Abstract
Theory predicts that animals in adverse conditions can decrease individual risks and increase long-term benefits by cooperating with neighbours. However, some empirical studies suggest that animals often focus on short-term benefits, which can reduce the likelihood that they will cooperate with others. In this experimental study, we tested between these two alternatives by evaluating whether increased predation risk (as a correlate of environmental adversity) enhances or diminishes the occurrence of cooperation in mobbing, a common anti-predator behaviour, among breeding pied flycatchers Ficedula hypoleuca. We tested whether birds would join their mobbing neighbours more often and harass a stuffed predator placed near their neighbours' nests more intensely in areas with a higher perceived risk of predation. Our results show that birds attended mobs initiated by their neighbours more often, approached the stuffed predator significantly more closely, and mobbed it at a higher intensity in areas where the perceived risk of predation was experimentally increased. In such high-risk areas, birds also were more often involved in between-pair cooperation. This study demonstrates the positive impact of predation risk on cooperation in breeding songbirds, which might help in explaining the emergence and evolution of cooperation.
Keywords: cooperation, predation risk, social behaviour, pied flycatcher
1. Introduction
The origin and evolution of cooperation among selfish individuals still represents an unexplained cornerstone of evolutionary biology (Dugatkin 1997; Nowak & Sigmund 1998; Wilson & Hölldobler 2005; Fletcher et al. 2006; Foster et al. 2006; Rockenbach & Milinski 2006; Ekman 2007). The concept of coexistence of organisms is usually based on the idea of minimum tolerance towards co-occurring species or individuals of the same species (Seed et al. 2008). Behavioural interactions, however, need not always be negative and they can show considerable plasticity depending on the local biotic and abiotic conditions (Agrawal 2001). Especially harsh and physiologically stressful environments have been suggested to enhance the occurrence of positive interactions (Greenbee & Callaway 1996; Callaway et al. 2002) and cooperation with neighbours (Bertness & Callaway 1994; Mönkkönen et al. 1997) and to decrease competition (Forsman et al. 2008) and delay dispersion (Kokko & Ekman 2002; Griesser et al. 2008). Although empirical evidence from plant and animal communities and human societies suggests that there is a correlation between adverse conditions and cooperation (e.g. Knight 1984; Wilkinson 1984; Rytkönen & Soppela 1995; Spinks et al. 2000; De Bono et al. 2002; Spieler 2003), it is not known whether adverse conditions actually cause and promote cooperation to evolve.
There is little experimental support that adverse environmental conditions enhance cooperation (Thomson et al. 2003). Moreover, some studies suggest that, in certain situations, animals value smaller, immediate benefits over large, future benefits (e.g. Mazur 1987). Since participation in cooperative behaviours is often costly over short time scales, it can reduce the proclivity of animals to cooperate with others (Stephens et al. 2002) explaining why adverse conditions might inhibit the evolution of cooperation. As a result, an alternative hypothesis is that adverse conditions such as the increased risk of predation might lead animals to focus on immediate direct benefits that do not rely on continued participation from others, with the result being that animals might actually be less likely to cooperate in adverse environments. In this case, the presence of cooperation in adverse environments occurs despite the adverse conditions, not because of them.
In many systems, predation is a strong selective pressure and can be one of the main factors responsible for mortality of prey individuals (Lima 2009). Organisms use a variety of strategies, such as alarm calling, distraction displays and attack responses to inhibit predators (e.g. Montgomerie & Weatherhead 1988). Prey individuals in some communities join together to mob a predator by cooperatively attacking it (Curio 1978). This communal defence can cause a predator to vacate its immediate foraging area, which reduces the threat to nearby prey individuals and allows them to resume their daily activities (Flasskamp 1994). Mobbing behaviour is most frequently seen in avian species (Curio 1978; Krams & Krama 2002; Olendorf et al. 2004; Templeton et al. 2005; Krams et al. 2006a; Griesser 2009), although it is also known to occur in other social animals such as mammals, fishes (Kirkwood & Dickie 2005; Solórzano-Filho 2006) and some invertebrates (Mori & Saito 2004). Aside from these benefits, anti-predator behaviours such as mobbing have costs (Montgomerie & Weatherhead 1988; Brunton 1990; Krams et al. 2007) and there appears to be a group size effect in mobbing, which indicates the importance of cooperation among prey individuals in driving predators away (Mori & Saito 2004; Krams et al. 2009).
In this study, we evaluated the response of breeding pied flycatchers Ficedula hypoleuca to a predator at their own nest as well as their response to a predator at a neighbour's nest under conditions of normal and increased predation risk. If increased perceived risk of predation leads to an elevated reliance on cooperation to alleviate risk, then we predict that birds should join nearby mobs more often and harass a stuffed predator placed near their neighbours' nests more intensely in areas with higher perceived risk of predation. Alternatively, if the presence of predators leads individuals to discount future rewards, then neighbouring birds should reduce their inclination to cooperate when predation risk is higher.
2. Material and methods
(a). Study site and general details
The field study was carried out in May and June 2004 and 2008 near Krāslava, southeastern Latvia (54°58′ N, 27°10′ E). The pied flycatcher, a small semi-colonially breeding migratory bird, is among the best-studied birds in the world (Lundberg & Alatalo 1992). We performed the field experiments in a population of pied flycatchers that was attracted to wooden nest-boxes placed in dry young pine forests. The nest-boxes were arranged in pairs (n = 28) and placed 36–43 m apart (40.12 ± 1.82 m, mean ± s.e.). This arrangement of the nest-boxes was not unnatural, because pied flycatchers provided with nest-boxes often exhibit semicolonial breeding behaviour. The adult flycatchers were neither trapped nor were they manipulated in any other way. They had marked themselves with colours of non-waterproof ink 2–6 days before the trials by touching a piece of ink-saturated foam-rubber while entering or leaving the entrance of their nest-boxes.
We restricted our study to pairs of nest-boxes, which were each occupied by pied flycatchers. Since some studies indicate that parents should take higher risks while defending more valuable and older offspring (Montgomerie & Weatherhead 1988; Rytkönen 2002; Tilgar & Kikas 2009), we monitored egg and fledgling ages to identify box pairs where the maximum difference in the age of nestlings in two neighbouring nest-boxes was 3 days. A total of 28 pairs of nest-boxes (56 individual nest-boxes) met these requirements. Fifteen pairs of nest-boxes were assigned to the experimental group and another 13 pairs served as a control group. A stuffed tawny owl Strix aluco—a common predator of small birds in northern Europe—served as a predator stimulus in all trials. When pied flycatchers detect this predator near their nests, they mob it while uttering characteristic calls that attract other hetero- and conspecific individuals (Curio 1975; Krama & Krams 2005). Our experiments consisted of two phases—(i) a training phase in which we increased the perceived risk of predation by exposing the experimental group to a predator stimulus; and (ii) a testing phase in which we determined whether birds in the experimental group mobbed more or less strongly and were more or less willing to mob at their neighbours' nests.
(b). Training
To increase the perceived risk of predation in the experimental group, we repeatedly placed a stuffed tawny owl 150 m from the nest-boxes of each experimental pair. To ensure that the predator was detected, we played back the alarm calls of pied flycatchers, which attracted the attention of both flycatcher pairs nearby. Using the same design, we presented a stuffed mistle thrush Turdus viscivorus in the vicinity of each pair of pied flycatchers in the control group. We demonstrated the predator (to the experimental group) and the thrush (to the control group) for 2 h on each of 5 days before the testing trials. Flycatchers in the experimental group did not mob the owl, but rather, continued their normal feeding activities. Although animals often habituate rapidly to dummy models, the birds in this study inspected the predator from a distance of 15–50 m while producing a few alarm calls during all of the training days, which suggests that they perceived the situation as risky (Desrochers et al. 2002). Since the density of breeding birds was very low in our study area located in a dry, young and tinned pine plantation, this area was rarely visited by any birds of prey. As a result, perceived risk of predation was relatively low in the vicinity of control nest boxes and relatively high at experimental nest boxes.
(c). Testing
We performed experimental trials as soon as the nestlings reached the age of 10 days. In both the experimental and control groups, the predator was mounted on a small platform 1.15 m above the ground between the two neighbouring nest-boxes, about 1.4 m from one of the nest-boxes, selected by a chance, and facing the nest. We observed and evaluated the behaviour of the birds from a small tent used as a ‘blind’ from a distance of 10 m. Before conducting the experiments, we marked nearby tree trunks and branches, which allowed us to accurately measure the birds' approach distances. The owl was presented when, to the best of our knowledge, no pied flycatcher was in the area. As soon as the owl was discovered by one of the nest owners, we began documenting the mobbing response of the nest owners and their neighbours. After 10 min of mobbing, we removed the decoy. We considered neighbours to have assisted the nest owners if they approached the owl closer than half of the distance between the two neighbouring nest-boxes. We did not consider the neighbours to have assisted if they remained within their own territory—even if they interrupted their feeding activities and continuously gave alarm signals. An analysis of variance was performed for minimum approach distance of males and females. Since this parameter appeared to be similar between both sexes in the case of nest owners (F1,27 = 1.03, p = 0.32) and neighbouring birds (F1,27 = 3.36, p = 0.072), we averaged the minimum approach distances of both the birds in each pair.
To obtain more specific information on the intensity of mobbing by both nest owners and their neighbours, we divided the mobbing response of pied flycatchers, according to their displays and vocal response, into four categories: (i) no response to the dummy predator (0 points)—birds investigated the predator from a distance usually without alarm calls while continuing activities such as foraging or singing; (ii) weak response (1 point)—frequent approaches and retreats to/from the predator; (iii) average response (2 points)—the birds tended to be close to the predator, and they moved restlessly around it by bowing, pivoting, tail-flicking or hovering in the air in front of it; (iv) strong response (3 points)—intense movements and display, which included frequent dive-attacks at the predator. During weak, average and strong responses, pied flycatchers used ‘pik’ calls (Curio 1975; Krams et al. 2006b). The observers of birds' responses were not blind to the hypothesis and treatment. We observed and scored the mobbing behaviour of each individual bird in both pairs (i.e. four adults). Since the mobbing intensity of males and females was similar both in the case of nest owners (Kruskal–Wallis test: χ2 = 0.33, p = 0.86) and neighbouring flycatchers (Kruskal–Wallis test: χ2 = 0.42, p = 0.52), we calculated the mean value and assigned it to each pair of adult pied flycatchers.
One hour later, we conducted ‘repeated trials’ in which we presented the owl for another 10 min at the nests of the former ‘neighbours’ in both control and experimental groups to test whether neighbouring birds engage in reciprocity when assisting one another. This part of the study was done to see if there is any repeatability in the antipredator behaviour of neighbouring pairs of pied flycatchers and to determine if nest owners that were assisted by neighbours in the initial trials support their neighbours in the future. The repeated owl test was carried out at 10 nests of the experimental group and at eight nests of the control group. The rest of the nests were rejected because we either observed predators in the vicinity of those nests or the neighbouring individuals were observed involved in territorial conflicts. We recorded neither mobbing intensity scores nor the distance of approach during these second trials. The repeated trials were carried out as a part of pilot study on ‘forgiving’ within the concept of tit-for-tat and these trials were not initially related to the experiment on cooperation under adverse conditions.
3. Results
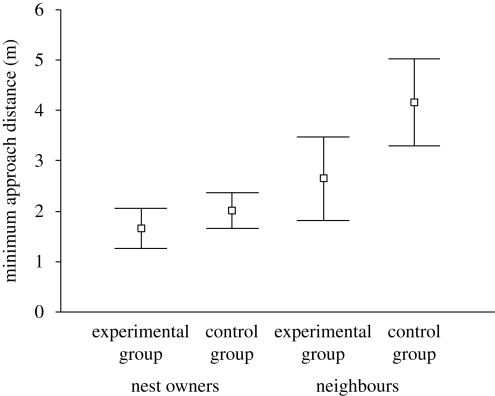
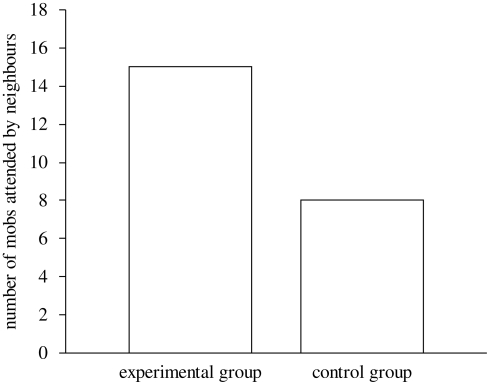
The intensity of mobbing by nest owners did not differ between the experimental and control groups. The behaviour by nest owners was scored as ‘average to strong’ in both the control (2.40 ± 0.13, mean and s.e., n = 13) and experimental groups (2.33 ± 0.12, mean and s.e., n = 15) and there was no difference between the groups (two-tailed Mann–Whitney U-test—U = 105.0, n1 = 13, n2 = 15, p = 0.709). Similarly, the nest owners' minimum approach distance to the predator did not differ significantly between the experimental and control groups (figure 1; two-tailed t-test, t = −1.341, d.f. = 26, p = 0.187). However, neighbouring birds in the experimental group were more likely to assist their neighbours (figure 2; two-tailed sign-test, p = 0.031). All of neighbouring pairs in the experimental treatment joined the mobbing while 61.5 per cent of the neighbours did so in the control treatment. Neighbouring birds in the experimental group approached the predator decoy more closely than neighbouring birds in the control group (figure 1; two-tailed t-test, t = −2.322, d.f. = 21, p = 0.030). Although the intensity of mobbing behaviour by neighbouring individuals was scored as ‘weak to average’ in both the control (1.38 ± 0.14, mean and s.e.) and experimental groups (1.85 ± 0.10, mean and s.e.), the behaviour of individuals in the experimental group was found to be more intense towards the predator (two-tailed Mann–Whitney U-test, U = 45.5, n1 = 13, n2 = 15, p = 0.018).
Figure 1.
Minimum approach distance from the stuffed owl by nest owners and neighbouring flycatchers. Whiskers denote s.e.
Figure 2.
Number of mobs attended by neighbouring pied flycatchers in experimental and control groups.
Neighbouring flycatchers appeared within 20–126 s after the initiation of mobbing by nest owners and neighbours always arrived in pairs. We did not find any difference between experimental (53.00 ± 6.72 s mean and s.e.) and control (51.30 ± 4.70 s, mean and s.e.) groups (F1 = 0.40, d.f. = 1, p = 0.84). Nest owners mobbed the predator more intensely than their neighbours in the control group (two-tailed Mann–Whitney U-test, U = 55.0, n1 = 13, n2 = 15, p = 0.01). In the experimental group, nest owners also responded significantly more strongly towards the predator placed near their nest-boxes than their neighbours (two-tailed Mann–Whitney U-test, U = 22.5, n1 = 13, n2 = 15, p < 0.001).
In the repeated trials, when the owl was demonstrated at the nests of former neighbours 1 h after the initial trials, 8 out of 10 pairs of pied flycatchers of the experimental group attended mobs while the birds of the control group attended only two out of eight mobs at the nest boxes of the former neighbouring pied flycatchers and this difference in response was significant (sign-test, p = 0.031). The two pairs of pied flycatchers of the control group that were assisted by their neighbours during the second trial behaved in a cooperative way during the first trial.
4. Discussion
There is evidence that mobbing provides direct fitness benefits, because predators that have been mobbed can be driven off (Curio 1978; Knight & Temple 1986) and that mobbing might increase the probability that the predator will not return to an area where it has been unsuccessful in obtaining prey (Tinbergen et al. 1967; Lima 2002). These results suggest a link between mobbing as a part of nest defence and the increased fitness of those individuals who mob their natural enemies. Mobbing in a group enhances these benefits, which helps explain why birds mob cooperatively (Pettifor 1990). Our results show that birds were more willing to cooperate with their neighbours after many consecutive encounters with predators, which increased their perceived risk of predation, and triggered cooperation as a way to increase their fitness. Pied flycatchers were more willing to mob at their neighbours' nests and mobbed more intensely in areas with an increased predation risk. Moreover, in areas with a higher perceived predation risk, birds approached predators at their neighbours' nests an average of 1.5 m more closely. This difference between treatments is biologically significant, because mobbers can be injured or eaten when they approach predators (Hoogland & Sherman 1976; Denson 1979; Curio & Regelmann 1985, 1986; McLean & Rhodes 1991). Through cooperation, prey individuals might significantly increase their effect on the behaviour of predators and alleviate the risks associated with engaging in mobbing behaviour, which might make mobbing in groups profitable even under increased predation risk.
The repeated trial experiments show that the increased tendency for birds to mob at the nests of neighbours in areas of increased perceived predation risk is maintained over a number of interactions: birds that have had neighbours mob at their own nests are also more likely to mob at the nests of neighbours in the future. This result accords with previous studies showing that pied flycatchers engage in reciprocal cooperation when mobbing—birds mob at the nests of their neighbours contingent on whether or not those same neighbours have helped them previously (Dugatkin 1997; Krams et al. 2006b; Krams et al. 2008; Wheatcroft & Price 2008; Wheatcroft & Krams 2009). Reciprocal cooperation, a widely discussed mechanism that facilitates the evolution of cooperation, produces benefits for participants only when they interact with other cooperators (Axelrod & Hamilton 1981; Dugatkin 1997; Nowak 2006). If the survival of one's neighbours were unpredictable—for example, owing to high mortality—then individuals might be less willing to engage in reciprocity-based cooperative behaviours, which generally require long-term partnerships among known individuals. For example, the experiments of Stephens et al. (2002) show that blue jays (Cyanocitta cristata) value small immediate benefits over large future ones and do not engage in reciprocal cooperation when they can receive immediate benefits. However, our results suggest that such ‘temporal discounting’ might not always hinder reciprocal cooperation. One explanation is that reciprocity requires participants to interact repeatedly during the course of relatively short breeding season (Axelrod & Hamilton 1981). In this context, frequent encounters with predators might supply the incentive to engage in such behaviours, assuming the chances that one's neighbours will survive until the next encounter are sufficiently high. The results of the trials support the idea that increased predation risk might promote reciprocity-based cooperation in breeding pied flycatchers: ‘neighbours’ who more frequently encountered predators were more willing to support ‘nest owners’, who, in turn, were more willing to join mobs at the nests of ‘neighbours’ in the future. Together with previous results showing that breeding pied flycatchers join mobs at the nests of their neighbours contingent on whether or not these neighbours helped them previously (Krams et al. 2006b; Krams et al. 2008; Wheatcroft & Price 2008; Wheatcroft & Krams 2009), these results provide support for the idea that repeated interactions between individuals (via increased predation risk) might explain the evolution of complex cooperative behaviours, such as reciprocal cooperation (Axelrod & Hamilton 1981).
The results of our experiment agree with the predictions of a theoretical study by Andras et al. (2003), which showed that resource adversity should promote cooperation. Our results support this theoretical prediction and suggest that birds facing a risky environment can reduce their individual risks by cooperating with others. The correlation between group augmentation and safety might explain why birds breed in clusters (Kokko et al. 2001). However, more data are badly needed to demonstrate how adversity and uncertainty might enhance reciprocity-based and mutualistic cooperation in different taxa by improving fitness prospects for cooperating individuals.
Overall, our results suggest the importance of non-lethal or non-consumptive effects of predation in ecological systems. The diversity of possible responses by prey species to increase their fitness stresses the importance of the interactions among prey organisms and a need to pay more attention to environmental features such as the probability of being attacked by predators. The risk of predation can have a direct effect on cooperation as is shown in our experimental study, which might help to explain the origin and evolution of cooperation.
Acknowledgements
All animal manipulations were approved by the ethical committee by the University of Daugavpils and they comply with the current laws in Latvia.
The study was supported financially by the Academy of Finland to I.K. and M.J.R., and by the Ministry of Education and Science of the Republic of Latvia to I.K. We thank Mikus Abolins-Abols for his helpful comments on the manuscript.
References
- Agrawal A. A.2001Phenotypic plasticity in the interactions and evolution of species. Science 294, 321–326 (doi:10.1126/science.1060701) [DOI] [PubMed] [Google Scholar]
- Andras P., Roberts G., Lazarus J.2003Environmental risk, cooperation and communication complexity. In Adaptive agents and multi-agent systems (ed. Alonso E. K. D.), pp. 49–65 Berlin, Germany: Springer-Verlag [Google Scholar]
- Axelrod R., Hamilton W. D.1981The evolution of cooperation. Science 211, 1390–1396 (doi:10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- Bertness M. D., Callaway R.1994Positive interactions in communities. Trends Ecol. Evol. 9, 191–193 (doi:10.1016/0169-5347(94)90088-4) [DOI] [PubMed] [Google Scholar]
- Brunton D. H.1990The effects of nesting stage, sex, and type of predator on parental defense by killdeer (Charudrius vociferous): testing models of avian parental defense. Behav. Ecol. Sociobiol. 26, 181–190 (doi:10.1007/BF00172085) [Google Scholar]
- Callaway R. M., et al. 2002Positive interactions among alpine plants increase with stress. Nature 417, 844–847 (doi:10.1038/nature00812) [DOI] [PubMed] [Google Scholar]
- Curio E.1975The functional organization of anti-predator behaviour in the pied flycatcher: a study of avian visual perception. Anim. Behav. 23, 1–115 (doi:10.1016/0003-3472(75)90056-1) [DOI] [PubMed] [Google Scholar]
- Curio E.1978The adaptive significance of avian mobbing. I. Teleonomic hypotheses and predictions. Z. Tierpsychol. 48, 175–183 [Google Scholar]
- Curio E., Regelmann K.1985The behavioural dynamics of great tits (Parus major) approaching a predator. Z. Tierpsychol. 69, 3–18 [Google Scholar]
- Curio E., Regelmann K.1986Predator harassment implies a real deadly risk: a reply to Hennessy. Ethology 72, 75–78 [Google Scholar]
- Denson R. D.1979Owl predation on a mobbing crow. Wilson Bull. 91, 133 [Google Scholar]
- De Bono M., Tobin D. M., Davis M. W., Avery L., Bargmann C. I.2002Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature 419, 899–903 (doi:10.1038/nature01169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers A., Bélisle M., Bourque J.2002Do mobbing calls affect the perception of predation risk by forest birds? Anim. Behav. 64, 709–714 (doi:10.1006/anbe.2002.4013) [Google Scholar]
- Dugatkin L. A.1997Cooperation among animals: an evolutionary perspective New York, NY: Oxford University Press [Google Scholar]
- Ekman J.2007Cooperation—the role of past and present. Behav. Process. 76, 118–119 (doi:10.1016/j.beproc.2006.12.017) [DOI] [PubMed] [Google Scholar]
- Flasskamp A.1994The adaptive significance of avian mobbing. V. An experimental test of the ‘move on’ hypothesis. Ethology 96, 322–333 [Google Scholar]
- Fletcher J. A., Zwick M., Doebeli M., Wilson D. S.2006What's wrong with inclusive fitness? Trends Ecol. Evol. 21, 597–598 (doi:10.1016/j.tree.2006.08.008) [DOI] [PubMed] [Google Scholar]
- Forsman J. T., Hjernquist M. B., Taipale J., Gustafsson L.2008Competitor density cues for habitat quality facilitating habitat selection and investment decisions. Behav. Ecol. 19, 539–545 (doi:10.1093/beheco/arn005) [Google Scholar]
- Foster K. R., Wenseleers T., Ratnieks F. L. W.2006Kin selection is the key to altruism. Trends Ecol. Evol. 21, 57–60 (doi:10.1016/j.tree.2005.11.020) [DOI] [PubMed] [Google Scholar]
- Greenbee J. T., Callaway R. M.1996Abiotic stress and the relative importance of interference and facilitation in montane bunchgrass communities in western Montana. Am. Nat. 148, 386–396 (doi:10.1086/285931) [Google Scholar]
- Griesser M.2009Mobbing calls signal predator category in a kin group-living bird species. Proc. R. Soc. B 276, 2887–2892 (doi:10.1098/rspb.2009.0551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesser M., Nystrand M., Eggers S., Ekman J.2008Social constraints limit dispersal and settlement decisions in a group-living bird species. Behav. Ecol. 19, 317–324 (doi:10.1093/beheco/arm131) [Google Scholar]
- Hoogland J. L., Sherman P. W.1976Advantages and disadvantages of bank swallow (Riparia riparia) coloniality. Ecol. Monogr. 46, 33–58 (doi:10.2307/1942393) [Google Scholar]
- Kirkwood R., Dickie J.2005Mobbing of a great white shark (Carcharodon carcharias) by adult male Australian fur seals (Arctocephalus pusillus doriferus). Mar. Mammal Sci. 21, 336–339 (doi:10.1111/j.1748-7692.2005.tb01234.x) [Google Scholar]
- Knight R. L.1984Responses of nesting ravens to people in areas of different human densities. Condor 86, 1345–1346 (doi:10.2307/1367010) [Google Scholar]
- Knight R. L., Temple S. A.1986Nest defence in the American goldfinch. Anim. Behav. 34, 887–897 (doi:10.1016/S0003-3472(86)80075-6) [Google Scholar]
- Kokko H., Ekman J.2002Delayed dispersal as a route to breeding: territorial inheritance, safe havens, and ecological constraints. Am. Nat. 160, 468–484 (doi:10.1086/342074) [DOI] [PubMed] [Google Scholar]
- Kokko H., Johnstone R. A., Clutton-Brock T. H.2001The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196 (doi:10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krama T., Krams I.2005Cost of mobbing call to breeding pied flycatcher, Ficedula hypoleuca. Behav. Ecol. 16, 37–40 (doi:10.1093/beheco/arh116) [Google Scholar]
- Krams I., Krama T.2002Interspecific reciprocity explains mobbing behaviour of the breeding chaffinches, Fringilla coelebs. Proc. R. Soc. Lond. B 269, 2345–2350 (doi:10.1098/rspb.2002.2155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krams I., Krama T., Igaune K.2006aAlarm calls of wintering great tits Parus major: warning of mate, reciprocal altruism or a message to the predator? J. Avian Biol. 37, 131–136 (doi:10.1111/j.0908-8857.2006.03632.x) [Google Scholar]
- Krams I., Krama T., Igaune K.2006bMobbing behaviour: reciprocity-based co-operation in breeding pied flycatcher Ficedula hypoleuca. Ibis 148, 50–54 (doi:10.1111/j.1474-919X.2006.00480.x) [Google Scholar]
- Krams I., Krama T., Igaune K., Mänd R.2007Long-lasting mobbing of the pied flycatcher increases the risk of nest predation. Behav. Ecol. 18, 1082–1084 (doi:10.1093/beheco/arm079) [Google Scholar]
- Krams I., Krama T., Igaune K., Mänd R.2008Experimental evidence of reciprocal altruism in the pied flycatcher. Behav. Ecol. Sociobiol. 62, 599–605 (doi:10.1007/s00265-007-0484-1) [Google Scholar]
- Krams I., Bērziņš A., Krama T.2009Group effect in nest defense of breeding pied flycatchers, Ficedula hypoleuca. Anim. Behav. 77, 513–517 (doi:10.1016/j.anbehav.2008.11.007) [Google Scholar]
- Lima S. L.2002Putting predators back into behavioral predator–prey interactions. Trends Ecol Evol. 17, 70–75 (doi:10.1016/S0169-5347(01)02393-X) [Google Scholar]
- Lima S. L.2009Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol. Rev. 84, 485–513 (doi:10.1111/j.1469-185X.2009.00085.x) [DOI] [PubMed] [Google Scholar]
- Lundberg A., Alatalo R. V.1992The pied flycatcher London, UK: T & A. D. Poyser [Google Scholar]
- Mazur J. E.1987An adjusting procedure for studying bird delayed reinforcement. The effect of delay and of intervening events on reinforcement value. In The effect of delay and intervening events on reinforcement value. Quantitative analyses of behavior (eds Commous M. L., Mazur J. E., Nevin J. A.), pp. 55–73 Hillsdale, NJ: Lawrence Erlbaum Associates [Google Scholar]
- McLean I. G., Rhodes G.1991Enemy recognition and response in birds. Curr. Ornithol. 8, 173–211 [Google Scholar]
- Mönkkönen M., Helle P., Niemi G. J., Montgomery K.1997Heterospecific attraction affects community structure and migrant abundances in northern breeding bird communities. Can. J. Zool. 75, 2077–2083 (doi:10.1139/z97-842) [Google Scholar]
- Montgomerie R. D., Weatherhead P. J.1988Risks and rewards of nest defence by parent birds. Q. Rev. Biol. 63, 167–187 (doi:10.1086/415838) [Google Scholar]
- Mori K., Saito Y.2004Variation in social behavior within a spider mite genus, Stigmaeopsis (Acari: Tetranychidae). Behav. Ecol. 16, 232–238 (doi:10.1093/beheco/arh157) [Google Scholar]
- Nowak M. A.2006Five rules for the evolution of cooperation. Science 314, 1560–1563 (doi:10.1126/science.1133755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M. A., Sigmund K.1998Evolution of indirect reciprocity by image scoring. Nature 393, 573–577 (doi:10.1038/31225) [DOI] [PubMed] [Google Scholar]
- Olendorf R., Getty R., Scribner K.2004Cooperative nest defence in red-winged blackbirds: reciprocal altruism, kinship or by-product mutualism? Proc. R. Soc. Lond. B 271, 177–182 (doi:10.1098/rspb.2003.2586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettifor R. A.1990The effects of avian mobbing on a potential predator, the European Kestrel, Falco tinnunculus. Anim. Behav. 39, 821–827 (doi:10.1016/S0003-3472(05)80945-5) [Google Scholar]
- Rockenbach E., Milinski M.2006The efficient interaction of indirect reciprocity and costly punishment. Nature 444, 718–723 (doi:10.1038/nature05229) [DOI] [PubMed] [Google Scholar]
- Rytkönen S.2002Nest defence in great tits Parus major: support for parental investment theory. Behav. Ecol. Sociobiol. 52, 379–384 (doi:10.1007/s00265-002-0530-y) [Google Scholar]
- Rytkönen S., Soppela M.1995Vicinity of sparrowhawk nest affects willow tit nest defense. Condor 97, 1074–1078 (doi:10.2307/1369550) [Google Scholar]
- Seed A. M., Clayton N. S., Emery N. J.2008Cooperative problem solving in rooks (Corvus frugilegus). Proc. R. Soc. B 275, 1421–1429 (doi:10.1098/rspb.2008.0111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solórzano-Filho J. A.2006Mobbing of Leopardus wiedii while hunting by a group of Sciurus ingrami in an Araucaria forest of Southeast Brazil. Mammalia 70, 156–157 (doi:10.1515/MAMM.2006.031) [Google Scholar]
- Spieler M.2003Risk of predation affects aggregation size: a study with tadpoles of Phrynomantis microps (Anura: Microhylidae). Anim. Behav. 65, 179–184 (doi:10.1006/anbe.2002.2030) [Google Scholar]
- Spinks A. C., Jarvis J. U. M., Bennett N. C.2000Comparative patterns of philopatry and dispersal in two common mole-rat populations: implications for the evolution of mole-rat sociality. J. Anim. Ecol. 69, 224–234 (doi:10.1046/j.1365-2656.2000.00388.x) [Google Scholar]
- Stephens D. W., McLinn C. M., Stevens J. R.2002Discounting and reciprocity in an iterated Prisoner's Dilemma. Science 298, 2216–2218 (doi:10.1126/science.1078498) [DOI] [PubMed] [Google Scholar]
- Templeton C. N., Greene E., Davis K.2005Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937 (doi:10.1126/science.1108841) [DOI] [PubMed] [Google Scholar]
- Thomson R. L., Forsman J. T., Mönkkönen M.2003Positive interactions between migrant and resident birds: testing the heterospecific attraction hypothesis. Oecologia 134, 431–438 [DOI] [PubMed] [Google Scholar]
- Tilgar V., Kikas K.2009Is parental risk taking negatively related to the level of brood reduction? An experiment with pied flycatchers. Anim. Behav. 77, 43–47 (doi:10.1016/j.anbehav.2008.08.027) [Google Scholar]
- Tinbergen N., Impekoven M., Franck D.1967An experiment on spacing-out as a defence against predation. Behaviour 28, 307–321 (doi:10.1163/156853967X00064) [Google Scholar]
- Wheatcroft D. J., Price T. D.2008Reciprocal cooperation in avian mobbing: playing nice pays. Trends Ecol. Evol. 23, 416–419 (doi:10.1016/j.tree.2008.04.011) [DOI] [PubMed] [Google Scholar]
- Wheatcroft D. J., Krams I.2009Response to Russell and Wright: avian mobbing. Trends Ecol. Evol. 24, 5–6 (doi:10.1016/j.tree.2008.09.002) [DOI] [PubMed] [Google Scholar]
- Wilkinson G. S.1984Reciprocal food sharing in the vampire bat. Nature 308, 181–184 (doi:10.1038/308181a0) [Google Scholar]
- Wilson E. O., Hölldobler B.2005Eusociality: origin and consequences. Proc. Natl Acad. Sci. USA 102, 13 367–13 371 (doi:10.1073/pnas.0505858102) [DOI] [PMC free article] [PubMed] [Google Scholar]