Abstract
Quaternary glacial–interglacial cycles create lasting biogeographic, demographic and genetic effects on ecosystems, yet the ecological effects of ice ages on benthic marine communities are unknown. We analysed long-term datasets to develop a niche-based model of southern Californian giant kelp (Macrocystis pyrifera) forest distribution as a function of oceanography and geomorphology, and synthesized palaeo-oceanographic records to show that late Quaternary climate change probably drove high millennial variability in the distribution and productivity of this foundation species. Our predictions suggest that kelp forest biomass increased up to threefold from the glacial maximum to the mid-Holocene, then rapidly declined by 40–70 per cent to present levels. The peak in kelp forest productivity would have coincided with the earliest coastal archaeological sites in the New World. Similar late Quaternary changes in kelp forest distribution and productivity probably occurred in coastal upwelling systems along active continental margins worldwide, which would have resulted in complex shifts in the relative productivity of terrestrial and marine components of coastal ecosystems.
Keywords: palaeoecology, giant kelp, Macrocystis pyrifera, productivity, holocene, temperate reefs
1. Introduction
During glacial–interglacial transitions, the distribution of habitat-forming terrestrial plants responds directly to changes in glaciation, global air temperatures, aridity and atmospheric concentrations of CO2, especially at temperate and boreal latitudes (Hewitt 2000; Davis & Shaw 2001; Walter & Eperson 2001). For example, at temperate latitudes in North America, the most recent post-glacial warming resulted in an expansion of oak (Quercus) at the expense of spruce (Picea), generating much of the contemporary population genetic and community structure of terrestrial forest ecosystems (Davis & Shaw 2001). Effects of the glacial–interglacial transition on genetic and community structure of these ecosystems have been cross-validated using fossil-pollen records (see examples in Davis & Shaw 2001). Owing to the lack of palaeo-records of the distribution, abundance and productivity of kelps and other marine algae (Graham et al. 2003), such an historical perspective is conspicuously absent for temperate benthic marine systems (but see Fraser et al. 2009 for a recent genetic study of ice age biogeography of a sub-Antarctic kelp).
Temperate kelp forests represent some of the most productive and diverse ecosystems on the planet (Mann 1973; Dayton 1985), owing primarily to the provision of energy and complex habitat by the kelps themselves. Spatial and temporal variability in kelp distribution and abundance can cause variability in the productivity and diversity of kelp-associated communities, with cascading effects on nearby ecosystems and human economies (Dayton 1985). The role of kelps as foundation species is especially apparent along the northeast Pacific coastal margin where diverse kelp forests dominate near shore marine systems (Graham 2004). In the Southern California Bight, present-day populations of the canopy-forming giant kelp Macrocystis pyrifera are the largest in the world, supporting more than 275 common species of macroscopic algae and animals (Graham 2004). Some of the most extensive and detailed evidence for human reliance on kelp forests comes from southern California, where Native American shell middens dating to the early Holocene have been discovered on the mainland and offshore islands (Erlandson 2002; Erlandson et al. 2005). Giant kelp is widely distributed along the offshore islands and mainland of this region, which encompasses the area from Pt. Conception, California to the US–Mexico border, bounded seaward by the outer edge of the continental slope. Despite the relatively high diversity and productivity of the contemporary southern California giant kelp system, however, recent studies indicate late Quaternary fluctuations in population structure of kelp-associated species (Bernardi 2000). Archaeological evidence from southern California also suggests that human interactions with kelp forests varied greatly over the Holocene (Erlandson et al. 2005), with possible consequences for economic diversification, trade, conflict, disease and other fundamental cultural changes (Erlandson 2002). Finally, the detailed palaeo-oceanographic record that has emerged from the varved sediments of the Santa Barbara Basin (Kennett & Ingram 1995) reveals a history of dramatic late Quaternary changes in ocean dynamics and productivity in this region. The Southern California Bight thus offers a rich context of ecological, genetic, archeological and palaeo-climatic information in which responses of kelp forest ecosystems to millennial climate forcing can be examined and interpreted.
In this paper, we reconstruct millennial-scale variability in the distribution and productivity of southern California giant kelp forests since the last glacial maximum (LGM). Kelps (Class Phaeophyceae, Order Laminariales) do not readily fossilize, limiting the application of traditional palaeoecological approaches for reconstruction of giant kelp distributions. Instead, we use niche-based reconstructions that link known environmental constraints of present-day giant kelp forest distribution and productivity (reviewed in Graham et al. 2007) with palaeo-oceanographic records of spatio-temporal variability in key environmental parameters; our implicit (and untestable) assumption is that the response of giant kelp to environmental forcing (i.e. ecophysiology) has remained relatively constant over the last 20 000 years. Using this integrative approach, we show that glaciation may cause variability in kelp forest distribution, abundance and connectivity over a range of spatial scales (tens to hundreds of kilometres) in the Southern California Bight, and estimate the magnitude and timing of this millennial variability. We discuss the impacts of major changes in kelp forest distribution in relation to associated communities, including early maritime peoples.
2. Material and methods
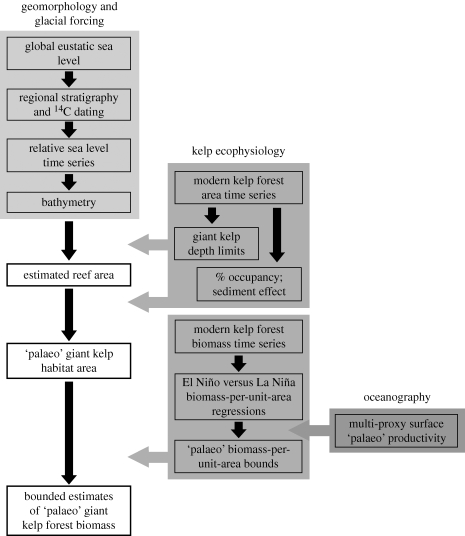
We aimed to reconstruct the past dynamics of southern California kelp forests based upon our understanding of their present-day distribution, dynamics and responses to environmental variation (figure 1). Although kelp forests are highly dynamic at small scales (<10 km), their spatial and temporal distribution at regional to global scales is known to be limited by a suite of environmental factors (Foster & Schiel 1985; Broitman & Kinlan 2006; Reed et al. 2008). The availability of rocky substrates within areas of sufficient irradiance and nutrients is the primary constraint on the distribution and productivity of present-day giant kelp (Macrocystis pyrifera) populations. In southern California, high irradiance requirements for sexual reproduction generally restrict giant kelp populations to depths of less than 25 m, where giant kelp is the competitive dominant throughout its range (Foster & Schiel 1985). Peak giant kelp productivity occurs during seasonal upwelling (Zimmerman & Robertson 1985), when local wind forcing brings deep, nutrient-rich water to the surface. Grazing by sea urchins (Strongylocentrotus purpuratus, S. franciscanus) can also locally limit giant kelp distribution in California (Behrens & Lafferty 2004), although not in the company of the primary urchin predator, sea otters (Enhydra lutris), which were present in the region prior to extirpation by Europeans in the 1700s (Kenyon 1969; VanBlaricom 1988); sea otter and sea urchin remains have been continuously observed in southern California archaeological records from 9800 to 220 years before present (Erlandson et al. 2005). Thus, at least until the regional loss of sea otters, the most important requirement for reconstructing palaeo-kelp forest distribution is a temporal sequence of maps that detail past distributions of rocky substrate, located at depths of less than 25 m.
Figure 1.
Primary methodological steps in the reconstruction of ‘palaeo’ forest areas, giant kelp habitat areas and giant kelp forest biomass. Shaded boxes indicate key geomorphological, ecophysiological and oceanographic inputs used in reconstructions.
The complex physiography of the Southern California Bight is caused by a variety of processes involved with the transformation of a subduction zone into the contemporary San Andreas strike-slip fault system, approximately 40 Myr before present (BP). Geological events episodically modify bathymetric features over short geological time scales (decades to millennia); however, typical rates of these vertical motions (uplift and subsidence) are small relative to that of the most recent sea level transgression (Graham et al. 2003). Therefore, millennial-scale variability in the availability of rocky substrates within 25 m depth can be approximated by superimposing temporal changes in sea level on a present-day high-resolution bathymetric map. We used extensive depth sounding surveys from the United States National Ocean Service (NOS) Hydrographic Survey (1920–2000) to produce such a map for the Southern California Bight (1 m vertical resolution, 100 m horizontal resolution grid, 1 m depth contours). Although the density of soundings declined offshore over deep basins, depth survey data were sufficiently dense in near-shore areas to warrant interpolation at the chosen resolution. All geospatial analyses were performed using ArcView GIS 3.2 and ArcGIS 8.2 (Environmental Systems Research Institute, Redlands, CA, USA).
To estimate changes in available giant kelp habitat as a function of variable sea level, the 0 m datum of the map was sequentially adjusted to reflect changes in sea level since the LGM (approx. 21 500 yr BP), and areas between 0 and 25 m depth were estimated for each 500 yr interval. The best available relative sea level curve for the southern California region was a synthesis from several sea level records (Masters 2006); the Masters (2006) sea level curve was digitized and linearly interpolated to extract sea levels at 500 yr intervals from the LGM to present. Published 14C dates were calibrated to calendar years according to Stuvier & Reimer (1993), Ingram & Southon (1996) and Hughen et al. (2004). The result was a digital reconstruction of late Quaternary changes in the available giant kelp habitat (forest area) for island and mainland regions in the Southern California Bight. To highlight scale and location dependence of kelp forest response to sea level rise, we also discriminated among different regional island groups owing to differences in geomorphology: a northern island group, ‘Santarosae’, consisted of four islands (San Miguel, Santa Rosa, Santa Cruz and Anacapa) that were contiguous during the LGM, whereas a ‘southern’ island group consisted of four islands (San Nicolas, Santa Barbara, Santa Catalina and San Clemente) that were separated from all other islands and from each other by deep water throughout the last glacial–interglacial cycle. To validate use of the 0–25 m depth band as potential kelp habitat, we determined kelp forest occupancy by analysing the contemporary fraction of 0–25 m forest area inhabited by giant kelp using high-resolution infrared aerial surveys conducted by the California Department of Fish and Game, Southern California Edison (data provided by L. Deysher, T. Dean and W. North), and ISP Alginates, Inc. (data provided by D. Glantz and the Santa Barbara Coastal LTER). Surveys occurred quarterly between 1968 and 2002 from Newport Beach, California to the US–Mexico border (32.5° N to 33.6° N; approx. 200 km of coast) and less frequently over the entire region. Infrared aerial photos and digital infrared images were georeferenced using ArcGIS 8.2, a landmask was applied and kelp canopies were classified by a standard vegetative index (Tucker 1979). This process allowed for the production of digital maps of kelp canopy distribution at approximately 2 m spatial resolution with a positional accuracy of approximately 2–10 m. Using a subset of surveys conducted over most or all of our study region (1977, 1978, 1989, 1999 and 2002–2005), we estimated kelp forest area occupancy as the fraction of predicted forest area (i.e. the 0–25 m depth band) in which kelp canopy was observed in at least one aerial survey (i.e. potential kelp habitat).
In addition to the areal extent of kelp populations, kelp forest productivity depends primarily on the biomass density of kelp canopies (Reed et al. 2008), nutrient flux to surface waters (Zimmerman & Robertson 1985) and incident solar irradiance. High-frequency oceanographic warming and cooling events (i.e. El Niño or La Niña) alter nutrient availability and can strongly and rapidly influence near-shore kelp biomass and productivity (Tegner et al. 1997; Dayton et al. 1999); as such, these events may provide a conservative analogue for the response of kelp systems to glacial–interglacial shifts in oceanographic productivity potential. Thus, to estimate variation in kelp biomass owing to fluctuations in area-specific productivity, we combined historical time series (1968–1999) from visual and infrared photographic surveys of (i) peak annual kelp forest canopy area and (ii) peak annual biomass in southern California to develop regressions describing the relationship between canopy area and standing crop (biomass) during different ENSO states (normal, El Niño and La Niña). Annual canopy survey methods were the same as described in the previous paragraph. For each year, the highest kelp canopy surface area was determined for each administrative kelp bed unit designated by the California Department of Fish and Game, to estimate peak annual kelp forest canopy area. ISP Alginates, Inc., a kelp harvesting company in San Diego, California, have surveyed kelp canopy biomass in the same administrative bed units on a monthly basis since 1958. Trained observers made visual determinations of biomass of the kelp surface canopy (defined as 0 to approx. 1 m depth) from fixed-wing aircraft, which were subsequently calibrated to harvest records. Surveys have been carried out continuously using the same methods since 1958; thus the 1968–2002 data were based on 10 years of initial calibration and method development from 1958–1968 (D. Glantz, ISP Alginates, Inc. 2004, personal communication). Diver surveys of whole-water-column kelp forest biomass in southern California subsequently confirmed that canopy biomass correlates well with both whole-forest biomass and annual net primary productivity (Reed et al. 2009). From this record, we selected biomass estimates corresponding to the peak annual kelp forest canopy area for each administrative bed unit to give the peak annual biomass. We then classified each year from 1968 to 1999 as ‘El Niño’, ‘La Niña’ or ‘Normal’ based on the multivariate Southern Oscillation Index (SOI; Pacific Fisheries Environmental Laboratory, http://www.pfeg.noaa.gov/products/PFEL/). We applied a 3-year moving-average filter to monthly values of this index to remove variation at frequencies higher than the ENSO period (approx. 4–7 years), and values were further averaged within each year to match the resolution of the canopy area data. El Niño years were operationally defined as years in which the smoothed, averaged SOI was <−1.5, La Niña years as SOI >1.5 and Normal years as −1.5≤SOI≤1.5. Both variables were square root transformed for normality and linearity, and regressed using a fixed intercept at (0,0). The regression statistics for canopy biomass (in kilogram wet mass) and canopy area (in m2) were as follows. La Niña: √canopy biomass = 1.52010 √canopy area, p < 0.0001, r2 = 0.635. Normal: √canopy biomass=0.99269 √canopy area, p < 0.0001, r2 = 0.603. El Niño: √canopy biomass=0.69908 √canopy area, p < 0.0001, r2 = 0.302.
In addition to biomass, the area-specific productivity of giant kelp strongly depends on the availability of nutrients, particularly nitrate, in shallow surface waters. In present-day southern California waters, nitrate concentration exhibits a strong, consistent relationship with sea temperature (Zimmerman & Robertson 1985; Dayton et al. 1999), and is correlated with the upwelling of cold sub-thermocline water driven by coastal winds. However, over millennial time scales, the supply of nutrients to surface waters also depends on the source of upwelled water. If source water below the photic zone is nutrient-depleted, surface waters may be low in nutrients even during the physical process of coastal upwelling. Thus, to estimate late Quaternary changes in giant kelp productivity potential we chose proxies for three environmental variables: sea surface temperature (SST), presence of nutrient-rich sub-photic water (δ15N) and surface water productivity (%Corg) from the varved sediments of the Santa Barbara Basin, one of the best-known regional records of late Quaternary climate change (Kennett & Ingram 1995). Data from two well-studied sediment cores in the Santa Barbara Channel region, Hole 1017E and Hole JPC76, were used to reconstruct periods of low or high productivity potential since the LGM (SST: Friddell et al. 2003; Hendy et al. 2004; δ15N: Hendy et al. 2004; %Corg: Hendy et al. 2002). These proxies provided independent measures of relative changes in temperature, nutrient delivery and productivity, three processes that are highly coupled at daily to decadal scales but may be decoupled at centennial to millenial and longer time scales. It should be noted that, owing to incomplete burn-down, the most recent 10 000 yr BP of the %Corg record should be considered an upper bound (Hendy et al. 2002). To approximate subsequent variation in kelp productivity, we applied (i) the normal-condition canopy biomass-area regression equation to the reconstructed time series of giant kelp forest areas to predict expected kelp forest canopy biomass; and (ii) either the El Niño or La Niña condition regression equations to predict the lower or upper limits of canopy biomass during the low and high productivity periods, respectively. That is, we bound our predictions with the assumption that millenial-scale conditions favourable to kelp productivity are similar to modern-day La Niña conditions, whereas unproductive conditions are similar to modern-day El Niño conditions.
3. Results
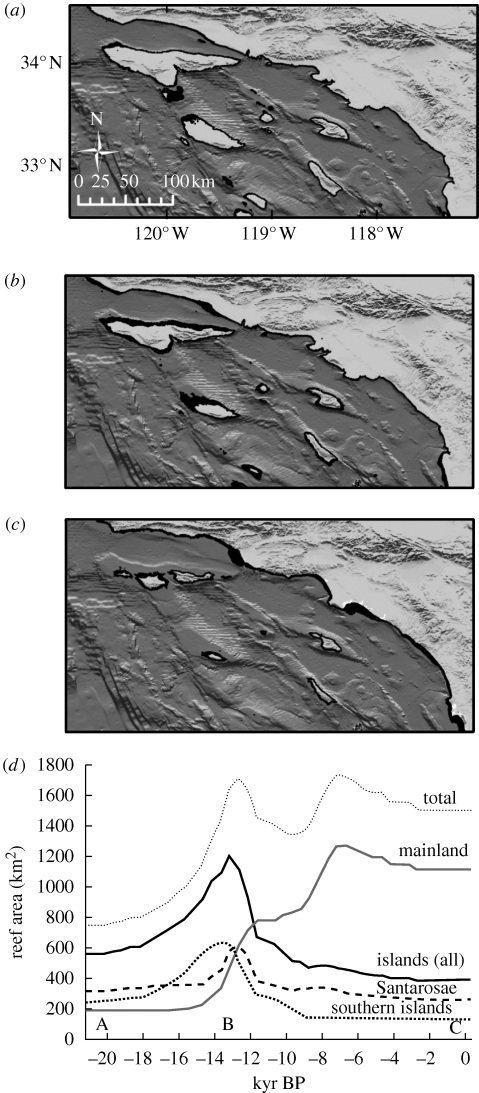
Predicted forest area in the Southern California Bight varied dramatically over the late Quaternary period. Unexpectedly, there were striking differences in the magnitude and timing of peaks in the predicted forested area both among islands and between islands and the mainland. These differences were because of the complex physiography of the region (described above and depicted in figure 2). Island forest area doubled during the first 7000 years of marine transgression, peaking approximately 13 500 yr BP owing to flooding of the broad wave-cut terraces that formerly lined the perimeters of the islands (figure 2). Despite this general increase, however, variability in forest area differed depending on location within the region, even among relatively proximate offshore islands (figure 2d). The principal differences are summarized by comparison of the Santarosae and southern island groups. After reaching a maximum around 14 000 yr BP that was 250 per cent above LGM forest area, southern island forest area decreased by 80 per cent through the end of the Younger Dryas cool period (approx. 9000 yr BP), and gradually thereafter, because of a rapid decrease in island perimeter. Santarosae island forest area reached a more recent maximum around 12 500 yr BP, 190 per cent above LGM forest area, owing to the flooding of an emergent marine terrace. This was followed by a decrease of 60 per cent by approximately 11 000 yr BP, when the sublittoral zone around the large aggregate island fragmented into the four present-day islands (figure 2b,c). The predicted size and distribution of present-day island forests closely matches known giant kelp distributions based on remote sensing surveys (86% concordance at 10 m resolution).
Figure 2.
(a–c) Late Quaternary variation in the distribution and area of southern California shallow regions (0–25 m; depicted in black) capable of supporting giant kelp forests (Macrocystis pyrifera). (a) Last glacial maximum (21 500 yr BP, (b) peak island reef area (13 500 yr BP) and (c) present (0 yr BP). (d) Time series of 0–25 m inhabitable forest areas for total (islands+mainland), mainland, all southern California islands, and Santarosae and southern island groups. Increments are 500 yr, continuous lines represent linear interpolations between data increments. Letters indicate time periods corresponding to maps (a–c).
In contrast to the islands, predicted mainland forest area gradually increased to a mid-Holocene (7500 yr BP) maximum 670 per cent above LGM levels (figure 2d). Predicted mainland forest area overestimated known mainland giant kelp distributions (only 22% concordance) owing to large sediment deposits offshore of the present-day southern California mainland that originated as sea level stabilized during the late Holocene around 4000–6000 yr BP (Kinlan et al. 2005); similar sediment deposition was much rarer around most of the arid offshore islands.
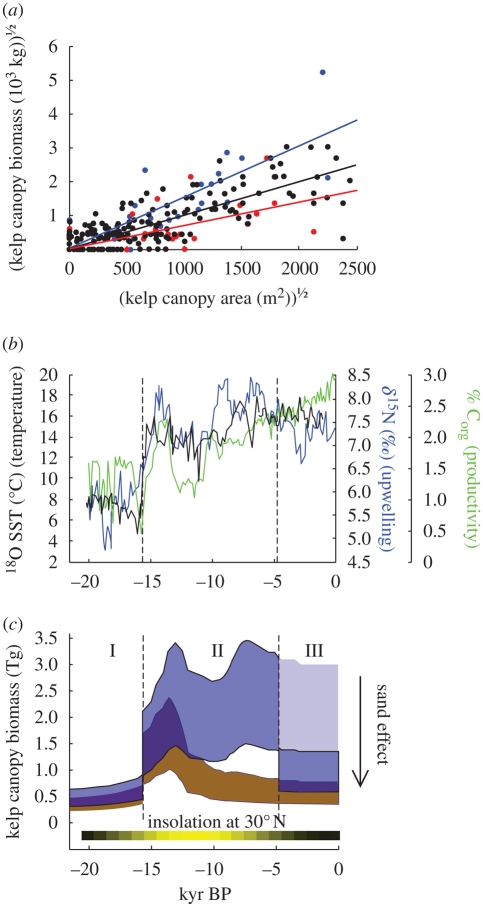
The strong response of modern-day kelp forest biomass to ocean climate fluctuation suggests that the effects of glacial–interglacial transitions on southern California kelp systems go beyond the simple redistribution of habitat and organisms. Analysis of southern California kelp canopy area and biomass records from 1968 to 2002 reveals predictably higher biomass density during periods of high productivity potential (La Niña) versus low productivity potential (El Niño; figure 3a), presumably owing to nutrient deficiency during El Niño events (Zimmerman & Robertson 1985; Tegner et al. 1997). Furthermore, recently published palaeo-oceanographic records (Hendy et al. 2002, 2004; Friddell et al. 2003) for the last 20 000 years reveal an abrupt shift in late Quaternary oceanographic productivity potential, from cold unproductive conditions at the LGM to warmer, more productive conditions from 15 600 yr BP to present (figure 3b). The low productivity potential of the LGM may be analogous to long-term El Niño conditions (Ortiz et al. 2004).
Figure 3.
Reconstruction of kelp forest productivity (change in biomass) since the last glacial maximum. (a) Annual maxima in giant kelp canopy biomass (wet weight, kg) versus giant kelp canopy surface area (m2) in southern California under prevailing El Niño (red), La Niña (blue) and Normal ocean climates (black). (b) Palaeo-oceanographic conditions in the Southern California Bight: sea surface temperature (SST; black), upwelling (δ15N; blue) and biological productivity (% organic carbon; green; value for most recent 10 000 yr BP is an upper bound). Left vertical reference line at 15 600 yr BP indicates the approximate timing of the Bolling-Allerod warming and marks the beginning of the high productivity period, whereas right vertical reference line at 4750 yr BP marks the approximate onset of mainland sand inundation. (c) Late Quaternary variation in giant kelp canopy biomass (wet weight, Tg) for southern California islands (brown) and total (islands+mainland; blue). Period I indicates low-productivity conditions; periods II and III indicate high-productivity conditions, before and after the beginning of mainland sand inundation. The large arrow and shading during period III indicates total giant kelp canopy biomass after removal of the ‘sand effect’ (70% reduction equivalent to modern difference in kelp occupancy between islands and mainland). At the bottom is an index of Northern Hemisphere solar insolation (Berger & Loutre 1991), with yellow as highest (520 W m−2) and black as lowest (460 W m−2).
The small inhabitable forest areas during the first 5500 years of marine transgression coincided with relatively poor oceanographic productivity, limiting potential increases in kelp biomass relative to LGM levels. Interestingly, the 13 500 yr BP peak in both island and mainland inhabitable forest area occurred after the oceanographic transition to more productive conditions around 15 600 yr BP, leading to a rapid increase in predicted kelp biomass to 150–325 per cent of LGM levels, dominated by large island-based kelp forests. This enhancement of kelp forest productivity probably lasted through most of the Holocene, although the dominant source of kelp biomass would have shifted from islands to the mainland around 11 000 yr BP. This maximum in kelp forest biomass was interrupted only by the mainland transition to a sand-dominated system approximately 4000–6000 yr BP (figure 3c; Kinlan et al. 2005), whereby mainland forests were probably inundated by sand, restricting the size of the available kelp habitat. We note that, although the direct relationship between solar insolation and kelp productivity is unknown, the pre- to mid-Holocene peak in kelp forest biomass coincides with the peak in late Quaternary solar insolation at 30° (figure 3c; Berger & Loutre 1991), which would probably have further enhanced kelp productivity during this period.
Finally, it is challenging to synthesize data records that span multiple temporal and spatial scales and that were collected with different levels of accuracy. Consequently, our results incorporate a degree of inherent error owing to the compounding of data records. Although we could not characterize the extent of such errors, two main sources are likely: (i) spatial errors created during the formation of the bathymetric map, which would affect interpretation of distribution and abundance patterns of kelp canopy/forest; and (ii) errors owing to temporal inaccuracies in the oceanographic and sea level records, which would primarily affect the timing of changes in reef area and kelp canopy biomass. The high spatial resolution of the bathymetric map and temporal resolution of the oceanographic and sea level records, however, suggest that subsequent errors would be small relative to the conspicuous predicted shifts in kelp forest reef area (hundreds of square kilometres) and the timing of such shifts (thousands of years). The generality of our conclusions does not hinge on finer-scale patterns. Instead, our aim is to represent broad-scale (>1 km) changes in the long-term (more than centennial) average distribution of kelp forest biomass.
4. Discussion
The overall pattern emerging from these reconstructions is one of climate-driven redistribution of coastal kelp forest resources since the LGM. Near the LGM, most inhabitable kelp forest areas existed around large offshore islands, due primarily to the steepness of the mainland coast during the low sea level stand. As such, the islands probably represented focal points for the aggregation of kelp forest organisms, similar to the glacial refugia of terrestrial systems (Hewitt 2000). With the transition to interglacial conditions, the productivity of these kelp systems probably increased owing to changes in oceanography, again similar to that of temperate forests (Davis & Shaw 2001); this pattern was because of an increase in upwelling despite increased warming of surface waters, evidenced by independent palaeo-oceanographic proxies of temperature and productivity. However, after the first 7500 years, the size of the island forests decreased rapidly, and they fragmented and increased in isolation distance, opposite to the commonly observed pattern of expansion and coalescence of terrestrial glacial refugia (Hewitt 2000).
Our reconstructions predict high fragmentation and recent isolation of Santarosae island giant kelp forests relative to their southern counterparts as a result of marine transgression. In contrast, coalescence of Santarosae island components would occur during regressive seas. Repetition of this pattern with each glacial–interglacial cycle (approx. 100 000 yr frequency) could result in greater genetic homogeneity among Santarosae island group populations of kelp forest organisms in comparison with southern ones. Southern Californian black surfperch (Embiotoca jacksoni), a near shore reef fish that lacks a pelagic larval stage and thus has limited ability to disperse among islands, exhibit a phylogeographic pattern consistent with this expectation (Bernardi 2000). Mitochondrial control-region sequences from populations in the southern California mainland and each of the eight present-day islands showed low average pairwise divergence (0.3%) and haplotype diversity (0.58) among the Santarosae island group relative to the southern islands (0.97 and 0.86%, respectively). Thus, similar to many terrestrial systems (Hewitt 2000), climate-forced habitat fragmentation/coalescence cycles may provide a useful framework for interpreting genetic signals in the structure of temperate marine species.
The predicted response of marine foundation species to glacial–interglacial fluctuations has important implications not only for predicting the long-term effect of past and future climate change on global distribution of biodiversity and productivity, but also the flux of resources across the land–sea interface to human and other communities. For instance, the redistribution of kelp forest resources implies that early human populations on the northern and southern islands (colonized by approx. 12 000 and 9000 yr BP, respectively; Erlandson 1994) experienced a 50–80 per cent decrease in the availability of kelp forest-based resources by the end of the mid-Holocene. Erlandson et al. (2005) showed a decline in the remains of sea urchins (Strongylocentrotus purpuratus, S. franciscanus) and red and black abalone (Haliotis cracherodii, H. rufescens) in island middens over the last 8000 years, suggesting decreased availability of these species owing to either decreasing kelp productivity (Graham 2004) or fishing (or both). Such changes could have contributed to an intensification and diversification of Native American fisheries evident on the California islands during the late Holocene (Kennett 2005).
Recent work in terrestrial palaeoecology has highlighted the strong regional variation of ecosystem responses to climate change. Our reconstructions of late Quaternary southern California kelp forest distribution reveal how different habitats within a region may exhibit distinct responses to climate forcing. Ecosystems at the interface between habitats, such as coastal areas, may therefore exhibit particularly complex responses to millennial-scale climate change. These responses can be traced to interactions and feedbacks among forcing processes in the atmosphere (precipitation, alongshore winds), ocean (sea level rise, nutrient flux) and lithosphere (uplift, subsidence, physiography, sediment deposition).
We have focused on the Southern California Bight because it supports the most productive, extensive and well-studied modern kelp forests in the world (Foster & Schiel 1985; Dayton et al. 1999; Graham et al. 2007) and, because of the availability of detailed bathymetric and oceanographic data, an excellent palaeo-oceanographic record (Kennett & Ingram 1995), and a wealth of archeological data documenting human use of kelp forest resources throughout the Holocene (Erlandson 2002; Erlandson et al. 2005; Kennett 2005). At present this combination of data does not exist for any of the other major kelp forest systems of the world. However, because the processes leading to kelp forest redistribution are general, glacial–interglacial cycles may have driven similar major fluctuations in kelp forest distribution and productivity in coastal upwelling systems worldwide. Moreover, the response of global kelp forest ecosystems to sea level change is probably characterized by strong regional signatures owing to differences in the physiography of coastal margins, geological processes that determine relative sea level and sediment deposition, and oceanographic processes that influence productivity. This point is supported by the extreme variability of present-day near-shore physiography among major coastal upwelling regions inhabited by canopy-forming kelps, with bathymetric profiles that contain unique features likely to influence palaeo-forest areas, including high or low relief shoals and offshore islands. The dependence of kelp forest responses to post-glacial change on non-linear bathymetries implies that near-shore benthic marine ecosystems generally will not follow the same post-glacial trajectories of abundance, productivity, diversity and connectivity as adjacent terrestrial or pelagic marine habitats. Although the impact of a predicted 1 m rise in future sea levels will probably be minimal on relatively linear temperate coastlines, the non-linear bathymetries of coastal islands may result in more conspicuous impacts to insular kelp forests; using our bathymetric map, predicted losses of kelp forest habitat in the northern Channel Islands are approximately 6 per cent for a 1 m sea level rise.
The decoupling of marine and terrestrial responses to sea level rise implies large changes in the relative importance of marine versus terrestrial production in coastal ecosystems over glacial–interglacial cycles. Flux of marine productivity across the coastal interface during periods of low terrestrial resource availability could alter patterns of coastal diversity and community structure as it does in arid coastal regions today (Polis & Hurd 1996), and may also have facilitated expansion of human settlements into otherwise unfavourable regions, including such fundamental events in human cultural evolution as the crossing from Asia to North America (Erlandson 2002; Erlandson et al. 2007). Given their continuing importance as centres of human economies, populations and impacts, the complexity of coastal ecosystem responses to global climate change, past and present, represents an important avenue of emerging research.
Acknowledgements
We thank the University of California Faculty Fellowship Program (M.H.G.) and the Fannie and John Hertz Foundation (B.P.K.) for funding, D. Glantz (ISP Alginates, Inc.), D. Reed and the Santa Barbara Coastal LTER (NSF OCE no. 9982105) for archiving historical kelp biomass data, the California Department of Fish and Game, Southern California Edison and L. Deysher for providing GIS maps of kelp canopy surveys, B. Turner, E. Weinberg, D. Silva and S. Smith for GIS support, and P. Masters, D. Inman, J. Erlandson, J. Estes, R. Steneck, K. Coale, L. Ferry-Graham, E. McPhee-Shaw and G. Bernardi for various discussions in support of this study.
References
- Behrens M. D., Lafferty K. D.2004Effects of marine reserves and urchin disease on southern Californian rocky reef communities. Mar. Ecol. Prog. Ser. 279, 129–139 (doi:10.3354/meps279129) [Google Scholar]
- Berger A., Loutre M. F.1991Insolation values for the climate of the last 10 million years. Quat. Sci. Rev. 10, 291–317 (doi:10.1016/0277-3791(91)90033-Q) [Google Scholar]
- Bernardi G.2000Barriers to gene flow in Embiotoca jacksoni, a marine fish lacking a pelagic larval stage. Evolution 54, 226–237 [DOI] [PubMed] [Google Scholar]
- Broitman B. R., Kinlan B. P.2006Spatial scales of benthic and pelagic producer biomass in a coastal upwelling ecosystem. Mar. Ecol. Prog. Ser. 327, 15–25 (doi:10.3354/meps327015) [Google Scholar]
- Davis M. B., Shaw R. G.2001Range shifts and adaptive responses to Quaternary climate change. Science 292, 673–679 (doi:10.1126/science.292.5517.673) [DOI] [PubMed] [Google Scholar]
- Dayton P. K.1985Ecology of kelp communities. Annu. Rev. Ecol. Syst. 16, 215–245 (doi:10.1146/annurev.es.16.110185.001243) [Google Scholar]
- Dayton P. K., Tegner M. J., Edwards P. B., Riser K. L.1999Temporal and spatial scales of kelp demography: the role of oceanographic climate. Ecol. Monogr. 69, 219–250 [Google Scholar]
- Erlandson J. M.1994Early hunter-gatherers of the California coast New York, NY: Plenum [Google Scholar]
- Erlandson J. M.2002Anatomically modern humans, maritime voyaging, and the Pleistocene colonization of the Americas. In The first Americans (ed. Jablonski N. G.), pp. 59–92 San Francisco, CA: Memoirs of the California Academy of Sciences [Google Scholar]
- Erlandson J. M., Rick T. C., Estes J. A., Graham M. H., Braje T. J., Vellanoweth R. L.2005Sea otters, shellfish, and humans: 10 000 years of ecological interaction on San Miguel Island, California. Proc. Calif. Isl. Symp. 6, 58–68 [Google Scholar]
- Erlandson J. M., Graham M. H., Bourque B. J., Corbett D., Estes J. A., Steneck R. S.2007The kelp highway hypothesis: marine ecology, the coastal migration theory, and the peopling of the Americas. J. Isl. Coast. Arch. 2, 161–174 (doi:10.1080/15564890701628612) [Google Scholar]
- Foster M. S., Schiel D. R.1985The ecology of giant kelp forests in California: a community profile. US Fish Wildl. Serv. Biol. Rep. 85, 1–152 [Google Scholar]
- Fraser C. I., Nikula R., Spencer H. G., Waters J. M.2009Kelp genes reveal effects of subantarctic sea ice during the last glacial maximum. Proc. Natl Acad. Sci. USA 106, 3249–3253 (doi:10.1073/pnas.0810635106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friddell J. E., Thunell R. C., Guilderson T. P., Kashgarian M.2003Increased northeast Pacific climatic variability during the warm middle Holocene. Geophys. Res. Lett. 30, 1560–1568 (doi:10.1029/2002GL016834) [Google Scholar]
- Graham M. H.2004Effects of local deforestation on the diversity and structure of southern California giant kelp forest food webs. Ecosystems 7, 341–357 (doi:10.1007/s10021-003-0245-6) [Google Scholar]
- Graham M. H., Dayton P. K., Erlandson J. M.2003Ice ages and ecological transitions on temperate coasts. Trends Ecol. Evol. 18, 33–40 (doi:10.1016/S0169-5347(02)00006-X) [Google Scholar]
- Graham M. H., Vásquez J. A., Buschmann A. H.2007Global ecology of the giant kelp Macrocystis: from ecotypes to ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 45, 39–88 [Google Scholar]
- Hendy I. L., Kennett J. P., Roark E. B., Ingram B. L.2002Apparent synchroneity of submillennial scale climate events between Greenland and Santa Barbara Basin, California from 30–10 ka. Quat. Sci. Rev. 21, 1167–1184 (doi:10.1016/S0277-3791(01)00138-X) [Google Scholar]
- Hendy I. L., Pedersen T. F., Kennett J. P., Tada R.2004Intermittent existence of a southern Californian upwelling cell during submillennial climate change of the last 60 kyr. Paleoceanography 19, 3007–3012 (doi:10.1029/2003PA000965) [Google Scholar]
- Hewitt G.2000The genetic legacy of the Quaternary ice ages. Nature 405, 907–913 (doi:10.1038/35016000) [DOI] [PubMed] [Google Scholar]
- Hughen K. A., et al. 2004Marine 04 marine radiocarbon age calibration, 0–26 cal kyr BP. Radiocarbon 46, 1059–1086 [Google Scholar]
- Ingram B. L., Southon J. R.1996Reservoir ages in eastern Pacific coastal and estuarine waters. Radiocarbon 38, 573–582 [Google Scholar]
- Kennett D. J.2005The island Chumash: behavioral ecology of a maritime society Berkeley, CA: University of California Press [Google Scholar]
- Kennett J. P., Ingram B. L.1995A 20 000-year record of ocean circulation and climate change from the Santa Barbara Basin. Nature 377, 510–512 (doi:10.1038/377510a0) [Google Scholar]
- Kenyon K. W.1969The sea otter in the eastern Pacific Ocean. N. Am. Fauna 68, 1–352 [Google Scholar]
- Kinlan B. P., Graham M. H., Erlandson J. M.2005Late-Quaternary changes in the size and shape of the California Channel Islands: implications for marine subsidies to terrestrial communities. Proc. Calif. Isl. Symp. 6, 119–130 [Google Scholar]
- Mann K. H.1973Seaweeds: their productivity and strategy for growth. Science 182, 975–981 [DOI] [PubMed] [Google Scholar]
- Masters P. M.2006Holocene sand beaches of southern California: ENSO forcing and coastal processes on millennial scales. Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 73–95 (doi:10.1016/j.palaeo.2005.08.010) [Google Scholar]
- Ortiz J. D., O'Connell S. B., DelViscio J., Dean W., Carriquiry J. D., Marchitto T., Zheng Y., van Geen A.2004Enhanced marine productivity off western North America during warm climate intervals of the past 52 ky. Geology 32, 521–524 (doi:10.1130/G20234.1) [Google Scholar]
- Polis G. A., Hurd S. D.1996Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am. Nat. 147, 396–423 (doi:10.1086/285858) [Google Scholar]
- Reed D. C., Rassweiler A., Arkema K. K.2008Biomass rather than growth rate determines variation in net primary production by giant kelp. Ecology 89, 2493–2505 (doi:10.1890/07-1106.1) [DOI] [PubMed] [Google Scholar]
- Reed D. C., Rassweiler A., Arkema K. K.2009Density derived estimates of standing crop and net primary production in the giant kelp Macrocystis pyrifera. Mar. Biol. 156, 2077–2083 (doi:10.1007/s00227-009-1238-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuvier M., Reimer P. J.1993Extended 14C database and revised CALIB 3.0 14 C age calibration program. Radiocarbon 35, 215–230 [Google Scholar]
- Tegner M. J., Dayton P. K., Edwards P. B., Riser K. L.1997Large-scale, low-frequency oceanographic effects on kelp forest succession: a tale of two cohorts. Mar. Ecol. Prog. Ser. 146, 117–134 (doi:10.3354/meps146117) [Google Scholar]
- Tucker C. J.1979Red and photographic infrared linear combinations for monitoring vegetation. Rem. Sens. Environ. 8, 127–150 (doi:10.1016/0034-4257(79)90013-0) [Google Scholar]
- VanBlaricom G. R.1988Effects of foraging by sea otters on mussel-dominated intertidal communities. In The community ecology or sea otters (eds VanBlaricom G. R., Estes J. A.), pp. 48–91 Berlin, Germany: Springer-Verlag [Google Scholar]
- Walter R., Epperson B. K.2001Geographic pattern of genetic variation in Pinus resinosa: area of greatest diversity is not the origin of postglacial populations. Mol. Ecol. 10, 103–111 (doi:10.1046/j.1365-294X.2001.01177.x) [DOI] [PubMed] [Google Scholar]
- Zimmerman R. C., Robertson D. L.1985Effects of El Niño on local hydrography and growth of the giant kelp, Macrocystis pyrifera, at Santa Catalina Island, California. Limnol. Oceanogr. 30, 1298–1302 [Google Scholar]