Abstract
When the optimal phenotype differs among environments, adaptive phenotypic plasticity can evolve unless constraints impede such evolution. Costs and limits of plasticity have been proposed as important constraints on the evolution of plasticity, yet confusion exists over their distinction. We attempt to clarify these concepts by reviewing their categorization and measurement, highlighting how costs and limits are defined in different currencies (and may describe the same phenomenon). Conclusions from studies that measure the costs of plasticity have been equivocal, but we caution that these conclusions may be premature owing to a potentially common correlation between environment-specific trait values and the magnitude of trait plasticities (i.e. multi-collinearity) that results in imprecise and/or biased estimates of the costs. Meanwhile, our understanding of the limits of plasticity, and how they may be underlain by the costs of plasticity, is still in its infancy. Based on our re-evaluation of these constraints, we discuss areas for future research.
Keywords: adaptation, canalization, constraint, cost of defence, homeostasis, phenotypic stability
1. Introduction
Phenotypes are determined by gene expression in particular environmental conditions, and understanding the factors that function in genotype–environment interactions is a central goal in evolutionary biology. In nature, where environmental conditions are perpetually variable, organisms face the challenge of maximizing fitness under heterogeneous conditions. Selection has solved this problem in numerous ways including environmental canalization, which reduces environmental influence on trait expression, and phenotypic plasticity, where a single genotype can produce multiple phenotypes under different environmental conditions. When a trait is canalized, it may be well-adapted to one environment, but when a trait is plastic it may be well-adapted to many environments (Bradshaw 1965; Pigliucci 2001). Yet, despite this benefit, plasticity remains far from ubiquitous. Consequently, there has been a great deal of interest in understanding how plasticity evolves, including the factors that favour and constrain its evolution, and the ecological importance of these outcomes (e.g. Schlichting 1986; Sultan 1987; DeWitt et al. 1998; Agrawal 2001; Pigliucci 2005; van Kleunen & Fischer 2005).
Numerous models have explored the evolution of plasticity either as a trait under direct selection or as the by-product of selection on distinct character states expressed in different environments (Scheiner 1993; Via et al. 1995; Berrigan & Scheiner 2004). While the manner in which plasticity evolves may depend on the trait in question and the source of environmental variation (Via et al. 1995), a general outcome of many theoretical models is that if the ability to be plastic carries with it some form of constraint, the evolution of plasticity may be impeded (van Tienderen 1991; DeWitt et al. 1998; Sultan & Spencer 2002; Ernande & Dieckmann 2004).
There is a wide range of factors that could potentially constrain the evolution of plasticity. Like any trait, plasticity may be constrained by a lack of genetic variation, allometric relationships among traits such that plasticity in one trait may constrain plasticity in another trait, environmental covariance such that certain types of plasticity cannot be expressed, or a phylogenetic history that may restrict the expression of particular trait values or plasticities (Schlichting & Pigliucci 1998). While all are worth considering, more interest has been placed on microevolutionary constraints called the ‘costs’ and ‘limits’ of plasticity (van Tienderen 1991; DeWitt et al. 1998; Pigliucci 2005; van Kleunen & Fischer 2005; Valladares et al. 2007).
Costs and limits of plasticity are theoretically championed, but remain empirically unclear owing to their current nebulous functional distinction. Over a decade ago, DeWitt et al. (1998, p. 78) defined a cost of plasticity as when ‘in a focal environment a plastic organism exhibits lower fitness while producing the same mean trait value as a fixed organism’. Hence, the costs of plasticity represent any fitness reduction incurred by a plastic individual compared with a non-plastic individual that expresses the same trait value. In contrast, limits of plasticity were defined as ‘evident when facultative development cannot produce a trait mean as near the optimum as can fixed development’. Hence, a limit of plasticity is detected when one observes that a plastic genotype cannot express the same phenotype as a non-plastic genotype. Therefore, costs and limits of plasticity are assessed using different currencies: costs are assessed in terms of fitness and limits are assessed in terms of other phenotypes.
Recently, van Kleunen & Fischer (2005, pp. 53–54) defined a cost of plasticity as a ‘reduction in the fitness of a genotype as a consequence of expressing a certain phenotype through plastic rather than fixed development’ while limits ‘differ from the costs of plasticity in that there is a cost of the trait value expressed in a single environment as a consequence of plasticity rather than a cost of having the potential for plastic development per se’. Van Kleunen & Fischer's (2005) definition of a cost is similar to that of DeWitt et al. (1998), although it is more restricted in that it focuses on the specific cost of producing a trait via plastic versus non-plastic means. In contrast, their definition of a limit confounds costs of plasticity and costs of the trait value. Given these different definitions, perhaps the time has come to re-evaluate the core concepts and determine whether costs and limits of plasticity are in fact distinct.
In this review, we discuss a number of issues related to distinguishing and measuring the costs and limits of plasticity. First, we review the distinction between various types of costs of plasticity and review methodology for their quantification. The majority of studies to date have revealed insignificant or weak costs of plasticity (van Kleunen & Fischer 2005, 2007; Van Buskirk & Steiner 2009), but we caution that a potentially widespread co-linearity between trait values and trait plasticities may complicate the empirical assessment of costs of plasticity. We highlight that the co-linearity between trait values and trait plasticities is necessarily environment-specific and, therefore, so may be the bias or imprecision in estimating the costs of plasticity. Second, we focus on the limits of plasticity, discussing their various potential types and empirical measurement. We highlight the fact that costs and limits may not be distinct and in many cases are probably two alternative views of the same constraint. Based on our review, we discuss avenues for future research aimed at improving our understanding of these constraints.
2. Re-evaluating the costs of plasticity
Phenotypically plastic organisms can incur costs in at least two different ways. First, there can be costs of expressing a suboptimal (or ‘wrong’) phenotype in a given environment (i.e. costs of phenotype–environment mismatching). In this case, the expressed phenotype is poorly matched to the environment, thereby preventing the organism from attaining the higher fitness that would be possible with a more optimal phenotype. Note that a non-plastic organism may frequently incur this type of cost when it finds itself in an environment to which it is poorly matched. Second, there can be costs of possessing the ability to be plastic (i.e. costs of plasticity per se). In this case, an individual experiences lower fitness not because of its phenotype, but simply owing to its ability to be plastic. While these two types of costs may be incurred simultaneously, they arise from distinct sources and therefore have different implications (Callahan et al. 2008). Statistical methods to address the distinction between the costs of phenotype–environment mismatching and costs of plasticity have been developed (DeWitt et al. 1998; Scheiner & Berrigan 1998) and are discussed further in the following section.
DeWitt et al. (1998) classified five potential kinds of costs of plasticity. Maintenance costs are energetic costs of sensory or regulatory systems needed to detect environmental conditions. Production costs are costs that plastic individuals incur to express a certain trait over-and-above the costs that a canalized individual pays to express the same trait. Information-acquisition costs are costs that are incurred by plastic individuals when obtaining information about environmental conditions (canalized individuals require no environmental information to construct their phenotype). Developmental-instability costs are costs associated with imperfect phenotype–environment matching owing to environmentally sensitive developmental processes. Finally, genetic costs are costs that result directly from linkage between loci affecting plasticity and loci with negative fitness effects, pleiotropic effects of loci affecting plasticity and other traits, or (negative) epistatic interactions among loci affecting plasticity and other loci. Recently, van Kleunen & Fischer (2005) echoed this classification scheme, but suggested that genetic costs should be more narrowly defined as ‘intrinsic genetic costs’ to reflect the fact that any of the five types of costs could be detected as negative correlations between plasticity and fitness, but only intrinsic genetic costs emerge from the mechanisms of linkage, pleiotropy or epistasis.
When considering these five costs of plasticity, it is important to consider whether they are distinct and tractable. Maintenance and production costs represent two fundamentally distinct potential costs of plasticity with different evolutionary implications because they are envisioned as environment-independent and environment-dependent, respectively (DeWitt et al. 1998; Scheiner & Berrigan 1998). For maintenance costs, plastic individuals must invest resources in maintaining the molecular/physiological ‘machinery’ needed to detect, monitor and respond to environmental conditions. Therefore, a plastic individual would need to maintain its sensory/regulatory mechanisms in all environments to accurately assess current environmental conditions. Canalized individuals need not make this investment. Alternatively, the magnitude of production costs of plasticity will depend upon the specific environment (Scheiner & Berrigan 1998; Sultan & Spencer 2002).
Because production costs should be environment-specific and maintenance costs should be environment-independent, these two costs of plasticity are predicted to differentially affect the evolution of plasticity (Sultan & Spencer 2002). While maintenance costs have been included in several models that demonstrate their potential role in preventing a population from evolving optimal plasticity (van Tienderen 1991; Moran 1992; León 1993), combinations of maintenance and production costs have only been incorporated into three models of plasticity evolution (Padilla & Adolph 1996; Sultan & Spencer 2002; Ernande & Dieckmann 2004). Padilla & Adolph (1996) modelled the evolution of reversible morphological plasticity and found that inclusion of maintenance and production costs did not qualitatively alter the outcome of their model. Alternatively, Sultan & Spencer (2002), in an extension of a model by Moran (1992), demonstrated that ‘global costs’ (e.g. maintenance costs) constrain the evolution of plasticity to a much greater extent than ‘local costs’ (e.g. production costs). Further, they argued that while local costs of plasticity should be less important in determining the fitness of a plastic genotype when migration occurs, local costs may be more common than global costs (DeWitt et al. 1998; Sultan & Spencer 2002). Ernande & Dieckmann (2004) also demonstrated that maintenance and production costs have different evolutionary consequences by varying the relative amount of these costs and showing that plasticity evolves as a compromise between an optimal and a cost-free (flat) reaction norm. Under only maintenance costs, the population evolves closer to the optimum in more frequently experienced environments. With only production costs, the evolution of the reaction norm is not affected by the environmental frequency. This outcome occurs because, by definition, production costs are only incurred in focal, inducing environments and, therefore, production costs counterbalance the benefits of expressing the optimal phenotype. Thus, maintenance and production costs represent two unique costs of plasticity that have different implications for the evolution of plasticity.
To evaluate environmental specificity of the costs of plasticity, we compiled a dataset (available upon request) of available studies containing 227 paired estimates of the cost of plasticity in two environments from 24 studies spanning 20 species. The majority of these (141 pairs; 62%) failed to detect significant costs of plasticity in either environment. Of the remaining 86 pairs, 5 per cent detected a cost of plasticity in both environments, 4 per cent detected a benefit of plasticity in both environments, 8 per cent detected a cost in one environment and a benefit in the other and 21 per cent found either a cost or a benefit in one environment and no selection in the other. Thus, among these same 86 pairs, 76 per cent found a change in selection gradient (i.e. change in sign or change from present to absent) across environments (χ2 = 22.51, d.f. = 1, p < 0.001), meaning that in most cases the selection gradient on plasticity changes across environments and that the majority of costs that have been detected are environment-specific. However, note that the majority of these studies estimate the cost of plasticity without deciphering whether the costs of plasticity stem from maintenance or production costs (but see Scheiner & Berrigan 1998).
One hypothesis for why costs of plasticity might differ among environments is that stress might increase the magnitude of the costs because of resource limitation (Scheiner & Berrigan 1998; Dorn et al. 2000; Steinger et al. 2003; Valladares et al. 2007). This hypothesis has attracted a lot of attention, resulting in a number of studies that estimate the costs of plasticity in two environments where one environment is envisaged as more ‘stressful’ than the other. Nonetheless, this hypothesis has received limited empirical support (Van Buskirk & Steiner 2009). Further theoretical and empirical work is needed to understand how and why the costs of plasticity may differ among environments.
We propose that two other types of costs of plasticity (i.e. information acquisition and developmental instability) may not represent distinct costs of plasticity. Information-acquisition costs (incurred when gathering information about the environment) are fundamentally similar to maintenance costs in that they should be incurred in all environments. In some cases, information acquisition may directly change the environment (e.g. behavioural inspection for predators may place prey into a predator environment), but this represents a cost of phenotype–environment mismatching rather than a cost of plasticity in that the phenotype (e.g. hiding) is no longer well matched to the environment (e.g. with predators). Developmental-instability costs result when plastic genotypes express inherently more variable development than canalized genotypes, thereby producing a number of less-than-optimal phenotypes (Palmer & Strobeck 1986; Scheiner et al. 1991). DeWitt (1998) and Relyea (2002) used systems of predator-induced snails and tadpoles, respectively, to estimate the developmental-instability cost by regressing within-environment variance for a family onto trait means and trait plasticities; neither of these studies found evidence for this kind of cost. We suggest that this cost represents an additional point of confusion between costs of plasticity and costs of phenotype–environment mismatching. If plasticity leads to more variable development and selection favours particular phenotypes from this distribution, then the costs associated with developmental instability actually emerge owing to the phenotype expressed, not from the ability to be plastic. Thus, costs associated with developmental instability are not true costs of plasticity.
3. How are costs of plasticity quantified?
Methods for quantifying the costs of plasticity were developed from methods for examining direct selection on a trait independent of selection on correlated traits (i.e. a multiple regression of fitness on trait values; Lande & Arnold 1983; van Tienderen 1991). Such an analysis can reveal direct selection on trait values while holding plasticity constant and vice versa. Therefore, a cost of plasticity is indicated by a negative partial regression coefficient for plasticity. Because the plasticity in a trait and the trait itself are unlikely to be completely independent, this approach can provide a useful means of estimating the former while controlling for the latter (DeWitt et al. 1998; Scheiner & Berrigan 1998).
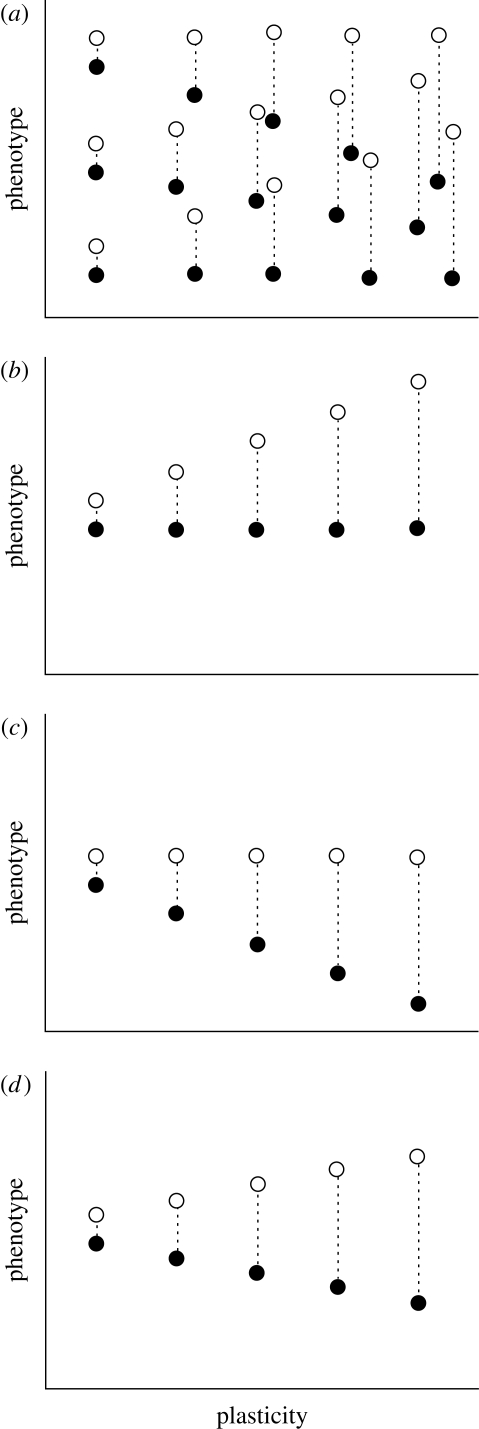
In applying the selection-gradient approach to quantifying costs of plasticity, little attention seems to have been given to the patterns of empirical data used in these tests. It has been previously noted that, in a selection-gradient analysis (as in any multiple regression), co-linearity between explanatory variables can result in a range of statistical problems (Lande & Arnold 1983; Mitchell-Olds & Shaw 1987, and references therein). However, this problem has been somewhat overlooked in studies measuring the costs of plasticity. While the original definition of a cost of plasticity is the cost of the ability to be plastic evaluated for genotypes that express the same trait values, the power of the multiple-regression approach is that it allows investigators to quantify costs of plasticity when different genotypes do not express the same phenotype in a given environment. Ideally, many combinations of phenotypic values and plasticities are possible, with extreme phenotypes being produced by both highly plastic genotypes and non-plastic genotypes (figure 1a). With such data, one can determine the independent contribution of each factor.
Figure 1.
Hypothetical relationships for genotypes that differ in phenotypic value and magnitude of plasticity. (a) A population of genotypes that shows independence between phenotypic values and magnitudes of plasticity. (b,c) Populations of genotypes that show independence between phenotypic values and magnitudes of plasticity in one environment but not in the other. (d) A population of genotypes showing no independence between phenotypic values and magnitudes of plasticity in either environment.
When phenotypic values are highly correlated with magnitudes of plasticity (e.g. open symbols in figure 1b, closed symbols in figure 1c, both symbols in figure 1d), two important statistical issues arise. First, if two highly correlated explanatory variables are both also correlated to fitness, including both explanatory variables produces much higher variation around the estimated selection coefficients. Although this does not bias the estimates of the selection coefficients, the greater variance around those estimates makes it inherently more difficult to conclude that a selection coefficient is different from zero. Mitchell-Olds & Shaw (1987) addressed the issue of multi-collinearity in multi-variate selection analysis and recommended removing one of the explanatory variables or developing a single composite variable. Given that we are interested in quantifying the separate effects of trait values and trait plasticities, neither of these recommended approaches would be satisfactory here.
The second issue arises when the two explanatory variables are highly correlated, but only one of them is actually correlated to fitness. In this case, a regression that simultaneously tries to estimate the separate effects of the two explanatory factors on fitness will produce a biased estimate for both factors. For example, if an environment contains genotypes that do not vary in their phenotypes but do differ in their magnitudes of plasticity (e.g. closed symbols in figure 1b or open symbols in figure 1c), then an unbiased estimate of the cost of plasticity can be made within that environment. However, if an environment contains genotypes that differ in phenotype and the genotypes with the most extreme trait values are also the genotypes with the most extreme plasticities (e.g. open symbols in figure 1b or closed symbols in figure 1c), estimates of the selection coefficients can be biased. For instance, consider the open symbols in figure 1b to represent the phenotypes produced by different genotypes. Even if we define a scenario in which higher trait values enjoy higher fitness but higher magnitudes of plasticity have no fitness effect, the regression will produce a positive selection coefficient for both trait value and plasticity. Thus, we would underestimate the positive effect of trait value and overestimate the positive effect of plasticity. This is just one of the several potential outcomes; the situation is even more troublesome when trait values are correlated with the plasticity in both environments (figure 1d). In general, the costs of plasticity can only be accurately estimated when trait values and plasticities are not highly correlated.
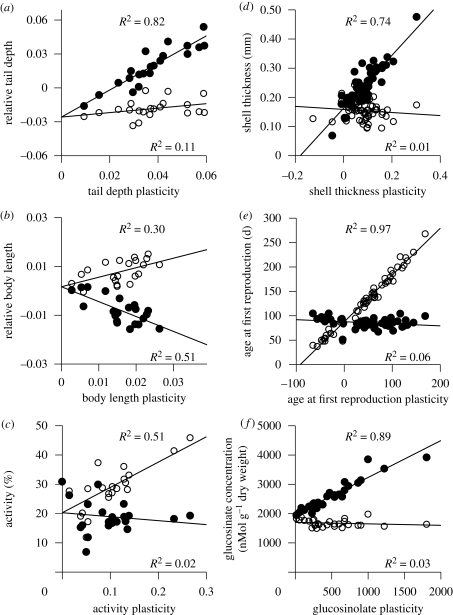
Given the importance of the relationship between phenotypic values and magnitudes of plasticity when trying to estimate the costs of plasticity, we need to examine whether empirical data ever contain such high correlations between trait values and trait plasticities. To do this, we examined the distributions from a variety of systems, not in an attempt to determine which patterns are more common, but to determine whether the situation might commonly exist. It appears that the problem of highly correlated trait values and trait plasticities occurs in all three systems in which we have worked (figure 2), including tadpoles responding to predators (Relyea 2002), freshwater snails responding to either predators or mates (J. R. Auld 2009, unpublished manuscript) and wild radishes responding to herbivory (Agrawal et al. 2002). In all three cases, we found the most plastic families produced the most extreme phenotypes and that, in many cases, plasticities and trait values were very highly correlated (up to an r2 = 0.97; see also Morris et al. 2006). Given the diversity of these systems, we suspect that highly correlated trait values and trait plasticities are common across many different taxa and inducing environments and, as a result, many of our current estimates of the costs of plasticity may possess unappreciated imprecision and bias. Just as costs of plasticity may be environment-specific, so may be these biases. While an immediate solution to this problem is not obvious, we urge a reinterpretation of the available data centred on the acknowledgement of the bias; it remains unclear how many current conclusions will hold upon re-evaluation of existing data. As a first step, we suggest a re-examination of the existing estimates of the costs of plasticity to reveal the extent of this problem. Subsequently, a new meta-analysis using only data where the estimates of costs of plasticity are not biased should be conducted.
Figure 2.
The relationship between trait values and trait plasticities (i.e. the difference in trait values across environments) for several traits in three species; all data points are family means. In all cases, the most plastic families exhibit the most extreme trait values. (a–c) Traits of larval wood frogs (Rana sylvatica) reared with (closed symbols) or without (open symbols) predators (Relyea 2002; plotted trait values are size-independent residuals from the linear regression of log-transformed trait values against log-transformed mass). (d) A morphological defence of freshwater snails (Physa acuta) reared with (closed) or without (open) predator cues and (e) a life-history trait from the same species reared with (closed) and without (open) mates (J. R. Auld 2009, unpublished manuscript). (f) A chemical-defence trait of wild radishes (Raphanus raphanistrum) when reared with (closed) and without (open) herbivore damage (Agrawal et al. 2002). Correlations between trait values and trait plasticities are provided as r2 values within each environment.
4. Re-evaluating the limits of plasticity
Six potential kinds of limits of plasticity have been proposed (DeWitt et al. 1998; Givnish 2002; van Kleunen & Fischer 2005; Valladares et al. 2007). Developmental-range limits occur when canalized development can produce more extreme phenotypes than plastic development. Information-reliability limits occur owing to imperfect sensory mechanisms or changing environmental conditions that prevent plastic organisms from accurately assessing environmental conditions. Lag-time limits occur when the expression of a plastic trait requires some amount of time, during which the organism expresses a suboptimal phenotype. Epiphenotype limits occur when plastic organisms that detect an environmental change late in ontogeny are not able to reorganize their phenotype adequately. Plasticity-history limits occur when the potential for a response to the current environment depends on previous responses where trait expression early in ontogeny may affect trait expression later in ontogeny. Finally, ecological limits occur when one environmental factor restricts the range of possible responses to other environmental factors.
One of the difficulties in understanding limits of plasticity is that the concept is inherently phenomenological; limits are identified when plastic and non-plastic genotypes differ in trait values. In our view, this is at the heart of the problem for empirically distinguishing costs of plasticity from limits of plasticity. First, because limits are defined in terms of trait values, there is the assumption that phenotypes and fitness show a direct correlation. Second, the underlying mechanism causing an observed limit may simply be a fitness reduction owing to costs of plasticity.
Van Kleunen & Fischer (2005) rejected the developmental-range limit by arguing that it must result from maintenance or production costs. We propose that various other limits of plasticity may stem from more general phenomena as well. For example, a high cost of plasticity could constrain the evolution of extreme phenotypes, such that a plastic genotype cannot achieve the same extreme phenotype as a non-plastic genotype. Thus, substantial fitness costs of carrying plasticity could prevent genotypes from evolving more extreme phenotypes, thereby placing a limit on phenotypic expression; other mechanisms could also be responsible for a limit to plasticity. Furthermore, both the epiphenotype limit (DeWitt et al. 1998) and the plasticity-history limit (Weinig & Delph 2001; van Kleunen & Fischer 2005) represent conditions whereby environmental induction early in ontogeny affects the potential for environmental induction later in ontogeny. Therefore, these two types of limits may stem from the same constraint—trait changes induced early in ontogeny that are not reversible (Schlichting & Pigliucci 1998). The information-reliability and lag-time limits stem from an imperfect ability to detect the environment and/or respond accordingly. Therefore, revealing the mechanism for these limits requires a demonstration of how the environment changes through time, how accurately the organism monitors these conditions and how quickly the organism can induce a response.
Of greater concern is that it may be impossible to distinguish ‘non-plastic’ genotypes from severely limited plastic genotypes. This is another situation where the costs of plasticity may be an important underlying cause for a limit of plasticity. For example, in resource-rich conditions, an organism may have the ability to incur both the maintenance and production costs of plasticity. However, resource-poor conditions may preclude an appropriate plastic response. Thus, limits of plasticity may result from underlying, environment-specific costs of plasticity (Valladares et al. 2007). In general, the ecological community within which natural populations are imbedded provides an important context for determining the scope of plasticity that can evolve. For example, if species are adapted to certain interspecific interactions or abiotic conditions, this may affect their potential plastic responses to other environmental variables.
5. How are limits of plasticity measured?
Several of the proposed limits of plasticity described by DeWitt et al. (1998) have been estimated empirically. For example, the developmental-range limit is confirmed if one finds that the most extreme phenotypes are produced by the least plastic individuals (or families). DeWitt (1998) found no support for developmental-range limits in freshwater snails. Instead, similar to the pattern described in the previous section (figure 2), the most plastic families often produced the most extreme trait values. The same result has been found in clonal herbs responding to competition (van Kleunen et al. 2000). This also appears to be a common result in plant-defence studies where species or genotypes with the highest level of defence also demonstrate the highest level of induction in response to an enemy (i.e. the most plastic are also the most defended; Morris et al. 2006). Hence, there appears to be no support for the developmental-range limit. Other limits of plasticity, such as the lag-time or epiphenotype limits, have been studied from a developmental perspective (Diggle 2002) and continue to be challenging concepts to understand mechanistically. However, it is clear that the timing of exposure to environmental cues can affect an organism's ability to respond to multiple environmental variables simultaneously (e.g. Weinig & Delph 2001; Relyea 2003; Cipollini 2004).
Researchers have investigated the ecological limits of plasticity by examining how a plastic response to one environment can limit the plastic response to a different environment. This has recently been reviewed by Valladares et al. (2007), but to highlight one early study that demonstrated such effects, Weinig (2000) showed that the photoperiod and temperature under which plants were grown significantly affected their elongation response to a change in light availability. Numerous other studies have demonstrated that response to one environment can alter the capacity for response to another, and these effects are determined, as mentioned earlier, by the ontogenetic stage that different environments are experienced (e.g. Weinig & Delph 2001; Cipollini 2004; Kurashige & Agrawal 2005). Collectively, as most organisms in nature probably detect and respond to multiple environments simultaneously and we are only beginning to understand how a response to one environment can influence responses to other environments, focusing on how responses to multiple environments are integrated seems crucial for understanding the basis of variation in plasticity and how it is constrained.
In general, many of the same factors that can constrain the expression and evolution of any trait can constrain plasticity (e.g. genetic correlations among plasticities, allometric relationships among plastic traits, phylogenetic history; Scheiner et al. 1991; Schlichting & Pigliucci 1998; Callahan & Pigliucci 2005). Ultimately, the developmental responses that generate plasticity can themselves be fundamental constraints on the expression and evolution of plasticity. Tonsor & Scheiner (2007) sketched a useful heuristic model for understanding how trait correlations may change across an environmental gradient, which can be readily extended to understand how one environmental factor may influence the expression of plasticity to a second environmental factor. Simply put, if a given resource (e.g. water, nitrogen) is shared among a set of biosynthetic pathways that result in the expression of several traits, the shared limiting resource can influence the pattern of trait correlations. When a shared resource is fully saturating and all pathways can function, the underlying developmental linkage (i.e. the dependence on the same resource) may not be revealed. However, if a shared resource is limiting, any change in flux through one pathway must result in a change in flux through another pathway. Thereby, the developmental mechanisms that result in the expression of plasticity are important for understanding environment-specific constraints on plasticity, specifically trade-offs among traits.
6. Experimental considerations for future studies
Central to a continued improvement of our understanding of the evolutionary constraints on plasticity will be a continued focus on how these constraints function in each environment and how they change among environments. We have highlighted that the correlation between trait values and trait plasticities can be environment-specific, leading to environment-specific sources of error in estimating the costs of plasticity. The current statistical framework for determining the magnitude of these costs can be used successfully provided that future studies explore the underlying relationship between trait values and trait plasticities in each environment and interpret the results accordingly. As some costs of plasticity are envisioned as environment-specific (e.g. production costs), this presents an intriguing problem for empiricists seeking to measure them. In general, this environment-specific focus represents a departure from previous work that has examined the relationship between the mean trait value across environments and plasticity (e.g. Pigliucci et al. 2003).
The apparently widespread underlying connection between trait values and trait plasticities that we have discussed is both a problem for our current methods of analysis and an important area for future studies to explore. For example, when the relationship between trait values and plasticities is flat in one environment and not in another environment (e.g. figure 2d–f), variation in plasticity is completely determined by variation in the phenotype in one environment. If this relationship gradually changes across the range of environmental variation, this suggests that plasticity itself may be the by-product of expressing different character states in different environments. Future studies that explore the genetic correlation between trait values and trait plasticities and how this relationship changes among environments will be useful for distinguishing the independent evolution of trait means and trait plasticities. Therefore, in lieu of focusing on the limits of plasticity per se in the future, we suggest that researchers focus on understanding variation in plasticity, whether it is independent from trait values expressed in each environment, and how this variation is affected by responses to additional environments.
In general, to explore the diversity of constraints on plasticity we need to consider a broad range of traits, including accurate measures of fitness. Most studies to date have been on morphological or structural traits that are hypothesized to be adaptive. Very few studies have examined physiological or biochemical traits despite the fact that these traits may be related to the developmental mechanisms for plasticity (Bradshaw 1965), and are therefore important for understanding maintenance or production costs. Future studies designed to evaluate selection on plasticity in physiological/biochemical traits, especially in response to multiple environmental factors, will be of great value for understanding the mechanisms that underlie the costs of plasticity. Furthermore, owing to the central role that fitness plays in estimating costs of plasticity, it is crucial to measure an accurate proxy for fitness that realistically approximates the contribution of an individual to the next generation.
In choosing the traits to study, it is particularly important for future studies to consider the genetic basis of plasticity and whether this is distinct from the genetic basis of the traits themselves, including how the genetic underpinnings of trait value and trait plasticity change across environments. Studies that have begun to unravel the genetic basis of plasticity through quantitative-genetic analyses, artificial selection and QTL analyses show a great amount of variation in the genetic basis of plasticity in the same trait across environments (e.g. Callahan & Pigliucci 2005; Lacaze et al. 2009). If plasticity in a given trait is influenced by ‘plasticity genes’ that are distinct from genes that affect trait expression in a particular environment (i.e. the epistasis model for the evolution of plasticity; Scheiner 1993), this may increase the likelihood that costs of plasticity exist.
Given that the costs of plasticity and the potential for bias in estimating these costs can be environment-specific and the environments that we select to assess plastic responses are typically selected from multiple possible points along multiple environmental continua, it may be important to assess the costs of plasticity at multiple points along the reaction norm to determine how increased magnitudes of plasticity affect our estimates of constraints. Here, is it worth noting that the selection-gradient approach discussed above is explicitly designed for estimating costs of plasticity in two contrasting environments. It is increasingly clear that patterns of trait correlations (and therefore their plasticities) can change across environments (e.g. Pigliucci & Preston 2004; Tonsor & Scheiner 2007), which can complicate the measurement and interpretation of constraints on plasticity and highlights the importance of evaluating how one environmental factor can alter the reaction norm to additional environmental factors. Additionally, the environmental specificity of a cost of plasticity should be considered in determining whether observed costs represent maintenance or production costs of plasticity. This point has been shown to be important in theoretical studies, but has been lost in the majority of empirical examinations (but see Scheiner & Berrigan 1998).
Our empirical understanding of the constraints on plasticity comes from a restricted sample of the biota (Van Buskirk & Steiner 2009). Most studies have focused on organisms collected from natural populations, but recently an argument has been made for crossing organisms from multiple natural populations to reshuffle the genes that affect the costs of plasticity and reveal constraints that may be masked in natural populations. Three recent studies have used such techniques, rearing plants from recombinant inbred lines (RILs; all from the Brassicaceae family) in multiple environments to explore whether these lines differ in costs of plasticity (Callahan et al. 2005; Weinig et al. 2006; Dechaine et al. 2007). Among these three studies the proportion of tests for costs of plasticity that was significant was higher compared to studies using genotypes from natural populations (van Kleunen & Fischer 2007). A benefit of using RILs is that subsequent work may be able to reveal the genetic basis of the costs of plasticity. However, if we can only detect costs of plasticity by creating artificially combined genotypes that have not been exposed to selection in nature, we must be cautious in extrapolating conclusions to the natural populations. Interestingly, comparisons of this type may be useful in determining whether there is any evidence for selection having operated in the past to reduce the prevalence of costs of plasticity in nature.
7. Conclusions
Despite a good deal of empirical research, we are only beginning to understand the constraints of plasticity and their ecological and evolutionary implications. Because costs of plasticity are more mechanistic descriptions of constraints whereas limits are generally phenomenological, costs of plasticity can be one of the mechanisms underlying limits of plasticity. As such, the concept of limits of plasticity may not be a particularly useful concept for understanding the evolutionary constraints on plasticity. Empirical studies quantifying costs of plasticity have found either no fitness effects or weak effects (both positive and negative) and these fitness effects are frequently environment-specific. The inherent imprecision and bias in many of these tests, owing to high correlations between trait values and trait plasticities, appears to have gone previously unnoticed but is of potentially large importance regarding our ability to draw any generalizations about the statistical significance, magnitude and direction of these costs. We need to re-examine the prevalence of this issue in past studies and consider the potential for this problem in future studies.
Why might costs of plasticity be so small and difficult to detect? As discussed above, selection may have acted to reduce the costs of plasticity. Nonetheless, detection of small or non-significant costs of plasticity does not necessarily mean that selection has operated to reduce them. Selection on trait plasticity may be weaker than selection on trait values and, therefore, this may mean that the costs of plasticity have always been relatively small. Van Buskirk & Steiner's (2009) meta-analysis suggests that this is not necessarily the case, but it remains to be determined how biased estimation of selection on plasticity may affect these conclusions. Finally, studying a diverse array of traits and considering whether adaptive plasticity evolves as the target or by-product of selection may reveal important information regarding the genetic basis of plasticity in particular traits. If plasticity in a given trait is not coded for by specific plasticity genes and has evolved as the by-product of selection, our experiments that measure costs of plasticity in this trait may yield results that are difficult to interpret. Only as we consider all of these issues will we have a reasonable sense for whether costs of plasticity are important in the evolution of plasticity. Clearly, understanding how organisms produce integrated, adaptive and environment-specific phenotypes is an important part of understanding evolution in natural populations, especially given the predicted changes in global climate.
Acknowledgements
We thank S. Sultan, J. Van Buskirk and several anonymous reviewers for comments on the manuscript. J.R.A. was supported by fellowships from the A.W. Mellon Foundation and received post-doctoral support from the French C.N.R.S. R.A.R. acknowledges the long-term support of his plasticity research from the National Science Foundation.
References
- Agrawal A. A.2001Phenotypic plasticity in the interactions and evolution of species. Science 294, 321–326 (doi:10.1126/science.1060701) [DOI] [PubMed] [Google Scholar]
- Agrawal A. A., Conner J. K., Johnson M. T. J., Wallsgrove R.2002Ecological genetics of an induced plant defense against herbivores: additive genetic variance and costs of phenotypic plasticity. Evolution 56, 2206–2213 [DOI] [PubMed] [Google Scholar]
- Berrigan D., Scheiner S. M.2004Modeling the evolution of phenotypic plasticity. In Phenotypic plasticity: function and conceptual approaches (eds DeWitt T. J., Scheiner S. M.), pp. 82–97 New York, NY: Oxford University Press [Google Scholar]
- Bradshaw A. D.1965Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115–155 (doi:10.1016/S0065-2660(08)60048-6) [Google Scholar]
- Callahan H. S., Pigliucci M.2005Indirect consequences of artificial selection on plasticity to light quality in Arabidopsis thaliana. J. Evol. Biol. 18, 1403–1415 (doi:10.1111/j.1420-9101.2005.00963.x) [DOI] [PubMed] [Google Scholar]
- Callahan H. S., Dhanoolal N., Ungerer M. C.2005Plasticity genes and plasticity costs: a new approach using an Arabidopsis recombinant inbred population. New Phytol. 166, 129–140 (doi:10.1111/j.1469-8137.2005.01368.x) [DOI] [PubMed] [Google Scholar]
- Callahan H. S., Maughan H., Steiner U. K.2008Phenotypic plasticity, costs of phenotypes, costs of plasticity. Ann. N. Y. Acad. Sci. 1133, 44–66 (doi:10.1196/annals.1438.008) [DOI] [PubMed] [Google Scholar]
- Cipollini D.2004Stretching the limits of plasticity: can a plant defend against both competitors and herbivores? Ecology 85, 28–37 (doi:10.1890/02-0615) [Google Scholar]
- Dechaine J. M., Johnston J. A., Brock M. T., Weinig C.2007Constraints on the evolution of adaptive plasticity: costs of plasticity to density are expressed in segregating progenies. New Phytol. 176, 874–882 (doi:10.1111/j.1469-8137.2007.02210.x) [DOI] [PubMed] [Google Scholar]
- DeWitt T. J.1998Costs and limits of phenotypic plasticity: tests with predator-induced morphology and life history in a freshwater snail. J. Evol. Biol. 11, 465–480 (doi:10.1007/s000360050100) [Google Scholar]
- DeWitt T. J., Sih A., Wilson D. S.1998Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81 (doi:10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- Diggle P. K.2002A developmental morphologist's perspective on plasticity. Evol. Ecol. 16, 267–283 (doi:10.1023/A:1019680527788) [Google Scholar]
- Dorn L., Pyle E. H., Schmitt J.2000Plasticity to light cues and resources in Arabidopsis thaliana: testing for adaptive value and costs. Evolution 54, 1982–1994 [DOI] [PubMed] [Google Scholar]
- Ernande B., Dieckmann U.2004The evolution of phenotypic plasticity in spatially structured environments: implications of intraspecific competition, plasticity costs and environmental characteristics. J. Evol. Biol. 17, 613–628 (doi:10.1111/j.1420-9101.2004.00691.x) [DOI] [PubMed] [Google Scholar]
- Givnish T. J.2002Ecological constraints on the evolution of plasticity in plants. Evol. Ecol. 16, 213–242 (doi:10.1023/A:1019676410041) [Google Scholar]
- Kurashige N. S., Agrawal A. A.2005Phenotypic plasticity to light competition and herbivory in Chenopodium album (Chenopodiaceae). Am. J. Bot. 92, 21–26 (doi:10.3732/ajb.92.1.21) [DOI] [PubMed] [Google Scholar]
- Lacaze X., Hayes P. M., Korol A.2009Genetics of phenotypic plasticity: QTL analysis in barley Hordeum vulgare. Heredity 102, 163–173 (doi:10.1038/hdy.2008.76) [DOI] [PubMed] [Google Scholar]
- Lande R., Arnold S. J.1983The measurement of selection on correlated characters. Evolution 37, 1210–1226 (doi:10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- León J.1993Plasticity in fluctuating environments. In Adaptation in stochastic environments (eds Yoshimura J., Clark C. W.), pp. 105–121 Berlin, Germany: Springer-Verlag [Google Scholar]
- Mitchell-Olds T., Shaw R. G.1987Regression analysis of natural selection: statistical inference and biological interpretation. Evolution 41, 1149–1161 (doi:10.2307/2409084) [DOI] [PubMed] [Google Scholar]
- Moran N.1992The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989 (doi:10.1086/285369) [Google Scholar]
- Morris W. F., Traw M. B., Bergelson J.2006On testing for a trade-off between constitutive and induced resistance. Oikos 112, 102–110 (doi:10.1111/j.0030-1299.2006.14253.x) [Google Scholar]
- Padilla D. K., Adolph S. C.1996Plastic inducible morphologies are not always adaptive: the importance of time delays in a stochastic environment. Evol. Ecol. 10, 105–117 (doi:10.1007/BF01239351) [Google Scholar]
- Palmer A. R., Strobeck C.1986Fluctuating asymmetry: measurement, analysis, patterns. Ann. Rev. Ecol. Syst. 17, 391–421 (doi:10.1146/annurev.es.17.110186.002135) [Google Scholar]
- Pigliucci M.2001Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: The Johns Hopkins University Press [Google Scholar]
- Pigliucci M.2005Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 20, 481–486 (doi:10.1016/j.tree.2005.06.001) [DOI] [PubMed] [Google Scholar]
- Pigliucci M., Preston K.2004Phenotypic integration: studying the ecology and evolution of complex phenotypes. New York, NY: Oxford University Press [Google Scholar]
- Pigliucci M., Pollard H., Cruzan M. B.2003Comparative studies of evolutionary responses to light environments in Arabidopsis. Am. Nat. 161, 68–82 (doi:10.1086/345460) [DOI] [PubMed] [Google Scholar]
- Relyea R. A.2002Costs of phenotypic plasticity. Am. Nat. 159, 272–282 (doi:10.1086/338540) [DOI] [PubMed] [Google Scholar]
- Relyea R. A.2003Predators come and predators go: the reversibility of predator-induced traits. Ecology 84, 1840–1848 (doi:10.1890/0012-9658(2003)084[1840:PCAPGT]2.0.CO;2) [Google Scholar]
- Scheiner S. M.1993Genetics and evolution of phenotypic plasticity. Ann. Rev. Ecol. Syst. 24, 35–68 (doi:10.1146/annurev.es.24.110193.000343) [Google Scholar]
- Scheiner S. M., Berrigan D.1998The genetics of phenotypic plasticity. VIII. The cost of plasticity in Daphnia pulex. Evolution 52, 368–378 (doi:10.2307/2411074) [DOI] [PubMed] [Google Scholar]
- Scheiner S. M., Caplan R. L., Lyman R. F.1991The genetics of phenotypic plasticity. III. Genetic correlations and fluctuating asymmetry. J. Evol. Biol. 4, 51–68 (doi:10.1046/j.1420-9101.1991.4010051.x) [Google Scholar]
- Schlichting C. D.1986The evolution of phenotypic plasticity in plants. Ann. Rev. Ecol. Syst. 17, 667–693 (doi:10.1146/annurev.es.17.110186.003315) [Google Scholar]
- Schlichting C. D., Pigliucci M.1998Phenotypic evolution: a reaction norm perspective Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- Steinger T., Roy B. A., Stanton M. L.2003Evolution in stressful environments. II. Adaptive value and costs of plasticity in response to low light in Sinapis arvensis. J. Evol. Biol. 16, 313–323 (doi:10.1046/j.1420-9101.2003.00518.x) [DOI] [PubMed] [Google Scholar]
- Sultan S. E.1987Evolutionary implications of phenotypic plasticity in plants. Evol. Biol. 21, 127–178 [Google Scholar]
- Sultan S. E., Spencer H. G.2002Metapopulation structure favors plasticity over local adaptation. Am. Nat. 160, 271–283 (doi:10.1086/341015) [DOI] [PubMed] [Google Scholar]
- Tonsor S. J., Scheiner S. M.2007Plastic trait integration across a CO2 gradient in Arabidopsis thaliana. Am. Nat. 169, E119–E140 (doi:10.1086/513493) [DOI] [PubMed] [Google Scholar]
- Valladares F., Gianoli E., Gómez J. M.2007Ecological limits to plant phenotypic plasticity. New Phytol. 176, 749–763 (doi:10.1111/j.1469-8137.2007.02275.x) [DOI] [PubMed] [Google Scholar]
- Van Buskirk J., Steiner U. K.2009The fitness costs of developmental canalization and plasticity. J. Evol. Biol. 22, 852–860 (doi:10.1111/j.1420-9101.2009.01685.x) [DOI] [PubMed] [Google Scholar]
- van Kleunen M., Fischer M.2005Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 166, 49–60 (doi:10.1111/j.1469-8137.2004.01296.x) [DOI] [PubMed] [Google Scholar]
- van Kleunen M., Fischer M.2007Progress in the detection of costs of phenotypic plasticity. New Phytol. 176, 727–730 (doi:10.1111/j.1469-8137.2007.02296.x) [DOI] [PubMed] [Google Scholar]
- van Kleunen M., Fischer M., Schmid B.2000Costs of plasticity in foraging characters of the clonal plant Ranunculus reptans. Evolution 54, 1947–1955 (doi:10.1554/0014-3820(2000)054[1947:COPIFC]2.0.CO;2) [PubMed] [Google Scholar]
- van Tienderen P. H.1991Evolution of generalists and specialists in spatially heterogeneous environments. Evolution 45, 1317–1331 [DOI] [PubMed] [Google Scholar]
- Via S., Gomulkiewicz R., de Jong G., Scheiner S. M., Schlichting C. D., van Tienderen P. H.1995Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212–217 (doi:10.1016/S0169-5347(00)89061-8) [DOI] [PubMed] [Google Scholar]
- Weinig C.2000Limits to adaptive plasticity: temperature and photoperiod influence shade-avoidance responses. Am. J. Bot. 87, 1660–1668 (doi:10.2307/2656743) [PubMed] [Google Scholar]
- Weinig C., Delph L. F.2001Phenotypic plasticity early in life constrains developmental responses later. Evolution 55, 930–936 (doi:10.1554/0014-3820(2001)055[0930:PPEILC]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Weinig C., Johnston J., German Z. M., Demink L. M.2006Local and global costs of adaptive plasticity to density in Arabidopsis thaliana. Am. Nat. 167, 826–836 (doi:10.1086/503530) [DOI] [PubMed] [Google Scholar]