Abstract
In the mature cerebral cortex of higher vertebrates, neurons are arranged in layers, each layer containing neurons of the same functional class. The cortical layering pattern is laid down during development by migration of young post-mitotic neurons along glial fibres to their correct positions in the cortical plate. The mechanics of whole-cell movement are well understood, but there is still uncertainty as to how a migrating neuron is instructed to leave its glial support when it reaches its destination. An intraneuronal signalling pathway initiated by reelin and containing apolipoprotein E receptor 2 (apoER2) is essential for normal cortical layering, and there is strong evidence that detachment of a migrating neuron is brought about by reelin-dependent downregulation of α3 integrin. But there remains the problem of how the reelin signal is switched on at a position in the cortex appropriate for each class of neuron. ApoER2 of placental mammals contains an amino acid sequence that is encoded in a separate exon in the apoER2 gene and is required for normal memory and spatial learning. The separate exon is not present in marsupials, birds or reptiles. The addition of this exon to the evolving apoER2 gene may have contributed to the success of placental mammals.
Keywords: apolipoprotein E receptor 2, apoER2 gene evolution, brain embryogenesis, integrins, reelin, spatial learning
1. Introduction
Apolipoprotein E receptor 2 (apoER2; also known as LRP8) is a cell-surface receptor essential for normal layering of neurons in the developing mammalian cerebral cortex and for the activity of a subclass of synapses in the adult mammalian brain (see Forster et al. 2006 for review). In this review, I discuss the effect of an evolutionary change in the apoER2 gene on brain function.
The review begins with a brief account of the migration of immature neurons into the embryonic cerebral cortex to form a layered structure, the control of this process by a reelin-activated signalling pathway that includes apoER2 and the molecular mechanisms underlying this control. Next, the role of apoER2 in the mature animal is considered. In adult mice, normal memory and learning require the presence of apoER2 containing an additional amino acid sequence encoded in an alternatively spliced exon in the apoER2 gene. The addition of this exon to the gene occurred at an early stage in the evolution of placental mammals and may have contributed to their success.
2. The formation of cerebral cortical layers during development
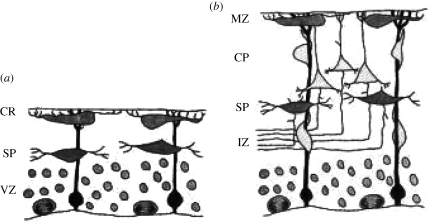
Formation of the layered cortex in the mammalian brain begins with the outward migration of post-mitotic neurons from a germinal layer, the ventricular zone (VZ) deep in the embryonic forebrain, to form a transient layered structure called the cortical plate (CP; figure 1). All the cells in a given layer of the CP eventually give rise to neurons of the same functional class in the corresponding layer of the mature cortex. The first cohort of migrating neurons forms the innermost layer of the CP, subsequent cohorts migrating past their predecessors in the CP to take up more superficial positions. This pattern of generating cortical layers, in which the oldest neurons form the deepest layers of the CP and those arriving later are more superficial, is known as inside-out. Migration of neurons takes place by crawling along glial fibres that span the width of the cortex from the VZ to the marginal zone (MZ) immediately beneath the pia (figure 1). The MZ contains a layer of specialized cells called Cajal–Retzius (CR) cells, which secrete reelin.
Figure 1.
Development of the cerebral cortex in normal mice. (a) The pre-plate stage. CR, Cajal–Retzius cells lying beneath the pia; SP, subplate cells; VZ, ventricular zone containing germinal neurons and glial cells. The glial fibres extend from the VZ to the outer margin of the pre-plate. (b) The cortical plate (CP) stage. Post-mitotic neurons leave the VZ and crawl along glial fibres, past the intermediate zone (IZ) of the cortex and the SP to form the CP between the SP and the marginal zone (MZ) containing the CR cells. Adapted from Rice & Curran (1999) with the permission of the authors.
The crawling of embryonic cortical neurons is driven by changes in the shape of the actin-rich layer of the cytoskeleton underlying the plasma membrane (the cell cortex). These changes are brought about by the controlled assembly and disassembly of actin filaments from soluble subunits in the cell cortex. The forward movement of a neuron along its glial support may be seen as a sequence of steps. First, the lagging edge is anchored to the glial fibre by focal adhesions (see below) while the plasma membrane at the leading edge is pushed forward by increased local formation of actin filaments. Next, focal adhesions are formed at the leading edge while those at the lagging edge are dismantled. Finally, the body of the cell is pulled forward, probably by contractile stress fibres attached to the focal adhesions at the leading edge. When a neuron has reached its laminar destination, it disengages from its glial support.
In addition to the predominant mode of migration of cortical neurons by glial-guided crawling, migration may also occur by nuclear translocation in which the neuron extends a leading process oriented radially towards the pia, followed by movement of the cell nucleus through the elongated process (Nadarajah et al. 2001). Nuclear translocation is most evident in the initial stage of cortical development, when the cortex is thin, and at the stage when neurons approach their final position in the CP.
The principal component of a focal adhesion is a cell-surface receptor (the integrin) with an intracellular domain bound to the cell cortex and an extracellular ligand-binding domain that binds to specific elements in the extracellular matrix (ECM) or on the surfaces of other cells. Each integrin is a heterodimer of α- and β-subunits, each type of subunit existing in several different forms. The affinity of an integrin for its ligand is regulated by signals transmitted from the cytoplasmic domain to the ligand-binding domain (Hynes 2002).
3. The reeler mutation and the reelin signalling pathway
(a). The reeler syndrome
In 1951, Falconer reported the spontaneous appearance of an autosomal recessive mutation in a colony of inbred mice at the Institute of Animal Genetics, Edinburgh (Falconer 1951). At about 18 days after birth, a mouse homozygous for the mutation begins to have difficulty holding its hindquarters upright, and when it tries to walk, it falls onto its side. The mutation was named ‘reeler’. The mutant allele at the reeler locus in the Edinburgh strain of reeler mice is denoted rl.
The pathological changes underlying the symptoms in reeler mice are most evident in the cerebral cortex and cerebellum (Caviness 1982). Migration of post-mitotic neurons from the VZ begins at the normal developmental stage and proceeds as far as the intermediate zone of the cortex (Caviness 1982). However, early-born neurons fail to advance into the CP region. The majority of neurons arriving later are unable to bypass their predecessors, resulting in the formation of a misplaced CP in which the oldest neurons are on the outside (outside-in pattern). The cerebellum in reeler mice is greatly reduced in size and the Purkinje cells, normally arranged as a single layer between the molecular and granular layers, fail to migrate from the inner layer of the cerebellar cortex.
(b). Reelin and the reelin gene
The mouse reelin gene spans about 450 kb of genomic DNA and has 65 exons encoding a protein with 3461 amino acids (Miao et al. 1994; D'Arcangelo et al. 1995; Royaux et al. 1997). The mutant gene in the Edinburgh strain of reeler has a 150 kb genomic deletion that includes a deletion of about 8 kb of 3′ coding sequence (Bar et al. 1995; D'Arcangelo et al. 1995).
(c). The reelin signalling pathway
At about the time when germinal neurons begin to migrate from the VZ, reelin activates a signalling pathway within the migrating neurons. This pathway is essential for the normal development of the inside-out layered cortex.
Reelin is secreted by CR cells into the ECM as a homodimer (Kubo et al. 2002; Strasser et al. 2004). Dimerized reelin binds to the extracellular binding domains of apoER2 and very-low-density-lipoprotein receptor (VLDLR), two receptors expressed on the surfaces of migrating neurons (D'Arcangelo et al. 1999; Strasser et al. 2004). Reelin binding to the receptor complex relays a signal across the plasma membrane that leads to tyrosine phosphorylation of disabled-1 (Dab1), a cytoplasmic adaptor protein (Hiesberger et al. 1999). Non-phosphorylated Dab1 binds to the phosphoinositide phosphatidylinositol-4,5-biphosphate (PI(4,5)P2) in the inner leaflet of the plasma membrane and to an NPXY amino-acid sequence in the cytoplasmic tails of apoER2 and VLDLR (Stolt et al. 2005). This binding is necessary for correct localization of Dab1 at the plasma membrane and for downstream transduction of the reelin signal from phosphorylated Dab1 (Stolt et al. 2005). Reelin binding to the receptor complex also leads to the activation of the cytoplasmic tyrosine kinases Fyn and Src, both of which phosphorylate Dab1 (Howell et al. 1997; Arnaud et al. 2003; Bock & Herz 2003). Phosphorylated Dab1 induces further activation of the two kinases (Bock & Herz 2003). The consequent auto-activation of Fyn and Src generates a tyrosine–kinase cascade. The targets of this cascade include proteins involved in the dynamic organization of the actin cytoskeleton (Chung et al. 2001). It is also possible that the Fyn/Src cascade synergizes with signals generated by ligand-engaged integrins at adhesion sites.
In addition to initiating the Fyn/Src tyrosine kinase cascade, reelin-dependent phosphorylation of Dab1 induces activation of the phosphatidylinositol 3′ kinase (PI3-K; Beffert et al. 2002). This enzyme catalyses the formation of phosphatidylinositol 3,4,5-triphosphate (PI(3,4,5)P3) from PI(4,5)P2 by phosphorylating the inositol ring at C-3. PI(3,4,5)P3 activates the monomeric GTPases, Rac and Rho, which control the assembly and organization of actin filaments at the leading edge of a moving cell (Chung et al. 2001). Bock et al. (2003) have also shown that the chemical inhibition of PI3-K prevents the formation of a layered cortex in embryonic mouse brain in vitro, indicating that PI3-K is a component of a branch of the reelin pathway.
Although apoER2 and the VLDL receptor act jointly to initiate the reelin pathway, the two receptors may have divergent roles in the migration of cortical neurons (Hack et al. 2007).
4. How does reelin control the layering of neurons?
It is now more than 50 years since the reeler mutation was discovered and more than a decade since the reelin gene was cloned, yet we still have no generally accepted explanation, in terms of molecular interactions, as to how reelin controls the layering of neurons in the mammalian embryonic cerebral cortex. We need to explain how reelin, secreted into the outermost layer of the cortex, transmits a signal to each migrating neuron to halt at its laminar destination and leave its glial support. Beffert et al. (2002) have shown that the reelin pathway is active in growth cones at the tips of the leading processes of migrating neurons. This suggests that reelin does not need to diffuse from the MZ through the ECM as far as the cell bodies of migrating neurons in order to influence their behaviour. In the reeler brain, neurons leave the VZ at their normal birth date and crawl along their glial supports as far as the intermediate zone of the cortex. Therefore, the reelin pathway is not needed for normal functioning of the steps involved in the initial stages of crawling.
(a). α3 integrins in migrating cortical neurons
The major integrin in neurons of developing mammalian brain is α3β1, the integrin responsible for neuron/glial adhesions. The essential role of the α3-subunit in brain development is shown by the absence of a layered cortex in gene-targeted α3−/− mice (Anton et al. 1999).
In the normal embryonic cerebral cortex, α3 is expressed strongly in neurons in the VZ and is downregulated in neurons that have migrated into the CP (Anton et al. 1999). In neuron–glial cultures, inactivation of α3 by anti-α3 antibodies stops migration of neurons and may cause them to disengage from their glia and adhere together in clusters. Anton et al. (1999) suggest that when migrating neurons reach the CP, expression of α3 is downregulated and that this leads to a change in the adhesive preference of CP neurons from glyophilic to neurophilic. This change would be expected to favour disengagement of migrating neurons from their glia and their assembly into cortical layers by neuron–neuron interaction. Observations on the behaviour of embryonic cortical neurons in mice with a null mutation in the Dab1 gene (scrambler mice) support this suggestion. Sanada et al. (2004) have shown that, in contrast to what occurs normally, in embryonic scrambler cortex the α3 integrin level in neurons that have migrated into the CP is not downregulated, and that these neurons do not disengage from their glia. In addition, Sanada et al. have shown that in the normal embryonic CP downregulation of α3 integrin and detachment of neurons from their glia require reelin-dependent phosphorylation of tyrosine 220 and tyrosine 232 of Dab1 in the reelin pathway (Sanada et al. 2004).
Dulabon et al. (2000) have shown that reelin inhibits migration of neurons and that this effect depends on the interaction of reelin with neuronal α3β1 integrin. They have proposed a model for the formation of the layered CP in which changes in ligand preference of α3β1 from glia to reelin play a significant role. Chai et al. (2009) have shown that inhibition of the migration of cortical neurons by reelin involves stabilization of the actin cytoskeleton in the leading processes of the neurons. Chai et al. have also provided evidence that this effect is due to inhibition of the breakdown of actin by n-cofilin, an actin-depolymerizing factor.
(b). Reelin as a positional signal
It has been suggested that reelin functions in the embryonic mammal to provide migrating cortical neurons with information as to their position. A positional signal would be expected to reach its target cells from a specific direction in relation to the path taken by the migrating neurons. Magdaleno et al. (2002) have generated a line of transgenic reeler mice in which reelin was expressed in the embryonic cortex exclusively by cells in the VZ. In these mice, reelin reached the neuron from a direction opposite to that in normal mice. The ectopic reelin in the transgenic reeler mice corrected some, but not all, of the cytological abnormalities of reeler. In particular, migrating neurons in the embryonic cortex reached the CP region to produce a CP normally positioned but without layering of its neurons.
The failure of ectopic reelin to bring about a layered CP in reeler brains is consistent with the supposition that reelin secreted in the MZ of the normal cortex activates the reelin pathway in migrating neurons, causing them to detach from their glia, perhaps by the Dab1/α3 integrin mechanism discussed above. However, this explanation leaves open the problem of how the reelin pathway is switched on in each migrating neuron as it reaches a position in the CP appropriate for the functional class to which the neuron belongs. One possibility is that a neuron recognizes its correct cortical layer by means of a class-specific surface receptor that binds to a layer-specific ligand in the cortex (see discussion in McConnell 1989). It is difficult to envisage a guidance system for cortical layering that does not include signals arising from the extracellular environment, as well as those arising from within the cell itself.
5. Reelin and apolipoprotein e receptor 2 in the adult mammal
(a). Long-term potentiation and synaptic plasticity
Expression of reelin in the CR cells of mammalian brain ceases shortly after birth and is replaced by expression at a subset of gamma-aminobutyric acid-ergic interneurons in the cortex and hippocampus. Reelin secreted at these sites takes on a new role in association with a group of proteins clustered beneath the post-synaptic membrane in a dense complex known as the post-synaptic density (PSD). The proteins of the PSD include the adaptors PSD-95 and Dab1, tyrosine kinases of the Src family, the cytoplasmic domain of N-methyl-d-aspartate (NMDA) receptors (a subset of glutamate-gated ion channels) and the cytoplasmic domain of apoER2 (Ziff 1997). In placental mammals, apoER2 is expressed in two different forms by alternative splicing of the RNA transcript. The ‘long’ form has an insert of 59aa containing three copies of a PXXP motif in the cytoplasmic tail. The insert is encoded in a separate exon (exon 19 in mice, exon 18 in primates); the ‘short’ form lacks the additional sequence.
(b). Reelin, synaptic plasticity and spatial learning
At some synapses in the mature hippocampus, a short burst of repetitive firing in pre-synaptic cells leads to a change in synaptic behaviour such that a subsequent single pre-synaptic action potential evokes a greatly increased response in the post-synaptic cells. This effect, known as long-term potentiation (LTP), may last for months or longer and is essential for some forms of learning, for the formation and retention of long-term memories, and for the experience-based remodelling of synaptic connections that takes place throughout the life of a mammal (synaptic plasticity).
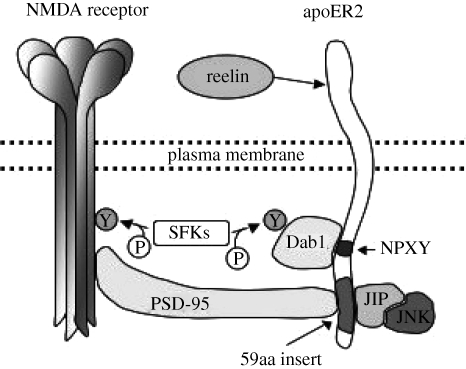
LTP is mediated by NMDA receptors and, in hippocampal slices from adult mice, is enhanced by reelin (Weeber et al. 2002). Reelin enhances LTP by inducing tyrosine phosphorylation of NMDA receptors in the presence of apoER2 containing the 59aa insert, but has no effect on LTP in hippocampal slices from mice in which exon 19 has been deleted from the apoER2 gene (apoER2(Δex19) mice; Beffert et al. 2005). Beffert et al. (2005) have also shown that apoER2 and the NMDA receptor form a functional complex in the PSD through which the reelin signal is transmitted from apoER2 to the NMDA receptor. The 59aa insert is not required for the physical interaction between the two receptors but is required for the transmission of the reelin signal. They suggest that the complex is formed by the binding of PSD-95 to a subunit of the NMDA receptor and to the insert in the tail of apoER2. In the model shown in figure 2, binding of reelin to apoER2 promotes the assembly of the apoER2/PSD-95/NMDA receptor complex and the recruitment of tyrosine kinases, leading to activation of NMDA receptors and enhancement of LTP.
Figure 2.
Model for activation of NMDA receptors by reelin binding to apoER2. The binding of reelin to apoER2 leads to the formation of a complex in which PSD-95 links the two receptors by binding to the NMDA receptor subunit 2A (NR2A) subunit of the NMDA receptor and the apoER2 tail. Formation of the complex induces recruitment of Src family tyrosine kinases (SFKs), leading to tyrosine phosphorylation of the NR2A subunit of the NMDA receptor. Adapted from Beffert et al. (2005) with the permission of the authors.
In apoER2(Δex19) mice, the failure of reelin to enhance LTP by activating NMDA receptors is accompanied by marked changes in behaviour. Fear-conditioned memory formation is defective and there is a marked deficit in spatial learning, as shown by tests of ability to use spatial cues to escape from a water maze by locating a submerged platform (Beffert et al. 2005). Despite these abnormalities in adult mice, the brains of apoER2(Δex19) embryos have normal inside-out layering of the cerebral cortex and normal arrangement of Purkinje cells in the cerebellum.
(c). Apolipoprotein E receptor 2 and the endocytosis of lipoproteins
ApoER2 expressed in Chinese hamster ovary (CHO) cells binds β-VLDL but does not mediate internalization and intracellular degradation of the bound lipoprotein (Sun & Soutar 1999). In the LDLR and in other receptors of the LDLR family, the NPXY motif acts as a signal for internalization of the surface-bound receptor via the clathrin-coated pit pathway, followed by the lysosomal degradation of its internalized ligand. So why does the NPXY motif in the cytoplasmic tail of apoER2 fail to operate in the same way? Sun & Soutar (2003) have constructed LDLR/apoER2 chimeras in which the extracellular and transmembrane domains were derived from the LDLR and the cytoplasmic domain was derived from apoER2 with or without the 59aa insert. When expressed in CHO cells, chimeras containing the normal insert in the cytoplasmic tail were unable to bind and internalize LDL. However, chimeras in which the insert was absent, or in which the proline residues in any of the three PXXP motifs were mutated, were able to bind and degrade LDL as efficiently as cells expressing normal LDLRs.
These observations suggest that the 59aa insert in apoEr2 masks the NPXY signal for the receptors to cluster in clathrin-coated pits. Stockinger et al. (2000) have shown that the 59aa insert binds the scaffold proteins JIP-1 and JIP-2. These proteins hold together the components of the c-Jun N-terminal kinase mitogen-activated protein kinase signalling module whose downstream targets include gene-regulatory proteins. JIP-1 also binds to RhoGEF (Meyer et al. 1999), leading to activation of RhoA, a monomeric GTPase. Activated RhoA promotes the formation of stress fibres and the assembly of focal adhesions (Hall 1993). Thus, the insert in apoER2 links the receptor to gene-regulatory proteins in the cell nucleus and to the actin cytoskeleton.
6. Aspects of the evolution of apolipoprotein e receptor 2 and the vertebrate brain
(a). The cortical plate
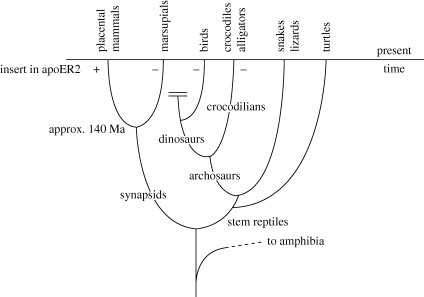
The forebrain of contemporary amniotes (reptiles, birds and mammals) has a layered cortex that develops by migration of immature neurons from the VZ to form a transient CP. In non-mammalian amniotes, reelin is expressed in the MZ and Dab1 is expressed in the CP. Expression of two major components of the mammalian reelin pathway in reptiles and birds suggests that a reelin pathway similar to that in mammals controls cortical layering in these two groups. Comparative studies of embryonic amniotes (Bar et al. 2000) have shown that the CP is rudimentary in turtles (thought to belong to the most ancient reptilian lineage), is more elaborate in birds and crocodilians, and is most elaborate in lizards and mammals (see figure 3 for amniote family tree). The formation of the CP in non-mammalian amniotes proceeds from outside to inside, not from inside to outside as in mammals. On the basis of these observations, Bar et al. (2000) suggest that in stem amniotes the development of a layered cortex involved the formation of a rudimentary CP by accretion of migrating neurons from outside to inside. The change to an inside-out mode of forming the CP in mammals could perhaps be seen as an evolutionary strategy for facilitating the remarkable outward expansion of the mammalian cortex by the addition of neurons generated in the deepest cortical layer.
Figure 3.
Simplified family tree of mammals, reptiles and birds. Synapsids are extinct mammal-like reptiles with a characteristic hole in the skull. Plus represents presence and minus represents absence of the exon encoding the 59aa in the cytoplasmic tail of apoER2.
(b). Apolipoprotein E receptor 2
ApoER2 is a member of the LDLR family. ApoER2 and the LDLR have the same domain structure, with an extracellular portion containing multiple binding repeats followed by a sequence of approximately 400 amino acids homologous to a segment of the epidermal growth factor (EGF) precursor, and a cytoplasmic tail containing the NPXY motif. The 400 amino acid sequences in apoER2, the LDLR and the EGF precursor are encoded by a similar block of eight exons. This sharing of exons by genes from different protein families is assumed to have come about by insertion of exons from somewhere in the genome into ancestors of contemporary genes (exon shuffling). In addition to the similar domain structure of the two receptors, the amino acid sequences within corresponding domains (excluding the 59aa insert in apoER2) are strongly homologous. These similarities indicate that the genes encoding apoER2 and the LDLR are descended from a common ancestor that duplicated at least once, giving rise to two or more lineages, one of which generated the apoER2 gene by mutation and the acquisition of exons by shuffling; another lineage generated the LDLR gene.
(c). The apolipoprotein E receptor 2 ligand-binding domain
ApoER2 is expressed in the brains of chickens and mice, and in both species the ligand-binding domain has eight repeats (Brandes et al. 1997); apoER2 of humans and marmosets (New World monkeys) has seven binding repeats. Kim et al. (1998) have shown that the human and marmoset apoER2 genes contain a pseudo-exon in the position corresponding to the exon encoding binding-repeat 8 in the mouse and the cow. A transcript of the pseudo-exon sequence is not spliced into the message owing to the presence of two point mutations in the 5′-splice donor adjacent to the pseudo-exon in the human and marmoset genes. Kim et al. (1998) suggest that at a recent stage in the evolution of the apoER2 gene, the above two mutations occurred in the gene of an ancestral primate, leading to skipping of the exon encoding the eighth binding repeat of the receptor in contemporary primates. Hence, the exon encoding the insert in the apoER2 tail is number 19 in non-primate placental mammals and number 18 in primates.
(d). The 59 amino acid insert in apolipoprotein E receptor 2
A highly conserved sequence of 59 amino acids is present in the cytoplasmic tail of apoER2 in all placental mammals that have been studied. The single exon encoding the insert is absent from the apoER2 gene in chickens (Brandes et al. 1997). I have excluded the presence of this exon in the apoER2 gene of Alligator mississippiensis by the analysis of genomic DNA from alligator embryos. Alligator DNA corresponding to the region spanning exon 17 to exon 19 in the human gene was amplified by polymerase chain reaction (PCR) with degenerate human primers. The PCR products contained sequences homologous to sequences in human exons 17 and 19, but there was no sequence homologous to human exon 18 in the intervening region (N. B. Myant 2004, unpublished observation). Sequences homologous to exons 17 and 19 of the apoER2 gene are present in Monodelphis domestica (a marsupial) in a region of chromosome 2 that is syntenic with a region of human chromosome 1 containing the apoER2 gene (http://www.ensembl.org/Monodelphis_domestica/Info/Index). Therefore, the apoER2 gene is present in the marsupial. However, the marsupial gene has no sequences homologous to human exon 18 (http://www.ensembl.org/Monodelphis_domestica/Info/Index).
Modern birds are descended from bipedal dinosaurs. Dinosaurs are descended from an ancient group of reptiles called Archosaurs, which also gave rise to crocodilians through a separate lineage (figure 3). The absence of the additional exon in the apoER2 gene of chickens and alligators, two species derived from two separate lineages, indicates that this exon was not present in the primordial gene in stem reptiles. The absence of the additional exon in a marsupial suggests that the exon was not added until after the placental–marsupial divergence in the mammalian lineage, thought to have occurred around 140 Ma (Dawkins 2004).
As discussed above, deletion of the additional exon in the mouse apoER2 gene (exon 19) leads to marked defects in long-term memory storage and spatial learning, probably by interruption of reelin-mediated activation of NMDA receptors. The addition of this exon to the evolving apoER2 gene in placental mammals may therefore have opened the way for the assembly of a signalling pathway involving reelin, the apoER2 cytoplasmic tail, PSD-95 and the NMDA receptor, with effects on behaviour that would have had a favourable influence on survival. In the evolution of amniotes, a change in the mode of formation of the CP from outside-in to inside-out occurred in mammals (Bar et al. 2000). The genetic changes underlying this event are not known. Insertion of the exon encoding the 59aa in the apoER2 gene may also have occurred at an early stage in the mammalian line (see above). However, the migration of cortical neurons and the formation of an inside-out CP in brain slices from apoER2(Δex19) mice are normal (Beffert et al. 2005). It is therefore unlikely that the addition of exon 19 to the apoER2 gene played any part in the evolutionary change to the inside-out CP of modern mammals.
Acknowledgements
I thank Professor Anne Soutar for help with the composition and preparation of this review and for allowing me to work in her laboratory as a retired worker. I also thank Dr X.-M. Sun for advice on sequencing the alligator apoER2 gene and Dr Naveenan Navaratnam for help with sequencing segments of the apoER2 gene in M. domestica DNA supplied by him. I thank Dr Christopher Healy (Guy's Hospital Dental Institute) for a gift of A. mississippiensis embryos. I am grateful to Georgina Going and her staff at the Hammersmith Campus Library (Delme Davies, Emma Hickman, Anita Quigley and Paul Stokes) for their help.
References
- Anton E. S., Kreidberg J. A., Rakic P.1999Distinct functions of α3 and αv integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron 22, 277–289 (doi:10.1016/S0896-6273(00)81089-2) [DOI] [PubMed] [Google Scholar]
- Arnaud L., Ballif B. A., Forster E., Cooper J. A.2003Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr. Biol. 13, 9–17 (doi:10.1016/S0960-9822(02)01397-0) [DOI] [PubMed] [Google Scholar]
- Bar I., Lambert De Rouvroit C., Royaux I., Krizman D. B., Dernoncourt C., Ruelle D., Beckers M. C., Goffinet A. M.1995A YAC contig containing the reeler locus with preliminary characterization of candidate gene fragments. Genomics 26, 543–549 (doi:10.1016/0888-7543(95)80173-J) [DOI] [PubMed] [Google Scholar]
- Bar I., Lambert de Rouvroit C., Goffinet A. M.2000The evolution of cortical development. An hypothesis based on the role of the Reelin signaling pathway. Trends Neurosci. 23, 633–638 (doi:10.1016/S0166-2236(00)01675-1) [DOI] [PubMed] [Google Scholar]
- Beffert U., Morfini G., Bock H. H., Reyna H., Brady S. T., Herz J.2002Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J. Biol. Chem. 277, 49 958–49 964 (doi:10.1074/jbc.M209205200) [DOI] [PubMed] [Google Scholar]
- Beffert U., et al. 2005Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor ApoER2. Neuron 47, 567–579 (doi:10.1016/j.neuron.2005.07.007) [DOI] [PubMed] [Google Scholar]
- Bock H. H., Herz J.2003Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 13, 18–26 (doi:10.1016/S0960-9822(02)01403-3) [DOI] [PubMed] [Google Scholar]
- Bock H. H., Jossin Y., Liu P., Forster E., May P., Goffinet A. M., Herz J.2003Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J. Biol. Chem. 278, 38 772–38 779 (doi:10.1074/jbc.M306416200) [DOI] [PubMed] [Google Scholar]
- Brandes C., Novak S., Stockinger W., Herz J., Schneider W. J., Nimpf J.1997Avian and murine LR8B and human apolipoprotein E receptor 2: differentially spliced products from corresponding genes. Genomics 42, 185–191 (doi:10.1006/geno.1997.4702) [DOI] [PubMed] [Google Scholar]
- Caviness V. S., Jr1982Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Dev. Brain Res. 4, 293–302 (doi:10.1016/0165-3806(82)90141-9) [DOI] [PubMed] [Google Scholar]
- Chai X., Förster E., Zhao S., Bock H H., Frotscher M.2009Reelin acts as a stop signal for radially migrating neurons by inducing phosphorylation of n-cofilin at the leading edge. Commun. Integr. Biol. 2, 375–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. Y., Funamoto S., Firtel R. A.2001Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem. Sci. 26, 557–566 (doi:10.1016/S0968-0004(01)01934-X) [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G., Miao G. G., Chen S. C., Soares H. D., Morgan J. I., Curran T.1995A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374, 719–723 (doi:10.1038/374719a0) [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G., Homayouni R., Keshvara L., Rice D. S., Sheldon M., Curran T.1999Reelin is a ligand for lipoprotein receptors. Neuron 24, 471–479 [DOI] [PubMed] [Google Scholar]
- Dawkins R.2004The ancestor's tale London, UK: Weidenfeld and Nicholson [Google Scholar]
- Dulabon L., Olson E. C., Taglienti M. G., Eisenhuth S., McGrath B., Walsh C., Kreidberg J., Anton E.2000Reelin binds α3β1 integrin and inhibits neuronal migration. Neuron 27, 33–34 (doi:10.1016/S0896-6273(00)00007-6) [DOI] [PubMed] [Google Scholar]
- Falconer D. S.1951Two new mutants, ‘trembler’ and ‘reeler’, with neurological actions in the house mouse (Mus musculus L.). J. Genet. 50, 192–201 (doi:10.1007/BF02996215) [DOI] [PubMed] [Google Scholar]
- Forster E., Jossin Y., Zhao S., Chai X., Frotscher M., Goffinet A. M.2006Recent progress in understanding the role of Reelin in radial neuronal migration, with specific emphasis on the dentate gyrus. Eur. J. Neurosci. 23, 901–909 (doi:10.1111/j.1460-9568.2006.04612.x) [DOI] [PubMed] [Google Scholar]
- Hack I., Hellwig S., Junghans D., Brunne B., Bock H. H., Zhao S., Frotscher M.2007Divergent roles of ApoER2 and VLDLr in the migration of cortical neurons. Development 134, 3883–3891 (doi:10.1242/dev.005447) [DOI] [PubMed] [Google Scholar]
- Hall A.1993Rho GTPases and the actin cytoskeleton. Science 274, 509–514 [DOI] [PubMed] [Google Scholar]
- Hiesberger T., Trommsdorff M., Howell B. W., Goffinet A., Mumby M. C., Cooper J. A., Herz J.1999Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 24, 481–489 (doi:10.1016/S0896-6273(00)80861-2) [DOI] [PubMed] [Google Scholar]
- Howell B. W., Gertler F. B., Cooper J. A.1997Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 16, 121–132 See http://www.ensembl.org/Monodelphis_domestica/Info/Index (doi:10.1093/emboj/16.1.121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O.2002Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (doi:10.1016/S0092-8674(02)00971-6) [DOI] [PubMed] [Google Scholar]
- Kim H. J., Kim D. H., Magoori K., Saeki S., Yamamoto T. T.1998Evolution of the apolipoprotein E receptor 2 gene by exon loss. J. Biochem. 124, 451–456 [DOI] [PubMed] [Google Scholar]
- Kubo K., Mikoshiba K., Nakajima K.2002Secreted Reelin molecules form homodimers. Neurosci. Res. 43, 381–388 (doi:10.1016/S0168-0102(02)00068-8) [DOI] [PubMed] [Google Scholar]
- Magdaleno S., Keshvara L., Curran T.2002Rescue of ataxia and preplate splitting by ectopic expression of Reelin in reeler mice. Neuron 33, 573–586 (doi:10.1016/S0896-6273(02)00582-2) [DOI] [PubMed] [Google Scholar]
- McConnell S. K.1989The determination of neuronal fate in the cerebral cortex. Trends Neurosci. 12, 342–349 (doi:10.1016/0166-2236(89)90041-6) [DOI] [PubMed] [Google Scholar]
- Meyer D., Liu A., Margolis B.1999Interaction of c-Jun amino-terminal kinase interacting protein-1 with p190 rhoGEF and its localization in differentiated neurons. J. Biol. Chem. 274, 35113–35118 (doi:10.1074/jbc.274.49.35113) [DOI] [PubMed] [Google Scholar]
- Miao G. G., Smeyne R. J., D'Arcangelo G., Copeland N. G., Jenkins N. A., Morgan J. I., Curran T.1994Isolation of an allele of reeler by insertional mutagenesis. Proc. Natl Acad. Sci. USA 91, 11 050–11 054 (doi:10.1073/pnas.91.23.11050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B., Brunstrom J. E., Grutzendler J., Wong R. O. L., Pearlman A. L.2001Two modes of radial migration in early development of the cerebral cortex. Nat. Neurosci. 4, 143–150 (doi:10.1038/83967) [DOI] [PubMed] [Google Scholar]
- Rice D. S., Curran T.1999Mutant mice with scrambled brains: understanding the signaling pathways that control cell positioning in the CNS. Genes Dev. 13, 2758–2773 (doi:10.1101/gad.13.21.2758) [DOI] [PubMed] [Google Scholar]
- Royaux I., Lambert de Rouvroit C., D'Arcangelo G., Demirov D., Goffinet A. M.1997Genomic organization of the mouse reelin gene. Genomics 46, 240–250 (doi:10.1006/geno.1997.4983) [DOI] [PubMed] [Google Scholar]
- Sanada K., Gupta A., Tsai L. H.2004Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron 42, 197–211 (doi:10.1016/S0896-6273(04)00222-3) [DOI] [PubMed] [Google Scholar]
- Stockinger W., Brandes C., Fasching D., Hermann M., Gotthardt M., Herz J., Schneider W. J., Nimpf J.2000The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J. Biol. Chem. 275, 25 625–25 632 (doi:10.1074/jbc.M004119200) [DOI] [PubMed] [Google Scholar]
- Stolt P. C., Chen Y., Liu P., Bock H. H., Blacklow S. C., Herz J.2005Phosphoinositide binding by the disabled-1 PTB domain is necessary for membrane localization and Reelin signal transduction. J. Biol. Chem. 280, 9671–9677 (doi:10.1074/jbc.M413356200) [DOI] [PubMed] [Google Scholar]
- Strasser V., et al. 2004Receptor clustering is involved in Reelin signaling. Mol. Cell. Biol. 24, 1378–1386 (doi:10.1128/MCB.24.3.1378-1386.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. M., Soutar A. K.1999Expression in vitro of alternatively spliced variants of the messenger RNA for human apolipoprotein E receptor-2 identified in human tissues by ribonuclease protection assays. Eur. J. Biochem. 262, 230–239 (doi:10.1046/j.1432-1327.1999.00394.x) [DOI] [PubMed] [Google Scholar]
- Sun X. M., Soutar A. K.2003The transmembrane domain and PXXP motifs of ApoE receptor 2 exclude it from carrying out clathrin-mediated endocytosis. J. Biol. Chem. 278, 19 926–19 932 (doi:10.1074/jbc.M302047200) [DOI] [PubMed] [Google Scholar]
- Weeber E. J., Beffert U., Jones C., Christian J. M., Forster E., Sweatt J. D., Herz J. M.2002Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 277, 39 944–39 952 (doi:10.1074/jbc.M205147200) [DOI] [PubMed] [Google Scholar]
- Ziff E. B.1997Enlightening the postsynaptic density. Neuron 19, 1163–1174 (doi:10.1016/S0896-6273(00)80409-2) [DOI] [PubMed] [Google Scholar]