Abstract
Many organisms have evolved inducible defences in response to spatial and temporal variability in predation risk. These defences are assumed to incur large costs to prey; however, few studies have investigated the mechanisms and costs underlying these adaptive responses. I examined the proximate cause of predator-induced shell thickening in a marine snail (Nucella lamellosa) and tested whether induced thickening leads to an increase in structural strength. Results indicate that although predators (crabs) induce thicker shells, the response is a passive by-product of reduced feeding and somatic growth rather than an active physiological response to predation risk. Physical tests indicate that although the shells of predator-induced snails are significantly stronger, the increase in performance is no different than that of snails with limited access to food. Increased shell strength is attributable to an increase in the energetically inexpensive microstructural layer rather than to material property changes in the shell. This mechanism suggests that predator-induced shell defences may be neither energetically nor developmentally costly. Positive correlations between antipredator behaviour and morphological defences may explain commonly observed associations between growth reduction and defence production in other systems and could have implications for the evolutionary potential of these plastic traits.
Keywords: antipredator behaviour, Cancer productus, Nucella lamellosa, plasticity, predation risk, trade-off
1. Introduction
Phenotypic plasticity is a major theme in studies of ecology and evolution (Miner et al. 2005; Pigliucci 2005). Proximate mechanisms underlying plastic responses have, however, received less attention, but can enhance our understanding of the adaptive value and evolution of these traits (Windig et al. 2003). Determining whether plasticity can evolve more easily in certain traits requires identifying the costs and constraints on the expression of these traits. This is particularly true for correlated traits, where adaptively plastic phenotypes could be passive by-products of correlated plastic responses, rather than direct responses to specific environmental cues.
Predator-induced defences are well-studied and ecologically important forms of phenotypic plasticity (Tollrian & Harvell 1998), where prey species show adaptive morphological shifts that increase resistance to predation in response to temporal or spatial heterogeneity in predation risk. These morphological changes are often accompanied by a reduction in feeding and growth. For example, the predator-induced production of defensive spines by marine bryozoans is associated with reduced growth (Harvell 1992), and predator-induced phenotypes of larval anurans grow more slowly than non-induced phenotypes (Van Buskirk 2000). Such growth reductions have been used to infer energetic or developmental trade-offs between allocation to defensive structures and growth (Harvell 1992; Van Buskirk 2000). However, the conclusion of costs or trade-offs requires careful scrutiny with regard to the exact causal chain between the predator cue and the phenotypic response. For example, in bryozoans, spines cause hydrodynamic interference with feeding currents, suggesting that costs of inducible defence are primarily a reduction in clearance rate rather than metabolic investment in spine construction (Grunbaum 1997). Moreover, growth costs associated with predator-induced defences in larval anurans are due in part to environmentally induced changes in feeding morphology rather than direct allocation shifts between growth and defence (Relyea & Auld 2005). Plastic changes in traits that affect foraging may be important mechanistic links between the inducing environmental cue and the formation of the plastic phenotype.
Marine snails are excellent organisms for examining mechanisms and costs of inducible defences. A long evolutionary history with shell-breaking predators has increased the resistance of gastropod shells to crushing (Vermeij 1987) both as constitutive and inducible defences (Appleton & Palmer 1988; Trussell & Smith 2000). Studies of shell thickening in marine snails in response to cues indicative of crab predation document a correlated reduction in feeding and/or somatic tissue growth (Appleton & Palmer 1988; Palmer 1990; Trussell 1996). Growth rates of snails are also slower in habitats where risk of predation by crabs is higher (Trussell & Smith 2000; Perez et al. 2009). In some cases, crab-induced shell thickening can be an actively modified morphological response to risk stimuli at the presumed expense of reduced soft tissue growth (Palmer 1990; Brookes & Rochette 2007). Whether this mechanism is widespread and whether reduced growth reflects a decrease in feeding activity or a direct trade-off with the cost of producing a predator-resistant shell remain open questions.
To test for trade-offs and underlying mechanisms that drive predator-induced shell changes, I examined somatic growth, shell growth and shell morphology in the marine snail Nucella lamellosa by: (i) experimental manipulation of food availability and (ii) exposure to cues from the predatory crab Cancer productus. This allowed me to distinguish between two alternative hypotheses: (i) shell thickening is a direct response to risk cues resulting from increased shell deposition or (ii) shell deposition rate remains constant and shell thickening is a by-product of reduced feeding activity and growth rate. If shell thickening is a direct response to risk cues, snails exposed to crab cues should produce thicker shells than food-limited snails. If shell thickening is a by-product of reduced feeding and growth, somatic growth reduction will restrict linear shell growth resulting in increased shell deposition perpendicular to the axis of linear shell growth, causing shell thickening with no additional energetic investment in the production of the defence. If this second mechanism is operating, then both predators and food limitation should induce similar shell-thickening responses. I also measured shell strength to assess whether shell thickening induced by predators or food availability produced differences in resistance to crushing.
2. Material and Methods
(a). Experimental set-up
On San Juan Island, WA, USA, juvenile N. lamellosa (14–24 mm shell length) were collected from two current-swept sites where crabs are rare (P. E. Bourdeau 2009, unpublished data) and snails would have limited previous exposure to crabs: Westside Preserve (48°30′26.76″ N, 123°8′35.20″ W) and San Juan County Park (48°32′29.92″ N, 123°9′35.99″ W). Prior to experimentation, snails were individually numbered (bee tags attached with cyanoacrylate glue), fed barnacles in flow-through sea water tables for 48 h and measured and weighed. Ten snails were randomly allocated to each of 16 plastic aquaria (4800 ml) that served as experimental units (Bourdeau 2009). For a complete description, see electronic supplementary material 1. Aquaria were randomly assigned to one of the four treatments corresponding to feeding frequency, in a non-orthogonal design: (i) snails fed 4 of 6 days (67%), (ii) 2 of 6 days (33%), and (iii) 1 of 6 days (16.7%). In treatment 4, snails were fed on a 67 per cent feeding schedule while exposed to cues from the predatory crab, C. productus. Hereafter, treatments will be referred to as ‘high-food’, ‘moderate-food’, ‘low-food’ and ‘crab-exposed’, respectively. Treatments were replicated four times. Snails did not differ in initial values of morphometric variables among treatments (analysis of variance (ANOVA), all p's > 0.15). Snails were fed barnacles, Balanus glandula, attached to small stones. Stones were replaced as barnacles were depleted. Crabs were fed pre-cracked N. lamellosa to ensure consumption. I used similar sized crabs in the replicates of the crab-exposed treatment (mean CW = 128.59). During the experiment, some crabs died or escaped; they were replaced within 48 h. The experiment ran for 84 days.
(b). Feeding
I quantified the barnacles eaten at the end of each 6-day feeding period to assess the effect of treatment on barnacle consumption. This was done by counting the number of barnacles missing opercular plates and removing ‘empty’ tests to ensure they were counted only once. Barnacles attached to stones kept in flow-through sea tables in the laboratory in the absence of predators did not experience any mortality over the course of the experiment.
(c). Growth and morphology
At the end of the experiment I quantified shell length, shell width, shell aspect ratio (length : width), shell mass and body mass for each experimental snail. Shell mass, a good estimate of overall shell thickness, was obtained with a non-destructive technique (Palmer 1982). For a full description, see electronic supplementary material 2. Shell length, width and lip thickness were measured to the nearest 0.01 mm with digital callipers.
(d). Performance
I estimated shell strength by measuring the force required to fracture shells in an Instron Universal Testing Machine (Instron Corporation, MA, USA), after I took morphometric measurements. Shells were placed aperture down, so force was applied perpendicular to the axis of coiling. This provides a representative measure of resistance to forces exerted during a crushing attempt (Zipser & Vermeij 1978), and allowed shells to be tested similarly.
The force required to break a shell will vary depending on shell cross-sectional area (i.e. the larger the cross-sectional area, the larger the breaking force), so I quantified shell cross-sectional area as lip thickness multiplied by shell width. In tensioning or loading a beam, the cross-sectional area is the width multiplied by the thickness (Bird & Ross 2002). Differences among treatments in shell strength corrected for shell cross-sectional area could indicate either differences in the relative amount of different microstructural layers or the material properties of the shell layers. Thus, I also tested for differences in the relative thickness of the crossed lamellar layer (inner shell layer) and the homogeneous layer (outer shell layer) among treatments. Low- and moderate-food treatments were employed to obtain an experimental treatment with similar feeding rates as the crab treatment; given the low-food treatment provided those conditions, I excluded the moderate-food treatment from this analysis.
I examined shell fragments of 20 snails from each treatment under a light microscope, after being crushed. Total shell thickness and microstructure layer thickness were measured to the nearest 0.01 µm from light microscope images (Image Pro Plus). This technique provides similar estimates of shell layer thickness as scanning electron microscopy (Avery & Etter 2006). To ensure microstructural layers were quantified from shell growth that occurred during the experiment, measurements were taken from the main body whorl at the leading edge of the aperture. Total shell thickness and crossed lamellar layer thickness were quantified by taking measurements perpendicular to the inner edge of the shell. Shells were lightly sanded to ensure a smooth surface. Homogeneous layer thickness was calculated as the difference between total thickness and crossed lamellar layer thickness (Avery & Etter 2006).
(e). Statistical analyses
Barnacle consumption was analysed with single-factor ANOVA with treatment as a fixed factor.
Variation in growth and morphology was analysed with analysis of covariance (ANCOVA) with treatment as a fixed factor; replicate aquaria as a random factor nested within treatment, and the appropriate covariate. Because initial size can affect growth, ANCOVA was performed on soft tissue growth and linear shell growth with the initial measurement of each variable as the covariate. I analysed variation in overall shell thickness and shell accretion with multiple ANCOVAs using final shell mass as the dependent variable. Shell mass provides a measure of overall shell thickness that reflects snail vulnerability to C. productus, a species that can peel shells or crush them outright (Zipser & Vermeij 1978; Palmer 1985). Body mass was the size covariate for the analysis of shell thickness because when analysing differences in the allocation of resources to shell defence, it may be a more appropriate variable for scaling size than shell length (Palmer 1990). For shell accretion, linear shell growth was the covariate because if snails are actively increasing the rate of shell deposition in the presence of crabs, snails exposed to crabs should have heavier shells for a given amount of linear shell growth than snails in the absence of predation cue. Alternatively, if shell is deposited at similar rates, final shell mass should be similar among treatments.
Final shell mass varied among treatments, thus data violated the ANCOVA assumption that the covariate is independent of treatment. Specifically, there was a range of shell masses that were present in the high-food treatment but were not present in the crab treatment, thus, adjusting shell mass for the covariate would have involved extrapolation beyond the range of values for which I had data in the crab treatment. Thus, I omitted observations within treatment groups that had particularly high or low covariate values and ran the analyses for a narrower range of covariates that were comparable among groups (Quinn & Keough 2002; see electronic supplementary material 4, table S2, for details on covariate ranges). Shell length was used as a size covariate for total apertural lip thickness and homogeneous layer thickness. Shell shape (aspect ratio) variation was analysed with nested ANOVA, with treatment as a fixed factor and replicate aquaria nested within treatment.
I analysed the effect of shell morphology on shell strength with multiple ANCOVAs using force to fracture as the dependent variable and shell mass and shell cross-sectional area as covariates. Increases in shell mass and cross-sectional area represent increased production of shell material and should produce similar increases in shell strength (i.e. slopes should be similar) if shell material properties are similar among treatments. However, if snails can increase shell strength by altering shell material properties, shell strength should increase differently among treatments with respect to the amount of shell produced (i.e. the slopes should differ). Differences in microstructure layers among treatments were analysed with a nested ANCOVA using final shell length as a size covariate.
All ANCOVAs were initially run with treatment × covariate interaction terms. If interaction terms were not significant, they were removed from the model (Hendrix et al. 1982; Engqvist 2005). Because a p-value larger than 0.1 may still indicate that slopes differ, I visually inspected plots of the slopes and removed interaction terms from the model if slopes appeared different (Huitema 1980; Engqvist 2005). For apertural lip thickness and homogeneous shell layer, p < 0.1 for the interaction between the treatment and covariate, thus I assumed slope heterogeneity and included the interaction terms in the model. I used the Wilcoxon modification of the Johnson–Neyman technique to determine the range of covariates over which treatments significantly differed from one another (Quinn & Keough 2002).
Adherence to the assumptions of normality and homoscedasticity were tested using Shapiro–Wilk's W and Cochran's test, respectively. All data were log +1 transformed to better meet these assumptions, with the exception of shell cross-sectional area, which was square-root transformed. Body mass remained heteroscedastic among treatments after transformation, but variances were not dramatically different, thus I used parametric tests because they are not particularly sensitive to violations of this assumption (Sokal & Rohlf 1995). Because I was interested in how crab-exposed snails differed from high-food snails and low-food snails, I used a reverse Dunnett's test to compare each food availability treatment with the crab treatment. Statistica v. 6.1 was used for all analyses.
3. Results
(a). Feeding
Access to food strongly influenced feeding rates (ANOVA, F1,3 = 34.76, p < 0.001; electronic supplementary material 3, table S1). High-food snails ate more barnacles than those in the lower food treatments (mean barnacles eaten ± 95% CI: high food—152.75 ± 44.96, medium food—155.75 ± 37.24, low food—41.25 ± 14.08, crab-exposed—73.50 ± 15.62). Crab-exposed snails ate 51.9 per cent fewer barnacles than high-food snails (Dunnett's, p < 0.001), but the average number of barnacles eaten between crab-exposed snails and low-food snails was not statistically different (Dunnett's, p = 0.09).
(b). Growth and morphology
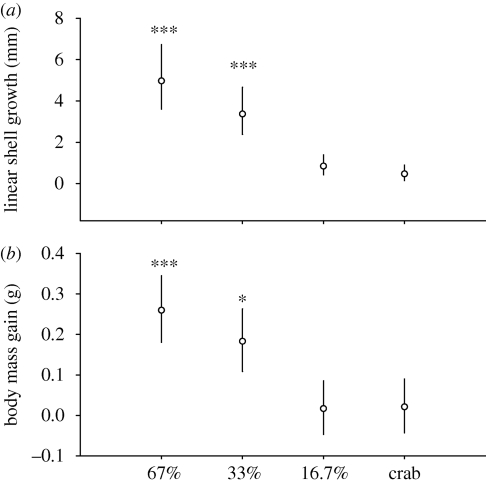
Snails grew exponentially as they consumed more barnacles for both linear shell growth (log(y) = log(−0.026 + 0.00 462 × x), r = 0.90, p < 0.001) and body mass gain (log(y) = log(−0.034 + 0.00 078 × x), r = 0.89, p < 0.001). Among treatments, both linear shell growth and soft tissue gain were significantly different (ANCOVA: linear shell growth, F3,12 = 32.61, p < 0.001; soft tissue gain, F3,12 = 14.07, p < 0.001; electronic supplementary material 4, table S2). Crab-exposed snails exhibited 88 per cent less linear shell growth and 93 per cent less body mass than high-food snails (Dunnett's: linear growth, p < 0.001; body growth, p < 0.001), but did not differ from low-food snails (Dunnett's: linear growth, p = 0.723; body growth, p = 0.990; figure 1a,b).
Figure 1.
Variation in (a) linear shell growth and (b) body mass gain in N. lamellosa provided with high (67%), moderate (33%) and low (16.7%) food availability and high food availability while exposed to predator cues (crab). Values are back-transformed least-squares means and 95% confidence intervals of log-transformed data computed for covariates (initial shell length and initial body mass, respectively) at their means. Asterisks indicate groups that are significantly different from crab treatment (Dunnett's, *p < 0.05, **p < 0.01, ***p < 0.001).
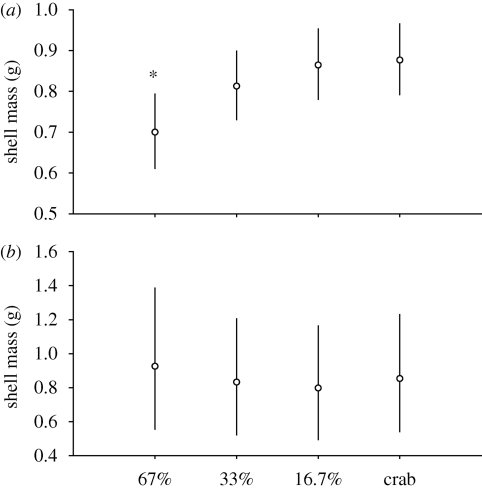
Treatment significantly affected shell thickness (ANCOVA, F3,11 = 3.94, p = 0.035; electronic supplementary material 4, table S2). For a given body mass, crab-exposed snails had heavier (i.e. thicker) shells than high-food snails (Dunnett's, p = 0.026; figure 2a). There was no difference in shell mass between crab-exposed snails and low-food snails (Dunnett's, p = 0.991; figure 2a). Treatment did not significantly affect shell deposition (ANCOVA, F3,11 = 0.248, p = 0.861; electronic supplementary material 4, table S2). Crab-exposed snails had similar shell mass to both high-food and low-food snails across the range of linear shell growth used for the analysis (figure 2b). Shell shape was invariant across treatments (ANCOVA, F3,12 = 2.09, p = 0.155; electronic supplementary material 4, table S2).
Figure 2.
Variation in shell mass in N. lamellosa provided with high (67%), moderate (33%) and low (16.7%) food availability and high food availability while exposed to predator risk cues (crab). Values are back-transformed least-squares means and 95% confidence intervals of log-transformed data computed for (a) final body mass and (b) linear shell growth at their means. Asterisks indicate groups that are significantly different from crab treatment (Dunnett's, *p < 0.05, **p < 0.01, ***p < 0.001).
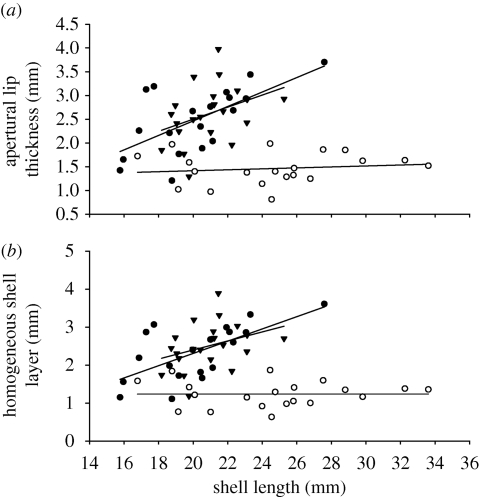
Analyses of apertural lip thickness and homogeneous shell layer thickness revealed potentially heterogeneous slopes for the covariate (shell length)—treatment relationships (ANCOVA: apertural lip thickness, F2,9 = 2.64, p = 0.08; homogeneous shell layer thickness, F2,9 = 2.89, p = 0.07; electronic supplementary material 4, table S2). Inspection of the slopes indicated that crab-exposed and low-food snails had thicker apertural lips (figure 3a) and thicker homogeneous shell layers (figure 3b) for a given shell length than high-food snails. Application of the Johnson–Neyman technique indicated that high-food snails had significantly thinner apertural lips than crab-exposed snails for shell lengths between 21.19 and 29.00 mm and a smaller amount of homogeneous shell layer than crab-exposed snails for shell lengths between 23.25 and 25.35 mm. There was no difference in lip thickness or homogeneous layer thickness between low-food and crab-exposed snails across the entire range of shell lengths.
Figure 3.
Relationship between (a) apertural lip thickness and final shell length and (b) homogeneous shell layer and final shell length of snails provided with high (67%) and low (16.7%) food availability and high food availability while exposed to predator risk cues (crab). Filled circles, 16.7%; open circles, 67%; filled triangles, crab.
(c). Performance
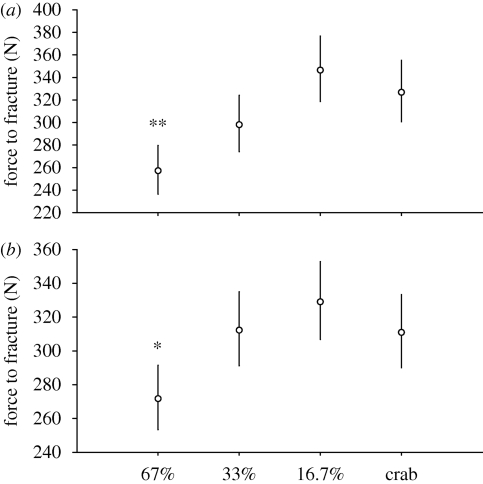
Treatment significantly affected shell strength (ANCOVA: shell mass covariate, F3,12 = 8.633, p = 0.002; cross-sectional area covariate, F3,12 = 5.99, p = 0.008; electronic supplementary material 5, table S3). Increases in shell mass and shell cross-sectional area produced similar increases in shell strength among treatments, indicating that shell strength was increasing at similar rates among treatments, relative to the amount of shell material produced (covariate × treatment interactions p's > 0.19). For a given shell mass, crab-exposed snails produced shells that were 36 per cent stronger than high-food snails (Dunnett's, p = 0.002; figure 4a), but did not produce shells any stronger than low-food snails (Dunnett's, p = 0.592; figure 4a). When corrected for shell cross-sectional area, crab-exposed snails produced 15 per cent stronger shells than high-food snails (Dunnett's, p = 0.029; figure 4b), but produced shells no stronger than low-food snails (Dunnett's, p = 0.478; figure 4b).
Figure 4.
Variation in force to fracture of N. lamellosa provided with high (67%), moderate (33%) and low (16.7%) food availability and high food availability while exposed to predator risk cues (crab). Values are back-transformed least-squares means and 95% confidence intervals of log-transformed data computed for (a) shell mass and (b) shell cross-sectional area at their means. Asterisks indicate groups that are significantly different from crab treatment (Dunnett's, *p < 0.05, **p < 0.01, ***p < 0.001).
4. Discussion
Predator-induced shell thickening in N. lamellosa is a passive consequence of reduced feeding rather than an active increase in calcification in response to predator risk. Snails exposed to risk cues ate fewer barnacles, grew less and produced thicker, more crab-resistant shells than frequently fed snails, but showed no difference in any of these traits when compared to food-limited snails. For a given amount of shell material, shell strength differed among treatments, suggesting that another factor, in addition to shell mass, was also contributing to shell strength. Shell shape was invariant across treatments and so could not be responsible for shell strength differences among treatments. It is unlikely that material properties of the shell are responsible as there were no differences among treatments in the properties of the shell material as they relate to shell strength. If such differences existed, one would expect significant differences in the slopes of force to fracture by size or cross-sectional area among treatments, which were not detected. Instead, an increase in the thickness of the homogeneous shell layer appears to be driving the observed pattern of shell strength. New shell in the crab-exposed and low-food snails was added perpendicular, rather than parallel, to the axis of coiling at the growing edge of the shell (i.e. the apertural lip was thickened rather than extended). As a consequence, the resulting shells of these snails were thicker and better defended against crushing forces. Although the homogeneous layer is mechanically weaker than the crossed-lamellar layer (Currey & Taylor 1974), its low organic content and passive production make it an inexpensive material for shell thickening (Palmer 1992; Avery & Etter 2006).
To my knowledge, this is the first example of a predator-induced morphological defence that is a passive by-product of a behavioural response to predators. This contrasts with previous work on other species of marine snails (Palmer 1990; Brookes & Rochette 2007), where there is evidence of active increase in the rate of calcification in response to predator cues. In a similar experiment on a north Atlantic congener, Nucella lapillus, Palmer (1990) found that shell thickness was amplified in snails exposed to crabs, over and above that in food-deprived snails. However, in Palmer's experiment, snails in the low-food treatment were starved rather than fed a similar number of barnacles to the crab cue treatment. As a consequence, stress-induced increases in metabolic rates caused by total starvation might account for the reduced morphological response in starved snails relative to crab-exposed snails in N. lapillus (Palmer 1990). In the present study, snails in the low-food treatment consumed similar numbers of barnacles to the crab treatment, making morphological responses in each treatment more directly comparable.
By quantifying food consumption, I determined actual differences in feeding behaviour among groups of snails exposed to different treatments and can conclude that the snails were eating fewer barnacles in both the crab treatment and the low-food treatment. This approach provides an advantage over previous studies of inducible defences in that it allows one to distinguish between morphological differences that are a by-product of behaviour and growth versus a direct response to risk cues. It also aids in the interpretation of studies of phenotypic plasticity where morphological relationships can change with body size, and body size often varies among treatments (McCoy 2007). For example, because of differences in growth, high-food and crab-exposed snails differed in final size, making differences in shell mass and shell deposition between high-food and crab treatments difficult to interpret. However, the addition of a low-food treatment provides an additional no-crab ‘control’ to which the crab treatment can be compared. Thus, similarities in size-adjusted shell mass and size-adjusted shell strength between the low-food snails and crab-exposed snails provide convincing evidence that shell thickening in N. lamellosa is a consequence of reduced feeding activity and growth.
The results of this study have profound implications for the ecology and evolution of inducible defences. That feeding and growth reductions often accompany predator-induced morphological responses in prey suggests that prey behaviour may play an important role in the development of morphological defences. Specifically, reductions in feeding alone, rather than energetic or developmental trade-offs, may be responsible for the expression of many observed inducible morphologies, and many predator-induced morphological traits thought to be direct responses to particular environmental cues may instead be by-products of developmentally correlated behavioural responses (West-Eberhard 2003). Thus, behaviour may be critical for shaping plastic phenotypes, as seen in the marked morphological changes in vertebrates owing to correlated changes in movement (Wimberger 1991). Such ‘plasticity cascades’ could provide a mechanism for the recent documentation of positive correlations among behavioural and morphological defences in other species (DeWitt et al. 1999).
Another intriguing possibility is that the observed morphological response is resource-mediated in the wild. Food availability can be limited in quiet-water habitats where crabs are abundant (Menge et al. 1994), so snails could be co-opting a pre-existing response (i.e. a thicker shell with slower growth in food-limited habitats) to respond adaptively to predation risk (Christy 1995; Emerson & Boyd 1999). Crab-induced suppression of snail feeding activity could produce even thicker shells that further increase resistance to predation, providing a positive feedback that would enhance the adaptive value of environmentally induced shell thickening. Such a mechanism could explain the common observation that muricids have thicker shells in quiet-water habitats, where crabs are abundant (Kitching 1976; Vermeij 1982; Crothers 1983). Crab-induced shell defence has been implicated in geographical patterns of shell thickness variation in littorine snails (Trussell & Smith 2000). However, for many muricids, as well as other snails, growth rates are slower on exposed shores (Menge 1978; Brown & Quinn 1988; Boulding & Vanalstyne 1993; Etter 1996; Brown et al. 2004) and the snails have thinner shells, suggesting that different mechanisms may be operating for different species.
These results also question the common conclusion that reduced somatic growth in marine snails is the result of a direct trade-off with the production of thicker shells in response to predation risk (Palmer 1990; Trussell & Nicklin 2002). First, the production of thicker shells in N. lamellosa does not appear to be energetically expensive (Palmer 1983, 1992). For example, increases in shell thickness of crab-exposed and food-limited snails were due to an increase in the less energetically expensive homogeneous shell layer, relative to the more expensive cross-lamellar shell layer. Energetic costs would predict a decrease in shell production when body growth slows or stops; yet this was not the case. For a given amount of linear shell growth, shell deposition remained relatively the same regardless of the presence or absence of crabs, indicating no additional metabolic or production costs to making a thicker shell in the presence of predators over and above the cost of producing a shell in a predator-free environment. Producing relatively more of the lighter shell layer may also reduce any energetic costs associated with continually depositing calcium carbonate and transporting a heavier shell in habitats with few crabs.
Secondly, that shell deposition remained similar among experimental treatments indicates that shell production is constant, regardless of body growth rate. Thus, the direction of shell deposition (i.e. linear shell growth versus shell thickening) is a consequence of body growth rate (Vermeij 2002). Passive shell thickening could impose developmental costs by restricting future somatic growth (Palmer 1981), but testing this hypothesis would require experiments examining growth rates of initially thick- and thin-shelled individuals with a common history of exposure to crabs. Nevertheless, decreased body growth in response to crabs appears to reflect feeding suppression rather than a direct developmental trade-off with producing a thicker shell (Palmer 1990; Trussell & Nicklin 2002). Growth reduction probably carries a fecundity cost, but the positive fitness effect of the behavioural response on survival (e.g. decreased detection by predators) may outweigh the cost of reduced growth. I cannot address these possibilities with the current experiment, thus further experimentation is needed to assess the relative importance of developmental constraints on future somatic growth, reduced feeding activity and growth and their net effect on fitness.
Why would natural selection favour the deposition of shell material under low-food conditions when there are not enough resources for somatic growth? In the absence of crabs, thicker shells may incur unnecessary developmental costs (Palmer 1981). However, any developmental cost associated with producing a thicker shell in the absence of crabs might be outweighed by mortality risk associated with producing a thinner, more susceptible shell when crabs are present. A snail's vulnerability to crabs depends on both shell thickness and size (Palmer 1985), thus Nucella must balance a trade-off between growing fast to decrease the amount of time spent small and growing slowly to increase shell strength. By growing fast, a snail can reach larger, less vulnerable sizes faster, but it will produce a thinner shell, susceptible to crushing. By depositing shell when feeding or somatic growth is slow or has stopped, the snail can reinforce the thinner shell produced during periods of rapid growth. Under this scenario, passive deposition of shell material may be adaptive.
Predator-induced shell thickening does appear to be an active physiological response to crab risk cues in Littorina obtusata, an herbivorous marine snail distantly related to N. lamellosa (Brookes & Rochette 2007). However, growth rate alone has been shown to affect shell thickening in another species of Littorina (Kemp & Bertness 1984). Thus, further experiments are needed to assess which mechanism is more common among other species of gastropods. My results, coupled with a recent study that demonstrated that environmental calcium modifies inducible defences in a freshwater snail (Rundle et al. 2004), suggest that adaptive inducible defences arising through environmental influences other than predation pressure, via phenotypic modulation (West-Eberhard 2003), may be more common than previously appreciated.
The findings of this study have important evolutionary implications because when one trait's performance depends on the response of the other, phenotypic linkages between the two traits may form via adaptive evolution (Henry et al. 2006). Trait correlations may in turn enhance the evolution of adaptive integration, especially if the traits are functionally related (Wagner 1988). The behavioural and morphological components of N. lamellosa's defensive phenotype are induced by crabs as an integrated unit and are likely to function as a unit as well, because both antipredator behaviour and shell defences can reduce snails’ susceptibility to crab predators at different stages of the predation process (Ramos-Jiliberto et al. 2007). Under this scenario, patterns of behavioural and morphological plasticity in the field should reflect the functional integration of behaviour and morphology favoured by correlated selection (Wagner 1996). Determining whether the observed link between feeding behaviour and shell morphology reflects underlying genetic correlations that were shaped by natural selection for a defensive function, or phylogenetically constrained and unchangeable developmental pathways, will require a comparison of behavioural and morphological plasticity among snails from spatially structured populations or closely related species.
Acknowledgements
I thank the director and staff at Friday Harbor Laboratories for logistical support. Emily Carrington provided use of the Instron and Megan Mach helped with the experiment. Dianna Padilla, Jeffrey Levinton, Massimo Pigliucci, Geerat Vermeij and three anonymous reviewers provided constructive criticism on previous versions of the manuscript. F. James Rohlf provided valuable statistical advice. A Ruth and Stephen Wainwright Fellowship supported this work. This is contribution number 1190 from the Department of Ecology and Evolution at Stony Brook University.
References
- Appleton R. D., Palmer A. R.1988Water-borne stimuli released by predatory crabs and damaged prey induce more predator-resistant shells in a marine gastropod. Proc. Natl Acad. Sci. USA 85, 4387–4391 (doi:10.1073/pnas.85.12.4387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery R., Etter R. J.2006Microstructural differences in the reinforcement of a gastropod shell against predation. Mar. Ecol. Prog. Ser. 323, 159–170 (doi:10.3354/meps323159) [Google Scholar]
- Bird J., Ross C.2002Mechanical engineering principles Oxford, UK: Newnes [Google Scholar]
- Boulding E. G., Vanalstyne K. L.1993Mechanisms of differential survival and growth of 2 species of Littorina on wave-exposed and on protected shores. J. Exp. Mar. Biol. Ecol. 169, 139–166 (doi:10.1016/0022-0981(93)90191-P) [Google Scholar]
- Bourdeau P. E.2009Prioritized phenotypic responses to combined predators in a marine snail. Ecology 90, 1659–1669 (doi:10.1890/08-1653.1) [DOI] [PubMed] [Google Scholar]
- Brookes J. I., Rochette R.2007Mechanism of a plastic phenotypic response: predator-induced shell thickening in the intertidal gastropod Littorina obtusata. J. Evol. Biol. 20, 1015–1027 (doi:10.1111/j.1420-9101.2007.01299.x) [DOI] [PubMed] [Google Scholar]
- Brown K. M., Quinn J. F.1988The effect of wave action on growth in 3 species of intertidal gastropods. Oecologia 75, 420–425 (doi:10.1007/BF00376946) [DOI] [PubMed] [Google Scholar]
- Brown K. M., McDonough M., Richardson T. D.2004Intraspecific life history variation in the southern oyster drill, Stramonita haemastoma: patterns and causes. J. Shellfish Res. 23, 149–155 [Google Scholar]
- Christy J. H.1995Mimicry, mate choice, and the sensory trap hypothesis. Am. Nat. 146, 171–181 (doi:10.1086/285793) [Google Scholar]
- Crothers J. H.1983Variation in dog-whelk shells in relation to wave action and crab predation. Biol. J. Linn. Soc. 20, 85–102 (doi:10.1111/j.1095-8312.1983.tb01591.x) [Google Scholar]
- Currey J. D., Taylor J. D.1974Mechanical-behavior of some molluscan hard tissues. J. Zool. 173, 395–406 (doi:10.1111/j.1469-7998.1974.tb04122.x) [Google Scholar]
- DeWitt T. J., Sih A., Hucko J. A.1999Trait compensation and cospecialization in a freshwater snail: size, shape and antipredator behaviour. Anim. Behav. 58, 397–407 (doi:10.1006/anbe.1999.1158) [DOI] [PubMed] [Google Scholar]
- Emerson S. B., Boyd S. K.1999Mating vocalizations of female frogs: control and evolutionary mechanisms. Brain Behav. Evol. 53, 187–197 (doi:10.1159/000006594) [DOI] [PubMed] [Google Scholar]
- Engqvist L.2005The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim. Behav. 70, 967–971 (doi:10.1016/j.anbehav.2005.01.016) [Google Scholar]
- Etter R. J.1996The effect of wave action, prey type, and foraging time on growth of the predatory snail Nucella lapillus (L). J. Exp. Mar. Biol. Ecol. 196, 341–356 (doi:10.1016/0022-0981(95)00139-5) [Google Scholar]
- Grunbaum D.1997Hydromechanical mechanisms of colony organization and cost of defense in an encrusting bryozoan, Membranipora membranacea. Limnol. Oceanogr. 42, 741–752 [Google Scholar]
- Harvell C. D.1992Inducible defenses and allocation shifts in a marine bryozoan. Ecology 73, 1567–1576 (doi:10.2307/1940010) [Google Scholar]
- Hendrix L. J., Carter M. W., Scott D. T.1982Covariance analyses with heterogeneity of slopes in fixed models. Biometrics 38, 641–650 (doi:10.2307/2530045) [PubMed] [Google Scholar]
- Henry L. M., Roitberg B. D., Gillespie D. R.2006Covariance of phenotypically plastic traits induces an adaptive shift in host selection behaviour. Proc. R. Soc. B 273, 2893–2899 (doi:10.1098/rspb.2006.3672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitema B. E.1980The analysis of covariance and alternatives New York, NY: John Wiley and Sons, Inc [Google Scholar]
- Kemp P., Bertness M. D.1984Snail shape and growth rates—evidence for plastic shell allometry in Littorina littorea. Proc. Natl Acad. Sci. USA 81, 811–813 (doi:10.1073/pnas.81.3.811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitching J. A.1976Distribution and changes in shell form of Thais spp. (Gastropoda) near Bamfield, BC. J. Exp. Mar. Biol. Ecol. 23, 109–126 (doi:10.1016/0022-0981(76)90135-0) [Google Scholar]
- McCoy M. W.2007Conspecific density determines the magnitude and character of predator-induced phenotype. Oecologia 153, 871–878 (doi:10.1007/s00442-007-0795-y) [DOI] [PubMed] [Google Scholar]
- Menge B. A.1978Predation intensity in a rocky intertidal community—effect of an algal canopy, wave action and dessication on predator feeding rates. Oecologia 34, 17–35 (doi:10.1007/BF00346238) [DOI] [PubMed] [Google Scholar]
- Menge B. A., Berlow E. L., Blanchette C. A., Navarrete S. A., Yamada S. B.1994The keystone species concept—variation in interaction strength in a rocky intertidal habitat. Ecol. Monogr. 64, 249–286 (doi:10.2307/2937163) [Google Scholar]
- Miner B. G., Sultan S. E., Morgan S. G., Padilla D. K., Relyea R. A.2005Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692 (doi:10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- Palmer A. R.1981Do carbonate skeletons limit the rate of body growth? Nature 292, 150–152 (doi:10.1038/292150a0) [Google Scholar]
- Palmer A. R.1982Growth in marine gastropods—a non-destructive technique for independently measuring shell and body-weight. Malacologia 23, 63–73 [Google Scholar]
- Palmer A. R.1983Relative cost of producing skeletal organic matrix versus calcification—evidence from marine gastropods. Mar. Biol. 75, 287–292 (doi:10.1007/BF00406014) [Google Scholar]
- Palmer A. R.1985Adaptive value of shell variation in Thais lamellosa—effect of thick shells on vulnerability to and preference by crabs. Veliger 27, 349–356 [Google Scholar]
- Palmer A. R.1990Effect of crab effluent and scent of damaged conspecifics on feeding, growth, and shell morphology of the Atlantic dogwhelk Nucella lapillus (L). Hydrobiologia 193, 155–182 (doi:10.1007/BF00028074) [Google Scholar]
- Palmer A. R.1992Calcification in marine mollusks—how costly is it. Proc. Natl Acad. Sci. USA 89, 1379–1382 (doi:10.1073/pnas.89.4.1379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez K. O., Carlson R. L., Shulman M. J., Ellis J. C.2009Why are intertidal snails rare in the subtidal? Predation, growth and the vertical distribution of Littorina littorea (L.) in the Gulf of Maine. J. Exp. Mar. Biol. Ecol. 369, 79–86 (doi:10.1016/j.jembe.2008.09.019) [Google Scholar]
- Pigliucci M.2005Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 20, 481–486 (doi:10.1016/j.tree.2005.06.001) [DOI] [PubMed] [Google Scholar]
- Quinn G. P., Keough M. J.2002Experimental design and data analysis Cambridge, UK: Cambridge University Press [Google Scholar]
- Ramos-Jiliberto R., Frodden E., Aranguiz-Acuna A.2007Pre-encounter versus post-encounter inducible defenses in predator—prey model systems. Ecol. Model. 200, 99–108 [Google Scholar]
- Relyea R. A., Auld J. R.2005Predator- and competitor-induced plasticity: how changes in foraging morphology affect phenotypic trade-offs. Ecology 86, 1723–1729 (doi:10.1890/04-1920) [Google Scholar]
- Rundle S. D., Spicer J. I., Coleman R. A., Vosper J., Soane J.2004Environmental calcium modifies induced defences in snails. Proc. R. Soc. Lond. B 271, S67–S70 (doi:10.1098/rsbl.2003.0106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R. R., Rohlf F. J.1995Biometry: the principles and practice of statistics in biological research New York, NY: W.H. Freeman and Co [Google Scholar]
- Tollrian R., Harvell C. D. (eds) 1998The ecology and evolution of inducible defenses Princeton, NJ: Princeton University Press [Google Scholar]
- Trussell G. C.1996Phenotypic plasticity in an intertidal snail: the role of a common crab predator. Evolution 50, 448–454 (doi:10.2307/2410815) [DOI] [PubMed] [Google Scholar]
- Trussell G. C., Nicklin M. O.2002Cue sensitivity, inducible defense, and trade-offs in a marine snail. Ecology 83, 1635–1647 [Google Scholar]
- Trussell G. C., Smith L. D.2000Induced defenses in response to an invading crab predator: an explanation of historical and geographic phenotypic change. Proc. Natl Acad. Sci. USA 97, 2123–2127 (doi:10.1073/pnas.040423397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk J.2000The costs of an inducible defense in anuran larvae. Ecology 81, 2813–2821 (doi:10.1890/0012-9658(2000)081[2813:TCOAID]2.0.CO;2) [Google Scholar]
- Vermeij G. J.1982Phenotypic evolution in a poorly dispersing snail after arrival of a predator. Nature 299, 349–350 (doi:10.1038/299349a0) [Google Scholar]
- Vermeij G. J.1987Evolution and escalation: an ecological history of life Princeton, NJ: Princeton University Press [Google Scholar]
- Vermeij G. J.2002Characters in context: molluscan shells and the forces that mold them. Paleobiology 28, 41–54 (doi:10.1666/0094-8373(2002)028<0041:CICMSA>2.0.CO;2) [Google Scholar]
- Wagner G. P.1988The influence of variation and of developmental constraints on the rate of multivariate phenotypic evolution. J. Evol. Biol. 1, 45–66 (doi:10.1046/j.1420-9101.1988.1010045.x) [Google Scholar]
- Wagner G. P.1996Homologues, natural kinds and the evolution of modularity. Am. Soc. Zool. 36, 36–43 [Google Scholar]
- West-Eberhard M. J.2003Developmental plasticity and evolution Oxford, UK: Oxford University Press [Google Scholar]
- Wimberger P. H.1991Plascity of jaw and skull morphology in the neotropical cichlids Geophagus brasilensis and G. steindachneri. Evolution 45, 1545–1563 (doi:10.2307/2409778) [DOI] [PubMed] [Google Scholar]
- Windig J. J., De Kovel C. G. F., De Jong G.2003Genetics and mechanics of plasticity. In Phenotypic plasticity: functional and conceptual approaches (eds DeWitt T. J., Scheiner S. M.), pp. 31–49 Oxford, UK: Oxford University Press [Google Scholar]
- Zipser E., Vermeij G. J.1978Crushing behavior of tropical and temperate crabs. J. Exp. Mar. Biol. Ecol. 31, 155–172 (doi:10.1016/0022-0981(78)90127-2) [Google Scholar]