Abstract
The extremes of dinosaur body size have long fascinated scientists. The smallest (<1 m length) known dinosaurs are carnivorous saurischian theropods, and similarly diminutive herbivorous or omnivorous ornithischians (the other major group of dinosaurs) are unknown. We report a new ornithischian dinosaur, Fruitadens haagarorum, from the Late Jurassic of western North America that rivals the smallest theropods in size. The largest specimens of Fruitadens represent young adults in their fifth year of development and are estimated at just 65–75 cm in total body length and 0.5–0.75 kg body mass. They are thus the smallest known ornithischians. Fruitadens is a late-surviving member of the basal dinosaur clade Heterodontosauridae, and is the first member of this clade to be described from North America. The craniodental anatomy and diminutive body size of Fruitadens suggest that this taxon was an ecological generalist with an omnivorous diet, thus providing new insights into morphological and palaeoecological diversity within Dinosauria. Late-surviving (Late Jurassic and Early Cretaceous) heterodontosaurids are smaller and less ecologically specialized than Early (Late Triassic and Early Jurassic) heterodontosaurids, and this ecological generalization may account in part for the remarkable 100-million-year-long longevity of the clade.
Keywords: Ornithischia, Heterodontosauridae, Jurassic, body size, palaeoecology
1. Introduction
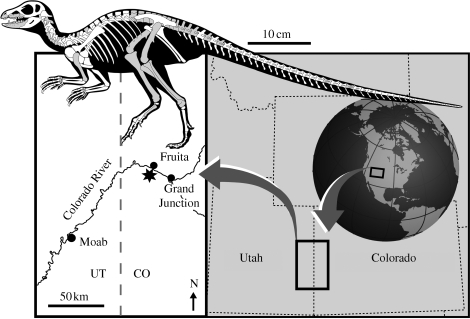
The iconic dinosaur is gigantic (e.g. Royo-Torres et al. 2006; Sander & Clauss 2008), and yet since the description of the carnivorous theropod Compsognathus in 1859 it has been known that some dinosaurs were very small (Wagner 1861; Xu et al. 2000; Turner et al. 2007). It has since been well established that the smallest (<1 m length) dinosaurs were saurischian theropods and that none of the predominantly herbivorous ornithischians (the other major division of dinosaurs) approached that range as adults—presumably because moderately large size is required to digest plants. Fully grown ornithischians ranged in size from just over a metre (e.g. Heterodontosaurus tucki, Early Jurassic, South Africa: Santa Luca 1980) up to 17 m (e.g. Shantungosaurus giganteus, Late Cretaceous, China: Seebacher 2001) in length and from approximately 2–22 500 kg in body mass (Seebacher 2001; Weishampel et al. 2004). The apparent absence of very small adult ornithischians is surprising given the diversity and abundance of small-bodied herbivorous and omnivorous mammals and reptiles in modern ecosystems (e.g. Damuth 1981). Here, we describe the fossils of a new diminutive dinosaur from North America (figure 1) that represents a late-surviving member of the ornithischian clade Heterodontosauridae (Santa Luca 1980; Norman et al. 2004), the first heterodontosaurid to be described from this continent. This new taxon is the smallest known ornithischian dinosaur and yields new insights into early ornithischian evolution and palaeoecology, which are currently poorly understood (Parker et al. 2005; Butler et al. 2007).
Figure 1.
Fruitadens haagarorum, locality map and skeletal reconstruction (postcranium based upon Heterodontosaurus tucki and redrawn and modified from Norman et al. 2004) showing body size of adult individuals (70 cm) and preserved elements (grey).
Heterodontosaurids are an enigmatic clade of small-bodied ornithischians known primarily from the Early Jurassic of southern Africa (Santa Luca 1980; Norman et al. 2004; Butler et al. 2008a); they have also been described from the Late Triassic of South America (Báez & Marsicano 2001) and the Early Cretaceous of Europe and Asia (Norman & Barrett 2002; Zheng et al. 2009). Recent work has demonstrated that heterodontosaurids may represent the most basal radiation of ornithischians and are of vital importance in understanding ornithischian phylogeny (Butler et al. 2007, 2008b). In addition, a recent discovery indicated that at least some heterodontosaurids possessed filamentous integumentary structures interpreted as possibly homologous with ‘protofeathers’ (Zheng et al. 2009). The group is thus integral to understanding the dynamics of ornithischian evolutionary history, as well as dinosaur origins and the earliest stages in the evolution of feathers.
2. Systematic palaeontology
Dinosauria Owen, 1842
Ornithischia Seeley, 1887
Heterodontosauridae Kuhn, 1966
Fruitadens haagarorum gen. et sp. nov.
(a). Etymology
Fruitadens, from Fruita (hypodigm locality) and dens (Latin, tooth); haagarorum, for Paul Haaga, Jr, Heather Haaga, Blythe Haaga, Paul Haaga III and Catalina Haaga, to honour their support of the Natural History Museum of Los Angeles County (LACM, Los Angeles, USA).
(b). Holotype
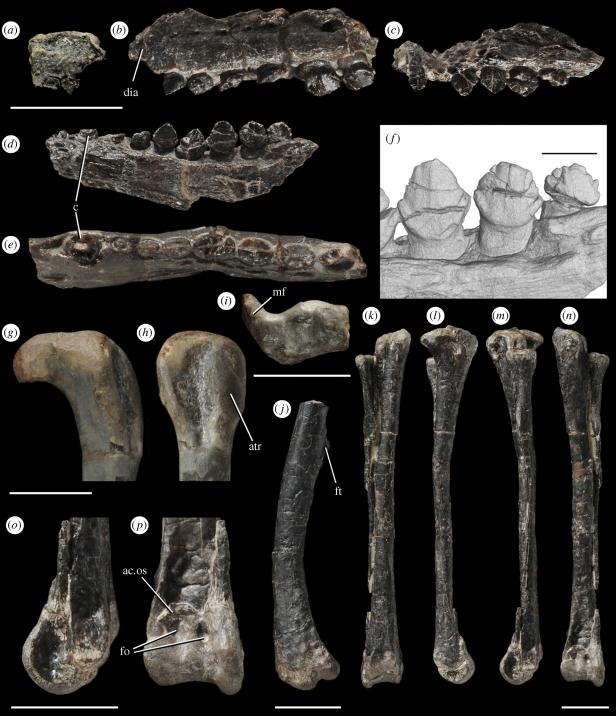
LACM 115747, associated jaws, vertebrae and limb bones of a nearly full-grown individual; includes incomplete maxillae and dentaries, disarticulated vertebrae including cervicals, dorsals, six sacral vertebrae and caudals, proximal end of the right femur, both ends of both tibiae, and partial right metatarsal I (figures 2b,e,i and 3a–c).
Figure 2.
Anatomy of Fruitadens haagarorum, (a,c,d,f) LACM 128258, (holotype: b,e,i) LACM 115747, (g,h) LACM 115727 and (j–p)LACM 120478. (a) Right premaxilla, lateral. (b) Left maxilla, lateral. (c) Left maxilla, lateral. (d) Right dentary, medial. (e) Right dentary, dorsal. (f) Close-up of posterior dentary teeth, lingual (µCT reconstruction). (g) Proximal right femur, posterior. (h) Proximal right femur, lateral. (i) Distal end of left tibia, distal. (j) Left femur, lateral. (k–n) Left tibia, fibula, astragalus and calcaneum: posterior (k); medial (l); lateral (m); anterior (n). (o,p) Distal end of left tibia, fibula, astragalus and calcaneum: lateral (o); anterior (p). Abbreviations: ac.os, ascending process of astragalus, formed by separate ossification; atr, anterior trochanter; c, caniniform tooth; dia, contribution of maxilla to arched diastema between premaxillary and maxillary tooth rows; fo, foramina; ft, broken base of fourth trochanter; mf, anteromedial flange. Scale bars equal 10 mm, except (f) 2 mm.
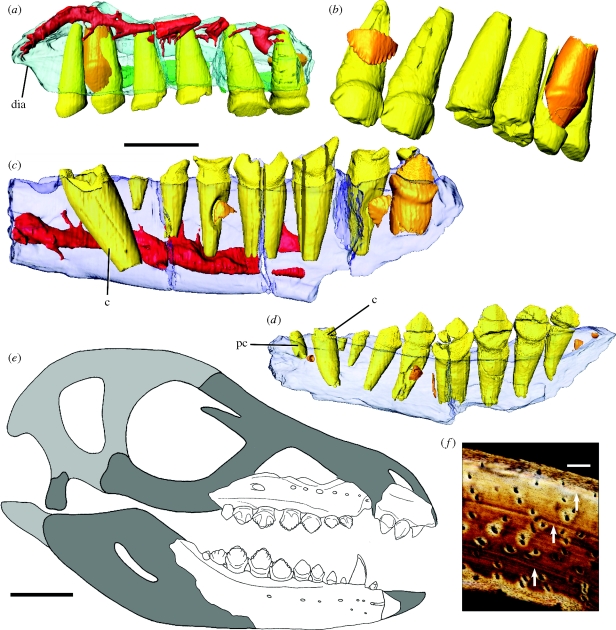
Figure 3.
(a)–(d) Fruitadens haagarorum, anatomy of the maxillae and dentaries reconstructed from µCT scan data, (e) cranial reconstruction and (f) osteohistology of the femur. Elements in the CT reconstructions are colour-coded as follows: maxilla, blue; dentary, violet; palatal fragment, green; functional teeth, yellow; replacement teeth, orange; internal canals, red. The maxilla and dentaries have been made transparent in order to better visualize their internal anatomy. (a) LACM 115747 (holotype), left maxilla. (b) LACM 115747 (holotype), left maxillary functional and replacement teeth (segmented out of maxilla), oblique posteromedial. (c) LACM 115747 (holotype), right dentary, medial. (d) LACM 128258, right dentary, medial. (e) Cranial reconstruction, missing areas based upon Tianyulong confuciusi (dark grey) and Heterodontosaurus tucki (light grey). (f) Transverse plane femoral diaphyseal histology of LACM 115727 viewed with polarized microscopy. Arrows denote growth lines. Abbreviations: c, caniniform tooth; dia, contribution of maxilla to arched diastema between premaxillary and maxillary tooth rows; pc, precaniniform tooth. Scale bars, (a,c,d) 5 mm; (e) 10 mm; (f) 0.15 mm. A scale bar is not shown for (b) because it is an oblique view.
(c). Referred material
LACM 115727, nearly full-grown individual, fragmentary cervical, dorsal and caudal vertebrae, partial femora, and partial tibiae with articulated astragalus/calcaneum (figure 2g,h); LACM 120478, juvenile individual, left humerus, partial left femur, and articulated left tibia, fibula and astragalus/calcaneum (figure 2j–p); LACM 128258, juvenile individual, right premaxilla, partial left maxilla, dentaries, dorsal vertebra, distal caudal vertebra (figures 2a,c,d,f and 3d).
(d). Horizon and locality
The base of the Brushy Basin Member of the Morrison Formation (Late Jurassic: early Tithonian) at the LACM Fruita Paleontological Area, northwest of Grand Junction, Colorado, USA (figure 1 and figure S1 in the electronic supplementary material; Kirkland 2006).
(e). Diagnosis
Small heterodontosaurid ornithischian characterized by the following unique combination of characters, including autapomorphies (*indicates character that is autapomorphic within Heterodontosauridae; **indicates character that is autapomorphic within Ornithischia): (1*) premaxillary crowns small and subequal in size, expanded labiolingually and mesiodistally above the root; (2) maxillary caniniform absent; (3) maxillary/dentary crowns low and triangular, with symmetrically distributed enamel; (4*) denticles extend over half of the maxillary/dentary crowns, not restricted to apical third; (5**) dentary caniniform present but apicobasal height does not exceed that of the largest dentary cheek tooth crown; (6**) small, unserrated, peg-like and procumbent tooth present anterior to the dentary caniniform; (7**) distal end of the tibia with anteromedial flange; (8**) apex of the ascending process of astragalus is formed by a separate ossification; (9**) two large foramina pierce anterior surface of the ascending process of astragalus.
(f). Description and comparisons
The three premaxillary teeth are small, subtriangular and expanded transversely and mesiodistally above the root. The second and third crowns are subequal in size to one another and not enlarged relative to the maxillary crowns. In other heterodontosaurids, the premaxillary crowns are conical and unexpanded above their roots, and the posterior crown is enlarged and caniniform (Thulborn 1970; Charig & Crompton 1974; Norman et al. 2004). An arched and recessed diastema for the dentary caniniform is present between the maxilla and the premaxilla, as in Heterodontosaurus (Norman et al. 2004; Butler et al. 2008a) and Tianyulong (Zheng et al. 2009). There is no maxillary caniniform, unlike the condition in Echinodon (Norman & Barrett 2002). The maxillary teeth resemble those of Echinodon and the basal ornithischian Lesothosaurus (Sereno 1991). The crowns are low and triangular, transversely and mesiodistally expanded above the root, possess enamel that is symmetrically distributed on labial and lingual surfaces and show coarse denticles along mesial and distal edges. Close dental packing and systematic tooth-on-tooth wear facets are absent. Unlike other heterodontosaurids (Norman & Barrett 2002), the denticles are not restricted to the apical third of the crown but extend over half of the apicobasal height.
The three partial dentaries, each containing between nine and 11 teeth, are dorsoventrally deepest below the middle teeth of the series, and taper anteriorly. The dentary dentition is heterodont and includes an unserrated anterior caniniform that is similar in size to the largest post-caniniform (‘cheek’) tooth crown. As in other heterodontosaurids, the first cheek tooth is substantially smaller than the succeeding teeth, which are similar in morphology to the maxillary teeth. An unserrated, procumbent, peg-like tooth (‘precaniniform’) is present anterior to the caniniform, a feature absent in other heterodontosaurids and all other ornithischians. Computed tomography (CT) data reveal replacement crowns in all of the maxillae and dentaries (figure 3), indicating active dental replacement (at least until young adulthood, see below). By contrast, replacement teeth are unknown in most other heterodontosaurid specimens, although the ontogenetic and palaeobiological significance of this is uncertain (Butler et al. 2008a).
Overall body proportions of Fruitadens appear to have been similar to those of Heterodontosaurus (Santa Luca 1980), with a forelimb that is short relative to the hindlimb and elongate distal leg bones, suggesting that the slenderly built Fruitadens was primarily bipedal and cursorial. Six sacral vertebrae are present. The appendicular elements show strong similarities to those of Heterodontosaurus (Santa Luca 1980). The articular head of the humerus is enlarged, subspherical and separated from the well-developed and thickened medial tubercle by a distinct notch. The deltopectoral crest is 40 per cent of the length of the humerus, as in Heterodontosaurus, but its anterior projection is less developed. All hindlimb elements are extensively hollowed, to a degree comparable with that seen in small theropod dinosaurs. The femur has a strongly inturned head that is not separated proximally from the anterior and greater trochanters. The fourth trochanter is pendant and rod-like, with a short base as in Heterodontosaurus and Abrictosaurus. The distal tibia does not resemble that of other ornithischians (with the possible exception of Heterodontosaurus in which the interpretation of this area is complicated by fusion with the proximal tarsals): it is only weakly expanded transversely relative to the shaft and is L-shaped in distal view as a result of a prominent anteromedially directed medial flange. The distal fibula is reduced to a very narrow splint and is backed by the lateral malleolus of the tibia. The fused astragalus and calcaneum form a narrow, pulley-like articular surface unlike that of other ornithischians, except Heterodontosaurus (Santa Luca 1980). The proximal part of the high, wedge-shaped ascending process of the astragalus is a separate ossification, as occurs in some basal theropods (Welles 1984; Tykoski 1998), and it articulates medially with the anteromedial flange of the distal tibia. Two prominent foramina pierce the anterior surface in the suture line of the ascending process, as in the basal theropod Dilophosaurus (Welles 1984).
3. Discussion
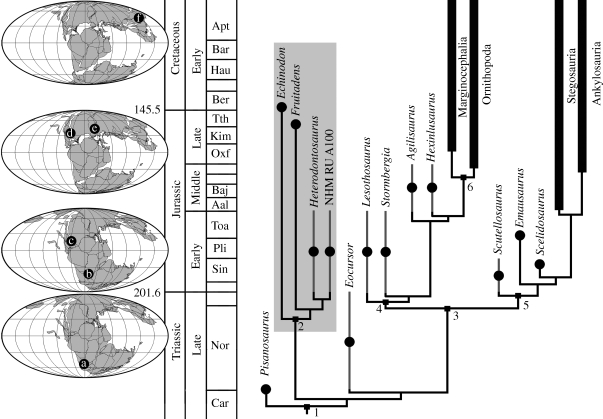
Phylogenetic analysis places Fruitadens within Heterodontosauridae (figure 4 and figures S3–4 in the electronic supplementary material); characters supporting this position include three premaxillary teeth, the presence of an arched and recessed diastema between the premaxillary and the maxillary tooth rows, a groove on the proximal surface of the humerus separating the head from the medial tubercle, a rod-like fourth trochanter, reduction of the distal end of the fibula and the fusion of the astragalus and calcaneum. Relationships within Heterodontosauridae remain poorly resolved, probably owing to both the fragmentary nature of the known material of most members of the clade and genuine character conflict (Butler et al. 2008b); however, Fruitadens appears to be more closely related to H. tucki from the Early Jurassic of South Africa than to Echinodon becklesii from the earliest Cretaceous of England, and shares with the former taxon the possession of a dentary caniniform. The description of the first heterodontosaurid taxon from North America provides further support for the cosmopolitan distribution of this clade (figure 4), now known from parts of Gondwana (Santa Luca 1980; Norman et al. 2004; Butler et al. 2008a) and all of the continents of Laurasia (Norman & Barrett 2002; Zheng et al. 2009).
Figure 4.
Phylogenetic relationships of basal ornithischian dinosaurs (see electronic supplementary material) calibrated to the stratigraphic record. Grey error bars indicate uncertainty in the stratigraphic dating of taxa. Numbers refer to major ornithischian clades: 1, Ornithischia; 2, Heterodontosauridae (highlighted within grey box); 3, Genasauria; 4, Neornithischia; 5, Thyreophora; 6, Cerapoda. Palaeogeographical maps show distribution of heterodontosaurids in the Late Triassic, Early Jurassic, Late Jurassic-earliest Cretaceous and late Early Cretaceous. A, unnamed taxon, Laguna Colorada Formation, Argentina (Báez & Marsicano 2001). B, Heterodontosaurus, Abrictosaurus, Lycorhinus, NHM RU A100, upper Elliot and Clarens formations, southern Africa (Santa Luca 1980; Norman et al. 2004; Butler et al. 2008a). C, undescribed taxon, Kayenta Formation, USA (Norman & Barrett 2002). D, Fruitadens, Morrison Formation, USA. E, Echinodon, Purbeck Formation, England (Norman & Barrett 2002). F, Tianyulong, Yixian Formation, China (Zheng et al. 2009).
Gross anatomical and osteohistological examination of the femur indicates that a referred specimen (LACM 115727), nearly identical in size to the holotype, represents a young adult individual in its fifth year in which growth was beginning to slow (figure 3f and figure S2 in the electronic supplementary material). The cortex of the femur is composed almost entirely of parallel-fibered bone, among the slowest growing bone tissues (Margerie et al. 2002) and typical of diminutive non-avian theropod dinosaurs such as the deinonychosaurian Mahakala (Turner et al. 2007). A smaller individual (LACM 120478), approximately 80 per cent of the size of the larger specimens, is inferred to represent a juvenile in its second year of growth. These data indicate that Fruitadens grew on a par if not slower than, and reached an adult size (approx. 65–75 cm in length and 0.5–0.75 kg body mass; see the electronic supplementary material) comparable with the smallest known dinosaurs, which are characterized as having undergone extreme miniaturization (Turner et al. 2007) just prior to the origin of birds. Indeed, Fruitadens was only slightly larger than the earliest bird, Archaeopteryx (58 cm body length, 0.50 kg body mass: Turner et al. 2007). The Cretaceous heterodontosaurids Echinodon and Tianyulong (Norman & Barrett 2002; Zheng et al. 2009) have been reported as similar in body size to Fruitadens, but precise information on the ontogenetic stage of these taxa is not available.
The absence of well-developed dental wear and the presence of a dentary caniniform and a more anteriorly positioned procumbent peg-like crown are suggestive of omnivory in Fruitadens. Adaptations to herbivory are less well developed than in Heterodontosaurus, which has nevertheless been suggested to represent a facultative omnivore (Barrett 2000; Butler et al. 2008a). As in extant lizards (Pough 1973; Cooper & Vitt 2002), energetic considerations resulting from small body size in Fruitadens (particularly juveniles) may have led to the consumption of some animal matter, although plants probably remained a significant component of the diet. The presence of omnivory in one of the most basal known ornithischian lineages provides further support for the hypothesis that the earliest dinosaur radiation may have been composed largely of facultative omnivores (Barrett 2000).
Early Jurassic heterodontosaurids such as Heterodontosaurus and Lycorhinus possessed sophisticated cranial adaptations to herbivory, including a closely packed and high-crowned dental battery and tooth wear suggesting the presence of a transverse power stroke (Hopson 1980; Crompton & Attridge 1986; Weishampel & Norman 1989; Norman et al. 2004; Porro 2007; Butler et al. 2008a). By contrast, the late-surviving heterodontosaurids Fruitadens, Echinodon and Tianyulong possessed considerably less sophisticated craniodental adaptations, with a plesiomorphic dentition, an inferred orthal jaw motion and an absence of systematic tooth-on-tooth wear. The small body size and possible omnivorous diet of Fruitadens, Tianyulong and Echinodon suggest that they were ecological generalists. This inferred ecological generalism may account in part for the 100 million year long longevity (Late Triassic–late Early Cretaceous: Báez & Marsicano 2001; Zheng et al. 2009) of Heterodontosauridae, which exceeds that of other early dinosaur clades such as prosauropods and coelophysoids. Late-surviving heterodontosaurids possessed less specialized craniodental adaptations than did the earliest known heterodontosaurids: this is in contrast to the evolutionary history of almost all other herbivorous dinosaur groups in which later taxa tend to possess more sophisticated dietary adaptations (Weishampel & Norman 1989).
Acknowledgements
Thanks to Paige Johnson and Samuel McLeod (LACM) for access to specimens of F. haagarorum. We thank Stig Walsh and Richard Abel (NHM) for assistance with CT data, Stephanie Abramowicz (LACM) for photography and illustrations, Ken Womble (FSU) for photography and illustrations for the developmental analysis and Paul Barrett, Roger Benson, Stephen Brusatte, Matthew Carrano, Ben Creisler, Randall Irmis, Marc Jones, Jim Kirkland and Susannah Maidment for useful discussion and information. R.J.B. was funded during the completion of this work by an Alexander von Humboldt Postdoctoral Research Fellowship. The histological research was funded by National Science Foundation grants NSF DBI 0446224 and EAR 04418649 to GME. Thanks to Bill Parker and Adam Yates for their helpful review comments.
References
- Báez A. M., Marsicano C. A.2001A heterodontosaurid ornithischian dinosaur from the Upper Triassic of Patagonia. Ameghiniana 38, 271–279 [Google Scholar]
- Barrett P. M.2000Prosauropod dinosaurs and iguanas: speculations on the diets of extinct reptiles. In Evolution of herbivory in terrestrial vertebrates: perspectives from the fossil record (ed. Sues H.-D.), pp. 42–78 Cambridge, UK: Cambridge University Press [Google Scholar]
- Butler R. J., Porro L. B., Norman D. B.2008aA juvenile skull of the primitive ornithischian dinosaur Heterodontosaurus tucki from the ‘Stormberg’ of southern Africa. J. Vert. Paleontol. 28, 702–711 (doi:10.1671/0272-4634(2008)28[702:AJSOTP]2.0.CO;2) [Google Scholar]
- Butler R. J., Smith R. M. H., Norman D. B.2007A primitive ornithischian dinosaur from the Late Triassic of South Africa, and the early evolution and diversification of Ornithischia. Proc. R. Soc. B 274, 2041–2046 (doi:10.1098/rspb.2007.0367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R. J., Upchurch P., Norman D. B.2008bThe phylogeny of the ornithischian dinosaurs. J. Syst. Palaeontol. 6, 1–40 (doi:10.1017/S1477201907002271) [Google Scholar]
- Charig A. J., Crompton A. W.1974The alleged synonymy of Lycorhinus and Heterodontosaurus. Ann. South Afr. Mus. 64, 167–189 [Google Scholar]
- Cooper W. E., Jr, Vitt L. J.2002Distribution, extent, and evolution of plant consumption by lizards. J. Zool. 257, 487–517 (doi:10.1017/S0952836902001085) [Google Scholar]
- Crompton A. W., Attridge J.1986Masticatory apparatus of the larger herbivores during Late Triassic and Early Jurassic times. In The beginning of the age of dinosaurs (ed. Padian K.), pp. 223–236 Cambridge, UK: Cambridge University Press [Google Scholar]
- Damuth J.1981Population density and body size in mammals. Nature 290, 699–700 (doi:10.1038/290699a0) [Google Scholar]
- Hopson J. A.1980Tooth function and replacement in early Mesozoic ornithischian dinosaurs: implications for aestivation. Lethaia 13, 93–105 (doi:10.1111/j.1502-3931.1980.tb01035.x) [Google Scholar]
- Kirkland J. I.2006Fruita Paleontological Area (Upper Jurassic, Morrison formation), Western Colorado: an example of terrestrial taphofacies analysis. In Paleontology and geology of the Upper Jurassic Morrison Formation (eds Foster J. R., Lucas S. G.), Bulletin vol. 36, pp. 67–95 Albuquerque, NM: New Mexico Museum of Natural History & Science [Google Scholar]
- Kuhn O.1966Die Reptilien, System und Stammesgeschichte Krailling bei München, Germany: Verlag Oeben [Google Scholar]
- Margerie E., de Cubo J., Castanet J.2002Bone typology and growth rate: testing and quantifying ‘Amprino's rule’ in the mallard (Anas platyrhynchos). Compt. Rendus Biol. 325, 221–230 (doi:10.1016/S1631-0691(02)01429-4) [DOI] [PubMed] [Google Scholar]
- Norman D. B., Barrett P. M.2002Ornithischian dinosaurs from the Lower Cretaceous (Berriasian) of England. Spec. Papers Palaeontol. 68, 161–189 [Google Scholar]
- Norman D. B., Sues H.-D., Witmer L. M., Coria R. A.2004Basal Ornithopoda. In The Dinosauria (eds Weishampel D. B., Dodson P., Osmólska H.), pp. 393–412 2nd edn.Berkeley, CA: University of California Press [Google Scholar]
- Owen R.1842Report on British fossil reptiles, part II. Rep. Br. Assoc. Adv. Sci. 1841, 60–294 [Google Scholar]
- Parker W. G., Irmis R. B., Nesbitt S. J., Martz J. W., Browne L. S.2005The Late Triassic pseudosuchian Revueltosaurus callenderi and its implications for the diversity of early ornithischian dinosaurs. Proc. R. Soc. B 272, 963–969 (doi:10.1098/rspb.2004.3047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro L. B.2007Feeding and jaw mechanism in Heterodontosaurus tucki using finite element analysis. J. Vert. Paleontol. 27(Suppl.), 131A [Google Scholar]
- Pough F. H.1973Lizard energetics and diet. Ecology 54, 837–844 (doi:10.2307/1935678) [Google Scholar]
- Royo-Torres R., Cobos A., Alcalá L.2006A giant European dinosaur and a new sauropod clade. Science 314, 1925–1927 (doi:10.1126/science.1132885) [DOI] [PubMed] [Google Scholar]
- Sander P. M., Clauss M.2008Sauropod gigantism. Science 322, 200–201 (doi:10.1126/science.1160904) [DOI] [PubMed] [Google Scholar]
- Santa Luca A. P.1980The postcranial skeleton of Heterodontosaurus tucki (Reptilia, Ornithischia) from the Stormberg of South Africa. Ann. South Afr. Mus. 79, 159–211 [Google Scholar]
- Seebacher F.2001A new method to calculate allometric length-mass relationships of dinosaurs. J. Vert. Paleontol. 21, 51–60 (doi:10.1671/0272-4634(2001)021[0051:ANMTCA]2.0.CO;2) [Google Scholar]
- Seeley H. G.1887On the classification of the fossil animals commonly named Dinosauria. Proc. R. Soc. Lond. 43, 165–171 [Google Scholar]
- Sereno P. C.1991Lesothosaurus, ‘fabrosaurids,’ and the early evolution of Ornithischia. J. Vert. Paleontol. 11, 168–197 [Google Scholar]
- Thulborn R. A.1970The systematic position of the Triassic ornithischian dinosaur Lycorhinus angustidens. Zoo. J. Linn. Soc. 49, 235–245 (doi:10.1111/j.1096-3642.1970.tb00739.x) [Google Scholar]
- Turner A. H., Pol D., Clarke J. A., Erickson G. M., Norell M. A.2007A basal dromaeosaurid and size evolution preceding avian flight. Science 317, 1378–1381 (doi:10.1126/science.1144066) [DOI] [PubMed] [Google Scholar]
- Tykoski R. S.1998The osteology of Syntarsus kayentakatae and its implications for ceratosaurid phylogeny. MSc thesis, University of Texas, TX [Google Scholar]
- Wagner A.1861Neue Beiträge zur Kenntnis der urweltlichen Fauna des lithographischen Schiefers; V. Compsognathus longipes Wagner. Abh. Bayer. Akad. Wissensch. 9, 30–38 [Google Scholar]
- Weishampel D. B., Dodson P., Osmólska H.2004The Dinosauria, 2nd edn.Berkeley, CA: University of California Press [Google Scholar]
- Weishampel D. B., Norman D. B.1989Vertebrate herbivory in the Mesozoic; jaws, plants, and evolutionary metrics. In Paleobiology of the dinosaurs, vol. 238 (ed. Farlow J. O.), pp. 87–100 Boulder, CO: Geological Society of America [Google Scholar]
- Welles S. P.1984Dilophosaurus wetherilli (Dinosauria, Theropoda) osteology and comparisons. Palaeontographica A 185, 85–180 [Google Scholar]
- Xu X., Zhou Z., Wang X.2000The smallest known non-avian theropod dinosaur. Nature 408, 705–708 (doi:10.1038/35047056) [DOI] [PubMed] [Google Scholar]
- Zheng X.-T., You H.-L., Xu X., Dong Z.-M.2009An Early Cretaceous heterodontosaurid dinosaur with integumentary structures. Nature 458, 333–336 (doi:10.1038/nature07856) [DOI] [PubMed] [Google Scholar]