Abstract
Exposure to potential mates triggers rapid elevations of testosterone and glucocorticoid concentrations in males of many non-human species, and preliminary studies support similar effects in human males. The human studies have all reported large individual differences in these responses, however, and the present study tested whether specific biological variables may help explain these differences. Replicating past research, the present study found that men's salivary testosterone and cortisol concentrations increased after a brief conversation with a young woman, but did not change (or slightly decreased) after a conversation with a young man. In addition, smaller numbers of CAG repeats in men's androgen receptor gene, and lower baseline cortisol concentrations, each predicted larger testosterone responses to the interactions with women. The CAG repeat finding demonstrates that a specific genetic polymorphism predicts physiological responses to social interactions that may in turn have important downstream consequences on men's mating behaviour. The effects of cortisol are consistent with past demonstrations of glucocorticoid inhibition of testosterone production and show that such inhibition also affects testosterone responses to social stimuli. In sum, the present study both confirms men's hormonal reactions to potential mates and identifies novel biological variables that predict individual differences in these responses.
Keywords: testosterone, cortisol, human mating, androgen receptor gene
1. Introduction
Males of many vertebrate species exhibit an endocrine mating response in which non-tactile exposure to potential mates triggers rapid (less than 45 min) increases in testosterone (Purvis & Haynes 1974; Batty 1978; Pfeiffer & Johnston 1994; Amstislavskaya & Popova 2004; Bonilla-Jaime et al. 2006) and glucocorticoid (Bronson & Desjardins 1982; Mendoza & Mason 1989; Bonilla-Jaime et al. 2006) concentrations. These responses are generally absent in sexually sated animals (Bronson & Desjardins 1982; Bonilla-Jaime et al. 2006) and after comparable exposure to conspecific males (Macrides et al. 1975; Pfeiffer & Johnston 1992; Amstislavskaya & Popova 2004), suggesting functional sensitivity to mating-relevant stimuli. Lesion studies have further demonstrated that both behavioural (for reviews, see Meisel & Sachs 1994; Paredes & Baum 1997) and hormonal (Kamel & Frankel 1978) responses to potential mates appear to be regulated by structures within a limbic–hypothalamic circuit, such as the medial amygdala and medial preoptic area. The phylogenetic conservation of this circuit raises the possibility that homologous mechanisms may be expressed in humans.
Preliminary studies have provided evidence that brief conversations with young women can in fact trigger rapid elevations of testosterone in human males (Roney et al. 2003, 2007; van der Meij et al. 2008), with two of these studies likewise demonstrating elevations of cortisol (Roney et al. 2007). In all of these studies, however, there have been large individual differences in the magnitude of hormonal responses. The present investigation was designed to test replication of men's hormonal responses to potential mates while also testing whether specific biological variables may help explain individual differences in the size of these responses.
Three classes of individual difference variables were targeted for investigation: (i) sexual experience, (ii) androgen receptor (AR) gene sequence polymorphism and (iii) basal concentrations of cortisol and testosterone. Sexually experienced male rodents exhibit larger hormonal responses to female stimuli than do inexperienced males (Pfeiffer & Johnston 1994; Bonilla-Jaime et al. 2006), and sexual experience likewise increases Fos immunoreactivity in the medial preoptic area in response to contact with females (Lumley & Hull 1999). These results suggest that sexual experience may induce changes in the limbic–hypothalamic circuit that sensitize its responses to mating-relevant stimuli. In humans, one study reported a marginally significant trend towards larger testosterone increases after interactions with women among men with sexual experience (Roney et al. 2003), though a subsequent study failed to confirm this effect (van der Meij et al. 2008). The present study allowed an additional investigation of this variable.
The human AR gene sequence is highly polymorphic (Edwards et al. 1992) and studies have shown that smaller numbers of CAG codon repeats in exon 1 are associated with both greater expression of AR protein (Choong et al. 1996) (i.e. larger numbers of receptors) and with enhanced transcriptional activity of the AR (Chamberlain et al. 1994). As a result, men with shorter CAG repeats appear to convert the same concentrations of androgen into larger physiological effects than do men with longer repeats (Campbell et al. 2007; Zitzmann & Nieschlag 2007), which may explain why shorter repeats have in some studies been associated with the risk of prostate cancer (Casella et al. 2001), enhanced spermatogenesis (von Eckardstein et al. 2001) and incidence of violent behaviour (Rajender et al. 2008). Importantly, the limbic–hypothalamic circuit that regulates responses to potential mates contains the highest density of AR of any brain region (Pfaff 1981), and blockade of AR in this pathway reduces or eliminates sexual responses to females (Harding & McGinnis 2004; Raskin et al. 2009). In addition, sexual satiety that eliminates behavioural and hormonal responses to females (Bronson & Desjardins 1982; Bonilla-Jaime et al. 2006) is associated with dramatic drops in AR density in this pathway (Fernandez-Guasti et al. 2003; Romano-Torres et al. 2007), and subsequent recovery of sexual responses over time is correlated with restoration of AR levels (Romano-Torres et al. 2007). These findings suggest that AR activity modulates the sensitivity of the limbic–hypothalamic pathway to cues from females. Men with shorter CAG repeats and thus greater AR activity might therefore be expected, ceteris paribus, to have more sensitized pathway structures that lead to greater hormonal responses to potential mates.
Finally, basal concentrations of men's cortisol and testosterone may be important predictors of individual differences in hormonal responses to potential mates. Glucocorticoids have been shown to suppress Leydig cell testosterone responses to luteinizing hormone (e.g. Sapolsky 1985; Dong et al. 2004), and, conversely, testosterone administration can reduce corticotropin-releasing hormone (CRH)-stimulated cortisol production (Rubinow et al. 2005). These results suggest that baseline concentrations of each hormone may negatively predict change scores in the other hormone. Baseline concentrations of each hormone are almost certainly negatively correlated with their own change scores as a mathematical consequence of regression to the mean (Campbell & Kenny 1999), and baseline concentrations were therefore controlled before examining the influence of other individual difference variables on hormone change scores.
In the present research, young men were randomly assigned to engage in a social interaction with either a young man (male condition) or a young woman (female condition). Saliva samples were collected just before and 40 min after the start of these interactions, from which cortisol and testosterone concentrations were assayed. Participants indicated their lifetime number of sex partners and their current mood states on a survey administered after the interactions. Participants also swished with mouthwash before expectorating into a test tube in order to obtain buccal cells from which DNA was extracted in order to assay the number of CAG repeats in the AR gene. Testosterone and cortisol concentrations were hypothesized to increase in the female condition relative to the male condition. In addition, sexual experience, number of CAG repeats and baseline hormone concentrations were expected to predict the magnitude of hormone responses specifically within the female condition.
2. Material and Methods
(a). Participants
A total of 149 men (mean age = 18.95 years; range 18–24 years) participated in the study for partial fulfilment of a course requirement, though sample sizes for data analyses were smaller owing to missing hormone data (§2d). Sixty-six per cent of the sample reported being white, 20 per cent Asian, 13 per cent Latino, and one per cent African-American. All men self-identified as heterosexuals.
(b). Materials
Participants completed background and mood surveys at the end of their testing session. Among the measures relevant to the present report, men self-reported their lifetime total number of sexual intercourse partners (mean = 2.84, s.d. = 5.85; 35% reported zero partners). The mood scale asked participants to indicate how well a series of terms described the way they felt at the present moment, using a 1–7 Likert scale. Because cortisol increases are often associated with negative and threatening events (for a review, see Dickerson & Kemeny 2004), we were interested in whether cortisol reactions to potential mates may represent an exception in which glucocorticoid elevations are associated with positive mood states. A factor analysis of 16 total items revealed a factor that indexed positive mood and contained the following items: happy, cheerful, lively, excited. Ratings across the four items were averaged to form a composite scale (α = 0.82) that was tested for associations with change in cortisol concentrations (see §3).
(c). Procedure
Sex of experimenter comprised the experimental manipulation. One third of the participants (n = 50) were randomly assigned to interact with a male experimenter (age 25). Two-thirds of the participants interacted with one of seven young women (mean age = 19 years; range 18–22 years). The female condition was initially split into two subconditions, one in which the participant interacted with a woman alone and the other in which a male observer was seated outside the testing room in which the interaction with a woman took place in order to test whether the presence of a potential competitor might affect hormone responses. Because there were no differences between these two subconditions for any of the variables reported here, they were combined into a single female exposure condition.
Experimental sessions began between 13.00 and 16.00. The study was described as an investigation of whether cortisol levels may affect the speed of word categorizations. After the informed consent process, men immediately provided a baseline saliva sample via passive drool. While the first computer program designed to measure word categorizations was ostensibly loading, the experimenter made conversation with the subject for approximately 5 min. Female experimenters attempted to be flirtatious and to signal interest throughout the session; male experimenters simply attempted friendly conversation. After completion of a computer-based reaction time task (approx. 5 min), a second 5 min waiting period allowed further interaction between the participant and the experimenter. Additional categorization tasks followed before a second saliva sample was collected at approximately 40 min after the first sample. As part of the debriefing process, men in the female condition rated the physical attractiveness of the women confederates on a 1–7 scale; the mean rating was 5.83 (s.d. = 0.94), which is higher than the ratings obtained in previous studies that have employed similar manipulations (Roney et al. 2003, 2007). Finally, participants swished with mouthwash before expectorating into collection vials for the purpose of obtaining buccal cells (see §2e).
(d). Hormone assays
Saliva samples were stored in polypropylene vials at −80°C before being shipped on dry ice to the Biomarkers Core Laboratory at the Yerkes National Primate Research Center, Atlanta, GA, USA. Salivary testosterone was assayed in triplicate using procedures described in Roney et al. (2007). Five samples had insufficient quantities of saliva for assay and six others returned values outside the standard curve, thus leaving n = 138 for analyses involving testosterone. To improve the precision of testosterone estimates, the mean of the closest two out of three triplicate values were used in data analyses; taking the closest two values improved the intra-assay coefficients of variation (CVs) from 10.95 to 6.91 per cent. The inter-assay CV was 15.60 per cent, but samples from the same individual were assayed within the same batch and experimental conditions were equally distributed across batches. Testosterone values were normally distributed after log transformation.
Saliva remaining after testosterone assay was shipped on dry ice to the Endocrine Core Laboratory at the California Regional Primate Research Center, Davis, CA, USA. Twenty samples had insufficient saliva for assay at this stage, leaving n = 129 for analyses involving cortisol. Salivary concentrations of cortisol were estimated in duplicate using procedures described in Roney et al. (2007). Intra- and inter-assay CVs were 3.60 and 4.05 per cent, respectively. Cortisol concentrations were estimated as the mean of duplicate values and were normally distributed after log transformation.
(e). Genotyping
The mouthwash/expectorate samples were stored at −80°C before being delivered on dry ice for DNA extraction to the Biological Samples Processing Core at UCLA. Extraction was done by Autopure LS (Qiagen Inc.) using the Puregene Buccal Cell Kit (Qiagen Inc.). After extraction, genotyping and sequencing were carried out by the UCLA Sequencing and Genotyping core. The forward and reverse primers used in the polymerase chain reaction (PCR) were: 5′-TCCAGAATCTGTTCCAGAGCGTGG-3′ and 5′-GCTCTGAAGGTTGCTGTTCCTCAT-3′, respectively. Procedures for the PCR reaction were identical to those in Gonzalez Hernandez et al. (2007), with the following modifications: reaction volume was reduced to 12.5 µl (using 50 ng of genomic DNA) and fragments were separated using an ABI 3730 Genetic Analyzer (Applied Biosystems). Repeat numbers were confirmed by sequencing a subset of samples with alleles of different lengths using the Big Dye Terminator Sequencing Kit (Applied Biosystems). Sequencing used reverse primer; sequencing reaction consisted of 30 cycles at 96°C for 10 s, 50°C for 5 s, 60°C for 4 min.
Mean CAG repeat length among men for whom DNA could be extracted (n = 138) was 21.74 (range 14–31 repeats). Ethnicity could not account for the relationship between CAG repeat length and men's testosterone responses since addition of dichotomous race categories to the regression model depicted in table 1 did not alter the effect size for CAG repeats (β = −0.15).
Table 1.
Multiple regression model predicting log-transformed post-test testosterone concentrations in the female condition. (For the full model: F3,73 = 31.83, p < 0.001. CAG repeat number and baseline cortisol jointly accounted for 10% of the residual variance in post-test testosterone that was not explained by baseline testosterone. Predictor variables were uncorrelated with each other)
| predictor variables | standardized β | ΔR2 |
|---|---|---|
| step 1 | 0.53** | |
| log baseline testosterone | 0.73 | |
| step 2 | 0.05* | |
| CAG repeat number | −0.15 | |
| log baseline cortisol | −0.15 |
*p < 0.05.
**p < 0.0001.
(f). Data analyses
Effects of the experimental manipulation on testosterone and cortisol concentrations were tested using repeated measures ANOVA. The influence of individual difference variables on change in hormones was tested using a regression approach in which baseline hormone concentrations were entered as the first step of models predicting post-test hormone concentrations, with individual difference variables entered at subsequent steps in the model. As expected, baseline hormone concentrations in the present sample negatively predicted raw change scores (for testosterone: r = −0.31, p < 0.001; for cortisol: r = −0.55, p < 0.0001), and the regression approach in effect controls for the influence of baseline values (Campbell & Kenny 1999). Associations between positive mood and change in cortisol were tested using Pearson correlations as well as partial correlations controlling for baseline cortisol. All significance tests were two-tailed.
3. Results
(a). Hormonal responses to potential mates
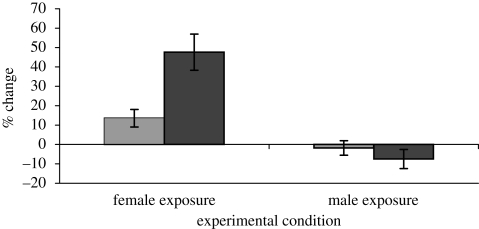
Figure 1 demonstrates that both testosterone and cortisol increased among men who spoke with women but declined among men who interacted with other men. Repeated measures ANOVAs demonstrated significant interactions between time of measurement (pre versus post) and experimental group (female versus male) for both testosterone (F1,136 = 3.92, p = 0.05) and cortisol (F1,127 = 18.90, p < 0.0001). These results replicate previous studies in showing that exposure to women produces rapid hormone increases in men that are absent after exposure to other men.
Figure 1.
Mean per cent change from baseline (±s.e.m.) for cortisol and testosterone concentrations within each experimental condition. Per cent changes in raw concentrations are depicted for presentation purposes, but statistics were performed on log-transformed values (see text). Light grey bars, testosterone; dark grey bars, cortisol.
Contrary to the possibility that the cortisol increase in the female condition represents a ‘stress response’, change in cortisol in the female condition predicted more positive mood states after the social interactions (r = 0.21, p = 0.06; though partial r = 0.16, p = 0.16 after controlling for baseline cortisol), whereas the opposite pattern was found in the male condition (r =−0.29, p = 0.05; partial r =−0.30, p = 0.04). In addition, mean positive mood ratings were above the neutral point of the scale in both the female (mean = 4.97, s.d. = 0.93) and male (mean = 4.78, s.d. = 1) conditions. These results provide no evidence that the large cortisol increases in the female condition were indicative of negative mood states, and suggest instead an example of cortisol elevation in response to hedonically positive social stimuli.
(b). Individual differences in hormonal responses
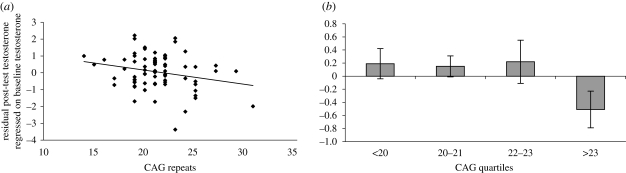
Table 1 presents the regression model in the female condition that best explained post-test testosterone values after controlling for baseline testosterone concentrations. It can be seen that CAG repeat number and baseline cortisol jointly accounted for significant residual variance in post-test testosterone; in addition, each variable explained significant variance when entered alone as the second step in the model: CAG repeats, β =−0.17, p = 0.04; baseline cortisol, β =−0.16, p = 0.04. Sexual experience (whether measured dichotomously or continuously as number of partners), by contrast, failed to explain significant variance when added at any step to the regression model (p > 0.35). Figure 2 visually depicts the influence of CAG repeats across both the full range of repeats and when repeat number was broken into quartiles; the quartile depiction suggests that the men with the longest repeats were especially likely to show smaller testosterone responses to the interactions with women.
Figure 2.
Relationship between CAG repeat number and change in testosterone in the female condition. The y-axis is the standardized residual of post-test testosterone regressed onto baseline testosterone in the female condition only; the zero point thus essentially represents the average change score in the female condition corrected for the influence of baseline values. (a) Scatterplot across the full range of CAG repeats. (b) The same data with CAG repeats divided into quartiles (mean ± s.e.m.).
Parallel regression models analysing cortisol responses in the female condition revealed no significant effects of any of the three individual difference variables (p > 0.40). Likewise, in the male condition, none of the individual difference variables were significant predictors of either post-test testosterone or cortisol concentrations after controlling for baseline concentrations of the respective hormones.
4. Discussion
The present results further demonstrate hormonal responses of men to social interactions with women and, when considered in conjunction with past studies (Roney et al. 2003, 2007; van der Meij et al. 2008), provide strong evidence that human males exhibit an endocrine mating response similar to that seen in non-human vertebrate species. Parallels between the human and non-human responses include the rapid timescale of the effects (within 20–40 min of first exposure to females), the presence of both testosterone and cortisol (or corticosterone) increases, and the absence of hormonal responses after comparable exposure to other males. The human responses can thus be seen within the context of a more general vertebrate pattern that may be generated by the operation of homologous brain mechanisms.
The possibility of homologous mechanisms is further supported by the present finding of larger testosterone increases among men with smaller numbers of CAG repeats. Shorter CAG repeats are associated with more active ARs (Chamberlain et al. 1994; Choong et al. 1996), and AR activity within a specific limbic–hypothalamic circuit is positively associated with non-human males' responses to females (Fernandez-Guasti et al. 2003; Harding & McGinnis 2004; Romano-Torres et al. 2007; Raskin et al. 2009). A larger hormone response among men with shorter repeats is therefore the expected pattern if the same brain mechanisms were conserved in humans. In addition, a recent study reported that men with shorter numbers of CAG repeats exhibited larger amygdala reactivity to motivationally significant stimuli (Manuck et al. in press), which provides further evidence that this genetic polymorphism predicts the degree to which structures within the limbic–hypothalamic circuit are responsive to particular classes of stimuli.
The likely function of the limbic–hypothalamic pathway that regulates responses to potential mates is to couple the expression of courtship and sexual behaviours to the conditions in which such behaviours are most adaptive (see Roney 2009); reactive hormone increases are thus expected to facilitate the successful execution of mate-seeking and/or copulatory behaviours. Cortisol increases promote short-term glucose availability (for a review, see Peters et al. 2004) and may thereby function as energy mobilizations to support courtship efforts. Past research suggests that cortisol can facilitate the production of physical behaviours, enhanced attention/concentration or enhanced memory consolidation in response to motivationally significant events (for a review, see Erickson et al. 2003), and each of these possible effects of cortisol reactions to potential mates could be tested in future research. Notice, furthermore, that the cortisol increases in the female condition of the present study were associated with positive mood states and thus do not appear to be ‘stress responses’ in the classic sense. This argues against the common tendency in the human psychology literature to infer from cortisol increases that stimuli have been perceived as aversive or threatening (see Dickerson & Kemeny 2004), and suggests instead a broader conceptualization in which cortisol directs energetic resources towards responding to both appetitive and aversive challenges (for similar arguments, see Flinn 2006).
Reactive testosterone increases, on the other hand, may promote mate-seeking via rapid effects on motivation and risk aversion. Rodents self-administer testosterone (e.g. Wood et al. 2004) and exhibit conditioned place preferences for settings in which testosterone was injected (e.g. Alexander et al. 1994); testosterone responses to female stimuli may therefore reinforce such stimuli and promote their pursuit. Such reinforcing effects of testosterone appear to involve dopamine release in the nucleus accumbens and are blocked by dopamine receptor antagonists (Schroeder & Packard 2000). Testosterone injections can also rapidly (within 30 min) reproduce the increased exploration of open spaces that occurs in male rodents after exposure to female urine (Aikey et al. 2002) and that presumably functions to reduce aversion to predation risk when mating opportunities increase the potential payoffs of exploratory behaviours. Aikey et al. (2002) additionally demonstrated a non-genomic mechanism for these results by showing that the risk-promoting effects of testosterone could be reproduced by testosterone metabolites known to act as γ-aminobutyric acid (GABA) agonists (such as 3-α-androstanediol), while also demonstrating that the effects of testosterone could be blocked by GABA antagonists. Some authors have further suggested that testosterone's effects on reinforcement and risk aversion may be mediated by similar mechanisms, with metabolites of testosterone promoting both effects via non-genomic influences on dopaminergic pathways (Aikey et al. 2002; Wood 2004).
In humans, a role for testosterone in promoting risk-taking via dopaminergic pathways is suggested by studies showing that baseline testosterone (Apicella et al. 2008) and the 7R+ allele of the DRD4 dopamine receptor gene (Dreber et al. 2009) have similar positive associations with men's propensity for risk-taking. Although these results pertain to baseline testosterone, acute testosterone increases could cause men to facultatively adopt the greater risk-taking proclivities of higher testosterone men. This in turn would probably be functional when the presence of potential mates alters the risk–reward ratio associated with intrasexual competition. Consistent with such a role for acute testosterone increases, research in humans has shown that the size of reactive testosterone responses to a competitive task predicts self-reports of willingness to compete again (Mehta & Josephs 2006; Carre & McCormick 2008). In addition, experimental administration of testosterone reduces human fear responses within a few hours of injection (van Honk et al. 2005; Hermans et al. 2006). In sum, human and non-human studies both support the conclusion that acute testosterone increases reduce risk aversion, which could support mate-seeking via exploratory behaviours in rodents and via competitive behaviours in men.
The above lines of evidence for functional effects of hormonal responses to potential mates provide a broader context in which to interpret individual differences in such responses. Baseline cortisol concentrations negatively predicted changes in testosterone after exposure to potential mates in the present sample. Testosterone increases may promote willingness to take competitive risks when mating opportunities are present, but risk-proneness should also be sensitive to the potential costs of competitive behaviours. The inhibitory effects of baseline cortisol on testosterone reactivity may help account for such costs, since many conditions that cause elevated cortisol (e.g. hypoglycaemia, immune activation) would probably make risky behaviours more costly and thus less functional. More generally, given that cortisol is elevated during energy shortages (see Laugero 2001; Peters et al. 2004), baseline cortisol may negatively index the energetic resources currently available for mating effort, while cortisol responses to potential mates may represent the marginal increases in energetic resources that can be devoted to such effort.
Reinforcing and risk-promoting effects of testosterone suggest that the relationship between CAG repeat lengths and testosterone reactivity may have important downstream consequences. Men with longer repeats may have less reinforcing interactions with women owing to smaller testosterone responses, for instance, which could in turn affect their subsequent behavioural patterns. Furthermore, because the AR regulates the expression of many genes via its role as a ligand-activated transcription factor, modulation of its activity by CAG repeat lengths could in principle cause functionally coordinated adjustments across many behavioural and morphological traits. One possibility consistent with the current findings is that shorter CAG repeat lengths predict greater physiological and behavioural investments in mating effort, which is also consistent with findings suggesting associations with outcomes such as sperm production (von Eckardstein et al. 2001). Further investigation of this possibility could provide important insights into the causes of individual differences in human mating psychology.
Contrary to our expectations, we found no evidence that sexual experience predicts the magnitude of men's hormonal responses to potential mates. We measured experience via self-reports of sexual intercourse, but it is possible that other sexual experiences that were not measured may be sufficient to cause the changes in hormone responses that can be experimentally induced by manipulating sexual access in non-human species. Alternatively, sexual experience may not have the same effects in humans that it has in rodents.
Although the present study is one of the first to identify variables that explain individual differences in hormonal responses to potential mates, it was still the case that baseline cortisol and CAG repeat number jointly accounted for only 10 per cent of the residual variance in post-test testosterone in the female condition, and thus other sources of variability remain to be identified. Hormonal responses to potential mates have heretofore been demonstrated only in students within controlled laboratory conditions, and a direction for future research might entail testing such effects in other populations situated in more naturalistic settings. Finally, analogous research could examine women subjects. Lopez et al. (2009) recently demonstrated that women exhibit reactive increases in both testosterone and cortisol after exposure to videos of an attractive man, and future research might examine predictors of individual differences in these responses.
In conclusion, the present study both confirms men's hormonal responses to potential mates and identifies novel biological variables that predict variance in these responses. The effect of AR gene sequence on testosterone responses, in particular, points to a potentially important variable for the explanation of individual differences in human mating psychology. The influence of this genetic polymorphism also adds evidence that a conserved limbic–hypothalamic pathway may play an important role in the regulation of men's responses to social interactions with women.
Acknowledgements
The procedures in this study were approved by the UCSB Institutional Review Board.
This work was supported by a UCSB Academic Senate Grant to the first author. The authors thank the research assistants who volunteered to be conversation partners, and Matt Cohen and Seth Miller for other assistance with data collection. Thanks also to two anonymous reviewers for helpful comments on a previous version of this manuscript.
References
- Aikey J. L., Nyby J. G., Anmuth D. M., James P. J.2002Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm. Behav. 42, 448–460 (doi:10.1006/hbeh.2002.1838) [DOI] [PubMed] [Google Scholar]
- Alexander G. M., Packard M. G., Hines M.1994Testosterone has rewarding affective properties in male rats: implications of the biological bases of sexual motivation. Behav. Neurosci. 108, 424–428 (doi:10.1037/0735-7044.108.2.424) [DOI] [PubMed] [Google Scholar]
- Amstislavskaya T. G., Popova N. K.2004Female-induced sexual arousal in male mice and rats: behavioral and testosterone response. Horm. Behav. 46, 544–550 (doi:10.1016/j.yhbeh.2004.05.010) [DOI] [PubMed] [Google Scholar]
- Apicella C. L., Dreber A., Campbell B., Gray P. B., Hoffman M., Little A. C.2008Testosterone and financial risk preferences. Evol. Hum. Behav. 29, 384–390 (doi:10.1016/j.evolhumbehav.2008.07.001) [Google Scholar]
- Batty J.1978Acute changes in plasma testosterone levels and their relation to measures of sexual behavior in the male house mouse (Mus musculus). Anim. Behav. 26, 349–357 (doi:10.1016/0003-3472(78)90053-2) [DOI] [PubMed] [Google Scholar]
- Bonilla-Jaime H., Vazquez-Palacios M., Arteaga-Silva M., Retana-Marquez S.2006Hormonal responses to different sexually related conditions in male rats. Horm. Behav. 49, 376–382 [DOI] [PubMed] [Google Scholar]
- Bronson F. H., Desjardins C.1982Endocrine responses to sexual arousal in the male. Endocrinology 111, 1286–1291 (doi:10.1210/endo-111-4-1286) [DOI] [PubMed] [Google Scholar]
- Campbell D. T., Kenny D. A.1999A primer on regression artifacts New York, NY: Guilford [Google Scholar]
- Campbell B. C., Gray P. B., Eisenberg D. T. A., Ellison P., Sorenson M. D.2007Androgen receptor CAG repeats and body composition among Ariaal men. Int. J. Androl. 32, 140–148 (doi:10.1111/j.1365-2605.2007.00825.x) [DOI] [PubMed] [Google Scholar]
- Carre J. M., McCormick C. M.2008Aggressive behavior and change in salivary testosterone concentrations predict willingness to engage in a competitive task. Horm. Behav. 54, 403–409 (doi:10.1016/j.yhbeh.2008.04.008) [DOI] [PubMed] [Google Scholar]
- Casella R., Maduro M. R., Lipshultz L. I., Lamb D. J.2001Significance of the polyglutamine tract polymorphism in the androgen receptor. Urology 58, 651–656 (doi:10.1016/S0090-4295(01)01401-7) [DOI] [PubMed] [Google Scholar]
- Chamberlain N. L., Driver E. D., Miesfeld R. L.1994The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 22, 3181–3186 (doi:10.1093/nar/22.15.3181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong C. S., Kemppainen J. A., Zhou Z., Wilson E. M.1996Reduced androgen receptor gene expression with first exon CAG repeat expansion. Mol. Endocrinol. 10, 1527–1535 (doi:10.1210/me.10.12.1527) [DOI] [PubMed] [Google Scholar]
- Dickerson S. S., Kemeny M. E.2004Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 130, 355–391 (doi:10.1037/0033-2909.130.3.355) [DOI] [PubMed] [Google Scholar]
- Dong Q., Salva A., Sottas C. M., Niu E., Holmes M., Hardy M.P.2004Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J. Androl. 25, 973–981 [DOI] [PubMed] [Google Scholar]
- Dreber A., Apicella C. L., Eisenberg D. T. A., Garcia J. R., Zamore R. S., Lum J. K., Campbell B.2009The 7R polymorphism in the dopamine receptor D4 gene (DRD4) is associated with financial risk taking in men. Evol. Hum. Behav. 30, 85–92 (doi:10.1016/j.evolhumbehav.2008.11.001) [Google Scholar]
- Edwards A., Hammond H. A., Jin L., Caskey C. T., Chakraborty R.1992Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics 12, 241–253 (doi:10.1016/0888-7543(92)90371-X) [DOI] [PubMed] [Google Scholar]
- Erickson K., Drevets W., Schulkin J.2003Glucocorticoid regulation of diverse cognitive functions in normal and pathological states. Neurosci. Biobehav. Rev. 27, 233–246 (doi:10.1016/S0149-7634(03)00033-2) [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A., Swaab D., Rodriguez-Manzo G.2003Sexual behavior reduces hypothalamic androgen receptor immunoreactivity. Psychoneuroendocrinology 28, 501–512 (doi:10.1016/S0306-4530(02)00036-7) [DOI] [PubMed] [Google Scholar]
- Flinn M. V.2006Evolution and ontogeny of stress response to social challenges in the human child. Dev. Rev. 26, 138–174 (doi:10.1016/j.dr.2006.02.003) [Google Scholar]
- Gonzalez Hernández A., Cabrera de León A., Dominguez Coello S., Almeida González D., Rodríguez Perez M. C., Brito Diaz B., Aguirre-Jaime A., Díaz-Chico B. N.2008Serum resistin and polymorphisms of androgen receptor CAGn and GGNn and aromatase TTTAn. Obesity 16, 2107–2112 (doi:10.1038/oby.2008.289) [DOI] [PubMed] [Google Scholar]
- Harding S. M., McGinnis M. Y.2004Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors. Physiol. Behav. 81, 671–680 (doi:10.1016/j.physbeh.2004.03.008) [DOI] [PubMed] [Google Scholar]
- Hermans E. J., Putman P., Baas J. M., Koppeschaar H. P., van Honk J.2006A single administration of testosterone reduces fear-potentiated startle in humans. Biol. Psychiatry 59, 872–874 (doi:10.1016/j.biopsych.2005.11.015) [DOI] [PubMed] [Google Scholar]
- Kamel F., Frankel A. I.1978The effect of medial preoptic area lesions on sexually stimulated hormone release in the male rat. Horm. Behav. 10, 10–21 (doi:10.1016/0018-506X(78)90020-X) [DOI] [PubMed] [Google Scholar]
- Laugero K. D.2001A new perspective on glucocorticoid feedback: relation to stress, carbohydrate feeding and feeling better. J. Neuroendocrinol. 13, 827–835 (doi:10.1046/j.1365-2826.2001.00706.x) [DOI] [PubMed] [Google Scholar]
- Lopez H. H., Hay A. C., Conklin P. H.2009Attractive men induce testosterone and cortisol release in women. Horm. Behav. 56, 84–92 (doi:10.1016/j.yhbeh.2009.03.004) [DOI] [PubMed] [Google Scholar]
- Lumley L. A., Hull E. M.1999Effects of a D1 antagonist and of sexual experience on copulation-induced Fos-like immunoreactivity in the medial preoptic nucleus. Brain Res. 829, 55–68 (doi:10.1016/S0006-8993(99)01338-4) [DOI] [PubMed] [Google Scholar]
- Macrides F., Bartke A., Dalterio S.1975Strange females increase plasma testosterone levels in male mice. Science 189, 1104–1106 (doi:10.1126/science.1162363) [DOI] [PubMed] [Google Scholar]
- Manuck S. B., Marsland A. L., Flory J. D., Gorka A., Ferrell R. E., Hariri A. R.In press Salivary testosterone and a trinucleotide (CAG) length polymorphism in the androgen receptor gene predict amygdala reactivity in men. Psychoneuroendocrinology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P. H., Josephs R. A.2006Testosterone change after losing predicts the decision to compete again. Horm. Behav. 50, 684–692 (doi:10.1016/j.yhbeh.2006.07.001) [DOI] [PubMed] [Google Scholar]
- Meisel R. L., Sachs B. D.1994The physiology of male sexual behavior. In The physiology of reproduction (eds Knobil E., Neill J. D.), pp. 3–105 New York, NY: Raven [Google Scholar]
- Mendoza S. P., Mason W. A.1989Behavioral and endocrine consequences of heterosexual pair formation in squirrel monkeys. Physiol. Behav. 46, 597–603 (doi:10.1016/0031-9384(89)90338-7) [DOI] [PubMed] [Google Scholar]
- Paredes R. G., Baum M. J.1997Role of the medial preoptic area/anterior hypothalamus in the control of masculine sexual behavior. Annu. Rev. Sex Res. 8, 68–101 [PubMed] [Google Scholar]
- Peters A., Schweiger U., Pellerin L., Hubold C., Oltmanns K. M., Conrad M., Schultes B., Born J., Fehm H. L.2004The selfish brain: competition for energy resources. Neurosci. Biobehav. Rev. 28, 143–180 (doi:10.1016/j.neubiorev.2004.03.002) [DOI] [PubMed] [Google Scholar]
- Pfaff D. W.1981Theoretical issues regarding hypothalamic control of reproductive behavior. In Behavioral studies of the hypothalamus (eds Morgane P. J., Panksepp J.), pp. 241–258 New York, NY: Marcel Dekker [Google Scholar]
- Pfeiffer C. A., Johnston R. E.1992Socially stimulated androgen surges in male hamsters: the roles of vaginal secretions, behavioral interactions, and housing conditions. Horm. Behav. 26, 283–293 (doi:10.1016/0018-506X(92)90048-Z) [DOI] [PubMed] [Google Scholar]
- Pfeiffer C. A., Johnston R. E.1994Hormonal and behavioral responses of male hamsters to females and female odors: roles of olfaction, the vomeronasal system, and sexual experience. Physiol. Behav. 55, 129–138 (doi:10.1016/0031-9384(94)90020-5) [DOI] [PubMed] [Google Scholar]
- Purvis K., Haynes N. B.1974Short-term effects of copulation, human chorionic gonadotropin injection and non-tactile association with a female on testosterone levels in the male rat. J. Endocrinol. 60, 429–439 (doi:10.1677/joe.0.0600429) [DOI] [PubMed] [Google Scholar]
- Rajender S., Pandu G., Sharma J. D., Gandhi K. P. C., Singh L., Thangaraj K.2008Reduced CAG repeats length in androgen receptor gene is associated with violent criminal behavior. Int. J. Legal Med. 122, 367–372 (doi:10.1007/s00414-008-0225-7) [DOI] [PubMed] [Google Scholar]
- Raskin K., de Gendt K., Duittoz A., Liere P., Verhoeven G., Tronche F., Mhaouty-Kodja S.2009Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J. Neurosci. 29, 4461–4470 (doi:10.1523/JNEUROSCI.0296-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-Torres M., Phillips-Farfan B. V., Chavira R., Rodriguez-Manzo G., Fernandez-Guasti A.2007Relationship between sexual satiety and brain androgen receptors. Neuroendocrinology 85, 16–26 (doi:10.1159/000099250) [DOI] [PubMed] [Google Scholar]
- Roney J. R.2009The role of sex hormones in the initiation of human mating relationships. In The endocrinology of social relationships (eds Ellison P. T., Gray P. B.), pp. 246–269 Cambridge, MA: Harvard University Press [Google Scholar]
- Roney J. R., Mahler S. V., Maestripieri D.2003Behavioral and hormonal responses of men to brief interactions with women. Evol. Hum. Behav. 24, 365–375 (doi:10.1016/S1090-5138(03)00053-9) [Google Scholar]
- Roney J. R., Lukaszewski A. W., Simmons Z. L.2007Rapid endocrine responses of young men to social interactions with young women. Horm. Behav. 52, 326–333 (doi:10.1016/j.yhbeh.2007.05.008) [DOI] [PubMed] [Google Scholar]
- Rubinow D. R., Roca C. A., Schmidt P. J., Danaceau M. A., Putnam K., Cizza G., Chrousos G., Nieman L.2005Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology 30, 1906–1912 (doi:10.1038/sj.npp.1300742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R. M.1985Stress-induced suppression of testicular function in the wild baboon: role of glucocorticoids. Endocrinology 116, 2273–2278 (doi:10.1210/endo-116-6-2273) [DOI] [PubMed] [Google Scholar]
- Schroeder J. P., Packard M. G.2000Role of dopamine receptor subtypes in the acquisition of a testosterone conditioned place preference in rats. Neurosci. Lett. 282, 17–20 (doi:10.1016/S0304-3940(00)00839-9) [DOI] [PubMed] [Google Scholar]
- van der Meij L., Buunk A. P., van de Sande J. P., Salvador A.2008The presence of a woman increases testosterone in aggressive dominant men. Horm. Behav. 54, 640–644 [DOI] [PubMed] [Google Scholar]
- van Honk J., Peper J. S., Schutter D. J. L. G.2005Testosterone reduces unconscious fear but not consciously experienced anxiety: implications for the disorders of fear and anxiety. Biol. Psychiatry 58, 218–225 [DOI] [PubMed] [Google Scholar]
- von Eckardstein S., Syska A., Gromoll J., Kamischke A., Simoni M., Nieschlag E.2001Inverse correlation between sperm concentration and number of androgen receptor CAG repeats in normal men. J. Clin. Endocrinol. Metab. 86, 2585–2590 (doi:10.1210/jc.86.6.2585) [DOI] [PubMed] [Google Scholar]
- Wood R. I.2004Reinforcing aspects of androgens. Physiol. Behav. 83, 279–289 [DOI] [PubMed] [Google Scholar]
- Wood R. I., Johnson L. R., Chu L., Schad C., Self D. W.2004Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology 171, 298–305 (doi:10.1007/s00213-003-1587-7) [DOI] [PubMed] [Google Scholar]
- Zitzmann M., Nieschlag E.2007Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J. Clin. Endocrinol. Metab. 92, 3844–3853 [DOI] [PubMed] [Google Scholar]