Abstract
In zones of sympatry between closely related species, species recognition errors in a competitive context can cause character displacement in agonistic signals and competitor recognition functions, just as species recognition errors in a mating context can cause character displacement in mating signals and mate recognition. These two processes are difficult to distinguish because the same traits can serve as both agonistic and mating signals. One solution is to test for sympatric shifts in recognition functions. We studied competitor recognition in Hetaerina damselflies by challenging territory holders with live tethered conspecific and heterospecific intruders. Heterospecific intruders elicited less aggression than conspecific intruders in species pairs with dissimilar wing coloration (H. occisa/H. titia, H. americana/H. titia) but not in species pairs with similar wing coloration (H. occisa/H. cruentata, H. americana/H. cruentata). Natural variation in the area of black wing pigmentation on H. titia intruders correlated negatively with heterospecific aggression. To directly examine the role of wing coloration, we blackened the wings of H. occisa or H. americana intruders and measured responses of conspecific territory holders. This treatment reduced territorial aggression at multiple sites where H. titia is present, but not at allopatric sites. These results provide strong evidence for agonistic character displacement.
Keywords: character displacement, agonistic character displacement, species recognition, competitor recognition, mate recognition, intrasexual competition
1. Introduction
When closely related species come into secondary contact, species recognition errors may arise in both intrasexual and intersexual contexts. Similarity between species in traits used in mate recognition can lead to maladaptive cross-species courtship and mating (Estrada & Jiggins 2008; Groening & Hochkirch 2008). Selection resulting from such mate recognition errors can cause mating signals and/or mate preferences to diverge in areas of sympatry compared with areas of allopatry, a process known as reproductive character displacement (RCD; Brown & Wilson 1956). Because multiple processes can lead to similar geographical patterns in mating signals, it is important to also test for shifts in mate preferences when evaluating putative cases of RCD (Gabor & Ryan 2001). Several studies have documented sympatric shifts in both mating signals and mate preferences (e.g. Ryan et al. 1996; Pfennig 2000; Ptacek 2000; Hobel & Gerhardt 2003; Hoskin et al. 2005; Rundle et al. 2005; Higgie & Blows 2008; Lemmon 2009).
A less explored consequence of secondary contact is the possibility of maladaptive interspecific aggression arising from similarity between species in traits used for recognizing conspecific competitors. Selection resulting from errors in competitor recognition can cause divergence in agonistic signals and/or competitor recognition functions between species in sympatry, a process known as agonistic character displacement (ACD; Grether et al. 2009). There are many putative examples of divergent ACD, but most involve displacement in traits that might also be subject to RCD (reviewed in Grether et al. 2009). For example, male pied flycatchers (Ficedula hypoleuca) occur in both brown and black morphs; the black morph resembles the competitively dominant collared flycatcher (F. albicollis). Males of the brown morph receive less territorial aggression from collared flycatchers than the black morph and are only found where the two species occur in sympatry (Alatalo et al. 1994). This geographical pattern has the effect of reducing competitive interference between the species in sympatry, but selection against cross-species mating provides a plausible alternative explanation. Indeed, while female pied flycatchers prefer black males over brown males at allopatric sites, this preference is reversed at sympatric sites (Saetre et al. 1997; Saether et al. 2007).
In another illustrative example, the average wing spot size of the damselfly Calopteyrx splendens decreases across populations with increasing relative abundance of the large-wing-spotted and competitively dominant congener, C. virgo, perhaps because territorial aggression from C. virgo increases with the size of the black spots on male C. splendens (Tynkkynen et al. 2004, 2005, 2006). Selection against cross-species mating offers a plausible alternative explanation for the geographical pattern, however, because female C. splendens appear to use wing pigmentation to discriminate between conspecific and heterospecific males (Svensson et al. 2007).
Many secondary sexual characters are used in both mate choice and mate competition contexts (reviewed in Berglund et al. 1996). Therefore, sympatric shifts in such signals (coloration, song, etc.) are often difficult to attribute to RCD or ACD alone. Sympatric shifts in recognition functions do not suffer from this ambiguity because they involve different behavioural contexts and have distinctly different outcomes (i.e. reductions in cross-species mating versus reductions in interspecific aggression). We tested for sympatric shifts in competitor recognition in the damselfly genus Hetaerina.
Territorial aggression between sympatric Hetaerina is best understood as a case of misidentification (Murray 1981; Schultz & Switzer 2001) rather than adaptive defence of a shared limiting resource (Cody 1969), as the following details of this system will clarify. Adult males compete for small mating territories along river margins where females oviposit (Alcock 1987; Weichsel 1987; Grether 1996). These clearly are not feeding territories because foraging activity peaks in the hours before males arrive on their territories in the morning and again after they abandon defence in the late afternoon (Grether & Grey 1996). Mating is initiated by tandem formation, in which the male clasps the female's prothorax using his caudal abdominal appendage (Corbet 1999; Cordoba-Aguilar & Cordero-Rivera 2005). Tandem pairs typically fly through multiple territories and only rarely oviposit in the territory of the female's mate (Grether 1996). Residents do not prevent tandem pairs from using oviposition sites (submerged vegetation) within their territories and do not abandon their territories if oviposition substrate is experimentally removed (Alcock 1987). Thus, a Hetaerina territory is essentially defended air space within which the resident male has priority of access to passing females (Weichsel 1987; Grether 1996). Logically, in such a system, males of different species should not waste time and energy competing for space.
We studied four sympatric species pairs—Hetaerina americana/H. titia, H. americana/H. cruentata, H. occisa/H. titia and H. occisa/H. cruentata—as well as allopatric populations of H. americana and H. occisa. Males of all species have red spots at the base of each wing, and the wing spot size is intrasexually selected, at least in H. americana (Grether 1996; Contreras-Garduno et al. 2006; Serrano-Meneses et al. 2007). Beyond the basal red spots, the wings of H. americana, H. occisa and H. cruentata are mostly transparent; H. occisa wings bear small apical red spots and the wing tips of H. cruentata are darkened (Westfall & May 1996). In contrast, H. titia has extensive black pigmentation on the wings (Cordoba-Aguilar et al. 2007). H. titia is also unique among Hetaerina in showing extensive variation in wing pigmentation both within and between populations (Johnson 1963; Garrison 1990). The hindwings of H. titia range from black only at the wing base (and otherwise transparent) to completely black, and there is geographical and seasonal variation in the extent of this variation (C. N. Anderson & G. F. Grether 2007, unpublished data). Despite the variation, we usually had no difficulty identifying males to species in the field based on wing coloration alone (verified by examination of abdominal claspers and other taxonomically diagnostic characters; Garrison 1990), but our results indicate that the damselflies themselves have trouble distinguishing species.
Our initial observations revealed that interspecific fights often occur where Hetaerina species are found in sympatry. Based on the assumption that interspecific aggression is maladaptive in this system (see above), we predicted that if males are able to distinguish between conspecific and heterospecific intruders, their ability to do so would be affected by variation in wing coloration and enhanced in sympatric compared with allopatric populations of the same species. Here we report the results of simulated intruder tests of two types: (i) tests in which territory holders were presented with tethered but otherwise unmanipulated males of two species; and (ii) tests in which territory holders were presented with conspecific males whose wing coloration was manipulated to resemble a congener and two types of controls (unmanipulated and sham-manipulated conspecifics). The second type of test was carried out at sites where the species occur in sympatry and also at allopatric sites, to test for sympatric shifts in competitor recognition.
2. Material and methods
(a). Sampling design and localities
Our sampling design is based on comparing sites where H. titia is sympatric with H. americana or H. occisa to sites where one of these species is present and H. titia is absent. Testing for character displacement patterns by comparing allopatric and sympatric populations requires some level of replication and phylogenetic independence within each of these categories (Schluter 2000). Based on the limited dispersal capabilities of damselflies, we guessed that geographically close populations would be more similar genetically than geographically distant populations and used this as a guide for selecting study sites. For each study site where H. titia occurs in sympatry with H. americana or H. occisa, we also sampled a nearby site where the congener occurs in allopatry from H. titia. Post hoc genetic analyses confirmed that we succeeded in sampling multiple, independent areas of sympatry (see electronic supplementary material).
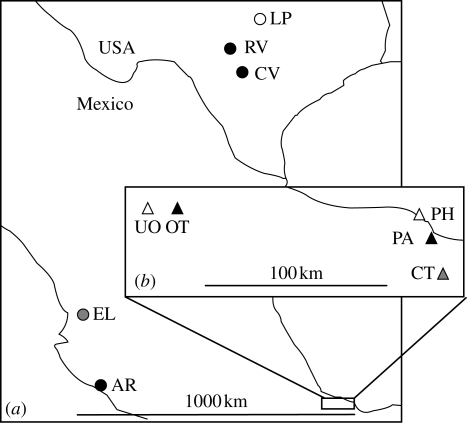
The experiments reported here were carried out in June–August 2006, May–July 2007 and April–May 2008 at 10 sites in Texas and Mexico (figure 1): Lampasas, Texas (LP: 31.07 N, 98.17 W); Ingram, Texas (RV: 30.07 N, 99.28 W); Castroville, Texas (CV: 29.34 N, 98.88 W); El Limon, Mexico (EL: 21.37 N, 104.62 W); Armeria, Mexico (AR: 18.96 N, 103.95 W); southwest of La Tinaja, Mexico (UO: 18.71 N, 96.57 W); southeast of La Tinaja, Mexico (OT: 18.69 N, 96.39 W); Playa Hermosa, Mexico (PH: 18.66 N, 95.13 W); La Palma, Mexico (PA: 18.56 N, 95.07 W); and Tebanca, Mexico (CT: 18.37 N, 95.00 W). From the standpoint of behavioural research, all of these sites are effectively one- or two-species sites, although other Hetaerina species were present at low densities at some sites. We visited each site multiple times between 2004 and 2008 and never observed H. titia at LP, EL, UO, PH or CT. We therefore classify these sites as allopatric from H. titia. H. cruentata was present at two of the latter sites (CT and EL). At three sites we performed replicate experiments in separate visits: EL (May 2007, May 2008), CV (April 2008, August 2008) and OT (June 2006, June 2007). In no cases did the results differ within sites between visits at p < 0.05; therefore, for most analyses, we present only the pooled results. The one exception (involving site CV) is explained below.
Figure 1.
(a) Distribution of the five H. americana (circles) study sites in Texas and Mexico. (b) Distribution of the five H. occisa (triangles) study sites in the Mexican state of Veracruz. The study sites were: Lampasas, Texas (LP); Ingram, Texas (RV); Castroville, Texas (CV); El Limon, Mexico (EL); Armeria, Mexico (AR); southwest of La Tinaja, Mexico (UO); southeast of La Tinaja, Mexico (OT); Playa Hermosa, Mexico (PH); La Palma, Mexico (PA); and Tebanca, Mexico (CT). Black triangles, H. occisa/H. titia; black circles, H. americana/H. titia; grey triangles, H. occisa/H. cruentata; grey circles, H. americana/H. titia; white triangles, H. occisa; white circles, H. americana.
(b). Experimental set-up and procedure
Study transects (50–100 m) were established along river margins. Each male damselfly was individually marked with a unique combination of three colours on its abdomen using paint pens. A male was classified as a territory holder if it was consistently seen at the same location (±1.5 m) on at least two consecutive days during the hours when males are territorial (10.00–18.00 h).
During a simulated territory intrusion test, an observer recorded the behaviour of the territory holder on a continuously running audio recorder, while a tethered male was presented using 0.3 m of fine transparent thread and a modified fishing pole. The pole was manoeuvred to keep the tethered male flying within the focal male's territory for 2 min. Each territory holder was tested with multiple tethered intruders within a 20–30 min window. A minimum of 5 min elapsed between consecutive tests on a territorial male and the order of treatments (see below) was varied systematically. This design allowed each individual territory holder to serve as his own control. Tethered males were captured outside the study transect and used for at most two tests before being released again outside the transect. All simulated intruder tests were carried out between 10.00–18.00 h under sunny or lightly clouded conditions (territory defence wanes under heavy cloudy cover).
The responses of territory holders to tethered intruders covered the full range of behaviours observed in natural territorial encounters. Aggressive responses that we recorded include chasing (flight towards the tethered male) and two forms of escalation that occur during chasing: ‘slams’, defined as attempts to ram the tethered intruder (whether successful or not), and ‘grabs’, defined as extended physical contact in which the resident lands on the intruder in flight.
(c). Responses to unmanipulated conspecific and heterospecific intruders
The main goal of this experiment, which was carried out on all four sympatric species pairs, was to determine whether territory holders discriminate between conspecific and heterospecific male intruders. Each territory holder was presented sequentially with both types of intruders, with the order of presentation alternating between trials. We conducted paired intrusion tests on 66 H. americana territory holders (AR: 16, CV: 33, EL: 17), 58 H. occisa territory holders (OT: 16, PA: 26, CT: 16), 79 H. titia territory holders (AR: 14, CV: 30, OT: 16, PA: 19) and 25 H. cruentata territory holders (CT: 15, EL: 10). A subset of these data was used to examine the effects of natural variation in the wing coloration of H. titia intruders on the responses of territory holders. We made an effort to use H. titia intruders representing the full range of wing spot variation present at each site.
Hetaernia titia wings were photographed with a digital camera (Canon 10D; Canon USA, Inc., Lake Success, NY, USA) equipped with a 100 mm macro lens and macro flash attachment (Canon MT-24EX). Measurements of total wing area and the extent of pigmentation were made using NIH Image software (US National Institutes of Health; available on the Internet at http://rsb.info.nih.gov/nih-image/). Wing spot size was measured as the proportion of total wing area covered with dark pigment.
(d). Responses to wing-colour-manipulated intruders
To directly examine the effects of an intruder's wing coloration on the responses of territory holders, while controlling for other traits that differ between species, we manipulated the wing colour of tethered intruders using ink. Specifically, we examined the effects of making the hindwings of H. americana and H. occisa intruders more closely resemble those of H. titia. We measured the responses of H. americana and H. occisa territory holders to colour-manipulated conspecifics at sites where H. titia was both present and absent. If present at a site, we also measured the responses of H. titia and H. cruentata territory holders.
The wing colour treatments were as follows. Clear: hindwings fully painted with colourless marker (Prismacolor PM-121; Sanford L.P., Oak Brook, IL, USA). Half Black: basal half of hindwings painted with black marker (Prismacolor PM-2) and distal half of hindwings painted with colourless marker. Black: hindwings fully painted with the black marker. The black marker used in this experiment was chosen because it closely matches the colour of H. titia wings, both to human eyes and as assessed with reflectance spectrometry. Each territory holder was presented sequentially with intruders of all three treatments, with the order of presentation alternating between trials. We conducted wing-colour-manipulated intrusion tests on 126 H. americana territory holders (AR: 16, CV: 33, RV: 17, EL: 44, LP: 16), 102 H. occisa territory holders (OT: 32, PA: 26, CT: 15, PH: 14, UO: 15), 103 H. titia territory holders (AR: 14, CV: 30, RV: 9, OT: 31, PA: 19) and 35 H. cruentata territory holders (CT: 19, EL: 16).
(e). Data analysis
Territorial aggression was quantified as the proportion of time the resident spent chasing the tethered intruder and by the rate of mid-air attacks: (number of slams + number of grabs)/trial duration. Attack rate was log (x + 0.01) transformed and proportion of time chasing was arcsine (√x) transformed to meet parametric assumptions.
We used paired t-tests to compare the response of territory holders to conspecific and heterospecific intruders, separately by species and site. The effects of natural variation in H. titia wing spot size were examined with generalized linear models. Each generalized linear model was fitted with a binomial error structure and logit link function, with the proportion of time chasing the H. titia intruder as the response variable, the size of the hindwing spot of the H. titia intruder fitted as the explanatory term and the proportion of time chasing the conspecific intruder as a covariate to control for individual variation in aggression. Because mean H. titia spot size at site CV differed between visits (t34 = 7.37, p < 0.0001), we present the results for each visit separately.
We used repeated-measures analyses of variance to analyse the results of the colour-manipulated intruder tests for each species separately. For H. americana and H. occisa, explanatory terms in the full model included the between-subjects factors sympatry with H. titia (levels: allopatric, sympatric) and site (nested within sympatry category), and the within-subjects factor intruder wing colour treatment (levels: clear, half, black). The interaction between sympatry status and intruder treatment was of particular interest because this tests for sympatric shifts in the response function. For H. titia and H. cruentata, all sites were of the same sympatry status, so this term was dropped from the models.
3. Results
(a). Responses to unmanipulated conspecific and heterospecific intruders
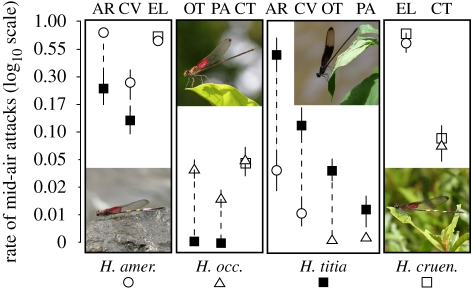
In most cases, territorial males attacked conspecific intruders at higher rates than heterospecific intruders, but the exceptions are informative (figure 2; table 1). Hetaerina titia territory holders were consistently more aggressive to conspecific intruders than to intruders of the sympatric congener, although the results for one site were not significant after a Bonferroni correction for multiple tests (table 1). Territorial H. americana and H. occisa attacked conspecific intruders more aggressively than H. titia intruders but did not distinguish between conspecific intruders and H. cruentata intruders. Hetaerina cruentata territory holders did not discriminate between conspecific intruders and either H. americana or H. occisa intruders. Compared with H. titia, H. cruentata is more similar to H. americana and H. occisa in wing coloration (figure 2). Therefore, these results support the idea that males use wing coloration to identify conspecific competitors and have difficulty distinguishing between males of similar-looking species.
Figure 2.
The average attack rate (attacks per second) ±s.e. of territory holders of four Hetaerina spp. to tethered conspecifics and sympatric congeners. Symbols identify species of the intruders (open circles, H. americana; open triangles, H. occisa; filled squares, H. titia; open squares, H. cruentata). Photographs are of (left to right) H. americana, H. occisa, H. titia and H. cruentata. Site codes are given above the corresponding symbols. Vertical dashed lines connect means for tests carried out at the same site.
Table 1.
Results from paired t-tests investigating the relative response to conspecific and heterospecific intruders. Territory holders of the focal species were presented with tethered intruders of their own species and also of the sympatric congener (see §2c for further details). Comparisons that were significant after sequential Bonferroni correction (Rice 1989) are denoted with an asterisk.
| focal species | sympatric congener | site | n | t | d.f. | p |
|---|---|---|---|---|---|---|
| H. americana | H. titia | AR | 16 | 3.6109 | 15 | 0.00257* |
| H. americana | H. titia | CV | 33 | 7.7795 | 32 | <0.0001* |
| H. americana | H. cruentata | EL | 17 | 0.0241 | 16 | 0.98107 |
| H. cruentata | H. occisa | CT | 15 | 0.714 | 14 | 0.48696 |
| H. cruentata | H. americana | EL | 10 | 0.8531 | 9 | 0.41573 |
| H. occisa | H. titia | OT | 16 | 7.3273 | 15 | <0.0001* |
| H. occisa | H. titia | PA | 26 | 4.9414 | 25 | 0.00004* |
| H. occisa | H. cruentata | CT | 16 | 0.1437 | 15 | 0.88765 |
| H. titia | H. americana | AR | 14 | 5.908 | 13 | 0.00005* |
| H. titia | H. americana | CV | 30 | 8.2647 | 29 | <0.0001* |
| H. titia | H. occisa | OT | 16 | 6.5646 | 15 | 0.00001* |
| H. titia | H. occisa | PA | 19 | 2.7945 | 18 | 0.01198 |
The proportional size of the hindspot on H. titia intruders was negatively related to the duration of chasing by H. occisa territorial holders (OT: z = −4.52, p < 0.001, n = 15; PA: z = −6.31, p < 0.001, n = 24) but not by H. americana territory holders (AR: z = 0.58, p = 0.559, n = 16; April 2008 CV: z = −0.61, p = 0.539, n = 16; August 2008 CV: z = −1.60, p = 0.111, n = 15).
(b). Responses to wing-colour-manipulated intruders
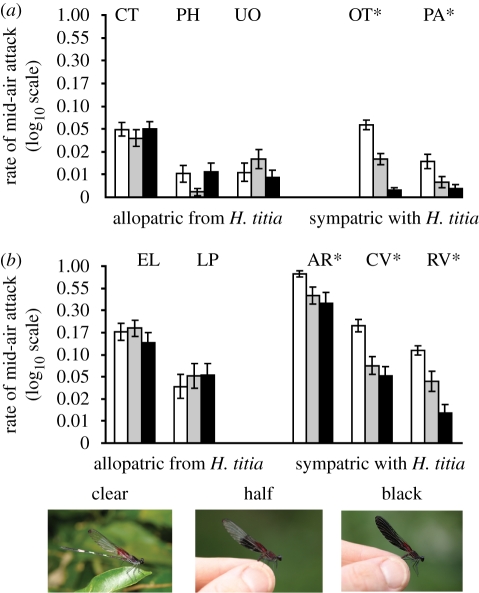
For both H. americana and H. occisa, there was a significant sympatry status by intruder treatment interaction (H. americana: F2,248 = 20.91, p < 0.001; H. occisa: F2,200 = 22.02, p < 0.001). Analysing sites separately showed that blackening the wings of intruders reduced the aggressive response of conspecific territory holders at sympatric sites (figure 3; H. americana: AR: F2,30 = 4.82, p = 0.0153; CV: F2,64 = 30.85, p < 0.0001; RV: F2,32 = 25.03, p < 0.001; H. occisa: OT: F2,62 = 78.08, p < 0.001; PA: F2,50 = 6.93, p = 0.0022) but not at allopatric sites (H. americana: EL: F2,86 = 2.22, p = 0.1151; LP: F2,30 = 0.90, p = 0.4176; H. occisa: CT: F2,28 = 1.27, p = 0.2959; PH: F2,26 = 2.83, p = 0.0776; UO: F2,28 = 1.97, p = 0.1584). The wing colour treatment also had no significant effect on the responses of H. cruentata territory holders at sites allopatric from H. titia (CT: F2,36 = 1.32, p = 0.28; EL: F2,30 = 0.38, p = 0.69).
Figure 3.
The average attack rate (attacks per second) ±s.e. towards conspecific intruder males with altered wing coloration for tests involving (a) H. occisa and (b) H. americana. Clear, half and black refer to the hindwing colour treatment (shown in photos). White bar, clear; grey bar, half; black bar, black. Site codes are given above the corresponding bars. Asterisks indicate significant (<0.05) p-values based on repeated-measures analyses of variance.
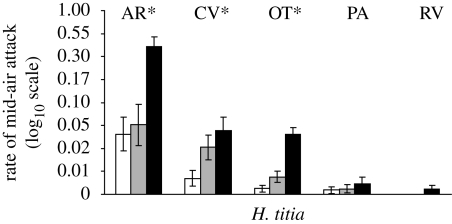
In contrast, blackening the wings of heterospecific (H. occisa or H. americana) intruders increased the attack rate of H. titia territory holders at three of five sites (figure 4; AR: F2,26 = 15.64, p < 0.001; CV: F2,58 = 12.00, p < 0.001; OT: F2,60 = 43.08; p < 0.0001; PA: F2,36 = 2.08, p = 0.1391; RV: F2,16 = 2.29; p = 0.1339). The non-significant results for sites PA and RV may reflect the fact that attack rates were low for all types of intruders at these sites. When the same model was tested with proportion of time chasing as the dependent variable, the wing colour treatment had highly significant effects on the response of H. titia territory holders at all sites (AR: F2,26 = 8.58, p = 0.001; CV: F2,58 = 8.29, p < 0.001; OT: F2,60 = 49.50; p < 0.0001; PA: F2,36 = 15.28, p < 0.001; RV: F2,16 = 21.12; p < 0.001).
Figure 4.
The average attack rate (attacks per second) ±s.e. towards heterospecific intruder males with altered wing coloration for tests involving H. titia. Clear, half and black refer to the hindwing colour treatment (see figure 3). White bar, clear; grey bar, half; black bar, black. Site codes are given above the corresponding bars. Asterisks indicate significant (<0.05) p-values based on repeated-measures analyses of variance.
The order in which the treatments were presented had no significant effects on aggressive responses (H. americana: F2,375 = 0.79, p = 0.454; H. cruentata: F2,102 = 0.63, p = 0.54; H. occisa: F2,303 = 1.49, p = 0.23; H. titia: F2,306 = 0.07, p = 0.93).
4. Discussion
Where sympatric with H. titia, both H. americana and H. occisa discriminated between conspecific and H. titia intruders, responding more aggressively to conspecifics (figure 2). Where sympatric with H. cruentata, however, both H. americana and H. occisa were equally aggressive towards conspecific and H. cruentata intruders (figure 2a,c). This mirrors Schultz & Switzer's (2001) finding that territorial amberwing dragonflies (Perithemis tenera) were likely to pursue heterospecific targets that were most similar in coloration to conspecifics.
We also investigated the effect of wing coloration on heterospecific aggression by using within-site variation in H. titia wing spot size. At the two H. occisa sites, we found a negative relationship between the proportional size of the hindwing spot of the H. titia intruder and the duration of time spent chasing by the H. occisa territory holder. That is, H. titia individuals with smaller areas of black wing pigmentation received greater aggression from H. occisa. We did not find the same pattern at H. americana sites, but these sites showed either extremely reduced variation in the size of H. titia wing spots (sites AR, CV in April 2008) or low rates of heterospecific chasing (CV in August 2008), diminishing our ability to detect a relationship between heterospecific aggression and wing spot size.
Tynkkynen et al. (2004) found that C. virgo exhibited greater heterospecific aggression towards C. splendens males who resembled C. virgo than towards more divergent forms of C. splendens. Our results are similar, but the relationship between resemblance and spot size is reversed: C. splendens with small wing spots escape aggression by C. virgo (a large wing-spotted damselfly), while H. titia with large wing spots escape aggression by H. occisa (a small wing-spotted damselfly). These results suggest that heterospecific territorial aggression in calopterygid damselflies is influenced by phenotypic similarity as opposed to a simple stimulus-based rule (e.g. attack larger spots more frequently).
Populations of H. americana and H. occisa that are sympatric with H. titia showed reduced aggression towards conspecific male intruders with experimentally blackened wings (which increased their resemblance to H. titia), and H. titia (always sympatric with a clear-winged congener in this study) showed the opposite response. In contrast, populations of H. americana, H. occisa and H. cruentata that are allopatric from H. titia responded with equal aggression towards blackened intruders and clear-winged controls. Sympatric shifts in competitor recognition have also been found in chaffinches (Lynch & Baker 1991) and dendrobatid frogs (Amezquita et al. 2006). These putative examples of ACD are unique in that RCD is not a plausible alternative explanation because shifts in recognition occur in potential competitors (same-sex individuals) rather than potential mates (opposite-sex individuals).
Decreased territorial responses to heterospecific coloration were reliably detected at multiple, independent sympatric sites in both H. americana and H. occisa (figure 3; electronic supplementary material). Parallel results such as these provide strong support for the role of selection over alternative mechanisms (Rundle et al. 2000). It is not yet clear, however, whether the geographic pattern that we detected reflects independent evolution of competitor recognition across multiple secondary contact scenarios or a common adaptive reaction norm. Experience has been shown to influence mate recognition in Enallagma civile damselflies (Fincke et al. 2007). If experience influences competitor recognition in Hetaerina, this could be an example of facultative character displacement (Pfennig & Murphy 2002).
Other outstanding questions raised by these results include the following. (i) Is character displacement in the recognition function matched by character displacement in the sexual signal? (ii) Is a reciprocal pattern of character displacement present or is character displacement asymmetric (Cooley et al. 2006)? (iii) What is the role of density and frequency dependence in this system? Preliminary evidence indicates that upon repeated visits to sites, neither average spot size nor relative species abundance are necessarily fixed features of a site (C. N. Anderson & G. F. Grether 2007, unpublished data). Nevertheless, the results presented here show that sympatric H. americana and H. occisa have reduced responses towards intruders with H. titia-like wing coloration regardless of whether H. titia is numerically dominant or exhibits fully black wing coloration.
In summary, we found evidence for character displacement of competitor recognition in the damselflies H. americana and H. occisa. Whether these population differences in competitor recognition reflect genetic divergence or adaptive phenotypic plasticity remains to be determined. Either way, these results demonstrate not only that divergence in wing coloration reduces territorial aggression between sympatric Hetaerina species but also that selection has probably shaped these responses. Hetaerina can be added to a growing list of examples of ACD (Grether et al. 2009).
Acknowledgements
We thank Simon Alarcon, Gaby Besne, Veronica Campos, Jasmine Loveland, Eliot Miller and Nick Taft for assistance with fieldwork. Zac Cheviron, Klaus-Peter Koepfli and John Pollinger provided genetic advice. Two anonymous reviewers, Daniel Blumstein, Tom Smith, Kerry Deere, Neil Losin and the UCLA Center for Tropical Research Group provided useful comments on the manuscript. We also thank the Statistical Consulting group in the UCLA Academic Technology Services for statistical advice. This work was supported by grants from UCMEXUS and UCLA Council on Research (G.F.G.), the Edward W. Pauley Fellowship, an NSF Graduate Research Fellowship and a UCMEXUS dissertation improvement grant (C.N.A.).
References
- Alatalo R. V., Gustafsson L., Lundberg A.1994Male coloration and species recognition in sympatric flycatchers. Proc. R. Soc. Lond. B 256, 113–118 (doi:10.1098/rspb.1994.0057) [Google Scholar]
- Alcock J.1987The effects of experimental manipulation of resources on the behavior of two calopterygid damselflies that exhibit resource-defense polygyny. Can. J. Zool.-Rev. Canad. De Zool. 65, 2475–2482 (doi:10.1139/z87-374) [Google Scholar]
- Amezquita A., Hodl W., Lima A. P., Castellanos L., Erdtmann L., De Araujo M. C.2006Masking interference and the evolution of the acoustic communication system in the Amazonian dendrobatid frog Allobates femoralis. Evolution 60, 1874–1887 (doi:10.1554/06-081.1) [PubMed] [Google Scholar]
- Berglund A., Bisazza A., Pilastro A.1996Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 58, 385–399 (doi:10.1111/j.1095-8312.1996.tb01442.x) [Google Scholar]
- Brown W. L., Wilson E. O.1956Character displacement. Syst. Zool. 5, 49–64 (doi:10.2307/2411924) [Google Scholar]
- Cody M. L.1969Convergent characteristics in sympatric species: a possible relation to interspecific competition and aggression. Condor 71, 222–239 (doi:10.2307/1366300) [Google Scholar]
- Contreras-Garduno J., Canales-Lazcano J., Cordoba-Aguilar A.2006Wing pigmentation, immune ability, fat reserves and territorial status in males of the rubyspot damselfly Hetaerina americana. J. Ethol. 24, 165–173 (doi:10.1007/s10164-005-0177-z) [Google Scholar]
- Cooley J. R., Marshall D. C., Hill K. B. R., Simon C.2006Reconstructing asymmetrical reproductive character displacement in a periodical cicada contact zone. J. Evol. Biol. 19, 855–868 (doi:10.1111/j.1420-9101.2005.01056.x) [DOI] [PubMed] [Google Scholar]
- Corbet P. S.1999Dragonflies: behavior and ecology of Odonata Ithaca, NY: Comstock Pub. Associates [Google Scholar]
- Cordoba-Aguilar A., Cordero-Rivera A.2005Evolution and ecology of Calopterygidae (Zygoptera: Odonata): status of knowledge and research perspectives. Neotropic. Entomol. 34, 861–879 (doi:10.1590/S1519-566X2005000600001) [Google Scholar]
- Cordoba-Aguilar A., Lesher-Trevino A. C., Anderson C. N.2007Sexual selection in Hetaerina titia males: a possible key species to understand the evolution of pigmentation in calopterygid damselflies (Odonata: Zygoptera). Behaviour 144, 931–952 (doi:10.1163/156853907781492672) [Google Scholar]
- Estrada C., Jiggins C. D.2008Interspecific sexual attraction because of convergence in warning colouration: is there a conflict between natural and sexual selection in mimetic species? J. Evol. Biol. 21, 749–760 (doi:10.1111/j.1420-9101.2008.01517.x) [DOI] [PubMed] [Google Scholar]
- Fincke O. M., Fargevieille A., Schultz T. D.2007Lack of innate preference for morph and species identity in mate-searching Enallagma damselflies. Behav. Ecol. Sociobiol. 61, 1121–1131 (doi:10.1007/s00265-006-0345-3) [Google Scholar]
- Gabor C. R., Ryan M. J.2001Geographical variation in reproductive character displacement in mate choice by male sailfin mollies. Proc. R. Soc. Lond. B 268, 1063–1070 (doi:10.1098/rspb.2001.1626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison R. W.1990A synopsis of the genus Hetaerina with descriptions of 4 new species (Odonata, Calopterygidae). Trans. Am. Entomol. Soc. 116, 175–259 [Google Scholar]
- Grether G. F.1996Intrasexual competition alone favors a sexually dimorphic ornament in the rubyspot damselfly Hetaerina americana. Evolution 50, 1949–1957 (doi:10.2307/2410753) [DOI] [PubMed] [Google Scholar]
- Grether G. F., Grey R. M.1996Novel cost of a sexually selected trait in the rubyspot damselfly Hetaerina americana: conspicuousness to prey. Behav. Ecol. 7, 465–473 (doi:10.1093/beheco/7.4.465) [Google Scholar]
- Grether G. F., Losin N., Anderson C. N., Okamoto K.2009The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biol. Rev. 84, 617–635 (doi:10.1111/j.1469-185X.2009.00089.x) [DOI] [PubMed] [Google Scholar]
- Groening J., Hochkirch A.2008Reproductive interference between animal species. Q. Rev. Biol. 83, 257–282 (doi:10.1086/590510) [DOI] [PubMed] [Google Scholar]
- Higgie M., Blows M. W.2008The evolution of reproductive character displacement conflicts with how sexual selection operates within a species. Evolution 62, 1192–1203 (doi:10.1111/j.1558-5646.2008.00357.x) [DOI] [PubMed] [Google Scholar]
- Hobel G., Gerhardt H. C.2003Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea). Evolution 57, 894–904 (doi:10.1111/j.0014-3820.2003.tb00300.x) [DOI] [PubMed] [Google Scholar]
- Hoskin C. J., Higgie M., McDonald K. R., Moritz C.2005Reinforcement drives rapid allopatric speciation. Nature 437, 1353–1356 (doi:10.1038/nature04004) [DOI] [PubMed] [Google Scholar]
- Johnson C.1963Interspecific territoriality in Hetaerina americana (Fabricius) and H. titia (Drury) (Odonata: Calopterygidae) with a preliminary analysis of the wing color pattern variation. Can. Entomol. 95, 575–582 [Google Scholar]
- Lemmon E. M.2009Diversification of conspecific signals in sympatry: geographic overlap drives multidimensional reproductive character displacement in frogs. Evolution 63, 1155–1170 (doi:10.1111/j.1558-5646.2009.00650.x) [DOI] [PubMed] [Google Scholar]
- Lynch A., Baker A. J.1991Increased vocal discrimination by learning in sympatry in 2 species of chaffinches. Behaviour 116, 109–126 (doi:10.1163/156853990X00383) [Google Scholar]
- Murray B. G.1981The origins of adaptive interspecific territorialism. Biol. Rev. Camb. Phil. Soc. 56, 1–22 (doi:10.1111/j.1469-185X.1981.tb00341.x) [Google Scholar]
- Pfennig K. S.2000Female spadefoot toads compromise on mate quality to ensure conspecific matings. Behav. Ecol. 11, 220–227 (doi:10.1093/beheco/11.2.220) [Google Scholar]
- Pfennig D. W., Murphy P. J.2002How fluctuating competition and phenotypic plasticity mediate species divergence. Evolution 56, 1217–1228 (doi:10.1111/j.0014-3820.2002.tb01433.x) [DOI] [PubMed] [Google Scholar]
- Ptacek M. B.2000The role of mating preferences in shaping interspecific divergence in mating signals in vertebrates. Behav. Process. 51, 111–134 (doi:10.1016/S0376-6357(00)00123-6) [DOI] [PubMed] [Google Scholar]
- Rice W. R.1989Analyzing tables of statistical tests. Evolution 43, 223–225 [DOI] [PubMed] [Google Scholar]
- Rundle H. D., Nagel L., Boughman J. W., Schluter D.2000Natural selection and parallel speciation in sympatric sticklebacks. Science 287, 306–308 (doi:10.1126/science.287.5451.306) [DOI] [PubMed] [Google Scholar]
- Rundle H. D., Chenoweth S. F., Doughty P., Blows M. W.2005Divergent selection and the evolution of signal traits and mating preferences. PLoS Biol. 3, 1988–1995 (doi:10.1371/journal.pbio.0030368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. J., Dries L. A., Batra P., Hillis D. M.1996Male mate preferences in a gynogenetic species complex of Amazon mollies. Anim. Behav. 52, 1225–1236 (doi:10.1006/anbe.1996.0270) [Google Scholar]
- Saetre G. P., Moum T., Bures S., Kral M., Adamjan M., Moreno J.1997A sexually selected character displacement in flycatchers reinforces premating isolation. Nature 387, 589–592 (doi:10.1038/42451) [Google Scholar]
- Saether S. A., et al. 2007Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science 318, 95–97 (doi:10.1126/science.1141506) [DOI] [PubMed] [Google Scholar]
- Schluter D.2000Ecological character displacement in adaptive radiation. Am. Nat. 156, S4–S16 (doi:10.1086/303412) [Google Scholar]
- Schultz J. K., Switzer P. V.2001Pursuit of heterospecific targets by territorial amberwing dragonflies (Perithemis tenera Say): a case of mistaken identity. J. Insect Behav. 14, 607–620 (doi:10.1023/A:1012223217250) [Google Scholar]
- Serrano-Meneses M. A., Cordoba-Aguilar A., Mendez V., Layen S. J., Szekely T.2007Sexual size dimorphism in the American rubyspot: male body size predicts male competition and mating success. Anim. Behav. 73, 987–997 (doi:10.1016/j.anbehav.2006.08.012) [Google Scholar]
- Svensson E. I., Karlsson K., Friberg M., Eroukhmanoff F.2007Gender differences in species recognition and the evolution of asymmetric sexual isolation. Curr. Biol. 17, 1943–1947 (doi:10.1016/j.cub.2007.09.038) [DOI] [PubMed] [Google Scholar]
- Tynkkynen K., Rantala M. J., Suhonen J.2004Interspecific aggression and character displacement in the damselfly Calopteryx splendens. J. Evol. Biol. 17, 759–767 (doi:10.1111/j.1420-9101.2004.00733.x) [DOI] [PubMed] [Google Scholar]
- Tynkkynen K., Kotiaho J. S., Luojumaki M., Suhonen J.2005Interspecific aggression causes negative selection on sexual characters. Evolution 59, 1838–1843 (doi:10.1554/04-716.1) [PubMed] [Google Scholar]
- Tynkkynen K., Kotiaho J. S., Luojumaki M., Suhonen J.2006Interspecific territoriality in Calopteryx damselflies: the role of secondary sexual characters. Anim. Behav. 71, 299–306 (doi:10.1016/j.anbehav.2005.03.042) [Google Scholar]
- Weichsel J. I.1987The life history and behavior of Hetaerina americana (Fabricus) (Odonata: Calopterygidae). PhD thesis, University of Michigan, Ann Arbor [Google Scholar]
- Westfall M. J., May M. L.1996Damselflies of North America Gainseville, FL: Scientific Publishers [Google Scholar]