi:10.1111/j.1469-7793.2001.0781h.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesi C., Colomo F., Nencini S., Piroddi N., Poggesi C.2000The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys. J. 78, 3081–3092 (doi:10.1016/S0006-3495(00)76845-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesi C., Colomo F., Nencini S., Piroddi N., Poggesi C.2002Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles. J. Physiol. 541, 187–199 (doi:10.1113/jphysiol.2001.013418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W., Lu Z., Moss R. L.1992Effect of Ca2+ on the kinetics of phosphate release in skeletal muscle. J. Biol. Chem. 267, 2459–2466 [PubMed] [Google Scholar]
- West T. G., Ferenczi M. A., Woledge R. C., Curtin N. A.2005Influence of ionic strength on the time course of force development and phosphate release by dogfish muscle fibres. J. Physiol. 567, 989–1000 (doi:10.1113/jphysiol.2005.087106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. D., Taylor E. W.1976Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry 15, 5818–5826 (doi:10.1021/bi00671a020) [DOI] [PubMed] [Google Scholar]
- White H. D., Belknap B., Webb M. R.1997Kinetics of nucleoside triphosphate cleavage and phosphate release steps by associated rabbit skeletal actomyosin, measured using a novel fluorescent probe for phosphate. Biochemistry 36, 11828–11836 (doi:10.1021/bi970540h) [DOI] [PubMed] [Google Scholar]
- Woledge R. C., Curtin N. A., Homsher E.1985Energetics aspects of muscle contraction. London, UK: Academic Press; [PubMed] [Google Scholar]
- Woledge R. C., Barclay C. J., Curtin N. A.2009Temperature change as a probe of muscle crossbridge kinetics: a review and discussion. Proc. R. Soc. B 276, 2685–2695 (doi:10.1098/rspb.2009.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Kawai M.1991Kinetic and thermodynamic studies of the cross-bridge cycle in rabbit psoas muscle fibers. Biophys. J. 67, 1655–1668 (doi:10.1016/S0006-3495(94)80638-1) [DOI] [PMC free article] [PubMed] [Google Scholar]