Abstract
We describe a new species of psittacosaur, Psittacosaurus gobiensis, from the Lower Cretaceous of Inner Mongolia and outline a hypothesis of chewing function in psittacosaurs that in many respects parallels that in psittaciform birds. Cranial features that accommodate increased bite force in psittacosaurs include an akinetic skull (both cranium and lower jaws) and differentiation of adductor muscle attachments comparable to that in psittaciform birds. These and other features, along with the presence of numerous large gastroliths, suggest that psittacosaurs may have had a high-fibre, nucivorous (nut-eating) diet.
Psittacosaurs, alone among ornithischians, generate oblique wear facets from tooth-to-tooth occlusion without kinesis in either the upper or lower jaws. This is accomplished with a novel isognathous jaw mechanism that combines aspects of arcilineal (vertical) and propalinal (horizontal) jaw movement. Here termed clinolineal (inclined) jaw movement, the mechanism uses posteriorly divergent tooth rows, rather than kinesis, to gain the added width for oblique occlusion. As the lower tooth rows are drawn posterodorsally into occlusion, the increasing width between the upper tooth rows accommodates oblique shear. With this jaw mechanism, psittacosaurs were able to maintain oblique shearing occlusion in an akinetic skull designed to resist high bite forces.
Keywords: psittacosaur, parrot-beaked dinosaurs, jaw mechanics, cranial kinesis, mesowear, nucivorous
1. Introduction
The skull in psittacosaurs, or ‘parrot-beaked’ dinosaurs, is characterized by its short, deep snout and ventrolaterally projecting cheek horns. In most ornithischian herbivores, kineticism within the lower and/or upper jaws allows the formation of oblique wear facets during isognathous occlusion. Adult psittacosaurs, in contrast, have a coossified mandibular symphysis and do not show any evidence of movable joints within either the upper or lower jaws. Yet, their dentition is characterized by well-formed oblique wear facets that truncate individual crowns at a significant angle from vertical, similar to those in other ornithischians with kinetic jaws. How psittacosaurs accomplished tooth wear of this sort has never been adequately explained.
Psittacosaur cranial morphology has been described in detail only recently (Sereno 1987, 2009; Averianov et al. 2006; Zhou et al. 2006; You et al. 2008), despite early finds of exceptional quality (Osborn 1923, 1924; Young 1958). Functional aspects of their jaws and dentition have only been briefly considered (Norman & Weishampel 1991). Psittacosaurs are restricted to central Asia in horizons of mid-Cretaceous (late Barremian-Albian) age. In the terrestrial faunas in which they occur, they are often the predominate vertebrate recovered, sometimes known from hundreds of specimens and multiple species (Zhou et al. 2006; Qi et al. 2007). At least eight valid species have been named within the genus Psittacosaurus that differ primarily in body-size and details of tooth and horn ornamentation (Sereno 2009).
Here we describe a new small-bodied species from the Gobi Desert of Inner Mongolia (figure 1a) and use its well preserved skull and worn dentition to gain a better understanding of psittacosaur cranial and dental form and function.
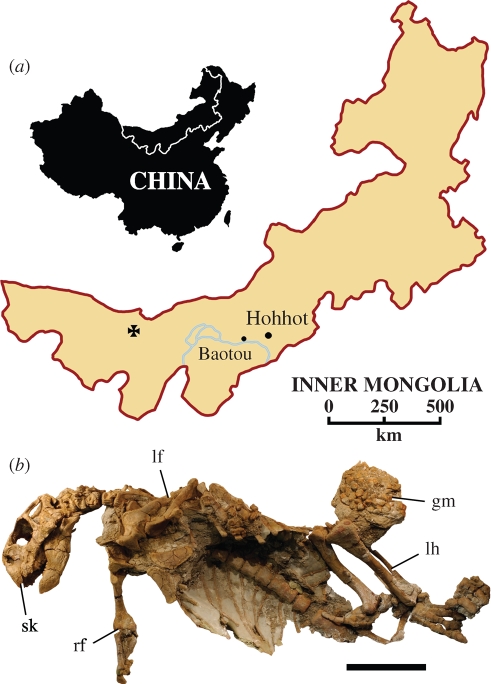
Figure 1.
Locality and holotypic specimen of Psittacosaurus gobiensis LH PV2. (a) Map showing locality (cross) southwest of Suhongtu in the Nei Mongol Autonomous Region of China. (b) Holotypic skeleton exposed in ventral view. Scale bar (b), 10 cm. Abbreviations: gm, gastrolith mass; lf, left forelimb; lh, left hind limb; rf, right forelimb; sk, skull.
2. A new psittacosaur
Dinosauria Owen, 1842
Ornithischia Seeley, 1888
Ceratopsia Marsh, 1890
Psittacosaurus Osborn 1923
Psittacosaurus gobiensis, sp. nov.
(a). Holotype
Skull and articulated postcranial skeleton with gastrolith mass, Long Hao Institute of Geology and Paleontology (LH) PV2 (figure 1b), lacking only portions of the right manus, some of the sacrum and pelvic girdle, and the right hind limb and tail. The holotype and more fragmentary remains of additional individuals nearby were discovered in 2001 during the first Chinese-American Dinosaur Expedition.
The holotypic skeleton of P. gobiensis represents a fully mature individual with coossification of many cranial sutures as well as all neurocentral sutures in the axial column (figure 1b). The mandibular symphysis, for example, is solidly fused. The rostral–premaxillary and predentary-dentary sutures, in addition, are obliterated by fusion, and many other cranial sutures, such as the frontal–frontal and frontal–parietal, can be traced but are fused. The remains of other individuals discovered near the holotypic site also confirm that P. gobiensis is a small-bodied species comparable to Psittacosaurus sinensis (Young 1958).
The trunk measures only 40 cm in length, corresponding to a body length of approximately 1 m. The well-preserved skull was turned to the side. The postcranial skeleton was preserved belly up with the pectoral girdles, forelimbs, sternal plates and ribcage preserved in three-dimensional articulation, suggesting that it was buried as a dried carcass. The gastrolith mass, which is intact with facets of adjacent stones fitted to one another, is positioned to the side, possibly the result of evisceration by a scavenger before burial.
(b). Etymology
Gobi, after the Gobi Desert; -ensis (Latin) belonging to.
(c). Localities and horizon
The holotype and potential referred specimens were found southwest of Suhongtu (40°59′42.4″ N, 104°3′53.8″ E) in the western Gobi Desert, Nei Mongol Autonomous Region, People's Republic of China (figure 1a) in the Lower Cretaceous (Aptian, ca. 115 Ma; Gradstein et al. 2004) Bayan Gobi Formation.
(d). Diagnosis
Small-bodied psittacosaur characterized by autapomorphies including: (i) pyramidal horn on the postorbital bar composed almost entirely of the postorbital, (ii) postorbital–jugal fossa, (iii) minimum width of the postorbital bar approximately 50 per cent the width of the base of the process, (iv) retroarticular process deflected posteromedially at an angle of 40° from the axis of the mandible, and (v) thin and restricted enamel on medial and lateral aspects of the maxillary and dentary crowns, respectively. None of these autapomorphies appear to be transient during growth or characterize juveniles alone (Sereno 2009).
Other features with limited distribution among psittacosaur species include a preorbital snout 35 per cent the length of the skull, a ventrolaterally projecting pyramidal jugal horn, a low quadratojugal eminence, absence of an external mandibular fenestra and maxillary and dentary tooth rows limited to eight and nine teeth, respectively.
A partial skull of the larger-bodied species Psittacosaurus mongoliensis (LH PV3) was also found in the same formation. Its longer skull proportions, higher tooth count, relatively smaller crowns, upturned prefrontal edge, primitive postorbital shape and ornamentation, straight retroarticular process and open sutures (despite its size) clearly show that it does not pertain to the new species.
Other species from other formations in Inner Mongolia include Psittacosaurus tingi, Psittacosaurus osborni, Psittacosaurus mazongshanensis, Psittacosaurus ordosensis and Psittacosaurus neimongoliensis. The first two species are described from very young juveniles and have been regarded as nomina dubia (Sereno 2009). Psittacosaurus mazongshanensis (Xu 1997) differs from P. gobiensis by its larger size, longer skull proportions, ovate crowns and posteriorly directed retroarticular process, although the now damaged holotype has also been regarded as a nomen dubium (Sereno 2009). Psittacosaurus ordosensis (Russell & Zhao 1996) differs from P. gobiensis in having an unusually short lower jaw as well as other features but as documented cannot be distinguished from P. sinensis (Sereno 2009). Psittacosaurus neimongoliensis, known from a complete subadult skull and nearly complete skeleton (Russell & Zhao 1996), also has a relatively short lower jaw and lacks the unusual postorbital shape and ornamentation and broad crown proportions present in P. gobiensis (Sereno 2009).
(e). Description
Psittacosaurus gobiensis represents yet another species in the speciose genus Psittacosaurus, differing in significant, but structurally minor, details from other species. In the skull (figure 2a,b), the horn on the postorbital bar is smooth and pyramidal in shape and formed primarily by the postorbital. The strong medial deflection of the retroarticular process (figure 2d) and the particularly asymmetrical distribution of enamel on upper and lower crowns (figure 3a–d) are unique features among psittacosaur species.
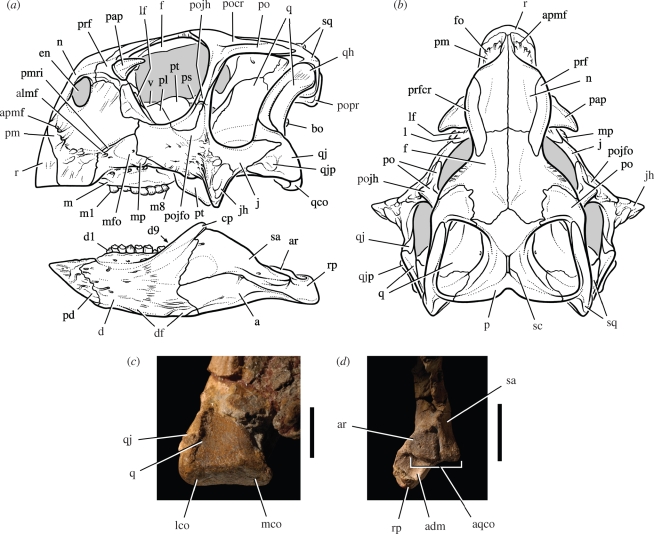
Figure 2.
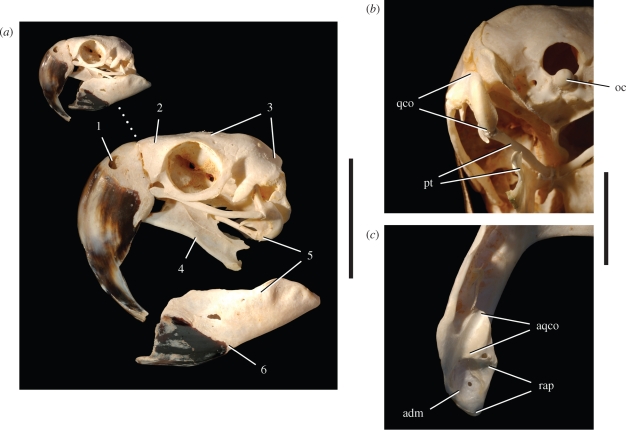
Skull and jaw joint in Psittacosaurus gobiensis (LH PV2). (a) Skull reconstruction in lateral view. (b) Skull reconstruction in dorsal view. (c) Left quadrate condyle in posteroventral view. (d) Posterior end of the right lower jaw in dorsal view. Scale bars, 1 cm in (c), 2 cm in (d). Abbreviations: a, angular; adm, attachment surface for the depressor mandibulae; almf, anterolateral maxillary foramen; apmf, anterior premaxillary foramen; aqco, articular surface for the quadrate condyles; ar, articular; bo, basioccipital; cp, coronoid process; d, dentary; d1, 9, dentary tooth 1 and 9; df, dentary flange; en, external naris; f, frontal; fo, foramen; j, jugal; jh, jugal horn; l, lacrimal; lco, lateral condyle; lf, lacrimal foramen; m, maxilla; m1, 8, maxillary tooth 1 and 8; mco, medial condyle; mfo, maxillary fossa; mp, maxillary protuberance; n, nasal; p, parietal; pap, palpebral; pd, predentary; pl, palatine; pm, premaxilla; pmri, premaxilla-maxilla ridge; po, postorbital; pocr, postorbital crest; pojfo, postorbital-jugal fossa; pojh, postorbital-jugal horn; popr, paroccipital process; prf, prefrontal; prfcr, prefrontal crest; ps, parasphenoid; pt, pterygoid; q, quadrate; qco, quadrate condyle; qh, quadrate head; qj, quadratojugal; qjp, quadratojugal protuberance; r, rostral; rp, retroarticular process; sa, surangular; sc, sagittal crest; sq, squamosal; v, vomer.
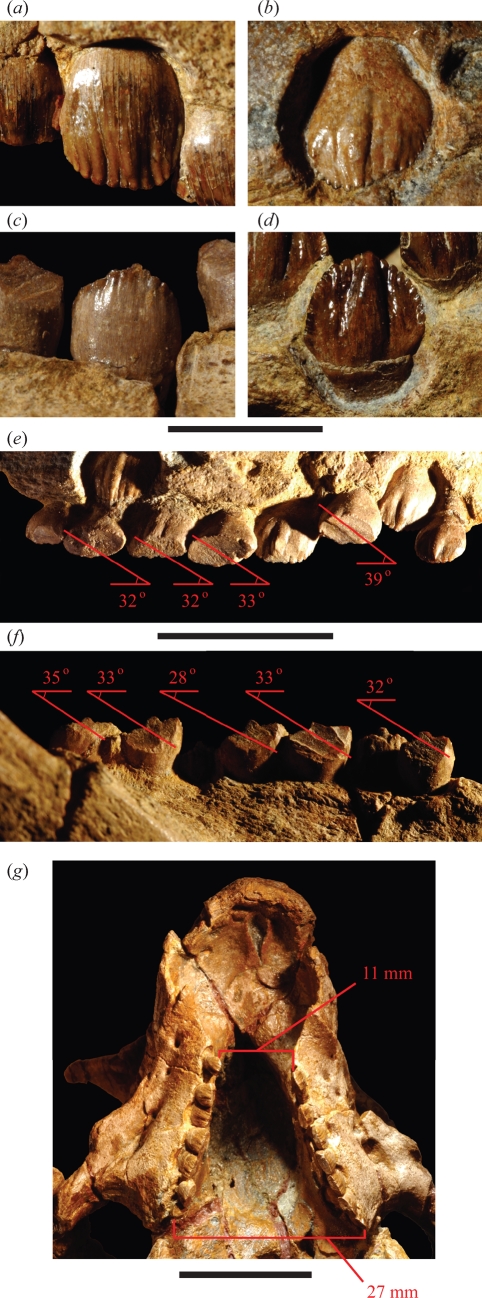
Figure 3.
Crown form and wear in Psittacosaurus gobiensis (LH PV2). Left maxillary crowns in lateral (a) and medial (b) views. Right dentary crowns in lateral (c) and medial (d) views. (e) Wear facets and striation angle in left maxillary crowns in medial view. (f) Wear facets and striation angle in right dentary crowns in lateral view. (g) Maxillary tooth rows in ventral view showing the distance between the tooth rows at anterior (mesial) and posterior (distal) ends. Scale bars, 5 mm in (a)–(d); 2 cm in (e), (f); 2 cm in (g).
The skull has a relatively short snout that is about 35 per cent of skull length (figure 2a), as in most species except the slightly longer-snouted P. mongoliensis. Both the rostral and predentary bones are coossified with the premaxillaries and dentaries, respectively. Psittacosaurus gobiensis lacks the fenestra that breaches the lacrimal canal on the snout sidewall in several other species. The squamosal participates in the lateral extremity of the horizontal shelf over the occiput formed by the fused parietals (figure 2b).
The basipterygoid processes are relatively short unlike those in Psittacosaurus major. The quadrate condyles are nearly flat and very poorly differentiated as in all psittacosaur species (figure 2c). Opposing these condyles is a flat articular surface on the lower jaw composed of the articular and surangular (figure 2d). The short, robust lower jaw has a prominent coronoid process that obscures the posteriormost dentary tooth in lateral view (figure 2a). As in other ceratopsians, a comma-shaped coronoid bone is present on the medial aspect of the coronoid process.
There are eight maxillary and nine dentary teeth on both sides (figure 2a). The crowns have a subcircular, rather than lobate shape as in some species (figure 3a–d). A prominent primary ridge is present on all but the lateral aspect of the dentary crowns. Secondary ridges extend for only a short distance from the denticles, unlike the longer secondary ridges in P. major (Sereno 2009). Enamel is asymmetrically distributed in all psittacosaur species, with thinner enamel at comparable crown height on the side that trails during occlusion. This feature is emphasized in the new species, in which enamel appears to be absent over most of the medial and lateral aspects of maxillary and dentary crowns, respectively (figure 3b–c).
Several features indicate a closer relationship between P. gobiensis and some species (Psittacosaurus sibiricus, P. neimongoliensis, P. sinensis) over others (P. mongoliensis, Psittacosaurus lujiatunensis, Psittacosaurus meileyingensis, P. major), including sutural contact between the premaxilla and jugal on the snout sidewall, a prominent postorbital–jugal horn, a posteriorly divergent temporal bar, a low dentary flange, broad maxillary crowns that are as broad as tall and a reduction of adult tooth number to less than 10 (figures 2 and 3) (Sereno 2009).
3. Psittacosaur skull structure and tooth wear
(a). Cranium
The unusual shape and structure of the psittacosaur cranium (figures 2, 4, 5) is argued here to be directly related to resistance to large bite forces generated by enhanced jaw musculature, as first suggested by Gregory (1927). Many of these features have direct osteological and muscular correlates in psittaciform birds (figure 6a, features 1–6), which are specialized durophagous feeders (Thompson 1899; Witmer & Rose 1991; Zusi 1993).
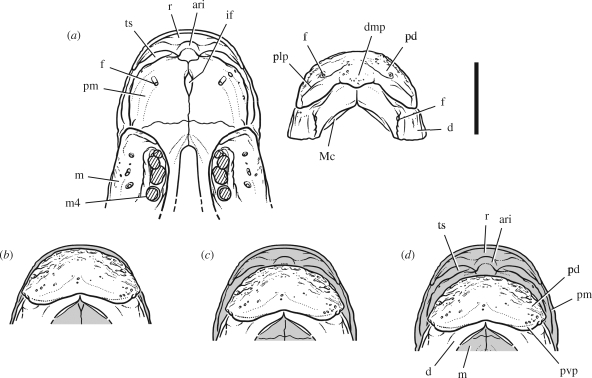
Figure 4.
Jaw articulation in subadult Psittacosaurus mongoliensis (American Museum of Natural History, AMNH 6540). (a) Upper and lower jaws, anterior end. (b) Articular relations at the anterior end of the jaws in ventral view showing three positions of the lower jaw. Scale bar, 1 cm. Abbreviations: ari, attachment ridge for the upper beak; d, dentary; dmp, dorsomedian process; f, foramen; if, incisive foramen; m, maxilla; m4, maxillary tooth 4; Mc, Meckel's canal; pd, predentary; plp, posterolateral process; pm, premaxilla; pvp, posteroventral process; r, rostral; ts, triturating surface.
Figure 5.
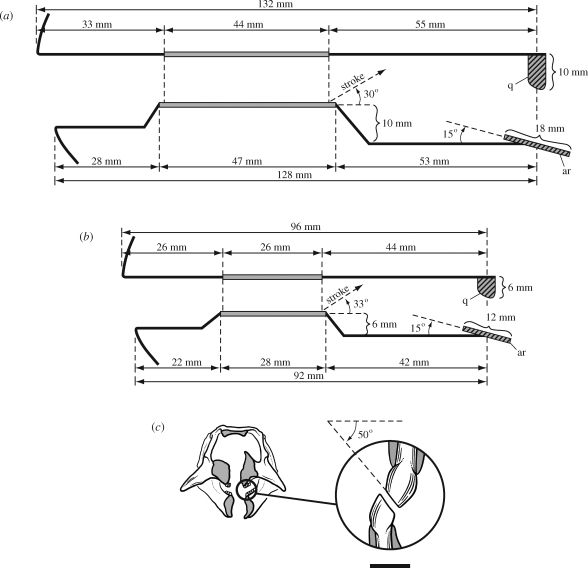
Schematized view of the jaws and jaw movement in lateral view and the wear facets in anterior view during occlusion in Psittacosaurus. (a) Jaw configuration in P. mongoliensis (AMNH 6534) with mesowear striation angle based on a second specimen (PI 698/1-3; Sereno 2009). (b) Jaw configuration and mesowear striation angle in P. gobiensis (LH PV2). (c) Computed tomographic cross-section of the snout of the skull of P. gobiensis (LH PV2) in anterior view with enlargement of worn maxillary and dentary crowns from the centre of the left upper and lower tooth rows showing the angle of oblique wear. Scale bar in (c), 2 mm. Abbreviations: ar, articular; q, quadrate.
Figure 6.
Skull and jaw joint morphology in the psittaciform bird Ara ararauna (blue and yellow macaw; University of Chicago Research Collection, UCRC R1). (a) Skull in lateral view. (b) Disc-shaped left quadrate condyle in posteroventral view. (c) Trough-shaped socket in left lower jaw for quadrate condyle in dorsal view. Skull morphology highlighted: 1, small, elevated external nares; 2, short, deep snout; 3, coossified cranial vault; 4, pendant, elongate pterygoid flanges for enhanced pterygoideus musculature; 5, jaw joint allowing fore-aft movement; 6, short, deep dentary ramus fused at the symphysis. Scale bars, 5 cm in (a) for enlarged view, 2 cm in (b) and (c). Abbreviations: adm, attachment for the depressor mandibulae; aqco, articular surface for quadrate condyles; oc, occipital condyle; pt, pterygoid; qco, quadrate condyle; rap, retroarticular process.
As in psittaciform birds, the preorbital portion of the cranium is greatly shortened to increase the bite force at the bill margin, the external nares are small and elevated, and the antorbital fenestra and fossa are closed (figures 2a and 6a). Besides these features, the psittacosaur snout is strengthened in several other ways not observed in any other plant-eating dinosaurs. First, the sidewall of the psittacosaur snout lacks any sutures and is composed entirely of an expanded ramus of the premaxilla. Second, the internarial bar also lacks the usual (premaxilla–nasal) suture and is composed only of the nasal (figure 2a). Third, the nasals extend ventrally to butt against the rostral, transferring forces from the upper bill directly to the dorsal skull roof (figure 2a,b).
Many of the sutures on the dorsal skull roof coossify with maturity, including nasal–prefrontal and interfrontal sutures. In the temporal region of the skull, other sutures are unusually long and/or coossified. The bones (postorbital, squamosal) forming the upper temporal bar, for example, overlap along the entire length of the bar rather than just at mid-section and often fuse as in the skull of P. gobiensis. Likewise, the bones (jugal, quadratojugal) forming the lower temporal bar have broadly overlapping surfaces that coossify.
The palate is rigid and reinforced. Anteriorly, the secondary palate is deeply arched with unique triturating surface formed by the edges of the premaxillae inside the bill margin (figure 4a). The posterior palate is uniquely immobilized and strengthened. The pterygoids have a neomorphic palatal ramus that fuses with its opposite in the midline below the primitive posterior palate (Sereno 2009).
(b). Lower jaw
The symphysis was stabilized by coossification of the predentary and dentaries with maturity. The middle portion of the dentary ramus is thickened laterally and ventrally (dentary flange) and covered with a rugose surface for muscle attachment. As in psittaciform birds, the coronoid process in P. gobiensis is particularly strong and anteriorly positioned, arising from the middle of the dentary tooth row (figures 2a and 6a).
Alone among nonavian dinosaurs, psittacosaurs have a flat jaw articulation (figure 2c,d). The articular surface on the lower jaw angles about 15° posteroventrally and about 5° ventrolaterally, as preserved in P. gobiensis and P. mongoliensis (figure 5a,b). The nearly flat, poorly differentiated quadrate condyles equal in transverse width the articular platform on the lower jaw but measure only about 5 per cent of its anteroposterior width. This differential allows the lower jaw to slide fore and aft against the quadrate, as in psittaciform birds (figure 6b,c).
When the lower jaw is positioned anteriorly as far as the jaw joint seems to allow, the U-shaped arc of the external edge of the predentary closely matches that of the opposing rostral (position 1, figure 4b). When the lower jaw is positioned with the quadrate condyles centred on the articular platform, the external edge of the predentary closely matches the location of a triturating edge formed by the premaxillaries on the anterior wall of the secondary palate (position 2, figure 4c). When the lower jaw is positioned posteriorly as far as the jaw joint seems to allow, the external edge of the predentary is located posterior to the triturating edge, its U-shaped external edge directed at the secondary palate (position 3, figure 4d).
The anterior position would be effective for cropping, with the cutting edges of upper and lower beaks aligned. Middle and posterior jaw positions would be effective for shelling seeds or crushing nuts against the premaxillary palate. The triturating surface and retracted positions of the lower jaw are comparable in psittaciform birds to an elevated, keratin-covered crushing platform on the upper bill jaw and similar positioning of the lower bill (figure 7a,b).
Figure 7.
Beak morphology in psittaciform birds. Upper bill in posterolateral (a) and posterior (b) views in Ara ararauna (blue and yellow macaw; UCRC R1). Skull in lateral view and beak in anterolateral view (c) in Melopsittacus undulatus (budgerigar; UCRC R2). Scale bars, 3 cm in (a) and (b), 1 cm in (c) for enlarged view. Abbreviations: ab, attachment surface for bill; ama, area of muscle attachment; b, bill; pl, platform; pt, pterygoid; sym, symphysis.
(c). Mesowear
Well-developed wear facets truncate the crowns of most of the teeth along both upper and lower tooth rows in P. gobiensis (figure 3e,f). The occlusal plane is oriented at approximately 50° from the horizontal when measured in situ (figure 5c). Striations on the wear facets angle at an average of 33° from the horizontal on both upper and lower crowns (as seen in lateral or medial views, respectively) (figures 3e,f and 5b). The power stroke during occlusion, thus, clearly involves posterodorsal displacement of the lower jaw, with posterior movement approximately twice that of vertical movement. The flat articular platform on the lower jaw must slide posteriorly against the quadrate condyles as upper and lower tooth rows converge.
The similar angle and nearly linear appearance of the striations within individuals and in other species (figure 5a) suggest a very controlled, deliberate power stroke. Because there are no edges from incomplete strokes or disengagement striations, the tooth rows must have sheared past each other before the jaw was dropped and repositioned for another power stroke. The lower tooth row has one more tooth and is slightly longer than the upper tooth row (figure 5a,b). At least in the middle of the tooth rows, each crown sheared against two crowns in the opposing tooth row, as the lower jaw was displaced posterodorsally.
4. Psittacosaur jaw mechanics
(a). Previous interpretations
The presence of oblique shearing wear facets in the akinetic psittacosaur skull was first noticed as a paradox by Norman & Weishampel (1991). How could oblique facets form during isognathus jaw closing without an accommodating transverse component of movement in either the upper or lower jaw? Propalinal (fore–aft, mesiodistal) jaw movement, they concluded, was the best explanation, pointing to the flat articular surface on the lower jaw. As seen above, however, simple propaliny is contradicted by the inclination of the power stroke (figure 3e,f) and by posteriorly divergent tooth rows (figure 3g), which would disengage with fore–aft movement alone.
Weishampel & Norman (1989, p. 92, fig. 1) and Reilly et al. (2001, fig. 4), in contrast, identified psittacosaurs as orthal slicers. The akinetic psittacosaur skull and the inclined power stroke, however, contradict strict orthal movement, which requires accommodating kinesis in either the cranium or lower jaws. Weishampel & Norman (1989, p. 92) observed in passing that tooth wear in psittacosaurs might be the result of a ‘combination of palinal and orthal movement of the lower jaws.’ We suggest below that such inclined movement is key to understanding the psittacosaur power stroke.
(b). Clinolineal shear
Reilly et al. (2001) differentiated two kinds of jaw mechanisms, propalinal and arcilineal, among living and fossil amniotes with isognathous (simultaneous) occlusion. These differ in shear direction, the former horizontal (anteroposterior) and the latter vertical (dorsoventral). Both mechanisms can generate oblique wear facets.
Vertical isognathous occlusion with an arcilinear jaw mechanism necessitates lateral displacement to generate planar or subplanar oblique wear facets (Norman & Weishampel 1985; Weishampel & Norman 1989). The transverse component during the power stroke must be accommodated by transverse movement of either (or both) upper or lower tooth rows. All ornithischians with oblique tooth wear except psittacosaurs maintain a mobile articulation at the mandibular symphysis to medially rotate (or wishbone) the lower tooth rows; some may have simultaneous lateral displacement of the snout sidewall and upper tooth rows.
Propalinal isognathous occlusion can also yield oblique wear facets along parasagittal tooth rows, although grinding against subhorizontal facets on molariform crowns or plates is more common (Throckmorton et al. 1981; Norman & Weishampel 1985; Reilly et al. 2001). To our knowledge, no tetrapod with simple leaf-shaped crowns like those in psittacosaurs (figure 3a–d) has been shown to have strictly propalinal jaw movement.
We suggest here that psittacosaurs generate oblique wear facets by a third isognathous jaw mechanism, here termed clinolineal (‘inclined line’), which involves both propalinal and arcilineal components (figure 5). Rather than transverse displacement of either upper or lower tooth rows, clinolineal occlusion uses the posterior divergence of the tooth rows to gain the added transverse width required to create oblique wear facets.
The transverse width of a typical wear facet (figure 5c) is approximately 1 mm, as measured in apical view of dentary and maxillary wear facets. The wear facet of each successive crown, in addition, is offset laterally (buccal) by approximately the same distance (1 mm). In upper jaws of P. gobiensis, for example, the posteriormost maxillary crown in the series (tooth 8) is 8 mm farther from the midline than the first maxillary crown (13.5 versus 5.5 mm from the midline or 27 versus 11 mm as measured between tooth rows) (figure 3g). Mesowear suggests that the posterodorsal power stroke results in shear across a little more than one crown (figure 3e,f), which closely matches the gain in width from the midline. The lower tooth row is also slightly longer than the upper tooth row (figure 5a,b).
Psittacosaurs, thus far, are the only herbivores of which we are aware with clinolineal shear. Similar striations have been described in ankylosaurid teeth that suggest a straight, posterodorsally inclined power stroke (Rybczynski & Vickaryous 2001), although more information is needed from individuals preserving the jaw articulation and intact dentitions.
5. Inferred jaw musculature
(a). Jaw-closing muscles
Judging from the relatively large volume of the adductor chamber between supra- and laterotemporal fenestrae (figure 2a,b), the adductor musculature must have been considerable in Psittacosaurus as in psittaciform birds (figure 8). Several features on the margins of the chamber confirm the presence of enhanced jaw-closing adductor musculature. Like other ceratopsians, the fossa marking the origin of the adductor mass on the skull roof extends medially to a low sagittal crest in the midline and posteriorly onto the parietal shelf (figure 2b). In dorsal view, the adductor fossa has an anteroposterior diameter equal to that of the orbit and the adductor chamber below is spacious. An external slip of adductor musculature also seems to have been attached to the lateral aspect of the supratemporal bar, which is slightly inset in P. mongoliensis and more deeply inset in P. gobiensis (figures 2a and 8b).
Figure 8.
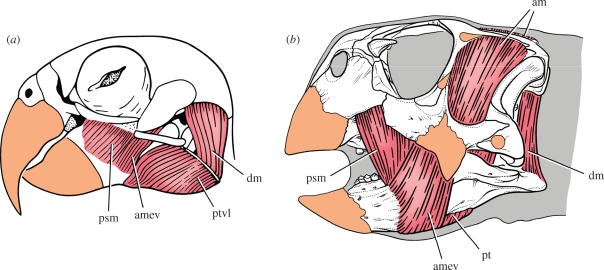
Jaw musculature (red) and keratin sheathing (orange) in psittaciform birds and psittacosaurs. (a) Pionites leucogaster (white-bellied parrot) (after Zusi 1993). (b) Jaw muscles and keratin inferred for Psittacosaurus gobiensis. Abbreviations: am, adductor mandibulae; amev, adductor mandibulae externus ventralis; dm, depressor mandibulae; psm, pseudomasseter; pt, pterygoideus; ptvl, pterygoideus ventralis lateralis.
A portion of the external adductor muscle also appears to have gained insertion on the lateral aspect of the dentary. A raised, rugose platform is present that extends ventrally to a dentary flange (figure 2a). In adult specimens of larger-bodied psittacosaurs, the platform and rugosities are more pronounced and the flange is pendant with a prominent anterior corner (Sereno 2009). These features provide a plausible anchor for enhanced posterodorsally inclined adductor musculature that may have originated on the medial aspect or ventral margin of the jugal.
The invariable presence in all psittacosaur species of a maxillary protuberance and an adjacent bony crest on the side of the cheek (premaxilla–maxilla crest) (figure 2a, pmri) may be indicative of attachment sites for a ‘pseudomasseter’ muscle similar to that in many psittaciform birds (figure 8a, psm). The presence of shallow fossae of unknown function on the maxilla and jugal in most species may also be related to muscle attachments. The pseudomasseter, a derivative of the adductor musculature in psittaciform birds, provides an anteriorly positioned jaw-closing muscle to assist in generating large-bite forces (figure 8a).
Hypertrophy of the pterygoid mandibular flange in psittacosaurs and the presence of an elongate, rugose muscle scar on the ventral margin of the splenial, indicates the presence of strong pterygoideus musculature for closing and possibly for protracting the lower jaw. In psittaciform birds, hypertrophied pterygoid flanges are also present for attachment of expanded pterygoideus musculature (figure 6a, number 4), which inserts on, and extends around, the ventral margin of the lower jaw (Hofer 1950; Burton 1974; Zusi 1993) (figure 8a, ptvl).
(b). Jaw-opening muscles
Unlike ornithopod ornithischians, the depressor mandibulae in psittacosaurs does not appear to originate on the squamosal posteroventral process or paroccipital process, which in all species of Psittacosaurus terminate laterally as subquadrate processes without hook-shaped ends (figure 2a). Instead, the ventral aspect of the parietal shelf is marked by distinctive fossae to each side of a vertical, plate-shaped nuchal crest. These parasagittal fossae, which are strongly marked in larger psittacosaur species (Sereno 2009), appear to constitute the areas of origin of a substantial depressor mandibulae, a muscle that inserts on the relatively long retroarticular process of the lower jaw (figure 2d, adm and figure 8b). Parrots also have a substantial depressor mandibulae muscle, which originates on the posterior aspect of the cranium and attaches to a fossa of similar form on the retroarticular process (Burton 1974; Zusi 1993) (figures 6c and 8a).
6. Psittacosaurs, nucivorous parrot analogues
Although originally named for the resemblance of their skull shape to that in psittaciform birds (Osborn 1923) and envisioned soon after as ‘nutcrackers’ (Gregory 1927), psittacosaur jaw mechanics and their shearing tooth wear have never been described in detail or satisfactorily explained. As outlined above, psittacosaurs were able to maintain oblique tooth wear in a skull characterized by reinforced, akinetic jaws by using a unique shearing motion (clinolineal), which takes advantage of the increasing width between posteriorly divergent tooth rows. In this way, psittacosaurs maintained oblique shearing action much like that in other basal ornithischians (figure 5c), despite having eliminated kinesis within both upper and lower jaws.
(a). Psittaciform skull morphology and function
The close parallel to psittaciform cranial form and function is strongly confirmed here by highlighting features of the skull related to (i) strengthening of the bill margins, snout, skull roof, palate and lower jaws to resist increased bite forces, (ii) the presence of a triturating edge on the anterior margin of the premaxillary palate (figure 4a,d,ts), and (iii) the presence of a sliding jaw articulation that allows adjustable positioning of the lower beak (figures 2d, 4b–d). Muscular features inferred from bony correlates also closely resemble the condition in psittaciform birds including (iv) enhancement of jaw-closing musculature (adductor, pterygoideus) and (v) differentiation of adductor musculature for mechanical advantage to insert on the lateral aspect of the dentary (pars externus ventralis) and cheek (pseudomasseter) (figure 8).
Upper and lower beaks in psittacosaurs have a semicircular contour of nearly equal size, judging from their supporting bones, the predentary and rostral (figure 4). Neither beak tapers to a recurved median tip as occurs in all other ceratopsians and in most birds including psittaciforms, where the form of the bill is reflected by the underlying bone (figure 7c). The psittacosaur upper and lower beak, which extends onto the premaxilla and dentary from the rostral and predentary, respectively (figure 8b), appears to have had a cropping function when aligned. When the lower beak was retracted and directed inside the upper beak, on the other hand, it appears to have had a shelling or nutcracking function (figure 4c,d).
(b). Gastric mill for durophagous material
Gastroliths are associated with nearly all well preserved psittacosaur skeletons (Osborn 1924; Xu 1997). In P. gobiensis there are more than 50 gastroliths adjacent to the ribcage with an average diameter in excess of 5 mm (figure 1b). Many gastroliths have a long dimension of at least 1 cm. We underscore here the regular presence of gastroliths and their large size in psittacosaurs, both of which are conspicuous (Wings 2004, p. 163). Ornithomimid herbivores of greater body mass, for example, have much smaller gastroliths, averaging about 1–2 mm in diameter (Kobayashi et al. 1999).
In birds, gastrolith size and body mass are correlated such that birds with less mass have smaller gastroliths (Gionfriddo & Best 1996). A small-bodied species like P. gobiensis, in other words, would be expected to have gastroliths of small, rather than unusually large, average diameter. Gastrolith size in birds, in addition, has been correlated with coarseness of diet (Farlow 1987, p. 63). Abundant gastroliths have been linked with diets consisting of ‘hard, coarse materials, especially seeds and other plant parts’ (Gionfriddo & Best 1996, p. 691; Meinertzhagen 1954). The unusual size and mass of the gastrolith mill in P. gobiensis and other psittacosaurs thus add support to the interpretation that nuts or seeds with a hard casing and high fibre content may have been a predominant component of their diet.
(c). Species diversity, morphological conservatism, limited body size and spatiotemporal range
Other less direct lines of evidence point toward a specialized and possibly high-caloric diet for psittacosaurs. Psittacosaurus is the most diverse dinosaurian genus with at least nine species after a conservative taxonomic revision (Sereno 2009). The genus is speciose because nearly all taxonomic differentia are limited to relatively minor tooth or cranial bone ornamentation. Variation in tooth replacement rate or jaw morphology is negligible compared to that seen in closely related basal neoceratopsians. The psittacosaur postcranial skeleton, in addition, is remarkably uniform. Characters linking two or more psittacosaur species are also scarce; only a handful of characters vary among psittacosaur species, providing minimal data to assess phylogenetic relationships (for review, see Sereno 2009). The genus Psittacosaurus, in other words, is exceptionally diverse in species but very conservative in dental, cranial, and postcranial morphology. Unlike nearly all other dinosaurian genera, in addition, there is increasing evidence at some locales for the coexistence of two psittacosaur species that differ most obviously in body size (e.g., P. gobiensis and P. mongoliensis). This species richness, conservative morphology, and co-occurrence favours an interpretation of dietary specialization with occasional size-related partitioning among species.
Psittacosaurs are all relatively small in body size, ranging from 1 to 2 m in body length. Their geographical range is limited to central Asia, and their temporal range may be as narrow as 10–20 million years in the mid-Cretaceous (Sereno 2009). Closely related basal neoceratopsians, in contrast, range in body size up to 4 m in length (e.g., Udanoceratops; Kurzanov 1992). Basal neoceratopsians also have a geographical range that includes North America and a temporal range spanning the Cretaceous (Ostrom 1978). The restricted body size and limited spatiotemporal range of psittacosaurs also favours their interpretation as dietary specialists that may have focused on plant resources of high caloric content such as nuts or seeds.
Acknowledgements
We thank C. Abraczinskas for the final draft of all of the figures, R. Masek, E. Fitzgerald, and E. Dong for fossil preparation, F. Moreau for discovering the specimen, and P. Makovicky, L. Porro, C. Ross, J. Whitlock, J. Wilson and X. Xing for comments that improved the manuscript. For permission and support of fieldwork, we are indebted to the Bureau of Land and Resources of the Nei Mongol Autonomous Region. For support of the research, we thank the National Geographic Society, the David and Lucile Packard Foundation, the Division of Biological Sciences of the University of Chicago, and the Long Hao Institute of Geology and Paleontology. This is Publication 2 of the Chinese-American Dinosaur Expeditions.
Footnotes
One contribution to a Special Issue ‘Recent advances in Chinese palaeontology’.
References
- Averianov A. O., Voronkevich A. V., Leshchinskiy S. V., Fayngertz A. V.2006A ceratopsian dinosaur Psittacosaurus sibiricus from the Early Cretaceous of west Siberia, Russia and its phylogenetic relationships. J. Systematic Palaeontol. 4, 359–395 (doi:10.1017/S1477201906001933) [Google Scholar]
- Burton P. J. K.1974Jaw and tongue features in Psittaciformes and other orders with special reference to the anatomy of the tooth-billed pigeon (Didunculus strigirostris). J. Zool. Lond. 174, 255–176 [Google Scholar]
- Farlow J. O.1987Speculations about the diet and digestive physiology of herbivorous dinosaurs. Paleobiology 13, 60–72 [Google Scholar]
- Gionfriddo J. P., Best L. B.1996Grit-use patterns in North American birds: the influence of diet, body size, and gender. Wilson Bull. 108, 685–696 [Google Scholar]
- Gradstein F. M., Ogg J. G., Smith A. G. (eds) 2004A geologic time scale 2004 Cambridge, UK: Cambridge University Press [Google Scholar]
- Gregory W. K.1927The Mongolian life record. Sci. Mon. 24, 169–181 [Google Scholar]
- Hofer H.1950Zur Morphologie der Kiefermuskulatur der Vögel. Zoologische Jahrbücher, Abteilung für Anatomie und Ontogenie der Tiere 70, 427–556 [Google Scholar]
- Kobayashi Y., Lu J. C., Dong Z. M., Barsbold R., Azuma Y., Tomida Y.1999Herbivorous diet in an ornithomimid dinosaur. Nature 402, 480–481 (doi:10.1038/44999)10591205 [Google Scholar]
- Kurzanov S. M.1992A giant protoceratopsid from the Upper Cretaceous of Mongolia. Palaeontol. J. 26, 103–116 [Google Scholar]
- Meinertzhagen R.1954Grit. Bull. Br. Ornithol. Club 74, 97–102 [Google Scholar]
- Norman D. B., Weishampel D. B.1985Ornithopod feeding mechanisms: their bearing on the evolution of herbivory. Am. Nat. 126, 151–164 (doi:10.1086/284406) [Google Scholar]
- Norman D. B., Weishampel D. B.1991Feeding mechanisms in some small herbivorous dinosaurs: processes and patterns. In Biomechanics in evolution (eds Rayner J. M. V., Wootton R. J.), pp. 161–181 Cambridge, UK: Cambridge University Press [Google Scholar]
- Osborn H. F.1923Two Lower Cretaceous dinosaurs from Mongolia. Am. Museum Novitates 95, 1–10 [Google Scholar]
- Osborn H. F.1924Psittacosaurus and Protiguanodon: two Lower Cretaceous iguanodonts from Mongolia. Am. Museum Novitates 127, 1–16 [Google Scholar]
- Ostrom J. H.1978Leptoceratops gracilis from the ‘Lance’ Formation of Wyoming. J. Paleontol. 52, 697–704 [Google Scholar]
- Qi Z., Barrett P. M., Eberth D. A.2007Social behaviour and mass mortality in the basal ceratopsian dinosaur Psittacosaurus (Early Cretaceous, People's Republic of China). Palaeontology 50, 1023–1029 (doi:10.1111/j.1475-4983.2007.00709.x) [Google Scholar]
- Reilly S. M., McBrayer L. D., White T. D.2001Prey processing in amniotes: biomechanical and behavioral patterns of food reduction. Comp. Biochem. Physiol. A 128, 397–415 (doi:10.1016/S1095-6433(00)00326-3) [DOI] [PubMed] [Google Scholar]
- Russell D. A., Zhao X.1996New psittacosaur occurrences in Inner Mongolia. Can. J. Earth Sci. 33, 637–648 [Google Scholar]
- Rybczynski N., Vickaryous M. K.2001Evidence of complex jaw movement in the Late Cretaceous ankylosaurid Euoplocephalus tutus. In The armored dinosaurs (ed. Carpenter K.), pp. 299–317 Bloomington, IN: Indiana University Press [Google Scholar]
- Sereno P. C.1987The ornithischian dinosaur Psittacosaurus from the Lower Cretaceous of Asia and the relationships of the Ceratopsia. Doctoral dissertation, Columbia University, New York [Google Scholar]
- Sereno P. C.2009Taxonomy, cranial morphology, and relationships of parrot-beaked dinosaurs (Ceratopsia: Psittacosaurus). In New perspectives on horned dinosaurs (eds Ryan M., Eberth D.), pp. 1–25 Bloomington, IN: Indiana University Press [Google Scholar]
- Thompson D'Arcy W.1899On characteristic points in the cranial osteology of the parrots. Proc. Zool. Soc. Lond. 1899, 9–46 [Google Scholar]
- Throckmorton G. S., Hopson J. A., Parks P.1981A redescription of Toxolophosaurus cloudi Olson, a Lower Cretaceous herbivorous sphenodontid reptile. J. Paleontol. 55, 586–597 [Google Scholar]
- Weishampel D. B., Norman D. B.1989Vertebrate herbivory in the Mesozoic: jaws, plants, and evolutionary metrics. Geol. Soc. Amer. Spec. Pap. 238, 87–100 [Google Scholar]
- Wings O.2004Identification, distribution, and function of gastroliths in dinosaurs and extant birds with emphasis on ostriches (Struthio camelus). Doctoral dissertation, Rheinischen Friedrich-Wilhelms-Universität Bonn, Bonn, Germany [Google Scholar]
- Witmer L. M., Rose K. D.1991Biomechanics of the jaw apparatus of the gigantic Eocene bird Diatryma: implications for diet and mode of life. Paleobiology 17, 95–120 [Google Scholar]
- Xu X.1997A new psittacosaur (Psittacosaurus mazongshanensis sp. nov.) from Mazongshan Area, Gansu Province, China. In Sino-Japanese silk road dinosaur expedition (ed. Dong Z.), pp. 48–67 Beijing: China Ocean Press [Google Scholar]
- You H. -L., Tanoue K., Dodson P.2008New data on cranial anatomy of the ceratopsian dinosaur Psittacosaurus major. Acta Palaeontol. Pol. 53, 183–196 [Google Scholar]
- Young C. C.1958The dinosaurian remains of Laiyang, Shantung. Palaeontol. Sin. (C) 16, 53–138 [Google Scholar]
- Zhou C., Gao K. Q., Fox R. C., Chen S.2006A new species of Psittacosaurus (Dinosauria: Ceratopsia) from the Early Cretaceous Yixian Formation, Liaoning, China. Palaeoworld 15, 100–114 (doi:10.1016/j.palwor.2005.11.001) [Google Scholar]
- Zusi R. L.1993Patterns of diversity in the avian skull. In The skull: patterns of structural and systematic diversity, vol. 2 (eds Hanken J., Hall B. K.), pp. 391–437 Chicago, IL: University of Chicago Press [Google Scholar]