Abstract
While the broad framework of deuterostome evolution is now clear, the remarkable diversity of extant forms within this group has rendered the nature of the ancestral types problematic: what, for example, does the common ancestor of a sea urchin and lamprey actually look like? The answer to such questions can be addressed on the basis of remarkably well-preserved fossils from Cambrian Lagerstätten, not least the celebrated Chengjiang Lagerstätte (Yunnan, China). This deposit is particularly important because of its rich diversity of deuterostomes. These include some of the earliest known representatives, among which are the first vertebrates, as well as more enigmatic groups, notably the vetulicolians and yunnanozoans. The latter groups, in particular, have been the subject of some radical divergences in opinion as to their exact phylogenetic placements. Here, we both review the known diversity of Chengjiang deuterostomes and in particular argue that the vetulicolians and yunnanozoans represent very primitive deuterostomes. Moreover, in the latter case we present new data to indicate that the yunnanozoans are unlikely to be any sort of chordate.
Keywords: Chengjiang, deuterostomes, yunnanozoans, vetulicolians, Cambrian, metazoan evolution
1. Introduction
Among the many trenchant points Darwin made in his epochal Origin was his observation that it was a futile exercise to attempt to envisage ancestral forms simply on the basis of the inspection of their descendants (Darwin 1860): organisms change, and often almost beyond recognition. Even 150 years after the publication of the Origin, this insight seems not always to be fully appreciated. Moreover, it gathers a special force when we come to consider the nature of the Cambrian fossil record. Nowhere is this more apparent than with respect to the material from the Chengjiang Fossil-Lagerstätte, as well as comparable deposits such as the Sirius Passet and the canonical Burgess Shale. Thus while the extraordinary wealth of soft-bodied material has provided new vistas into the nature of the Cambrian explosion, it has also presented a series of major evolutionary challenges. This is most obvious with respect to the interpretations of ostensibly enigmatic taxa (Gould 1989; Conway Morris 2003).
Looking back over the investigations in this area during the last decade it appears that a very interesting polarization has emerged. Broadly, there seem to be two approaches. While understandable, arguably, these serve to cripple further investigation. The first is to compare a given taxon, as closely as possible, to one or other of the extant phyla, or even some major group within a given phylum. One such example will be examined in more detail below, specifically the assignment of the yunnanozoans to craniates (Mallat & Chen 2003). This we will argue represents a ‘shoe-horning’, inconsistent with both our prior observations and new evidence (see also Shu et al. 2003a,b; Conway Morris 2006). In such a schema, therefore, the fossils that are all agreed certainly possess enigmatic features and are fitted to a template of what seem to be prior expectations. As the subtitle of the article by Mallat & Chen (2003) proclaims: ‘Predicted and Found’. In principle, such an exercise is necessary if any fossil is to be correctly attributed, but in the case of not only the yunnanozoans but also arguably other groups such as the halkieriids (Vinther & Nielsen 2005), in reality, the process runs a real risk of being procrustean. Structure X looks (sometimes very approximately) similar to structure Y, therefore X and Y must be the same.
The alternative approach seems more ecumenical, but arguably leads to an even greater degree of intellectual paralysis. Under this schema, there is an underlying scepticism that any feature in a fossil group is phylogenetically reliable. The consequence is that the taxon in question is either bundled into the wasteland of ‘extinct phyla’ (which thereby renders it largely immune to any sensible analysis) or it is compared with a plethora of other forms. Here too such an approach is not necessarily illogical. Any biologist can think of major transformations of body form that, in the absence of adequate knowledge (all too frequent in the fossil record), would appear to be remote from what in reality are closely related forms (and in at least some cases we now know involve trivial genetic changes). Among extant biotas at lower taxonomic levels, such examples are commonplace, but so too do investigators enjoy access to molecular phylogenetic data and usually much better sampling of their diversity. In the case of the fossil record, however, the identification of supposed homologies may seem more similar to guess work, at least to the outsider. Indeed, this problem is related to the even more serious danger of homoplasy: yes, two structures may look quite similar but if not recognized as convergent this will lead to a series of entirely erroneous placements. Combine this with what typically is a paucity of characters and/or ones found in an ‘unexpected’ (even ‘bizarre’) combination (again a more frequent occurrence in the early diversification of major groups than is perhaps realized), then phylogenetic analysis may face the possibility of total collapse (Conway Morris 1991). In principle, just such a case could apply to another group of Cambrian animals that we discuss below, the vetulicolians.
Accordingly, this polarization of approaches represents two endpoints: those of triumphalist certainty versus radical scepticism. Ironically, both are essential in any scientific endeavour. Paradoxically, we have to believe in something, but must equally keep our minds open to the possibility that we may be gloriously misinformed. And it is from this perspective that we will attempt to look at the early record of the deuterostomes. The present state of play needs little introduction. Deuterostomes appear to be monophyletic (Philippe et al. 2005; Bourlat et al. 2008; Dunn et al. 2008), but they show a remarkable diversity of forms, ranging from pelagic holothurians to elephants, and colonial tunicates to graptolites. The broad framework of deuterostome relationships also seems to be secure. Thus, two major clades are identified. First are the Ambulacraria that comprise the hemichordates and echinoderms (e.g. Bromham & Degnan 1999; Bourlat et al. 2008; see also Swalla & Smith 2008), along with the otherwise enigmatic xenoturbellids (e.g. Fritzsch et al. 2008). Second are the Chordata, which encompass the cephalochordates, tunicates (or urochordates) and craniates (or vertebrates). In some of these groups, notably among the echinoderms (e.g. Mallatt & Winchell 2007; see also Swalla & Smith 2008) and tunicates (e.g. Yokobori et al. 2005; Zeng et al. 2006; see also Swalla & Smith 2008), relationships are apparently robust. That, however, is the exception rather than the rule. For example, within the hemichordates the paraphyly or otherwise the pterobranchs to enteropneusts remains uncertain (but see Mallatt & Winchell 2007), while the potential trichotomy within the three chordate groups continues to generate considerable discussion (see Stach 2008).
The contribution of the fossil record to these debates is variable. In the obvious case of the vertebrates and the echinoderms, each well-mineralized, the geological history is good (if not excellent), but in the remaining groups it is either only locally informative (as with the graptolites) or very sparse. Moreover, when we approach the base of the deuterostome tree then at least from a palaeontological perspective matters become controversial. Nor is this surprising. To our eyes, many of the forms have bizarre anatomies and even when attributable to a known group, as for example the peculiar stylophorans are to the echinoderms, they still provoke discussion. Moreover, the fossils can be rare, are sometimes incomplete, and are assigned to clades such as vetulicolians, vetulocystids and yunnanozoans, which are alien concepts to the great majority of biologists.
Here, from the perspective of the Chengjiang material, we provide a brief overview of early deuterostome relationships. It summarizes many years work in Xi'an and Cambridge, and as before, it comes to some markedly different conclusions from those reached by other groups of investigators. We concentrate in some detail on the controversial interpretations of the phylogenetic relationships of the strange-looking vetulicolians and yunnanozoans. We make no apology for this, and for two reasons. First, because we query the claim by Swalla & Smith (2008) that is just because there are disagreements of interpretation, this somehow makes the identification of soft-tissue structures hopelessly tenuous. There may be difficulties, but as indicated above in terms of philosophy of approach any interpretation is driven by prior, and perhaps unavoidable, assumptions. Ideally, we need to know which characters are primitive, but as in other areas of science, circularities are a constant pit-fall and we may need to be content with the working hypotheses. Also while we entirely agree that the palaeontological interpretations must rely on phylogenies that employ neontological (and especially molecular) data, the obvious scepticism expressed by Swalla & Smith (2008) as to the status of the vetulicolians and yunnanozoans, has the risk of shutting the door on what we suggest could be central insights into the nature of primitive deuterostomes. New fossils will certainly modify all current positions, but here we propose that an overall framework of understanding is already in position.
2. The earliest deuterostomes: vetulicolians
To date there is no consensus as to the appearance of the first deuterostomes, and even their position in the wider scheme of metazoan phylogeny (e.g. Philippe et al. 2005; Bourlat et al. 2008; Dunn et al. 2008) provides us with few useful clues. Perhaps the only point of general agreement is that the animal possessed pharyngeal openings equivalent to gill slits. This could imply some sort of enlarged head. If so, conceivably, the body was bipartite (so echoing an earlier suggestion of Romer 1972) and the nervous systems may have been diffused. Given that the extant xenoturbellans may represent basal deuterostomes (Fritzsch et al. 2008), in this context their diffuse nerve net may well be significant (e.g. Stach et al. 2005), but their remarkable simplicity renders the xenoturbellans morphologically uninstructive when it comes to envisaging further steps in the evolution of early deuterostome. In fact, we have proposed (Shu et al. 2001b; Shu 2005) that the vetulicolians are currently the best candidates for the earliest deuterostomes, but given the very peculiar nature of these animals, unsurprisingly, this has proved to be controversial. Moreover, even though the geographical range (Butterfield 2005) and diversity of this group (e.g. Chen et al. 2003a,b; Shu 2005; Caron 2006) are now known to be quite considerable, with the range of forms strongly pointing towards a monophyletic identity, their very coherence seems to have rendered them less informative as to their possible wider relationships.
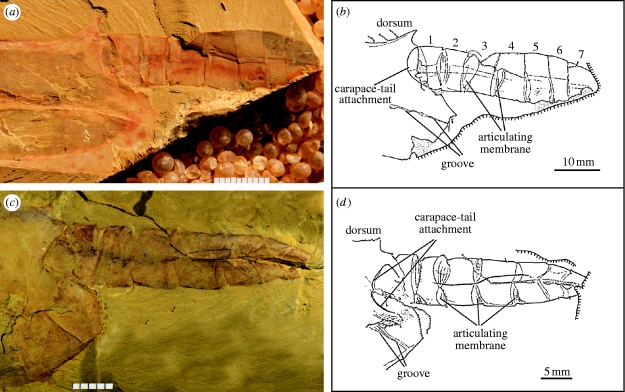
As has long been realized, the vetulicolians, and especially Vetulicola, have some striking similarities to the arthropods (e.g. Hou 1987; Caron 2001), notably a carapace-like anterior and a prominent segmented tail with arthrodial-like membranes (figure 1 a–d). The description of Skeemella clavula from the Middle Cambrian of Utah as a putative vetulicolian (Briggs et al. 2005), but which is even more arthropod-like than Vetulicola itself, might serve to support an assignment to this phylum. Skeemella, however, is only known from a single specimen and its affinities to the vetulicolians must be regarded as provisional (Shu 2005). While one can always argue that the diversity and evolutionary capacities of the Cambrian arthropods exceed our present expectations, two things need to be observed at this juncture. First, the impressive advances in our understanding of early arthropod evolution (e.g. Budd 2002; Chen et al. 2008; Harvey & Butterfield 2008; Hendricks & Liebermann 2008; Liu et al. 2008; cf Caron 2006) provide schemes of phylogeny into which the vetulicolians cannot easily be accommodated. Second, material of Vetulicola is abundant (thousands of specimens in at least five Chinese collections) and the capacious carapace-like structure is not only frequently filled with sediment, but variously broken open, and as has often been observed neither jointed appendages nor eyes have been identified. In addition, and apparently yet more fatal to the arthropod hypothesis, are the five prominent structures along the midline of either side. These structures were interpreted as gills with external openings by Shu et al. (2001b) (see figure 1 in the electronic supplementary material), leading to their conclusion that vetulicolians were early deuterostomes.
Figure 1.
Vetulicola cuneata: possible stem-group Deuterostomia. (a–d) Details of the posterior body. ELI-0000301, posterior of body to show (a) articulation of the tail, (b) with camera-lucida interpretation; ELI-0000302, (c) tail in approximately ventral orientation; note absence of fin, (d) with camera-lucida interpretation. All scale bars millimetric. Abbreviation in this and figure 2: ELI, Early Life Institute, Northwest University, Xi'an, China.
(a). Vetulicolian anatomy
In an overview of the morphology and possible relationships of the vetulicolians, this interpretation was effectively accepted by Aldridge et al. (2007). Their paper appears, however, to contain some misunderstandings of our interpretation (Shu et al. 2001b), and also arrives at conclusions on a number of aspects of vetulicolian anatomy that are in conflict with our observations. With regard to the identification of the gill slits, it first needs to be observed that while the majority of specimens show the associated external rhombic structures, termed lappets by Shu et al. (2001b; see figure 1c in the electronic supplementary material), relatively few reveal any details of the structure of the gills (and when they do, most usually, it is as filaments, see figure 1a,d in the electronic supplementary material; see also, e.g. Aldridge et al. 2007, Pl. 3, fig. 8; Text-fig. 3, Chen 2004, fig. 499). Moreover, those specimens that do show more or less complete details from the interior (see figure 1b in the electronic supplementary material; also Shu et al. 2001b, Figs. 3k,l, 4b–d) and exterior (see figure 1 a,c,d in the electronic supplementary material; also Shu et al. 2001b, Figs. 3e–j, 4e; see also Shu 2008, fig. 7B,C) are extremely rare (and also require appropriate excavation). This point may not have been appreciated by Aldridge et al. (2007) given that they wrote: ‘It is not clear how water was dispelled … in Vetulicola there appears to be a single longitudinal slit, with each of the internal pouches closed off by a rhombic covering … The specimens illustrated by Shu et al. (2001b, fig. 3g–i) do appear to show elliptical openings surrounded by fine filaments, although the published photographs are unclear; these openings are being viewed from the inside and there is no evidence as to their external expression’ (p. 152, our italics).
In order to clarify our interpretation, it may be useful first to stress that even from an examination of the illustrations in Aldridge et al. (2007, especially Pl. 1, fig. 10; see also Pl. 1, fig. 9; Pl. 2, figs. 6, 10; Pl. 3, fig. 7), and indeed those provided in earlier publications (e.g. Chen & Zhou 1997, figs. 134, 135; Chen 2004, figs. 496, 497; see also Shu et al. 2001b, fig. 4f), it is misleading to say that the rhombic coverings (or lappets) ‘cover’ the openings; rather they flank them (and the associated groove; see figure 1c in the electronic supplementary material). Most likely, they served as cuticular thickenings to support this area. Second, we earlier identified ‘exhalant openings’ (see figure 1a,c,d in the electronic supplementary material), and these were also illustrated in explanatory camera-lucida drawings (for a similar example see also Chen 2004, fig. 497B). These openings lie within the groove, immediately beneath the lappets, have an oval configuration and are sediment-filled (see figure 1c,d in the electronic supplementary material; a feature that in other vetulicolians Aldridge et al. (2007) employ in the description of the gill slits; see in particular their text-figs. 7 and 8). Also given that beneath these openings the gill structures form conspicuous internal pouches and have what appear to be anteriorly directed apertures (see figure 1b in the electronic supplementary material; interpreted as inhalant by Shu et al. 2001b; fig. 3k,l), then on the existing evidence we see no reason to revise our overall reconstruction of these complex structures. In passing, we should also note that the remark by Aldridge et al. (2007) that on the carapace ‘the lateral grooves do not extend to the posterior edge’ (p. 134) is incorrect. Although relatively subdued, a definite discontinuity can be traced to the posterior margin (figure 1 a–d; see also Chen & Zhou 1997, fig. 134; Chen 2004, fig. 497A). This further emphasizes how being composed of four plates, the anterior section differs in arrangement from any known arthropodan carapace.
All authors agree that the tail section of Vetulicola (and indeed other taxa such as Didazoon (Shu et al. 2001b, fig. 1a,d) and presumably Banffia (Caron 2006)), housed a gut, sometimes with a prominent infill that on occasion is strikingly coiled. Aldridge et al. (2007, p. 152), however, also tentatively identify a ‘notochord’ (see also Swalla & Smith 2008, p. 1561). If correct, this would be of considerable importance in terms of not only a deuterostome relationship, but specifically suggest that Vetulicolia might be better regarded as a tunicate (Lacalli 2002; see also Gee 2001). It is, however, not clear what relevance an elastic rod, for such is the basic construction of the notochord, would have in the context of a vetulicolian tail whose articulation seems unlikely to require a myotomal-like arrangement. As these workers also note, to judge from the broad inter-segmental spaces (evidently housing the equivalent of the arthrodial membranes) separating the first three segments, the greatest degree of flexibility lay in the proximal region (figure 1 a,b; see also Chen 2004, fig. 500). The suggestion, however, that these segments could concertina (Aldridge et al. 2007) is functionally problematic. In addition, the articulation between the carapace and tail is across a large hemispherical articulation (figure 1 a,b), and the tail occurs in a wide degree of attitudes varying from steeply downwards to gently upwards (Aldridge et al. 2007; see also Chen & Zhou 1997, fig. 136). In the more distal segments, narrower intersegmental boundaries suggest a limited degree of individual movement, consistent with the propulsive stroke being concentrated in the distal region.
Aldridge et al. (2007) proposed that in life the prominent fin was symmetrical and deployed horizontally, suggesting that its generally asymmetrical appearance was the result of a ‘twisting’ of the distal region (see their Pl. 1, figs. 1, 2). Examination of other material, however, does not appear to support this conclusion. In this respect one specimen (figure 1 c,d) is particularly instructive, especially with regard to its tail segments. The specimen is evidently obliquely buried because the inter-segmental membrane that serves to separate segments 1 and 2, does not show the normal lensoid arrangement (figure 1 a,b), but rather is displaced upwards and its opposite number (transversely wrinkled presumably because of the angle of burial) is visible towards the lower side of the tail. As is to be expected, this confirms that in life, the intersegmental membranes were bilaterally disposed with the membranes narrowing towards the midlines. Presumably, in this specimen, we have a ventral view, and as one moves distally, this bilateral disposition of the intersegmental membranes becomes more obvious (with the last two membranes being orientated forward; again consistent with the reduced flexibility of the distal tail). Also, note that in this specimen, the tail has a narrower aspect, whereas in lateral view the tail is relatively broad, as indeed is the laterally compressed carapace (Shu et al. 2001, fig. 4g). The crucial point is that in such a specimen (figure 1 c,d) this is exactly the orientation in which a horizontally deployed tail would be the most obvious. Its absence provides, therefore, strong support for its having an originally vertical orientation, and this would also be consistent with the greatest flexibility of the tail being in a lateral direction. Indeed, given the lensoid configurations of the anterior inter-segmental membranes as seen in lateral preservation, it is difficult to see how the propulsive force could have been in an up and down direction (although as noted the articulations around the tail clearly allowed some movement in this direction). There is also an unremarked dimorphism (possibly sexual) in as much as the first unit of the fin may arise on either the third (figure 1 a,b, Aldridge et al. Pl. 2, figs 1, 7) or fourth segment (Aldridge et al. 2007, Pl. 1, figs. 1, 3; see also Chen 2004, fig. 500), although in each case the tail has a total of seven segments.
Segmented tails characterize all the other vetulicolians, although the differences (most obviously expressed in Banffia (Caron 2006) and Heteromorphus (see Aldridge et al. 2007)) suggest a variety of functions that await detailed investigation. They also have taxonomic implications. Thus Aldridge et al. (2007) synonymized Xidazoon (Shu et al. 1999a,b, 2001) with Pomatrum, remarking ‘there are indeed very few differences between the type specimens of the type species of these two genera’ (p. 145). While there are certainly similarities, there are also obvious differences (Shu et al. 2001b, p. 421). Thus, in Pomatrum the tail is narrower (especially in the holotype [sic] of P. cf ventralis (Aldridge et al. 2007, Pl. 5, figs. 1, 2; text-fig. 8)), has conspicuously more segments (see also Aldridge et al. Pl. 4, figs. 6, 8), and its point of insertion into the carapace appears to be more dorsally situated. In addition, the anterior-most gill of Xidazoon is conspicuously larger. In our opinion, these differences would warrant generic differentiation.
(b). Vetulicolian relationships
What, therefore, of the wider relationships of the vetulicolians? The possibilities are reviewed at length by Aldridge et al. (2007), but despite their wide-ranging survey, the net results are somewhat inconclusive. Nor is this surprising given the uncertainties of character homologies that underpin this cladistic analysis, most obviously ‘segmentation’ (their character 4), which is probably convergent between protostomes and deuterostomes. More significant, however, are the identification of character states in the vetulicolians themselves. Thus, as we have seen ‘Lateral slit not reaching the posterior end of the anterior body’ (their character 32) is not valid in Vetulicola, nor it is possible to see how it can be scored as ‘present’ in Pomatrum, Didazoon (and Xidazoon; see above) given that the gills form discrete, isolated pores along the length of the anterior body. Similarly, reference to the body of Vetulicolia being twisted along its axis (character 3) is not supported by the evidence presented above (nor indeed is it evident in at least Didazoon and Xidazoon). The difficulties of such a cladistic exercise will be apparent, but in the quest to find a secure home for this group, Aldridge et al. (2007) have left few phylogenetic stones unturned. Accordingly, while we agree with their scepticism as to any arthropodan affinity, their proposal of a possible affinity to the kinorhynchs deserves brief mention. Three principal synapomorphies are mentioned, but two are surely open to question. Thus, ‘Muscle bands around the body’ (Aldridge et al. 2007, p. 157; character 25) is too generalized to be useful, and not only takes little account of the actual musculature of the kinorhynchs (e.g. Kristensen & Higgins 1991, pp. 394–397), but needs to be assessed in the light of the radically different body-plan of these interstitial and highly segmented animals. So too, the shared character of bifid terminations of kinorhynchs and Skeemella presuppose the latter is a vetulicolian (see above), but even if this was the case, it seems to be a rather minor character to employ in such a phylogenetic context. Aldridge et al. (2007), however, give their principal emphasis to an ‘oral disc’. Now, although kinorhynchs have a ‘mouth surrounded by a circlet of plates’ (character 18), there is no evidence that the mouth plates in Xidazoon (and close relatives) were cuticularized in the manner of the kinorhynch placids (see Adrianov & Malakhov 1996, fig. 2). Indeed, the organizational states of these circum-oral structures in either group suggest the similarity is more likely to be superficial. Nor, of course, do the vetulicolians have any structure that corresponds to the kinorhynch introvert.
We conclude (Shu et al. 2001b; Conway Morris & Shu 2003; Shu 2005, 2006, 2008) that placing the vetulicolians in the deuterostomes remains the best hypothesis, a conclusion that is followed by many workers (Chen et al. 2003a; Benton 2005; Luo et al. 2005, Steiner et al. 2005) and the one ultimately reached by Aldridge et al. (2007). But where precisely in this group? As already noted the barrel-like anterior and segmented tail of some vetulicolians invite comparison to the tunicates (Gee 2001; Lacalli 2002), either in the form of the larvae or as the adult appendicularians. Aldridge et al. (2007) address some of the difficulties with this hypothesis, but offer support on the basis of the supposition of a twisted tail in Vetulicola, which as already observed is not supported by our observations. These authors also invoke the possible presence of a notochord. As noted, however, not only is there no evidence for any such structure in Vetulicola, but more importantly its functional context in an appendage that evidently operated in a manner very much similar to that of an arthropod is problematic. Moreover, in as much as tunicates form part of the chordate trichotomy, the accommodation of the vetulicolians in these controversial phylogenies (Stach 2008) does not appear straightforward. We argue, therefore, that not only are vetulicolians deuterostomes, but existing evidence is more consistent with their having a basal position. In discussing this particular possibility Aldridge et al. (2007) engage in what seems to be effectively a circular argument. Thus, they propose that ‘a bipartite body, segmentation, gill slits, a differentiated gut (and) a stiffened body wall’ are characters ‘that must have developed along the deuterostome stem lineage before any advent of vetulicolians’ (p. 159) without providing a reason why vetulicolians themselves fail to qualify as this stem-group lineage.
3. Another cambrian headache: the yunnanozoans
If vetulicolians are controversial, then so too are the yunnanozoans (figure 2 a–f, see figure 2c,d in the electronic supplementary material). Unlike the former group where affinities as diverse as kinorhynchs and tunicates have been proposed, in the case of the yunnanozoans (represented by Haikouella and Yunnanozoon, and differing most obviously in the nature of the gills) the dichotomy of opinion is stark. On the one hand there is the craniate interpretation (Mallat & Chen 2003; see also Chen et al. 1995, 1999; Chen 2004), but by contrast there is the proposal that they are more primitive, with affinities to the vetulicolians (Shu et al. 2003a, Shu & Conway Morris 2003, see also Steiner et al. 2005, p. 148) and possibly the stem-group ambulacrarians (Shu et al. 2004; Shu 2005, 2008). In the case of the vetulicolians, all agree (Shu et al. 2001b; Aldridge et al. 2007) that the key argument concerns the gill slits, whose full identity is only evident in a small number of exceptionally preserved specimens (in palaeontology by no means an unfamiliar occurrence). The case of the yunnanozoans is somewhat different. Here, we have an abundance of well-preserved material, but the same structures are identified in a diametrically opposed fashion. Thus, while both schools identify the head region, what Mallat & Chen (2003) refer to as a massive ‘mandibular artery’ is reconstructed by us as a skirt-like arrangement supported by a dorsal cuticular bar that recurves around the anterior end and then dips downward on either side to define an oral cavity. At first sight, this dichotomy of interpretations would seem to support the complaint of Swalla & Smith (2008) that progress is effectively impossible because all views are too tenuous to allow any sensible discussion. Here, we present new evidence that suggests a way forward.
Figure 2.
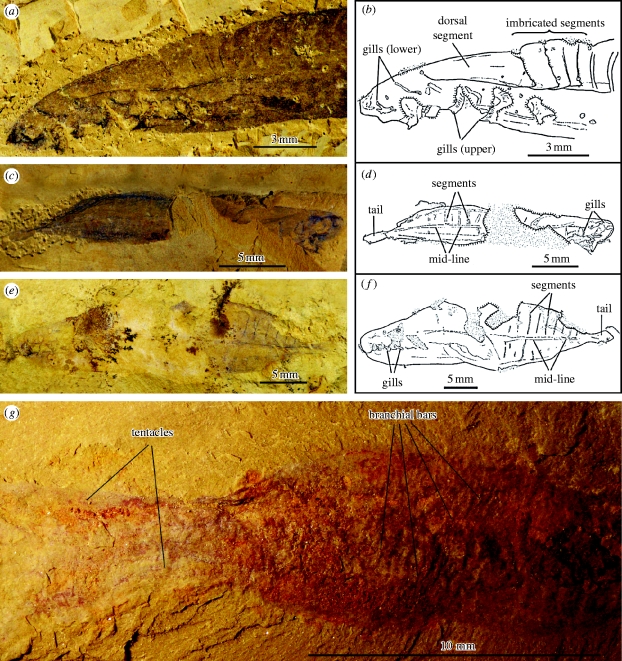
(a–f) Yunnanozoans: possible stem-group Ambulacraria and (g) a shankouclavid; ELI- EC-016, anterior of Haikouella, showing (a) details of dorsal segmentation including obvious rotation and imbrication of first four segments, (b) with camera-lucida interpretation; ELI-EC-021, dorsally preserved Haikouella with (c) tail and straight trunk segments separated by midline, (d) with camera-lucida interpretation; NWU93-1418, dorsally preserved Yunnanozoon with (e) tail and straight trunk segments separated by midline, (f) with camera-lucida drawing; (g) ELI-2005-SK-001, note the similarity of the body to Shankouclava (see Chen et al. 2003a,b), with possible branchial structures but also distal tentacles. All scale bars millimetric.
(a). Do yunnanozoans possess myomeres?
Our approach here is to choose one key feature, specifically the prominent dorsal segments, and to demonstrate that contrary to Mallat & Chen (2003; see also Chen 2004) these structures have no discernible similarity to any known myomere. That the identification of myomeres is central to the proposed chordate status of Yunnanozoon is unequivocally spelt out by Chen & Li (1997) when they write ‘The recognition of myosepta is one of the most critical pieces of evidence for a euchordate affinity for [Yunnanozoon]’ (p. 265). Thus, we believe it reasonable to suggest that on this point alone the craniate hypothesis can either stand or fall. Nor do we repeat our earlier criticisms (Shu et al. 1996a, 2003a,b; Conway Morris 2006; Shu 2005, 2006, 2008), but present new data.
While the great majority of yunnanozoan specimens are preserved laterally, presumably because of compression of the original body, occasionally material is orientated dorsally in both Yunnanozoon (figure 2 e,f) and Haikouella (figure 2 c,d). Such specimens look relatively unfamiliar, but in both cases, their identity is confirmed by the gills. Both the specimens discussed here are fusiform and although one of Haikouella is incomplete, it evidently has a narrower cross section. This, along with the much more prominent gills than those possessed by Yunnanozoon would be consistent with a more active mode of life, including faster locomotion through the water. In addition, in both specimens the tail is spatulate (rhombic in Yunnanozoon, more elongate in Haikouella) and apparently separated from the trunk by a slight constriction. This is presumably equivalent to the ‘caudal process’ (Chen et al. 1999, fig. 4b) or ‘tail’ (Mallat & Chen 2003; see also Chen 2004, fig. 542), but its orientation and shape are unlike any known chordate. Nor is there any evidence that it is some sort of isocercal tail that has been rotated. This conclusion is supported both on the basis of the specimens illustrated here, and also the reasonable assumption that such a characteristic outline would be more readily identifiable in at least some of the much more numerous laterally preserved specimens. More important is the fact that in these dorsoventral specimens, the segments are transverse, and show no sign of the classic v-shaped terminations that characterize all myomeres. In addition, note that the segments do not join, but are separated by a median zone. In passing, we should also note that this is very unlikely to be any sort of notochord given it has a position that is far too dorsal.
This median separation (figure 2 c–f) strongly suggests, therefore, that these segments and presumably the associated musculature form effectively separate blocks. Some additional evidence for this supposition comes from a specimen previously illustrated (see Chen et al. 2003a, fig. 1c; Shu 2003, fig. 3h), where the two sides of the trunk are seen to be clearly displaced. To the best of our knowledge such a feature has never been observed in other soft-bodied chordates from Chengjiang (e.g. Shu et al. 1999a,b; Shu 2003; see also Zhang & Hou 2004) and seems to be difficult to reconcile with a myotomal construction. Nor do the problems stop there. Consider the anterior-most segments (figure 2 a,b). This has a distinctive triangular shape, again to the best of our knowledge unlike any known myomeral arrangement (e.g. Shu 2003b; Conway Morris 2006). It has also been pointed out (e.g. Conway Morris 2006) that unlike any known chordate the segments evidently had a cuticular covering (with self-evident wrinkles; see also Mallat & Chen 2003, fig. 10). Striking confirmation of this cuticular composition comes from a specimen (figure 2 a,b) where the four most anterior segments are clearly imbricated and also rotated anti-clockwise; such characteristic would not be expected in any myomere. We do not, therefore, regard the rejection of the myomere hypothesis as ‘tenuous’; at least four lines of evidence (segments that are dorsally transverse not chevron, comprise laterally isolated units, have a cuticular exterior, and are capable of rotation) support it. The role, therefore, of the structure identified as a notochord (Chen et al. 1999; Mallat & Chen 2003) is necessarily problematic, as well as being difficult to reconcile with the extreme curvature of the body (e.g. Smith et al. 2001). This is echoed by Valentine (2004) who in his magisterial overview on the origin of phyla concludes that ‘The presence of a notochord [in yunnanozoans] now seems unlikely’ (p. 417).
(b). The yunnanozoan head
Other arguments against placing the yunnanozoans in the chordates have been rehearsed elsewhere. Nevertheless, it is important to stress that the large number of specimens and their exquisite preservation give some confidence to reconstructions. Thus, the head structure consists of a dark bar (presumed to be cuticular) which is oval to semicircular in dorsal view (Chen et al. 1999, fig. 4a; Mallat & Chen 2003, fig. 6; Shu et al. 2003, 3G–J), but evidently recurved ventrally and supporting a skirt-like structure (Shu et al. 2003, figs. 3A–F). Chen and co-workers see the same structure, but identify it as a massive blood vessel (either anterior branchial (Chen et al. 1999) or mandibular (Mallat & Chen 2003)) that occupies about a fifth of the head. All are agreed, therefore, as to the configuration of this relatively complex structure, but the interpretations are obviously divergent. Nevertheless, in a way that echoes our conclusions as to the non-myomeral nature of the dorsal segments, we are unable to see any feature in this well-defined anterior structure that is similar to any known chordate. So too, all are agreed that the mouth is enclosed, but to refer to ‘upper and lower lips’ presupposes a chordate relationship. Again transmuting a recurved cuticular bar into a specific chordate character (that is blood vessels) is, we suggest, based on prior expectations. In the large collections available to us, we also find no convincing evidence of eyes (a conclusion also reached by both the various visitors to our laboratory in Xi'an, and workers in other institutions). So too, we regard the evidence for nostrils (Mallat & Chen 2003, fig. 7) as inconclusive.
The one point on which all are agreed is the presence of gills, and it is that almost alone that underpins a deuterostome relationship. Mallat & Chen (2003) reconstruct them on a chordate model, but fortunately, the arrangement of the gills can be inferred to some degree of accuracy from the fact that the specimens are typically buried in several discrete levels. Not only does this allow ready distinction between left and right gills, but our evidence indicates that they were external to at least the bulk of the body (which included a relatively capacious pharyngeal cavity), occurring as they do on a distinct layer of sediment (see especially fig. 2G, H in Shu et al. 2003; figure 2 a,b). Accordingly, to label them as branchial bars, etc (Mallat & Chen 2003) seems speculative, as does the proposal that there were associated hearts. In conclusion, while we accept a deuterostome affinity for these curious animals, we propose that the evidence points to a more basal position. One possibility, connected to more effective locomotion, is that the bipartite body plan seen in the vetulicolians was modified so that the transition to a yunnanozoan involved the posterior region ‘advancing’ dorsally over the anterior gill-bearing section. As previously noted some tentative support for this comes from a specimen of Yunnanozoon where the dorsal segments are largely detached from the more ventral unit that extends to the anterior (see figure 2c,d in the electronic supplementary material; see also Shu et al. 1996a, figs. 1h, 2e; Shu et al. 2001b, fig. 6, Shu 2008), suggesting that structurally these two areas were distinct.
4. An overview of the other Chengjiang deuterostomes
Given the phylogenetic importance of the vetulicolians and yunnanozoans, and current controversies surrounding their interpretations, this area must remain the focus of our review. Nevertheless, it is important to stress that the overall diversity of the deuterostomes is impressive. In the electronic supplementary material, we provide an analysis of current data, but here we briefly review four key topics:
The vetulicystids (see figure 2a,b in the electronic supplementary material) are at present interpreted as pre-echinoderms (Shu et al. 2004; Shu 2008). If correct, they would be ancestral to key innovations in the echinoderm body plan, notably the diagnostic calcitic stereom and water-vascular system. So too, the curious asymmetrical arrangement of the vetulicystids finds a counterpart in the pre-radial echinoderms.
The best candidate for a cephalochordate is Cathaymyrus (Shu et al. 1996b); its interpretation as a crushed specimen of Yunnanozoon (Chen & Li (1997)) seems unlikely.
With respect to the tunicates Cheungkungella (see figure 3d in the electronic supplementary material) is known from a single specimen (Shu et al. 2001a). It is morphologically comparable to extant examples, and although partly similar to Phlogites lacks tentacles. In this context, although widely accepted as a tunicate, the discovery of a new shankouclavid (figure 2 g), combined with a re-examination of the original specimens of Shankouclava (Chen et al. 2003a,b), suggests that this taxon possessed tentacle-like structures. If correct this suggests that the shankouclavids are unlikely to be tunicates.
Vertebrates are represented by Myllokunmingia fengjiaoa, Haikouichthys ercaicunensis and Zhongjianichthys rostratus (see figure 4 in the electronic supplementary material). They show a number of convincing features, including branchial structures, eyes, myomeres and a notochord. These three taxa are distinctive, and Myllokunmingia is unlikely to be synonymous with Haikouichthys (Shu et al. 1999b, 2003b; Shu 2003).
5. Conclusions
Work on the Chengjiang Fossil-Lagerstätte continues to be pursued actively by several groups. We can be sure that new finds will modify, although we hope not overturn, the conclusions reached here (see also Halanych 2004). Nevertheless, we suggest that the evidence continues to point to the primitive status of the vetulicolians and yunnanozoans within the deuterostomes, and in particular the difficulties in accommodating the latter group in the much more advanced craniates as typified by the unequivocal Chengjiang agnathans. So far as early deuterostomes are concerned, Chengjiang very much remains the lodestar for future prospects. The large new collections of the Burgess Shale made by the Royal Ontario Museum have yielded numerous additional specimens of Pikaia, but apparently no new deuterostomes other than a possible ambulacrarian (Caron J.-B., Conway Morris, S. & Shu D.-G. In preparation). So too Metaspriggina walcotti has been redescribed as a chordate, possibly of agnathan grade (Conway Morris 2008), but it is only known from two specimens. To date the third most important Burgess Shale-type fauna, the Sirius Passet assemblage from North Greenland, has not yielded any unequivocal deuterostome material. Yunnanozoans, vetulicystids and myllokunmingids remain unique to Chengjiang, and although Vetulicola is recorded from the Lower Cambrian of Canada (Butterfield 2005), the material is relatively fragmentary and to date has not necessitated any re-thinking of existing hypotheses.
With respect to the critiques offered here, while we may be sure that they will be subject to further scrutiny, at least with respect to the vetulicolians (and their gill structures) and yunnanozoans (and their segmentation), we believe our observations can only lead to further constructive dialogue. There is, moreover, a more general point that emerges from these divergences of interpretation. Although Aldridge et al. (2007) and Mallat & Chen (2003) approach the respective questions of the affinities of vetulicolians and yunnanozoans from the two perspectives outlined at the beginning of this article, both emphasize the importance of character states for cladistic analysis. As a methodology, cladistics no doubt cannot be faulted, but the reality as it pertains to early deuterostome evolution for the present remains more problematic. As indicated, in a number of cases (and others could be included), the identification of a character in its supposed phylogenetic context is open to question. These, of course, are matters for continued discussion. There is, however, a more general point that may escape note. Body plans emerge by transformation, and although some characters will be de novo, in other cases one will transform into another (scales into feathers, etc). If, for example, a bipartite animal with a large anterior (bearing a gill slit) and a segmented tail known as a vetulicystid was to transform into a bipartite animal with a large anterior (bearing a gill slit) and a segmented tail, but with the mesodermal novelty of stereom, we would not be surprised. Whether such a transformation, of profound evolutionary importance, is amenable to current cladistic analyses is less certain.
Acknowledgements
We are grateful to Profs J. Rong, X. Xu and Z. Luo inviting us to present this work. Thanks are due to two referees for their valuable and insightful comments. Vivien Brown, M.-R. Cheng and J.-P. Zhai are thanked for their assistance in secretarial and laboratory work. This work is supported by the Natural Science Foundation of China (grant nos. 40830208, 40332016, 40602003, 40702003), MOST Special Fund from the State Key Laboratory of Continental Dynamics in Northwest University, Ministry of Science and Technology of China (2006CB806401), Cowper-Reed Fund and St John's College, Cambridge in the UK.
Footnotes
One contribution to a Special Issue ‘Recent advances in Chinese palaeontology’.
References
- Adrianov A. V., Malakhov V. V.1996The phylogeny and classification of the class Kinorhyncha. Zoosyst. Ross 4, 23–44 [Google Scholar]
- Aldridge R. J., Hou X.-G., Siveter D. J., Siveter D. J., Gabbott S. E.2007The systematics and phylogenetic relationships of vetulicolians. Palaeontology 50, 131–168doi:10.1111/j.1475-4983.2006.00606.x [Google Scholar]
- Benton M. J.Vertebrate palaeontology. 3rd edn.2005Oxford, UK:Blackwell [Google Scholar]
- Bourlat S. J., Nielsen C., Economou A. D., Telford M. J.2008Testing the new animal phylogeny: a phylum level analysis of the animal kingdom. Mol. Phylogenet. Evol 49, 23–31doi:10.1016/j.ympev.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Briggs D. E. G., Lieberman B. S., Halgedahl S. L., Jarrard R. D.2005A new metazoan from the Middle Cambrian of Utah and the nature of the Vetulicolia. Palaeontology 48, 681–686doi:10.1111/j.1475-4983.2005.00489.x [Google Scholar]
- Bromham L. D., Degnan B. M.1999Hemichordates and deuterostome evolution: robust molecular phylogenetic support for a hemichordate + echinoderm clade. Evol. Dev 1, 166–171doi:10.1046/j.1525-142x.1999.99026.x [DOI] [PubMed] [Google Scholar]
- Budd G. E.2002A palaeontological solution to the arthropod head problem. Nature 417, 271–275doi:10.1038/417271a [DOI] [PubMed] [Google Scholar]
- Butterfield N. J.2005Vetulicola cuneata from the lower Cambrian Mural Formation, Jasper National Park, Canada. Palaeontol. Assoc. Newsl 60, 17 [Google Scholar]
- Caron J.-B.2001The limbless animal Banffia constricta from the Burgess Shale (Middle Cambrian, Canada), a stem-group arthropod?. Paleobios 21, Suppl. 239 [Google Scholar]
- Caron J.-B.2006Banffia constricta, a putative vetulicolid from the Middle Cambrian Burgess Shale. Trans. R. Soc. Edinb. Earth Sci 96, 95–111 [Google Scholar]
- Chen J.-Y.Dawn of animal life 2004Nanjing, China:Jiangsu Science and Technical Press; [In Chinese.] [Google Scholar]
- Chen J.-Y., Li C.-W.1997Early Cambrian chordate from Chengjiang, China. Bull. Natl Mus. Nat. Sci. Taiwan 10, 257–273 [Google Scholar]
- Chen J.-Y., Zhou G.-Q.1997Biology of the Chengjiang fauna. Bull. Natl Mus. Nat. Sci. Taiwan 10, 11–105 [Google Scholar]
- Chen J.-Y., Dzik J., Edgecombe G. D., Ramsköld L., Zhou G.-Q.1995A possible early Cambrian chordate. Nature 377, 720–722doi:10.1038/377720a0 [Google Scholar]
- Chen J.-Y., Huang D.-Y., Li C.-W.1999An early Cambrian craniate-like chordate. Nature 402, 518–522doi:10.1038/990080 [Google Scholar]
- Chen A.-L., Feng H.-Z., Zhu M.-Y., Ma D.-S., Ming L.A new vetulicolian from the early Cambrian Chengjiang fauna in Yunnan of China. Acta Geol. Sin 77, 2003a281–287 [Google Scholar]
- Chen J.-Y., Huang D.-Y., Peng Q.-Q., Chi H.-M., Wang X.-Q., Feng M.The first tunicate from the early Cambrian of South China. Proc. Natl Acad. Sci. USA 100, 2003b8314–8318doi:10.1073/pnas.1431177100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-Y., Waloszek D., Maas D., Braun A., Huang D.-Y., Wang X.-Q., Stein M.2008Early Cambrian Yangtze Plate Maotianshan Shale macrofauna biodiversity and the evolution of predation. Palaeogeogr. Palaeoclimatol. Palaeoecol 254, 250–272doi:10.1016/j.palaeo.2007.03.018 [Google Scholar]
- Conway Morris S.Problematic taxa: a problem for biology or biologists?. In The early evolution of Metazoa and the significance of problematic taxa Eds Simonetta A. M., Conway Morris S.1991. pp. 19–24 Cambridge, UK:Cambridge University Press [Google Scholar]
- Conway Morris S.The crucible of creation: The Burgess Shale and the rise of animals. 2003Oxford, UK:Oxford University Press [Google Scholar]
- Conway Morris S.2006Darwin's dilemma: the realities of the Cambrian ‘explosion’. Phil. Trans. R. Soc B 361, 1069–1083doi:10.1098/rstb.2006.1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway Morris S.2008A redescription of a rare chordate, Metaspriggina walcotti Simonetta and Insom, from the Burgess Shale the (Middle Cambrian), British Columbia, Canada. J. Paleontol 82, 424–430doi:10.1666/06-130.1 [Google Scholar]
- Conway Morris S., Shu D.-G.Deuterostome evolution. In McGraw-Hill yearbook of science & technology 2003. pp. 79–82New York, NY:McGraw-Hill [Google Scholar]
- Darwin C.On the origin of species by natural selection, etc. 2nd edn.1860London, UK:John Murray [Google Scholar]
- Dunn C. W., et al. 2008Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749doi:10.1038/nature06614 [DOI] [PubMed] [Google Scholar]
- Fritzsch G., Böhme M. U., Thorndyke M., Nakano H., Israelsson O., Stach T., Schlegel M., Hankeln T., Stadler P. F.2008PCR survey of Xenoturbella bocki Hox genes. J. Exp. Zool. Mol. Dev. Evol 310, 278–284doi:10.1002/jez.b.21208 [DOI] [PubMed] [Google Scholar]
- Gee H.2001On being vetulicolian. Nature 414, 407–409doi:10.1038/35106680 [DOI] [PubMed] [Google Scholar]
- Gould S. J.Wonderful life: The Burgess Shale and the nature of history. 1989New York, NY:Norton [Google Scholar]
- Halanych K. M.2004The new view of animal phylogeny. Annu. Rev. Ecol. Evol. Syst 35, 229–256doi:10.1146/annurev.ecolsys.35.112202.130124 [Google Scholar]
- Harvey T. H. P., Butterfield N. J.2008Sophisticated particle-feeding in a large Early Cambrian crustacean. Nature 452, 868–871doi:10.1038/nature06724 [DOI] [PubMed] [Google Scholar]
- Hendricks J. R., Liebermann B. S.2008New phylogenetic insights into the Cambrian radiation of arachnomorph arthropods. J. Paleontol 82, 585–594doi:10.1666/07-017.1 [Google Scholar]
- Hou X.-G.1987Early Cambrian large bivalved arthropods from Chengjiang, eastern Yunnan. Acta Palaeontol. Sin 26, 286–298[In Chinese, with English summary.] [Google Scholar]
- Kristensen R. M., Higgins R. P.Kinorhyncha. In Microscopic anatomy of the invertebrates, Volume 4: Aschelminthes Eds Harrison F. W., Ruppert E. E.1991. pp. 377–404New York, NY:Wiley-Liss [Google Scholar]
- Lacalli T. C.2002Vetulicolians—are they deuterostomes? chordates?. BioEssays 24, 208–211doi:10.1002/bies.10064 [DOI] [PubMed] [Google Scholar]
- Liu J.-N., Shu D.-G., Hun J., Zhang Z.-F., Zhang X.-L.2008Origin, diversification, and relationships of Cambrian lobopods. Gondwana Res 14, 277–283doi:10.1016/j.gr.2007.10.001 [Google Scholar]
- Luo H.-L., Fu X. P., Hu S. X., Li Y., Chen L. Z., You T., Liu Q.2005New vetulicoliids from the Lower Cambrian Guanshan Fauna, Kunming. Acta Geol. Sin 79, 1–6 [Google Scholar]
- Mallat J., Chen J.-Y.2003Fossil sister group of craniates: predicted and found. J. Morph 258, 1–31doi:10.1002/jmor.10081 [DOI] [PubMed] [Google Scholar]
- Mallat J., Winchell C. J.2007Ribosomal RNA genes and deuterostome phylogeny revisited: more cyclostomes, elasmobranchs, reptiles, and a brittle star. Mol. Phylogenet. Evol 43, 1005–1022doi:10.1016/j.ympev.2006.11.023 [DOI] [PubMed] [Google Scholar]
- Philippe H., Lartillot N., Brinkmann H.2005Multigene analyses of bilateran animals corroborate the monophyly of Ecdysozoa, Lophotrochozoa, and Protostomia. Mol. Biol. Evol 22, 1246–1253doi:10.1093/molbev/msi111 [DOI] [PubMed] [Google Scholar]
- Romer A. S.1972The vertebrate as a dual animal-somatic and visceral. Evol. Biol 6, 121–156 [Google Scholar]
- Shu D.-G.2003A paleontological perspective of vertebrate origin. Chin. Sci. Bull 48, 725–733doi:10.1360/03wd0026 [Google Scholar]
- Shu D.-G.2005On the phylum Vetulicolia. Chin. Sci. Bull 50, 2342–2354doi:10.1360/982005-1081 [Google Scholar]
- Shu D.Preliminary study on phylogeny of Chengjiang deuterostomes. In Origination, radiations and biodiversity changes—evidences from the Chinese fossil record Eds Rong J., Fang Z., Zhou Z., Zhang R., Wang X., Yuan X.2006. pp. 109–124Beijing, China:Science Press; [In Chinese.] and 841–844. (English summary) [Google Scholar]
- Shu D.2008Cambrian explosion: birth of tree of animals. Gondwana Res 14, 219–240doi:10.1016/j.gr.2007.08.004 [Google Scholar]
- Shu D.-G., Conway Morris S.2003Response to comment on “A new species of yunnanozoan with implications for deuterostome evolution”. Science 300, 1372d.doi:10.1126/science.1085573 [DOI] [PubMed] [Google Scholar]
- Shu D.-G., Zhang X., Chen L.Reinterpretation of Yunnanozoon as the earliest known hemichordate. Nature 380, 1996a428–430doi:10.1038/380428a0 [Google Scholar]
- Shu D., Conway Morris S., Zhang X.-L.A Pikaia-like chordate from the Lower Cambrian of China. Nature 384, 1996b156–157doi:10.1038/384157a0 [Google Scholar]
- Shu D.-G., Conway Morris S., Zhang X.-L., Chen L., Li Y., Han J.A pipiscid-like fossil from the Lower Cambrian of South China. Nature 400, 1999a746–749doi:10.1038/23445 [Google Scholar]
- Shu D.-G., et al. Lower Cambrian vertebrates from South China. Nature 402, 1999b42–46doi:10.1038/46965 [Google Scholar]
- Shu D.-G., Chen L., Han J., Zhang X.-L.An early Cambrian tunicate from China. Nature 411, 2001a472–473doi:10.1038/35078069 [DOI] [PubMed] [Google Scholar]
- Shu D.-G., Conway Morris S., Han J., Chen L., Zhang X.-L., Zhang Z.-F., Liu H.-Q., Li Y., Liu J.-N.Primitive deuterostomes from the Chengjiang Lagerstätte (Lower Cambrian, China). Nature 414, 2001b419–424doi:10.1038/35106514 [DOI] [PubMed] [Google Scholar]
- Shu D.-G., Conway Morris S., Zhang Z.-F., Liu J.-N., Han J., Chen L., Zhang X.-L., Yasui K., Li Y.A new species of yunnanozoan with implications for deuterostome evolution. Science 299, 2003a1380–1384doi:10.1126/science.1079846 [DOI] [PubMed] [Google Scholar]
- Shu D.-G., et al. Head and backbone of the Early Cambrian vertebrate Haikouichthys. Nature 421, 2003b526–529doi:10.1038/nature01264 [DOI] [PubMed] [Google Scholar]
- Shu D.-G., Conway Morris S., Han J., Zhang Z.-F., Liu J. N.2004Ancestral echinoderms from the Chengjiang deposits of China. Nature 430, 422–427doi:10.1038/nature02648 [DOI] [PubMed] [Google Scholar]
- Smith M. P., Sansom I. J., Cochrane K. D.The Cambrian origin of vertebrates. In Major events in early vertebrate evolution Eds Ahlberg P. E.2001. pp. 67–84 London, UK:Taylor & Francis [Google Scholar]
- Stach T.2008Chordate phylogeny and evolution: a not so simple three-taxon problem. J. Zool 276, 117–141doi:10.1111/j.1469-7998.2008.00497.x [Google Scholar]
- Stach T., Dupont S., Israelson O., Fauville G., Nakano H., Kånnerby T.2005Nerve cells of Xenoturbella bocki (phylum uncertain) and Harrimania kupferri (Enteropneusta) are positively immunoreactivce to antibodies raised against echinoderm neuropeptides. J. Mar. Biol. Assoc. UK 85, 1519–1524doi:10.1017/S0025315405012725 [Google Scholar]
- Steiner M., Zhu M.-Y., Zhao Y.-L., Erdtmann B.-D.2005Lower Cambrian Burgess Shale-type fossil associations of south China. Palaeogeogr. Palaeoclimatol. Palaeoecol 220, 129–152doi:10.1016/j.palaeo.2003.06.001 [Google Scholar]
- Swalla B. J., Smith A. B.2008Deciphering deuterostome phylogeny: molecular, morphological and palaeontological perspectives. Phil. Trans. R. Soc. B 363, 1557–1568doi:10.1098/rstb.2007.2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J. W.On the origin of phyla. 2004Chicago, IL:University of Chicago Press [Google Scholar]
- Vinther J., Nielsen C.2005The early Cambrian Halkieria is a mollusc. Zool. Scr 34, 81–89doi:10.1111/j.1463-6409.2005.00177.x [Google Scholar]
- Yokobori S.-i., Oshima T., Wada H.2005Complete nucleotide sequence of the mitochondrial genome of Doliolum nationalis, with implications for evolution of urochordates. Mol. Phylogenet. Evol 34, 273–283doi:10.1016/j.ympev.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Zeng L.-Y., Jacobs M. W., Swalla B. J.2006Coloniality has evolved once in stolidobranch ascidians. Integr. Comp. Biol 46, 255–268doi:10.1093/icb/icj035 [DOI] [PubMed] [Google Scholar]
- Zhang X.-G., Hou X.-G.2004Evidence for a single median fin-fold and tail in the Lower Cambrian vertebrate, Haikouichthys ercaicunensis. J. Evol. Biol 17, 1162–1166doi:10.1111/j.1420-9101.2004.00741.x [DOI] [PubMed] [Google Scholar]