Abstract
The mutualism between fungus-growing termites (Macrotermitinae) and their mutualistic fungi (Termitomyces) began in Africa. The fungus-growing termites have secondarily colonized Madagascar and only a subset of the genera found in Africa is found on this isolated island. Successful long-distance colonization may have been severely constrained by the obligate interaction of the termites with fungal symbionts and the need to acquire these symbionts secondarily from the environment for most species (horizontal symbiont transmission). Consistent with this hypothesis, we show that all extant species of fungus-growing termites of Madagascar are the result of a single colonization event of termites belonging to one of the only two groups with vertical symbiont transmission, and we date this event at approximately 13 Mya (Middle/Upper Miocene). Vertical symbiont transmission may therefore have facilitated long-distance dispersal since both partners disperse together. In contrast to their termite hosts, the fungal symbionts have colonized Madagascar multiple times, suggesting that the presence of fungus-growing termites may have facilitated secondary colonizations of the symbiont. Our findings indicate that the absence of the right symbionts in a new environment can prevent long-distance dispersal of symbioses relying on horizontal symbiont acquisition.
Keywords: symbiont transmission mode, long-distance dispersal, mutualism, Microtermes, Termitomyces
1. Introduction
The establishment of a mutualistic relationship between two species allows both species to find a faster adaptive solution to a problem than would be possible on their own, owing to the synergistic combination of their traits (Maynard Smith & Szathmáry 1997). However, obligate mutualistic interactions also constrain dispersal, as that requires either that both partners disperse together or that they are able to find each other after independent dispersal. Here, we explore long-distance dispersal to the island of Madagascar by a mutualistic symbiosis between insects and fungi: macrotermitine termites and Termitomyces fungi.
The mutualism between fungus-growing termites and their fungal symbionts originated in Africa (Aanen & Eggleton 2005) and is obligate for both partners (Wood & Thomas 1989; Aanen et al. 2002; Froslev et al. 2003). The termites (Isoptera, Termitidae, Macrotermitinae) provide a constant, highly regulated, growth environment for the fungi, and the fungi (genus Termitomyces) provide the termites with a processed food source. Entering the symbiosis has allowed the fungi to overcome unfavourable ecological conditions (such us temperature and moisture fluctuations) and the termites to efficiently exploit complex and diverse plant substrates (such as lignocellulose). The Termitomyces fungi are reared on a special structure, the fungus comb, built from dry dead plant material that has been chewed and quickly passed through the termite gut. Within a nest, a single strain monoculture of Termitomyces is present and the fungal propagation is always asexual, via vegetative spores (Leuthold et al. 1989; Aanen 2006; Aanen et al. in press). In contrast to this asexual fungal propagation within a colony, the between-nest fungal symbiont transmission mode is horizontal for most species of fungus-growing termites. This means that, to establish the fungus garden of a new colony, foraging workers have to collect Termitomyces sexual spores (actively or passively) from the environment around the nest (Korb & Aanen 2003). These spores (basidiospores) are produced from sexual fruiting bodies (basidiocarps or mushrooms) that occasionally arise from nodules and are wind-dispersed.
This mode of symbiont transmission is likely to represent the ancestral state (Aanen et al. 2002). Only two exceptions are known where the termites show vertical transmission of their symbiont (i.e. where Termitomyces vegetative spores are transported in the gut of alates from a parental colony and used to inoculate the fungus comb of the newly founded colonies). This vertical transmission mode is uniparental in the fungus-growing termites: (i) via the female alate in Microtermes species and (ii) via the male alate in the species Macrotermes bellicosus (Grassé & Noirot 1955; Johnson 1981; Johnson et al. 1981; Aanen et al. 2002).
We hypothesize that horizontal transmission presents a severe constraint on long-distance colonization as it implies that the right fungal symbiont must be available in the new environment and that it is acquired by the termite colonizer upon arrival. Long-distance dispersal is a stochastic process and the likelihood that the disperser arrives, survives and reproduces to establish a new population is even smaller if the two symbiotic partners start the journey independently. Dispersing as a unit (with vertical symbiont transmission) is thus expected to enhance the chance of successful colonization.
A further challenge for successful colonization by a sexually reproducing species is the need of mate-finding after dispersal. Termites provide a clear contrast to other social insects in this respect. For example, in the fungus-growing ants, mate-finding after dispersal is not a critical constraint as the female disperses and founds a nest on her own, after having been inseminated. The case of the fungus-growing ant population of Guadeloupe is a good example, as it seems to have been founded by a single introduction of a lone multiply mated female, or perhaps a group of sisters (Mikheyev 2008). However, in the case of fungus-growing termites, finding a suitable mate in the new environment poses an additional severe constraint, as colonies are founded by a pair of reproductives.
Several successful out-of-Africa migrations by fungus-growing termites have occurred. Only 3 of the 11 genera of fungus-growing termites were previously reported in Madagascar: Odontotermes, Ancistrotermes and Microtermes (Eggleton & Davies 2003). Madagascar broke off from Gondwana around 165 Ma, reached its current position with respect to Africa at least 118 Ma and the most widely accepted date for the breaking off from India is 80 Ma (Yoder & Nowak 2006). While the proximity to Africa has allowed multiple colonizations, the distance separating these two landmasses, and the prevalent ocean currents that minimize transport on floating trees and other flood debris, has meant that colonizations have been rare, allowing Madagascar to develop a highly distinctive flora and fauna. The extant flora and fauna of Madagascar is a mixture of groups resulting from ancient vicariant events and groups originating from transoceanic dispersal (Monaghan et al. 2005; Kodandaramaiah & Wahlberg 2007; Orsini et al. 2007). For fungus-growing termites, previous work suggests that their presence on Madagascar is not due to vicariance but to secondary transoceanic dispersal (Aanen & Eggleton 2005). This is also consistent with the estimates obtained by Brandl et al. (2007), who dated the origin of fungus-growing termites to around 62 Ma (30–108 Myr as credibility interval). Given this age estimate, all extant genera have evolved after the separation of Madagascar from Africa and probably even from India.
Here we investigate (i) the number and timing of colonization events of Madagascar by fungus-growing termites and their fungal symbionts, and (ii) the degree of co-speciation between termite and fungus colonizers. The presence of fungus-growing termites on this isolated island provides us with a unique opportunity to study long-distance dispersal by organisms living in obligate symbiosis and showing variation in symbiont transmission mode.
2. Material and methods
(a). Taxa sampling and data collection
Fungus-growing termites were sampled in South Africa and Madagascar, at a range of locations (see table S1, electronic supplementary material) between 2000 and 2006. Most of the Malagasy samples consisted of foragers collected from dead wood while feeding in closed-canopy forest habitats, while most of the South African and some of the Malagasy samples were retrieved directly from the nest. Upon collection, they were preserved in 100 per cent alcohol.
Termite workers were allowed to dry on filter paper prior to DNA extraction. DNA was extracted separately from individual heads and abdomens using the Chelex method. The DNA extracted from the head capsules was used for the termites’ analyses and, as no fungus combs were sampled, Termitomyces DNA was obtained from the termites’ abdomen. All extraction products were stored at −20°C.
(b). Termite sequences and phylogenetic analyses
The mitochondrial gene cytochrome oxidase subunit 1 (COI) and part of the nuclear ribosomal internal transcribe spacer (ITS2) region (Jenkins et al. 2001; Aanen et al. 2002) were amplified using a standard PCR reaction (for details, see electronic supplementary material). All PCR products were then purified using the GenElute PCR clean-up kit (Sigma) and directly sequenced with the amplification primers. Sequencing was performed by MWG Biotech Germany.
The alignment of COI was straightforward, as no insertions/deletions had to be inferred. For the ITS2 region an iterative refinement method, which accounts for larger gaps in the sequences (E-INS-i) as implemented in the program MAFFT (Katoh & Toh 2008) was used to align the sequences. The optimal model of evolution was selected using MrModeltest v. 2.2 (Posada & Crandall 1998). Bayesian inference was conducted using MrBayes v. 3.0 (Huelsenbeck & Ronquist 2001; Ronquist & Huelsenbeck 2003). The default settings of MrBayes were used and with Markov chain Monte Carlo (MCMC) runs being repeated twice as a safeguard against spurious results. The first 1000 trees were discarded as burn-in, and the remaining trees were used to calculate a majority rule consensus tree. Stationarity was confirmed by analysis of the log likelihoods and the consistency between runs.
We used a relaxed molecular clock to estimate the age of the Malagasy fungus-growing termite node(s) by using the penalized likelihood function implemented on the program r8s v. 1.7 (Sanderson 2002). The analysis was based on the topology of the majority rule consensus COI tree of the trees sampled in the Bayesian analysis, with non-fungus-growing termite taxa being pruned, prior to age calculations. The Odontotermes node was constrained to a minimum age of 7 Ma according to the dating of the fossilized fungus comb reported in Duringer et al. (2007). A second constrained node was used simultaneously, with a minimum age of 3.4 Ma, and corresponding to the age for the most recent common ancestor (MRCA) of Macrotermes jeanneli according to Darlington (2005). The truncated Newton algorithm was employed and a coarse scale cross-validation (with parameters n1 = 0, n2 = 0.3 and k = 12) was undertaken to select the optimal smoothing parameter. The mean node age and its s.d. were calculated using 50 non-parametric bootstrap replicates of the dataset generated by PHYLIP v. 3.68 (Felsenstein 1989), followed by re-estimation of branch lengths and divergence times for each replicate (Sanderson 2002).
(c). Termitomyces sequences and phylogenetic analyses
Part of the nuclear ribosomal region—including the first internal transcribed spacer (ITS1), the 5.8S RNA gene and the second internal transcribed spacer (ITS2)—and the nuclear 25S RNA gene were amplified in a standard PCR reaction, using the ITS1FT and 25S4R primers described in Aanen et al. (2007; for details, see electronic supplementary material). Samples were selected for analysis on the basis of the host COI-based phylogeny (i.e. a minimum of two samples per clade were chosen for Termitomyces analysis). All desired PCR products were then purified using the GenElute PCR clean-up kit (Sigma). Purified PCR products were directly sequenced with both amplification primers. For some Termitomyces strains, two copies were present in the PCR products, differing by a small length mutation, which has been found previously (De Fine Licht et al. 2005). Sequencing was performed by MWG Biotech Germany, using both forward and reverse primers, and, whenever needed, the intermediate primers ITS4 (White et al. 1990) and/or its reverse complement.
Sequences were aligned in MAFFT (Katoh et al. 2005) under the option L-INS-I (iterative refinement method incorporating local pairwise alignments; gap opening penalty: 1.5 and gap extension penalty 0.14; 1PAM/κ = 2 scoring matrix for nucleotide sequences). Added to the dataset were sequences of Termitomyces symbionts of several Macrotermitinae (table S2, electronic supplementary material) from Aanen et al. (2002), whenever both ITS and 25S sequences were available from the same specimen or the specimen was still available for further analysis. A representative of each Termitomyces haplotype found in Aanen et al. (2007) was also included. The MCMC ran according to the models inferred with MrModeltest and using the default settings of MrBayes as described for the termite analysis.
3. Results and discussion
(a). The colonization of Madagascar by fungus-growing termites and their fungi
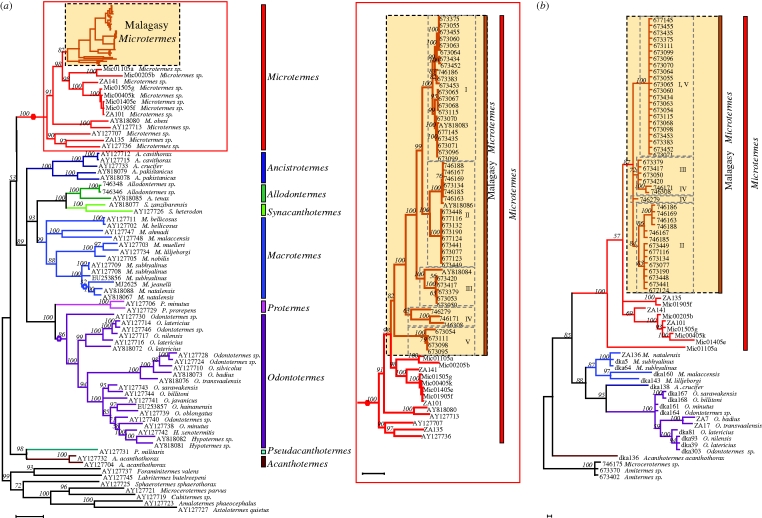
The fungus-growing termites of Madagascar are monophyletic on our tree (99% posterior probability), nested within the genus Microtermes (rooted majority rule consensus Bayesian tree; figure 1a). This analysis was based on the aligned dataset, for 108 fungus-growing termites taxa, of 929 bp of the COI region. The monophyly of the Malagasy Microtermes based on the mitochondrial COI gene, which is inherited via the maternal lineage only, is corroborated by the biparentally inherited ITS gene tree (figure 1b; total of 298 positions sequenced). Consistent with the different inheritance patterns of COI and ITS2, the tree topologies based on these genes are not totally congruent (e.g. the non-sister clades I and V in the COII tree form a monophyletic group with no substructure in the ITS2 tree), although the intergeneric relationships are kept.
Figure 1.
Phylogenetic relationship between Microtermes and other fungus-growing termites, including GenBank specimens when available (accession code given on tree). The phylogenies correspond to the majority-rule consensus tree of fungus-growing termites of trees sampled in a Bayesian analysis. The numbers above the branches refer to the Bayesian posterior probability of the nodes (more than 50%) and were derived from 19 500 Markov chain Monte Carlo-sampled trees. Other Termitidae species were used to root the tree. (a) Phylogeny based on COI; on the right side of this panel, the relationships within the genus Microtermes are enlarged. (b) Phylogeny based on ITS2 region. Scale bars, 0.05. The two nodes used as calibration points for the dating are indicated with a white dot (see text for details).
This result shows that the fungus-growing termites of Madagascar all originate from a single colonization event, estimated to have occurred at 13.18 Ma (bootstrapped mean: 11.04 ± 7.77 Ma). Given Madagascar's geographical history, with the last conceivable connection to other landmasses being dated from 80 Ma (Yoder & Nowak 2006), the current presence of Malagasy fungus-growing termites must be the result of long-distance dispersal and not of vicariance. The colonization of Madagascar occurred in the Upper/Middle Miocene, a time in which overseas dispersal would have been necessary. Our finding that all extant species of fungus-growing termites on Madagascar belong to a single clade within the genus Microtermes contradicts the previous report based on morphology that Odontotermes and Ancistrotermes species are also present on the island (Eggleton & Davies 2003). Whether the fungus-growing termites from Madagascar originate from a single female, a group of sisters or from multiple co-founding reproductives from a single species can not be inferred from the present data.
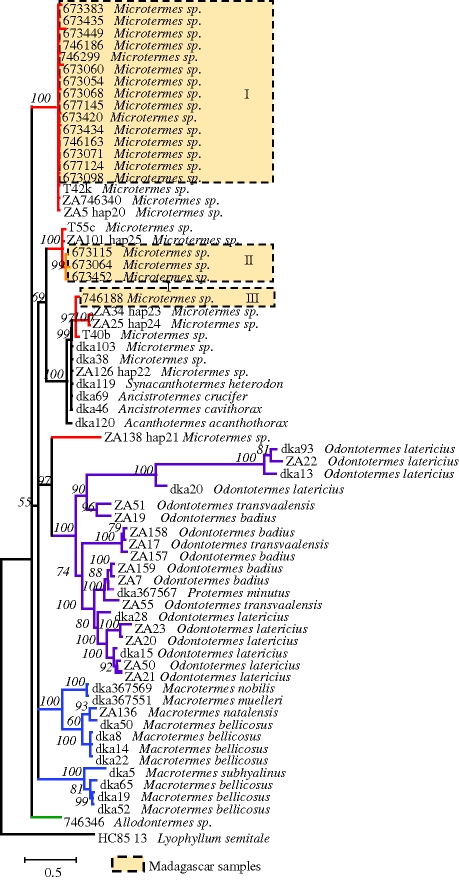
In contrast to their termite hosts, the Termitomyces symbionts of Madagascar are not monophyletic based on our reconstructed phylogeny (figure 2). Moreover, the degree of co-speciation between the two partners is very low. These findings confirm earlier results showing that specificity exists mainly at the generic level but not within genera (Aanen et al. 2002, 2007). Termitomyces symbionts associated with Malagasy Microtermes belong to three different clades and have in general identical or very similar haplotypes to known continental African symbionts (figure 2): (I) a large well-supported cluster containing the majority of the Madagascar haplotypes belonged to a clade together with three South African samples; (II) the only exclusively Madagascar clade is closely related to South African haplotypes; and, finally, (III) a single Madagascar haplotype is very similar to South African representatives. Coalescence theory predicts that ancestral haplotypes will be the most frequent sequences sampled in a population-level study (Crandall & Templeton 1993; Posada & Crandall 2001). If predictions from coalescence theory are to be applied to this particular case, then the first cluster (I) may represent the ancestral Termitomyces. However, this clade has a low diversity, which argues against this interpretation.
Figure 2.
Phylogenetic relationship of the Termitomyces symbionts of Microtermes included in this study and from Aanen et al. (2002, 2007; for details, see table S3, electronic supplementary material). The phylogeny was rooted using the free-living fungus Lyophyllum semitale (GenBank no. AF357049 and AF042581) as outgroup. The numbers above the branches refer to the Bayesian posterior probability of the nodes (more than 50%) and were derived from 19 500 Markov chain Monte Carlo-sampled trees. Scale bar, 0.5.
The generally low level of differentiation observed between Termitomyces from Madagascar and from continental Africa is striking. Similar results (i.e. nearly identical sequences of mixed Malagasy and continental African origin) were also observed on a molecular phylogenetic study of fungi of the genus Lactarius in Madagascar (Buyck et al. 2007). The very low endemism observed for both fungi suggests regular exchange between Madagascar and continental Africa preventing strong genetic differentiation. We can infer a minimum of three transoceanic migrations of the fungal symbionts of Microtermes onto Madagascar to explain the reconstructed Termitomyces phylogeny. The occurrence of several dispersion events of Termitomyces into Madagascar is not surprising, given what is known, mainly from studies on plant pathogens, about long-distance dispersal of fungi (Nagarajan & Singh 1990; Brown & Hovmoller 2002; Viljanen-Rollinson et al. 2007). Mainly the wind, but also water, animals or plants can act as passive spore dispersers.
(b). The significance of vertical transmission for long-distance colonization
In all studied Microtermes species, the female reproductive ingests conidia (asexual spores) from the comb before leaving the nest and transports the conidia in her digestive tube (Johnson 1981). This means that a colony can be established even if no fruiting fungus is present near the new colony. Presumably, vertical transmission therefore reduces the risk of fungus comb failure compared with most species of fungus-growing termites, which rely on the presence of spores in the environment of the new nest. Our results are compatible with the hypothesis that vertical transmission provided an advantage for long-distance colonization. However, as we have documented only one colonization event, we cannot say that the observed colonization by Microtermes does any more than provide a single example of support for the vertical transmission hypothesis.
Nonetheless, we can more generally hypothesize that Microtermes species with vertical transmission were readily able to disperse within Madagascar, and found there free niche space that has allowed them to diversify on the island into Ancistrotermes-like and Odontotermes-like morphotypes. This would explain the claims that members of these genera occur on Madagascar (Eggleton & Davies 2003). It is well accepted that, given a suitable unoccupied environment, the success of any given natural colonization event will depend largely on the availability of ecological space (Gillespie & Roderick 2002). The hypothesis that Microtermes has undergone an adaptive radiation on Madagascar needs to be tested in future studies using detailed morphological data.
Even though all of the five species of Microtermes studied so far have vertical transmission, earlier studies (Aanen et al. 2002, 2007) have indicated that occasional events of horizontal transmission also occur in the genus Microtermes. Such occasional horizontal transmission must explain the present result that multiple non-sister lineages of Termitomyces are associated with the fungus-growing termites of Madagascar.
Long-distance dispersal is an important strategy and the ability of fungal spores to use atmospheric pathways for rapid spread into new areas is a key factor for fungal success (Viljanen-Rollinson et al. 2007). However, a severe constraint for Termitomyces to be established on Madagascar (and thus to colonize it) is the need of spores to colonize fungus gardens: presumably aided by foraging termites, the spores need to be brought into the colony and become established in the fungus comb. A recent study shows that positive frequency-dependent selection leads to single-strain monocultures of Termitomyces within single nests and prevents subsequent colonization by other strains (Aanen et al. in press). Presumably, the establishment of a fungus comb by a new, horizontally transmitted, symbiont strain is therefore most likely to succeed in the initial stages of the colony formation, when the fungus still has a low biomass. Future experimental studies should focus on the details of symbiont transmission and the frequency of horizontal transmission in this group of fungus-growing termites.
4. Conclusions
We provide strong evidence that all extant fungus-growing termites on Madagascar result from a single overseas colonization event. This colonizer belongs to one of the only two clades within fungus-growing termites known to have vertical symbiont transmission, suggesting that transmission mode is a relevant pre-adaptation for long-distance dispersal of obligate symbioses. Vertical transmission couples the dispersal of the partners, thereby maximizing the probability of associating with a suitable partner species. In contrast to the single colonization event by their termite hosts, the symbiotic fungi have repeatedly colonized Madagascar secondarily, suggesting that the initial termite colonizer has facilitated subsequent colonizations of fungal symbionts. Our findings indicate that the absence of the right symbionts in a new environment can be a limiting factor for the success of long-distance dispersal of symbioses that rely on horizontal symbiont acquisition.
Acknowledgements
This research was supported by a Marie Curie Intra-European Fellowship within the 7th European Community Framework Programme (T.N.; IEF Project No. 220077) and by the Netherlands Organization for Scientific Research (D.K.A.; VIDI).
References
- Aanen D. K.2006As you reap, so shall you sow: coupling of harvesting and inoculating stabilizes the mutualism between termites and fungi. Biol. Lett. 2, 209–212 (doi:10.1098/rsbl.2005.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aanen D. K., Eggleton P.2005Fungus-growing termites originated in African rain forest. Curr. Biol. 15, 851–855 (doi:10.1016/j.cub.2005.03.043) [DOI] [PubMed] [Google Scholar]
- Aanen D. K., Eggleton P., Rouland-Lefevre C., Guldberg-Froslev T., Rosendahl S., Boomsma J. J.2002The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc. Natl Acad. Sci. USA 99, 14 887–14 892 (doi:10.1073/pnas.222313099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aanen D. K., Ros V., de Fine Licht H., Mitchell J., de Beer Z. W., Slippers B., Rouland-LeFevre C., Boomsma J.2007Patterns of interaction specificity of fungus-growing termites and Termitomyces symbionts in South Africa. BMC Evol. Biol. 7, 115 (doi:10.1186/1471-2148-7-115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aanen D. K., De Fine Licht H. H., Debets A. J. M., Kerstes N. A. G., Hoekstra R. F., Boomsma J. J.In press High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science [DOI] [PubMed] [Google Scholar]
- Brandl R., et al. 2007Divergence times in the termite genus Macrotermes (Isoptera: Termitidae). Mol. Phylogenet. Evol. 45, 239–250 (doi:10.1016/j.ympev.2007.07.007) [DOI] [PubMed] [Google Scholar]
- Brown J. K. M., Hovmoller M. S.2002Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297, 537–541 (doi:10.1126/science.1072678) [DOI] [PubMed] [Google Scholar]
- Buyck B., Verbeken A., Eberhardt U.2007The genus Lactarius in Madagascar. Mycol. Res. 111, 787–798 (doi:10.1016/j.mycres.2007.04.006) [DOI] [PubMed] [Google Scholar]
- Crandall K. A., Templeton A. R.1993Empirical tests of some predictions from coalescent theory with applications to intraspecific phylogeny reconstruction. Genetics 134, 959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington J.2005Distinctive fossilised termite nests at Laetoli, Tanzania. Insectes Sociaux 52, 408–409 (doi:10.1007/s00040-005-0830-0) [Google Scholar]
- De Fine Licht H. H., Andersen A., Aanen D. K.2005Termitomyces sp. associated with the termite Macrotermes natalensis has a heterothallic mating system and multinucleate cells. Mycol. Res. 109, 314–318 [DOI] [PubMed] [Google Scholar]
- Duringer P., Schuster M., Genise J. F., Mackaye H. T., Vignaud P., Brunet M.2007New termite trace fossils: galleries, nests and fungus combs from the Chad basin of Africa (Upper Miocene-Lower Pliocene). Palaeogeogr. Palaeoclimatol. Palaeoecol. 251, 323–353 (doi:10.1016/j.palaeo.2007.03.029) [Google Scholar]
- Eggleton P., Davies R. G.2003Isoptera, termites. In The natural history of Madagascar (eds Goodman S. M., Benstead J. P.), pp. 654–660 Chicago, IL: University of Chicago Press [Google Scholar]
- Felsenstein J.1989PHYLIP—phylogeny inference package (version 3.2). Cladistics 5, 164–166 [Google Scholar]
- Froslev T. G., Aanen D. K., Laessoe T., Rosendahl S.2003Phylogenetic relationships of Termitomyces and related taxa. Mycol. Res. 107, 1277–1286 (doi:10.1017/S0953756203008670) [DOI] [PubMed] [Google Scholar]
- Gillespie R. G., Roderick G. K.2002Arthropods on islands: colonization, speciation, and conservation. Annu. Rev. Entomol. 47, 595–632 (doi:10.1146/annurev.ento.47.091201.145244) [DOI] [PubMed] [Google Scholar]
- Grassé P.-P., Noirot C.1955La fondation de nouvelles sociétés par Bellicositermes natalensis Hav. Insectes Sociaux 2, 213–220 (doi:10.1007/BF02224382) [Google Scholar]
- Huelsenbeck J. P., Ronquist F.2001MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (doi:10.1093/bioinformatics/17.8.754) [DOI] [PubMed] [Google Scholar]
- Jenkins T. M., Dean R. E., Verkerk R., Forschler B. T.2001Phylogenetic analyses of two mitochondrial genes and one nuclear intron region illuminate European subterranean termite (Isoptera: Rhinotermitidae) gene flow, taxonomy, and introduction dynamics. Mol. Phylogenet. Evol. 20, 286–293 (doi:10.1006/mpev.2001.0966) [DOI] [PubMed] [Google Scholar]
- Johnson R.1981Colony development and establishment of the fungus comb in Microtermes sp. nr. usambaricus (Sjöstedt) (Isoptera: Macrotermitinae) from Nigeria. Insectes Sociaux 28, 3–12 (doi:10.1007/BF02223617) [Google Scholar]
- Johnson R. A., Thomas R. J., Wood T. G., Swift M. J.1981The inoculation of the fungus comb in newly founded colonies of some species of the Macrotermitinae (Isoptera) from Nigeria. J. Nat. Hist. 15, 751–756 (doi:10.1080/00222938100770541) [Google Scholar]
- Katoh K., Toh H.2008Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 9, 286–298 (doi:10.1093/bib/bbn013) [DOI] [PubMed] [Google Scholar]
- Katoh K., Kuma K.-I., Toh H., Miyata T.2005MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 33, 511–518 (doi:10.1093/nar/gki198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodandaramaiah U., Wahlberg N.2007Out-of-Africa origin and dispersal-mediated diversification of the butterfly genus Junonia (Nymphalidae: Nymphalinae). J. Evol. Biol. 20, 2181–2191 (doi:10.1111/j.1420-9101.2007.01425.x) [DOI] [PubMed] [Google Scholar]
- Korb J., Aanen D. K.2003The evolution of uniparental transmission of fungal symbionts in fungus-growing termites (Macrotermitinae). Behav. Ecol. Sociobiol. 53, 65–71 [Google Scholar]
- Leuthold R. H., Badertscher S., Imboden H.1989The inoculation of newly formed fungus comb with Termitomyces in Macrotermes colonies (Isoptera, Macrotermitinae). Insectes Sociaux 36, 328–338 (doi:10.1007/BF02224884) [Google Scholar]
- Maynard Smith J., Szathmáry E.1997The major transitions in evolution Oxford, UK: Oxford University Press [Google Scholar]
- Mikheyev A.2008History, genetics and pathology of a leaf-cutting ant introduction: a case study of the Guadeloupe invasion. Biol. Invasions 10, 467–473 (doi:10.1007/s10530-007-9144-7) [Google Scholar]
- Monaghan M. T., Gattolliat J.-L., Sartori M., Elouard J.-M., James H., Derleth P., Glaizot O., de Moor F., Vogler A. P.2005Trans-oceanic and endemic origins of the small minnow mayflies (Ephemeroptera, Baetidae) of Madagascar. Proc. R. Soc. B 272, 1829–1836 (doi:10.1098/rspb.2005.3139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan S., Singh D. V.1990Long-distance dispersion of rust pathogens. Annu. Rev. Phytopathol. 28, 139–153 (doi:10.1146/annurev.py.28.090190.001035) [DOI] [PubMed] [Google Scholar]
- Orsini L., Koivulehto H., Hanski I.2007Molecular evolution and radiation of dung beetles in Madagascar. Cladistics 23, 145–168 (doi:10.1111/j.1096-0031.2006.00139.x) [DOI] [PubMed] [Google Scholar]
- Posada D., Crandall K. A.1998MODELTEST: testing the model of DNA substitution. Bioinformatics 14, 817–818 (doi:10.1093/bioinformatics/14.9.817) [DOI] [PubMed] [Google Scholar]
- Posada D., Crandall K. A.2001Intraspecific gene genealogies: trees grafting into networks. Trends Ecol. Evol. 16, 37–45 (doi:10.1016/S0169-5347(00)02026-7) [DOI] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J. P.2003MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (doi:10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- Sanderson M. J.2002Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol. Biol. Evol. 19, 101–109 [DOI] [PubMed] [Google Scholar]
- Viljanen-Rollinson S. L. H., Parr E. L., Marroni M. V.2007Monitoring long-distance spore dispersal by wind—a review. NZ Plant Prot. 60, 291–296 [Google Scholar]
- White T., Bruns T., Lee S., Taylor J.1990Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: a guide to methods and applications (eds Innis M. A., Gelfand D., Shinsky J. J., White T. J.), pp. 315–322 San Diego, CA: Academic Press [Google Scholar]
- Wood T. G., Thomas R. J.1989The mutualistic association between Macrotermitinae and Termitomyces. In Insect–fungus interaction (eds Wilding N., Collins N. M., Hammond P. M., Webber J. F.), pp. 69–92 London, UK: Academic Press [Google Scholar]
- Yoder A. D., Nowak M. D.2006Has vicariance or dispersal been the predominant biogeographic force in Madagascar? Only time will tell. Annu. Rev. Ecol. Evol. Syst. 37, 405–431 (doi:10.1146/annurev.ecolsys.37.091305.110239) [Google Scholar]