Abstract
Intersexual conflicts over mating can engender antagonistic coevolution of strategies, such as coercion by males and selective resistance by females. Orangutans are exceptional among mammals for their high levels of forced copulation. This has typically been viewed as an alternative mating tactic used by the competitively disadvantaged unflanged male morph, with little understanding of how female strategies may have shaped and responded to this behaviour. Here, we show that male morph is not by itself a good predictor of mating dynamics in wild Bornean orangutans but that female conception risk mediated the occurrence and quality of male–female interactions. Near ovulation, females mated cooperatively only with prime flanged males who they encountered at higher rates. When conception risk was low, willingness to associate and mate with non-prime males increased. Our results support the hypothesis that, together with concealed ovulation, facultative association is a mechanism of female choice in a species in which females can rarely avoid coercive mating attempts. Female resistance, which reduced copulation time, may provide an additional mechanism for mate selection. However, coercive factors were also important as prime males were frequently aggressive to females and females used mating strategies consistent with infanticide avoidance.
Keywords: orangutan, sexual coercion, female choice, infanticide avoidance, paternity certainty, post-conception mating
1. Introduction
Differences in the optimal reproductive strategies of males and females create sexual conflict over when, how frequently and with whom matings will occur (van Schaik et al. 2004; Arnqvist & Rowe 2005). In highly dimorphic species, females face particular challenges to develop strategies that increase the probability of obtaining a high-quality sire while minimizing the potential costs of infanticide and other forms of sexual coercion (Smuts & Smuts 1993). Promiscuous mating by females of many primate species appears to be an effective strategy to reduce the probability of infanticide (Hrdy 1979); however, it can exacerbate harassment from males with conflicting interests (van Schaik et al. 2004). Concealed or obscured ovulation offers females the potential to develop counterstrategies that exploit imperfect male knowledge of fecundity (Dickemann 1979; Nunn 1999; Hestermann et al. 2001). Thus, recent studies have highlighted female primate mating strategies that blend promiscuity with subtle behavioural or morphological cues that allow direct or indirect manipulation of male mating access in accordance with fecundity or social context (Nunn 1999; Semple & McComb 2000; van Schaik et al. 2004; Engelhardt et al. 2005; Stumpf & Boesch 2005). Here, we examine mating dynamics in Bornean orangutans (Pongo pygmaeus) in order to understand whether such female counterstrategies are expressed in a species with demonstrated extremes of sexual dimorphism and sexual coercion.
Orangutan males occur in two sexually mature morphs: flanged and unflanged. Prime flanged males are very large (approx. 86 kg), possess fleshy, protruding cheek flanges or pads, produce long call vocalizations and are dominant to unflanged males (MacKinnon 1974; Rijksen 1978; Rodman 1979; Galdikas 1985a; Mitani 1985; Knott & Kahlenberg 2007). Unflanged males have not developed these secondary sexual characteristics, yet are sexually mature as they have sired offspring in both the wild and captivity (Kingsley 1982; Maggioncalda et al. 1999; Utami et al. 2002; Goossens et al. 2006). Some flanged males reach a ‘past-prime’ condition, having shrivelled cheek pads and poor body condition. Early studies emphasized the use of forced copulation by unflanged males, suggesting that it was an alternative mating strategy necessary to fertilize avoidant females (Rijksen 1978; Galdikas 1985b; Schürmann & van Hooff 1986). While larger samples gleaned from multiple populations support a female preference for flanged males, they reveal that these males also frequently use force to obtain copulations, whereas unflanged males are often successful in copulating without the use of aggression (Mitani 1985; Fox 2002; Utami et al. 2002; Knott & Kahlenberg 2007). These studies suggest that, rather than being a behavioural trait of the unflanged male morph, forced copulation is a contingent response of male orangutans to resistance by females, who may vary in their willingness to copulate with males even within the developmental categories, with respect to previous mating interactions, or in response to their current reproductive condition (Mitani 1985; Fox 2002). Intriguingly, a study of mating patterns in Sumatran orangutans revealed that female resistance was not expressed consistently within particular male–female dyads (Fox 1998). Here, we test the hypothesis that female resistance to copulation is contingent upon the costs of being fertilized by an undesirable male. We predict that female conception risk, in addition to male morph, influences the distribution of social and sexual encounters, and the expression of male and female sexual behaviour.
Unlike other great apes, orangutans do not have a sexual swelling as an external indicator of the periovulatory period (Galdikas 1981) and empirical data on mating behaviour in captivity and in the wild support the view that ovulation is effectively concealed from males (Nadler 1988; Fox 1998). This lack of information on the female periovulatory period has likewise hampered our understanding of orangutan mating behaviour. Thus, in order to investigate how conception risk might influence sexual behaviour, we assayed urine samples collected non-invasively from wild Bornean orangutans, and compared ovarian hormone profiles with the sexual behaviour of females (proceptive or resistant behaviours) and males (genital inspections or aggressive behaviours).
We examined two major predictions: first, if female orangutans exert an overall preference for prime flanged males as potential sires, they should preferentially copulate with these males during times of high conception risk. Second, female willingness to copulate with non-prime males should increase in non-conceptive periods, which may function to manipulate paternity certainty and/or to minimize aggression received when costs of mating are low. Whereas both explanations are supported by increased acquiescence to male mating attempts, only a paternity confusion strategy would be supported by active solicitation of males during periods of low conceptive risk. Finally, because female resistance has been found to be relatively ineffective in averting unwanted copulations (Knott & Kahlenberg 2007), we tested the hypothesis that female resistance reduces copulatory duration.
2. Material and methods
(a). Study population and long-term data
Data were collected at the 2100 ha Cabang Panti Research Site in Gunung Palung National Park, West Kalimantan, Indonesia. This 90 000 ha national park contains a resident population of approximately 2500 wild orangutans (Johnson et al. 2005). No ex-captives have been released in this park. Behavioural data were collected from 1994 until 2003 during focal follows by the first author and assistants of the Gunung Palung Orangutan Project. During this period, over 45 500 h of observation were recorded on wild orangutans and 2460 follows were conducted on females that were reproductively mature (i.e. that had established menstrual cycling). During these follows, 378 encounters occurred between mature males and females. An encounter was defined as an association with another orangutan within 50 m and of any duration (Knott et al. 2008). We could determine both male type and female reproductive status for 153 encounters. The majority of encounters involved animals that were individually recognized (female known in 96% of encounters, male in 77%). The identities of unknown individuals were resolved based on consensus of field observers, with consideration of distinguishing features and temporal and spatial location of sightings. Thus, encounters involved approximately 15 females (sampled in 56 female-months) and 27 males. During these encounters, 21 matings, involving 10 males and 7 females, occurred. An additional 21 matings were observed during follows of females of uncertain reproductive status and were included in some portions of the analysis (see below). One additional mating with a male of undetermined status was also observed.
(b). Behavioural data
The characterization of force versus cooperation during mating is problematic as matings are lengthy and often contain elements of each (Rijksen 1978; Galdikas 1981; Fox 2002). Thus, categorizing matings as simply forced or cooperative can obscure the complexity of these interactions. We addressed this by breaking down the components of each mating into discrete acts by the male and female, representing four behaviour types: resistance, proceptivity, attraction/genital inspection and aggression. Each female act was classified as indicating either proceptivity or resistance (Fox 2002). We quantified male acts that indicated attraction (genital inspection) or aggression. The intensity of the interaction was reflected by the number of individual acts within the behaviour type that occurred. Each act could occur more than once during the mating interaction and 63 separate types of acts were defined. While we recognize that all acts within a category such as ‘aggression’ are not equivalent, weighting such behaviours is problematic and we found that summing the number of acts provided a necessary distinction between fleeting and more persistent expressions of the behaviour type. Generally, mating interactions featuring qualitatively more intense expressions of a behaviour type also contained a greater number of acts of that behaviour type (e.g. male aggression scores were higher when contact aggression occurred; Mann–Whitney U, z = −5.214, NC = 17, NNC = 25, p < 0.001). The total number of defined behaviours ranged from 1 to 28 acts (median 7) per encounter, so the number of acts in each defined category was often small and skewed in its distribution. Variation in number of acts observed was not statistically associated with either observation time (Spearman's ρ = 0.10, p = 0.662) or mating duration (as described in the following paragraph; Spearman's ρ = 0.00, p = 0.996), so the use of behavioural rates did not seem warranted.
Duration of mating was recorded as the time of physical contact until time of physical separation, quantified for 39 mating events. A resistance score was calculated by dividing the total number of resistance acts by the total number of all female mating acts observed. Likewise, a proceptive score was calculated by dividing the total number of proceptive acts by the total number of all female mating acts observed. Matings with a resistance score of over 50 per cent were classified as resistant, matings with a proceptive score of over 50 per cent were classified as proceptive. Matings in which there was no resistant or proceptive behaviour observed during the mating were recorded as unresisted.
(c). Endocrine data
Endocrine data were derived from 990 urine samples collected from 10 of the females involved in encounters. Samples were collected non-invasively, preserved on filter paper (Knott 1996, 2005) and analysed using enzyme immunoassay for urinary metabolites of oestradiol (E1C) and progesterone (PdG; Emery Thompson 2005; reagents provided by CJ Munro, UC Davis). Intra-assay coefficients of variation (CVs) were 4 and 4.7 per cent for E1C and PdG (n = 6 replicates, average of high and low sample), respectively; inter-assay CVs were 13.9 and 14.1 per cent for E1C and PdG (n = 77 assays). Pregnancy was determined by back-dating 245 days from the offspring's birth (Graham 1988). In four cases, we resolved encounters near to the probable time of conception by determining whether hormonal data were consistent with those of pregnant females, whose mean E1C and PdG levels averaged more than 10 s.d. above other females. In consideration of gamete survival times, we defined the periovulatory period as any time between an unambiguous surge in E1C, indicative of the immediate pre-ovulatory condition, and the onset of peak PdG levels, indicative of luteal phase onset (Czekala et al. 1988); ‘peak’ levels were defined as those more than 2 s.d. above each female's baseline. Females were known to be non-periovulatory when (i) peaks in E1C and/or PdG were detected and indicated that the date was outside of the possible periovulatory period, (ii) ovarian data were available and no peaks in E1C and/or PdG were detected near the date of interest, and (iii) a female had an infant under the age of 2 years or was known through endocrine data not to have resumed cycling following lactational amenorrhoea. Females who were known to be in lactational amenorrhoea did not show differences in their mating behaviour from those known to be cycling and non-periovulatory thus we combined them to increase the statistical power of our tests. Of the 153 encounters with female reproductive category, 100 were non-periovulatory (14 females), 27 were pregnant (6 females) and 26 were periovulatory (4 females).
(d). Statistical analyses
To account for non-independence of observations owing to repeated observations of the same females, we employed one of two forms of mixed (also called ‘hierarchical’) regression. All random effects were intercept only and used female identity as the subject identifier. The use of female identity rather than male or dyad identity to characterize non-independence was justified and technically necessary both because females were selectively followed (whereas male inclusion in these observations was not under researcher control) and because the female-specific variable of interest (reproductive status) varies substantially more than the male-specific variable (prime versus non-prime type). Nevertheless, results should be interpreted with some caution given pseudoreplication resulting from the inclusion of repeated observations of some males.
For binary outcomes, like the type of male encountered and the occurrence of mating, we used mixed logistic models (logit.mixed procedure in R; Bailey & Alimadhi 2007). The results of logistic analyses are expressed as odds ratios, which are the ratio of the odds of one event versus another (dependent variable) in one group versus a reference group (independent variable). Events were defined for three analyses: (1) considering all encounters between adult females and males, was the male prime? (2) Considering all encounters between adult females and males, did a mating occur? In this case, male type is taken as an independent variable rather than an event type. (3) Considering only matings, was the male prime? The results of analyses (2) and (3) differ in that (2) gives the odds ratios for a mating occurring given that an encounter had occurred, whereas (3) gives the crude odds ratios contrasting the male types.
For continuous outcomes, like the number of resistant behaviours observed, we used mixed linear models (MIXED procedure in SPSS 17.0). We also used linear mixed modelling to investigate the associations between male and female behaviour during mating interactions. As the number of behaviours in each category, and also the duration of mating, were skewed, log-transformed variables were used for analysis, but returned to linear values for reporting of estimates.
In both types of models, we investigated the main effects of female reproductive status and male type. In separate models, we also included the interaction of female reproductive status and male type to test our primary hypotheses. We tested main effects and interactions estimated from linear mixed models using F-tests, and those estimated from logistic mixed models using likelihood ratio tests. The likelihood ratio statistic is distributed as χ2 with degrees of freedom determined by the difference in degrees of freedom between two alternative nested models. Contrasts were between specific levels of a variable (or of the reproductive status by male type interaction). In logistic mixed models, odds ratios were evaluated using t-values on individual parameters.
For analyses of encounters, the sample size with known male type and female reproductive status was sufficient for analyses. However, to investigate the distribution of male and female behaviours within matings, it was necessary to use a more inclusive dataset, in which samples of unclassified reproductive state were assumed to be non-periovulatory, the state in which females spend the vast majority of their long interbirth intervals. The primary reason for this was that, while nine males were observed mating in the conservative dataset, none of these was with a non-periovulatory or lactating female. We therefore report results from the inclusive dataset to avoid including a category with zero observations and to increase the sample size for our multivariate comparison. This procedure carries a small risk of miscategorizing periovulatory samples as non-periovulatory. However, we note that: (i) the mating characteristics of unclassified females fell within the range of non-periovulatory females; (ii) significant findings regarding female resistance and male inspection were unchanged from an analysis of the conservative dataset; and (iii) while findings for male aggression and female proceptivity became significant in the larger dataset, neither should have been affected by misclassified samples, as the former concerned only male type and the latter concerned divergent behaviour by pregnant females.
3. Results
(a). Mating and female reproductive state
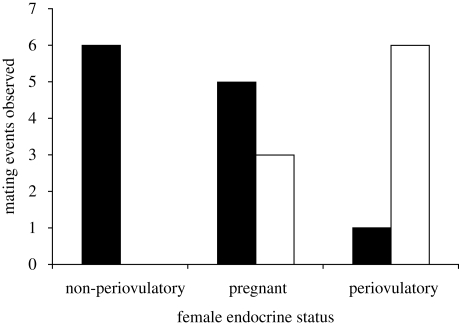
The distribution of matings was strongly predicted by the interaction of male type and female reproductive status. Non-periovulatory females mated most frequently with non-prime males, whereas periovulatory females mated more frequently with prime males (figure 1), though a single highly resisted mating with a past-prime male was observed. This pattern was so extreme that it could not be evaluated statistically in our conservative dataset (logit.mixed procedure could not reach convergence) owing to the lack of mating observations between non-periovulatory females and prime males. To address this problem, we increased the sample by assuming that 21 uncategorized matings without female endocrine data (16 with non-prime males and 5 with prime males) were most likely to have involved females who were lactating or otherwise non-periovulatory. Under this assumption, the model is estimable and the effect highly significant (likelihood ratio χ2 for global effect = 425, d.f. = 2, p < 0.001).
Figure 1.
Distribution of matings with respect to male type and female ovulatory status. Black bar, non-prime male; white bar, prime male.
(b). Probability of encounters and mating
Because orangutans are normally not group-living, mating is dependent on finding a mate, either by chance or design. Thus, we examined the pattern of mating outcomes discussed above in light of two components: (i) likelihood of encounter between females of known reproductive state and prime or non-prime males, and (ii) likelihood of mating during these encounters.
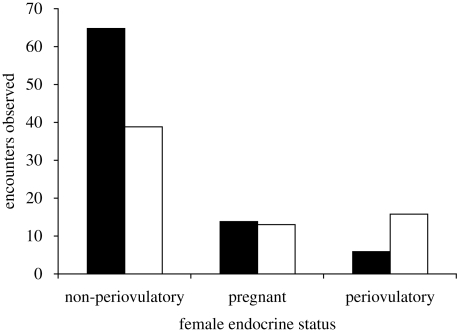
Periovulatory females may have mated disproportionately with prime males because they were most likely to encounter them. The odds of encountering a prime versus non-prime male increased 4.4 times (95% confidence interval: 1.6–12.3, p = 0.004) when females were periovulatory than when non-periovulatory (likelihood ratio χ2 for global effect = 10, d.f. = 2, p = 0.007; figure 2). Given that an encounter had occurred, periovulatory females had 9.7 (95% confidence interval: 2.6–35.9, p < 0.001) and pregnant females 7.5 (95% confidence interval: 2.3–24.5, p < 0.001) times greater odds of mating than non-periovulatory females. The pattern of mating was modified by male type (likelihood ratio χ2 for interaction = 6, d.f. = 2, p = 0.05), indicating that the apparent difference in mating patterns of prime versus non-prime males were driven both by the significantly higher rate of encounters between prime males and periovulatory females and by a higher chance of mating during those encounters.
Figure 2.
Distribution of female encounters with males with respect to male type and female ovulatory status. Black bar, non-prime male; white bar, prime male.
(c). Male–female mating interactions
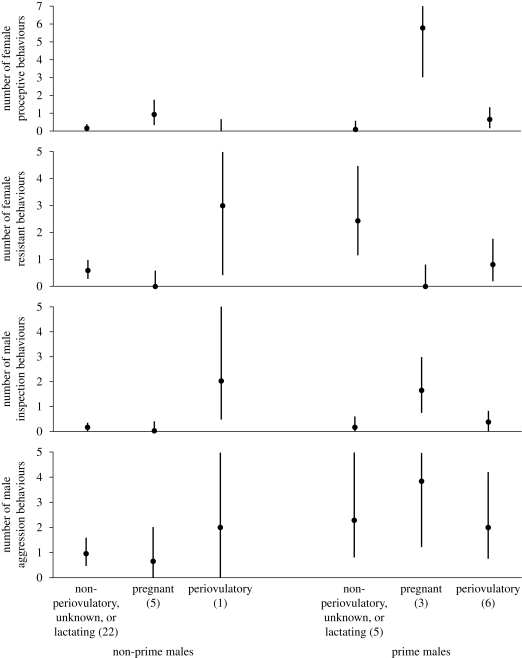
We next examined the type of male–female interactions in mating encounters using linear mixed models analysis (see table 1 and figure 3). Both resistant and proceptive female behaviours were predicted by a significant interaction of reproductive state with male type (resistance: F2,42 = 3.6, p = 0.035; proceptivity: F2,39 = 8.6, p = 0.001). Periovulatory females were less resistant to prime males than to non-prime males, but non-periovulatory females were less resistant to non-prime males. The most proceptive behaviour was shown by pregnant females (main effect of reproductive state: F2,35.4 = 12.9, p < 0.001) and was directed particularly towards prime males (figure 3). Some extremely proceptive behaviours, such as the female licking or putting the male's penis in her mouth, were only exhibited by pregnant females. Pregnant females were also the least resistant to male mating attempts, as demonstrated by a significant main effect of reproductive state (F2,42 = 4.2, p = 0.021). All copulations involving pregnant females were non-resisted.
Table 1.
Results of linear mixed model analysis of female and male behaviours observed during copulation.
| response variables | explanatory variables |
||
|---|---|---|---|
| main effects |
interaction |
||
| female reproductive status | male type | reproductive status by male type | |
| female proceptive behaviours | F2,35.4 = 12.9, p < 0.001 | F1,21.2 = 2.7, p = 0.117 | F2,39 = 8.6, p = 0.001 |
| female resistant behaviours | F2,42 = 4.2, p = 0.021 | F1,42 = 2.7, p = 0.110 | F2,42 = 3.6, p = 0.035 |
| male inspection behaviours | F2,17.7 = 0.9, p = 0.419 | F1,9.1 = 0.4, p = 0.527 | F2,42 = 7.7, p = 0.001 |
| male aggressive behaviours | F2,42 = 0, p = 0.966 | F1,42 = 5.1, p = 0.029 | F2,42 = 0.8, p = 0.458 |
Figure 3.
Estimated number of behaviours observed in each of four categories during mating observations, with 95% confidence intervals. Confidence intervals truncated at 0 and 5 or 7. In parentheses, the total number of observations, from 15 females, of social interactions with males that included mating.
(c). Male aggression towards females and genital inspection
In contrast to other reports, we found that male aggressive behaviours in the context of mating, such as chasing, pulling and restraining the female, were performed more often by prime than non-prime males (main effect: F1,42 = 5.1, p = 0.029); these were not directed significantly more towards any particular female reproductive class. While there was generally a positive association between male aggression and female resistance (F1,37 = 19.2, p < 0.001), prime males used some form of aggression in 86 per cent of their copulations, including matings with no resistance and even with proceptive female initiation. Male inspection of female genitalia was predicted by a significant interaction between male type and female reproductive status (F2,42 = 7.7, p = 0.001), with a high frequency of inspections of pregnant females by prime males.
(d). Length of matings
Matings lasted an average of 7.9 min (range: 1–31 min). We found that resisted matings were significantly shorter than matings in which the female was proceptive: 5.1 min (95% confidence interval: 3.5–7.1) versus 13.1 min (95% confidence interval: 8.7–19.5); F2,36 = 7.7, p = 0.002.
4. Discussion and conclusion
These results reflect a complex interaction between female endocrine status and male morph in orangutan mating patterns. Our results show clearly that prime males obtained preferential mating access with the most fecund females. Is this evidence of female choice? Prime males are approximately twice the size of females and, in our study, used more aggression or physical restraint in their mating interactions, suggesting that apparent female preference could instead be the result of intimidation. However, we found that one of the primary mechanisms for increased mating frequency was an increased encounter rate, and specific features of orangutan social behaviour suggest that this is primarily a female-driven outcome, indicating female choice. Because search costs are so high for a large-bodied organism in a low-density population, prime flanged males have been described as using a ‘sit-and-wait’ mating strategy, giving long calls that attract receptive females over a long distance (MacKinnon 1974; Horr 1975; Rijksen 1978; Utami & Setia 1995; Utami et al. 2002; Delgado 2006; Mitra Setia & van Schaik 2007). Unflanged males, on the other hand, do not give long calls and spend more time travelling each day in a probable search for mates (Mitani 1985; Utami et al. 2002; Morrogh-Bernard et al. 2009). Thus, increased encounter rates between ovulating females and prime males are probably the result of females finding these males.
Assuming that both prime and non-prime males are equally motivated and equally successful in their efforts to encounter periovulatory females, we would expect no difference in encounter rates. In fact, given their greater search effort we would expect unflanged males to have increased success rates in encountering periovulatory females. However, we find the opposite, despite equal to higher densities of non-prime males (particularly unflanged) compared with prime males (Knott 1999), suggesting that skewed encounter rates and resulting mating bias are the result of female strategies. The single case in which a non-prime male was able to mate with a periovulatory female was a forced copulation, supporting the view that females generally reject non-prime males as potential sires. Paternity data are not yet available for Gunung Palung, though data from other wild orangutan populations indicate that non-prime males can still succeed in siring offspring (Utami et al. 2002; Goossens et al. 2006).
Why should females prefer flanged males? Developing into the flanged male stage is energetically costly as these males are significantly larger, and expend and intake significantly more energy (Rodman 1979; Knott 1998). Flanged males can receive significant aggression from competing flanged males, evidenced by their high level of wounding and occasional fatalities (Knott & Kahlenberg 2007). As not all males are able to attain flanged status, it is a reasonable hypothesis that males that are able to develop into and maintain the flanged male morph are of higher genetic quality. Males provide no direct material benefits to females or their offspring, thus preference for prime flanged males is most consistent with selection for good genes (Trivers 1972; Kokko et al. 2003). This preference may be reinforced by the coercive behaviour of males, which can lead females to evolve cost-minimizing strategies (Kokko 2005).
If females do not prefer non-prime males, why do they sometimes mate cooperatively with them? Females mated most frequently with unflanged males overall, but they did so exclusively when conception risk was low. A single periovulatory mating with a past-prime male was highly resisted, while non-periovulatory matings met less resistance, and pregnant matings were not resisted at all. If a mating is unlikely to lead to conception, females may reduce resistance simply to avoid the costs of male aggression (Smuts & Smuts 1993). However, aggression within the mating context in orangutans has not been reported to lead to physical wounding of females (MacKinnon 1974; Rijksen 1978; Galdikas 1981; Schürmann 1982; Mitani 1985; Schürmann & van Hooff 1986; Fox 2002; Utami et al. 2002; Knott & Kahlenberg 2007); thus the costs of resistance appear to be low (Knott 2009).
Lowered mate selectivity outside the periovulatory period is consistent with another form of risk avoidance: the anti-infanticide strategy of paternity confusion (Hrdy 1979; van Schaik 2000a; Stumpf & Boesch 2005; Stumpf et al. 2008). This strategy, wherein females mate with potentially infanticidal males in order to increase their perception of paternity probability, is common in catarrhine primates as well as some species of carnivores and rodents (van Schaik et al. 2004). While females may adopt a strategy of general promiscuity to spread the probability of paternity, recent empirical and theoretical reports suggest that many primate females alternate between behaviours that bias paternity during times of high conception risk and obfuscate it at other times (Nunn 1999; Stumpf & Boesch 2005; Stumpf et al. 2008).
The existence of anti-infanticide counterstrategies is further supported by the post-conception sexual behaviour of females. Post-conception mating is seen in numerous other primates, as well as some felids, rodents and possibly dolphins, and is thought to be a low-risk strategy to provide the perception of paternity to non-sires and/or reinforce paternity certainty in dominant sires (van Noordwijk & van Schaik 2000). In our study, pregnant females lowered their resistance to non-prime males and became highly proceptive, particularly to prime males. It may be especially important to increase the paternity certainty of prime flanged males, who are more likely than other males to be resident and in the vicinity of a female when an offspring is born. Prime males performed frequent genital inspections of pregnant females, suggesting that they have an interest in determining the reproductive state of females rather than mating indiscriminately. Pregnant orangutans eventually display a small white or reddish labial swelling (Galdikas 1981), which would be detectable by males, but matings occurred in early pregnancy prior to the swelling's appearance.
It is possible that elevated female proceptivity is a side-effect of the high ovarian hormone levels experienced in pregnancy (Saayman 1975). However, it is notable that non-fertile mating, and particularly post-conception proceptive behaviour, is a phenomenon that occurs most commonly in primates (van Noordwijk & van Schaik 2000; Ziegler 2007), species with mating behaviour that is less strictly regulated by hormonal controls and more flexible with regard to social context than in other mammals (Wallen 2001). Moreover, proximate hormonal influences do not rule out an adaptive role for non-fertile mating (Engelhardt et al. 2007) and may themselves be situation-dependent, promoting paternity confusion at times of high infanticide risk (Connor et al. 1996; Pazol 2003). High progesterone levels in the luteal phase, which mostly resembles the hormonal environment of pregnancy, have been linked with decreased, rather than increased, sexual response in captive orangutans (Nadler 1988).
Notably, infanticide has not yet been observed in wild orangutans (Beaudrot et al. 2009), but at least one infanticide has occurred in captivity (Mallinson 1984). However, infanticide is rarely observed in primates, even in vulnerable species, despite decades of research, and the majority of documented cases have occurred under very specific circumstances such as a takeover of the alpha male position (van Noordwijk & van Schaik 2000; van Schaik 2000b). Slow reproduction and low association rates lower infanticide opportunities in orangutans, but also reduce the probability that an infanticide could be observed. Female orangutans demonstrate intense investment in infants, and high-infant survivorship is linked with the longest interbirth interval of any mammal (Galdikas & Wood 1990; Knott 2001; Wich et al. 2004). The high potential cost of an infant loss may facilitate counterstrategies to infanticide even when the probability of such an event is low. While orangutan life history features predict high vulnerability of females to infanticide (van Schaik 2000a,b), it has been argued that a lack of female counterstrategies suggests low infanticide risk (Beaudrot et al. 2009). We present evidence for such counterstrategies. Infanticide risk in orangutans is also supported by the avoidance of strange male long calls by females with young infants (Delgado 2003), and females in Sumatra have been shown to mate with non-preferred males during periods of male rank instability (Utami et al. 2002), supporting the existence of paternity confusion behaviours.
Resistance to mating attempts is predicted to evolve when females face high direct costs from mating frequently, even if resistance itself is costly (Gavrilets et al. 2001; Chapman et al. 2003). The use of resistance behaviour by females is nonetheless surprising given that female orangutans are rarely successful at preventing copulations (Fox 2002). Resistance may produce other advantages. We found that resisted matings were significantly shorter than proceptive matings. Thus, if long matings are necessary to achieve ejaculation in orangutans, resistance may be effective at decreasing the likelihood of insemination. This possibility remains to be tested.
In the context of coevolutionary sexual conflict, females are expected to evolve strategies that minimize costs while promoting their own reproductive interests (Chapman et al. 2003; Arnqvist & Rowe 2005). This can result in strategies that are subtle, complex and difficult to disentangle from the more overt coercive strategies employed by males. Our data indicate that female orangutans modify their behaviour in accordance with conception risk and, in doing so, appear to exert important influences on the distribution and duration of matings, despite the considerable size and coercive aggression of males. These findings allow for greater appreciation of the diversity and commonalities of female reproductive strategies among the apes, and the development of a testable framework for understanding female reproductive strategies more generally. Moreover, our study demonstrates that female mating behaviour in orangutans is not an unencumbered expression of choice, instead reflecting constraints from and counterstrategies to male behaviour. Females frequently received aggression, even from those males with whom they mated preferentially, and it is likely that the avoidance of aggression, intimidation, harassment and infanticide have critical influences on female behaviour. These data not only provide resolution to the perplexing patterns of female resistance and forced copulation in orangutans, specifically, but contribute an important empirical example to the developing study of male–female sexual conflict and coevolution (van Schaik et al. 2004; Arnqvist & Rowe 2005).
Acknowledgements
We thank the Directorate of Nature Conservation (PHKA) in Indonesia for permission to conduct research in Gunung Palung National Park and the Indonesian Institute of Sciences (LIPI), the Center for Research and Development in Biology (PPPB) and PHKA for their sponsorship. We are grateful to the many field assistants and project managers who contributed to the data presented here. Comments from two anonymous reviewers improved the quality of the manuscript. Grants from the National Geographic Society, the Leakey Foundation, the Conservation, Food and Health Foundation, the Orangutan Conservancy, the US Fish and Wildlife Service, the National Science Foundation, the Wenner-Gren Foundation and Harvard University made this work possible. The research was approved by the Standing Committee on the Use of Animals in Research and Teaching at Harvard University, Protocol no. 95-04.
References
- Arnqvist G., Rowe L.2005Sexual conflict Princeton, NJ: Princeton University Press [Google Scholar]
- Bailey D., Alimadhi F.2007logit.mixed: mixed effects logistic model. In Zelig: everyone's statistical software (eds Imai K., King G., Lav O.). http://gking.harvard.edu/zelig [Google Scholar]
- Beaudrot L. H., Kahlenberg S. M., Marshall A. J.2009Why male orangutans do not kill infants. Behav. Ecol. Sociobiol. 63, 1549–1562 (doi:10.1007/s00265-009-0827-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T., Arnqvist G., Bangham J., Rowe L.2003Sexual conflict. Trends Ecol. Evol. 18, 41–47 (doi:10.1016/S0169-5347(02)00004-6) [Google Scholar]
- Connor R. C., Richards A. F., Smolker R. A., Mann J.1996Patterns of female attractiveness in Indian Ocean bottlenose dolphins. Behaviour 133, 1–109 [Google Scholar]
- Czekala N. M., Shideler S. E., Lasley B. L.1988Comparisons of reproductive hormone patterns in the hominoids. In Orang-utan biology (ed. Schwartz J. H.), pp. 117–132 Oxford, UK: Oxford University Press [Google Scholar]
- Delgado R. A.2003. The function of adult male long calls in wild orangutans (Pongo pygmaeus). PhD dissertation, Duke University, Durham, NC [Google Scholar]
- Delgado R. A.2006Sexual selection in the loud calls of male primates: signal content and function. Int. J. Primatol. 27, 5–25 (doi:10.1007/s10764-005-9001-4) [Google Scholar]
- Dickemann M.1979Female infanticide, reproductive strategies and social stratification. In Evolutionary biology and human social behavior (eds Chagnon N., Irons W.), pp. 321–367 Duxbury, MA: North Scituate [Google Scholar]
- Emery Thompson M.2005Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): methodological considerations and the role of hormones in sex and conception. Am. J. Primatol. 67, 137–158 (doi:10.1002/ajp.20174) [DOI] [PubMed] [Google Scholar]
- Engelhardt A., Hodges J. K., Niemitz C., Heistermann M.2005Female sexual behavior, but not sex skin swelling, reliably indicates the timing of the fertile phase in wild long-tailed macaques (Macaca fascicularis). Horm. Behav. 47, 195–204 (doi:10.1016/j.yhbeh.2004.09.007) [DOI] [PubMed] [Google Scholar]
- Engelhardt A., Hodges J. K., Heistermann M.2007Post-conception mating in wild long-tailed macaques (Macaca fascicularis): characterization, endocrine correlates and functional significance. Horm. Behav. 51, 3–10 (doi:10.1016/j.yhbeh.2006.06.009) [DOI] [PubMed] [Google Scholar]
- Fox E. A.1998The function of female mate choice in the Sumatran orangutan (Pongo pygmaeus abelii). PhD dissertation, Duke University, Durham, NC [Google Scholar]
- Fox E.2002Female tactics to reduce sexual harassment in the Sumatran orangutan (Pongo pygmaeus abelii). Behav. Ecol. Sociobiol. 52, 93–101 (doi:10.1007/s00265-002-0495-x) [Google Scholar]
- Galdikas B. M. F.1981Orangutan reproduction in the wild. In Reproductive biology of the great apes (ed. Graham C. E.), pp. 281–300 New York, NY: Academic Press [Google Scholar]
- Galdikas B. M. F.1985aAdult male sociality and reproductive tactics among orangutans at Tanjung Puting. Folia Primatol. 45, 9–24 (doi:10.1159/000156188) [Google Scholar]
- Galdikas B. M. F.1985bSubadult male orangutan sociality and reproductive behavior at Tanjung Puting. Am. J. Primatol. 8, 87–99 (doi:10.1002/ajp.1350080202) [DOI] [PubMed] [Google Scholar]
- Galdikas B. M. F., Wood J. W.1990Birth spacing patterns in humans and apes. Am. J. Phys. Anthropol. 83, 185–191 (doi:10.1002/ajpa.1330830207) [DOI] [PubMed] [Google Scholar]
- Gavrilets S., Arnqvist G., Friberg U.2001The evolution of female mate choice by sexual conflict. Proc. R. Soc. Lond. B 268, 531–539 (doi:10.1098/rspb.2000.1382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens B., Setchell J. M., James S. S., Funk S. M., Chikhi L., Abulani A., Ancrenaz M., Lackman-Ancrenaz I., Bruford M. W.2006Philopatry and reproductive success in Bornean orang-utans (Pongo pygmaeus). Mol. Ecol. 15, 2577–2588 (doi:10.1111/j.1365-294X.2006.02952.x) [DOI] [PubMed] [Google Scholar]
- Graham C.1988Reproductive physiology. In Orang-utan biology (ed. Schwartz J. H.), pp. 91–103 Oxford, UK: Oxford University Press [Google Scholar]
- Hestermann M., Ziegler T., van Schaik C. P., Launhardt K., Winkler P., Hodges J. K.2001Loss of oestrus, concealed ovulation, and paternity confusion in free-ranging Hanuman langurs. Proc. R. Soc. Lond. B 268, 2445–2451 (doi:10.1098/rspb.2001.1833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horr D. A.1975The Borneo orangutan: population structure and dynamics in relationship to ecology and reproductive strategy. In Primate behavior: developments in field and laboratory research, vol. 4 (ed. Rosenblum L. A.), pp. 307–323 New York, NY: Academic Press [Google Scholar]
- Hrdy S. B.1979Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol. Sociobiol. 1, 13–40 [Google Scholar]
- Johnson A., Knott C., Pamungkas B., Pasaribu M., Marshall A.2005A survey of the orangutan Pongo pygmaeus pygmaeus population in and around Gunung Palung National Park, West Kalimantan, Indonesia based on nest counts. Biol. Conserv. 121, 495–507 (doi:10.1016/j.biocon.2004.06.002) [Google Scholar]
- Kingsley S.1982Causes of non-breeding and the development of the secondary sexual characteristics in the male orang utan: a hormonal study. In The orang utan: its biology and conservation The Hague, The Netherlands: Dr W. Junk [Google Scholar]
- Knott C. D.1996Field collection and preservation of urine in orangutans and chimpanzees. Tropic Biodivers. 4, 95–102 [Google Scholar]
- Knott C. D.1998Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. Int. J. Primatol. 19, 1061–1079 (doi:10.1023/A:1020330404983) [Google Scholar]
- Knott C. D.1999. Reproductive, physiological and behavioral responses of orangutans in Borneo to fluctuations in food availability. PhD dissertation, Harvard University, Cambridge, MA [Google Scholar]
- Knott C. D.2001Female reproductive ecology of the apes: implications for human evolution. In Reproductive ecology and human evolution (ed. Ellison P.), pp. 429–463 New York, NY: Aldine de Gruyter [Google Scholar]
- Knott C. D.2005Radioimmunoassay of estrone conjugates from urine dried on filter paper. Am. J. Primatol. 67, 121–135 (doi:10.1002/ajp.20173) [DOI] [PubMed] [Google Scholar]
- Knott C. D.2009Orangutans: sexual coercion without sexual violence. In Sexual coercion in primates: an evolutionary perspective on male aggression against females (eds Muller M. N., Wrangham R. W.), pp. 81–111 Cambridge, MA: Harvard University Press [Google Scholar]
- Knott C. D., Kahlenberg S.2007Orangutans in perspective: forced copulations and female mating resistance. In Primates in perspective (eds Bearder S., Campbell C. J., Fuentes A., MacKinnon K. C., Panger M.), pp. 290–305 Oxford, UK: Oxford University Press [Google Scholar]
- Knott C., Beaudrot L., Snaith T., White S., Tschauner H., Planansky G.2008Female–female competition in Bornean orangutans. Int J Primatol 29, 975–997 (doi:10.1007/s10764-008-9278-1) [Google Scholar]
- Kokko H.2005Treat 'em mean, keep 'em (sometimes) keen: evolution of female preferences for dominant and coercive males. Evol Ecol 19, 123–135 (doi:10.1007/s10682-004-7919-1) [Google Scholar]
- Kokko H., Brooks R., Jenions M., Morley J.2003The evolution of mate choice and mating biases. Proc. R. Soc. Lond. B 270, 653–664 (doi:10.1098/rspb.2002.2235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon J. R.1974The behaviour and ecology of wild orang-utans (Pongo pygmaeus). Anim. Behav. 22, 3–74 (doi:10.1016/S0003-3472(74)80054-0) [Google Scholar]
- Maggioncalda A. N., Sapolsky R. M., Czekala N. M.1999Reproductive hormone profiles in captive male orangutans: implications for understanding developmental arrest. Am. J. Phys. Anthropol. 109, 19–32 (doi:10.1002/(SICI)1096-8644(199905)109:1<19::AID-AJPA3>3.0.CO;2-3) [DOI] [PubMed] [Google Scholar]
- Mallinson J.1984Breeding of great apes at the Jersey Wildlife Preservation Trust and a look into the future. Zoo Biol. 3, 1–11 (doi:10.1002/zoo.1430030102) [Google Scholar]
- Mitani J.1985Mating behaviour of male orangutans in the Kutai Game Reserve, Indonesia. Anim. Behav. 33, 391–402 [Google Scholar]
- Mitra Setia T., van Schaik C. P.2007The response of adult orang-utans to flanged male long calls: inferences about their function. Folia Primatol. 78, 215–226 (doi:10.1159/000102317) [DOI] [PubMed] [Google Scholar]
- Morrogh-Bernard H. C., et al. 2009Orangutan activity budgets and diet. In Orangutans: geographic variation in behavioral ecology and conservation (eds Wich A., Utami S. S., Mitra Setia T., van Schaik C.), pp. 119–134 Oxford, UK: Oxford University Press [Google Scholar]
- Nadler R. D.1988Sexual and reproductive behavior. In Orang-utan biology (ed. Schwartz J. H.), pp. 31–51 Oxford, UK: Oxford University Press [Google Scholar]
- Nunn C. L.1999The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim. Behav. 58, 229–246 (doi:10.1006/anbe.1999.1159) [DOI] [PubMed] [Google Scholar]
- Pazol K.2003Mating in the Kakamega Forest blue monkeys (Cercopithecus mitis): does female sexual behavior function to manipulate paternity assessment? Behaviour 140, 473–499 (doi:10.1163/156853903322127940) [Google Scholar]
- Rijksen H. D.1978A field study on Sumatran orang-utans (Pongo pygmaeus abelii, Lesson 1827): ecology, behaviour, and conservation Wageningen, The Netherlands: H. Veenman & Zonen B.V [Google Scholar]
- Rodman P. S.1979Individual activity patterns and the solitary nature of orangutans. In The great apes, vol. 5 (eds Hamburg D. L., McCown E. R.), pp. 234–255 London, UK: W. A. Benjamin [Google Scholar]
- Saayman G.1975The influence of hormonal and ecological factors upon sexual behavior and social organization in Old World primates. In Socioecology and psychology of primates (ed. Tuttle R. H.). The Hague, The Netherlands: Mouton [Google Scholar]
- Schürmann C.1982Mating behaviour of wild orangutans. In The orang utan: its biology and conservation (ed. de Boer L.). The Hague, The Netherlands: W. Junk [Google Scholar]
- Schürmann C. L., van Hooff J. A. R. A. M.1986Reproductive strategies of the orang-utan: new data and a reconsideration of existing sociosexual models. Int. J. Primatol. 7, 265–287 (doi:10.1007/BF02736392) [Google Scholar]
- Semple S., McComb K.2000Perception of female reproductive state from vocal cues in a mammal species. Proc. R. Soc. Lond. B 267, 707–712 (doi:10.1098/rspb.2000.1060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuts B. B., Smuts R. W.1993Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv. Study Behav. 22, 1–63 (doi:10.1016/S0065-3454(08)60404-0) [Google Scholar]
- Stumpf R. M., Boesch C.2005Does promiscuous mating preclude female choice? Female sexual strategies in chimpanzees (Pan troglodytes verus) of the Tai National Park, Côte d'Ivoire. Behav. Ecol. Sociobiol. 57, 511–524 (doi:10.1007/s00265-004-0868-4) [Google Scholar]
- Stumpf R. M., Emery Thompson M., Knott C.2008A comparison of female mating strategies in Pan troglodytes and Pongo spp. Int. J. Primatol. 29, 865–884 (doi:10.1007/s10764-008-9284-3) [Google Scholar]
- Trivers R. M.1972Parental investment and sexual selection. In Sexual selection and the descent of man, 1871–1971 (ed. Campbell B.), pp. 136–179 Chicago, IL: Aldine [Google Scholar]
- Utami S., Setia T. M.1995Behavioral changes in wild male and female Sumatran orangutans (Pongo pygmaeus abelii) during and following a resident male take-over. In The neglected ape (eds Nadler R. D., Galdikas B. F. M., Sheeran L. K., Rosen N.), pp. 183–190 New York, NY: Plenum Press [Google Scholar]
- Utami S. S., Goossens B., Bruford M. W., de Ruiter J. R., van Hooff J. A. R. A. M.2002Male bimaturism and reproductive success in Sumatran orang-utans. Behav. Ecol. 13, 643–652 (doi:10.1093/beheco/13.5.643) [Google Scholar]
- van Noordwijk M. A., van Schaik C. P.2000Reproductive patterns in eutherian mammals: adaptations against infanticide? In Infanticide by males (eds van Schaik C. P., Janson C. H.), pp. 322–360 Cambridge, UK: Cambridge University Press [Google Scholar]
- van Schaik C. P.2000aInfanticide by male primates: the sexual selection hypothesis revisited. In Infanticide by males (eds van Schaik C. P., Janson C. H.), pp. 27–60 Cambridge, UK: Cambridge University Press [Google Scholar]
- van Schaik C. P.2000bVulnerability to infanticide by males: patterns among mammals. In Infanticide by males (eds van Schaik C. P., Janson C. H.), pp. 61–71 Cambridge, UK: Cambridge University Press [Google Scholar]
- van Schaik C. P., Pradhan G. R., van Noordwijk M. A.2004Mating conflict in primates: infanticide, sexual harassment and female sexuality. In Sexual selection in primates: new and comparative perspectives (eds Kappeler P. M., van Schaik C. P.), pp. 131–150 Cambridge, UK: Cambridge University Press [Google Scholar]
- Wallen K.2001Sex and context: hormones and primate sexual motivation. Horm. Behav. 40, 339–357 (doi:10.1006/hbeh.2001.1696) [DOI] [PubMed] [Google Scholar]
- Wich S. A., Utami-Atmoko S. S., Setia T. M., Rijksen H. D., Schürmann C., van Hooff J. A. R. A. M., van Schaik C. P.2004Life history of wild Sumatran orangutans (Pongo abelii). J. Hum. Evol. 47, 385–398 (doi:10.1016/j.jhevol.2004.08.006) [DOI] [PubMed] [Google Scholar]
- Ziegler T. E.2007Female sexual motivation during non-fertile periods: a primate phenomenon. Horm. Behav. 51, 1–2 (doi:10.1016/j.yhbeh.2006.09.002) [DOI] [PubMed] [Google Scholar]