Abstract
Transmission of infectious diseases is strongly influenced by who contacts whom. Despite the global distribution of tuberculosis (TB) in free-living wild mammal populations, little is known of the mechanisms of social transmission of Mycobacterium bovis between individuals. Here, I use a network approach to examine for correlations between five distinct types of intra- and intergroup social interaction and changes in TB status of 110 wild meerkats (Suricata suricatta) in five social groups over two years. Contrary to predictions, the most socially interactive animals were not at highest risk of acquiring infection, indicating that in addition to contact frequency, the type and direction of interactions must be considered when quantifying disease risk. Within social groups, meerkats that groomed others most were more likely to become infected than individuals who received high levels of grooming. Conversely, receiving, but not initiating, aggression was associated with M. bovis infection. Incidence of intergroup roving by male meerkats was correlated with the rovers themselves subsequently testing TB-positive, suggesting a possible route for transmission of infection between social groups. Exposure time was less important than these social interactions in influencing TB risk. This study represents a novel application of social network analysis using empirical data to elucidate the role of specific interactions in the transmission of an infectious disease in a free-living wild animal population.
Keywords: epidemiology, meerkat, Mycobacterium bovis, social network analysis, Suricata suricatta, tuberculosis
1. Introduction
The social structure of animal populations significantly influences the transmission dynamics and persistence of infectious diseases (Altizer et al. 2003; Perkins et al. 2008; Woodroffe et al. 2009). Knowledge of host interaction patterns is critical if we are to understand infectious disease transmission within a population (Cross et al. 2005). Despite this, few studies have examined the role of specific social interactions in the transmission of infectious agents within free-ranging populations of wild animals. Consequently, disease transmission models rarely go beyond a theoretical exploration of the influence of host ecology on disease transmission (e.g. Keeling 2005; Lloyd-Smith et al. 2005). This is particularly true for wildlife species owing to inherent difficulties with collecting empirical data. The role of social interaction in infectious disease transmission is an area which is crucial to understand if effective management strategies for diseases such as tuberculosis (TB) in wild animal populations are to be developed (Cross et al. 2009). In particular, for social animals, we need to know which interactions are associated with the transmission of infection within and between groups. We may then use this information to predict which individuals are more likely to transmit or receive, and which might (by their behaviour) be more susceptible to, or protected from, infection.
The effects of network structure on disease dynamics is an important and developing area of research (Corner et al. 2003a; Keeling 2005; Read et al. 2008; Perkins et al. 2009). For social animals, an individual's position in a group may influence risk of infection. In an infection–transmission study of TB caused by Mycobacterium bovis in captive brushtail possums (Trichosurus vulpecula), individuals who became infected were found to have greater closeness and flow-betweenness scores—that is, they were better connected to other possums in a network of den-sharing events—than those that remained free of infection (Corner et al. 2003a). Standard statistical analyses gave similar results but the network-specific measures were more precise and had the added benefit that they could be compared across time and between groups (Corner et al. 2003a). However, while different types of social interaction often produce different structures of contact network (Drewe et al. 2009c; Madden et al. 2009), with direct implications for disease transmission, this study was limited to the investigation of only one interaction type (den-sharing) and did not address intergroup transmission (Corner et al. 2003a). Further, the contact structure of a free-living population of wild possums is likely to be different from that of a captive population.
Quantification of the rates of social interactions between animals is the first step to determining their role in disease transmission. Recently, proximity loggers revealed all Tasmanian devils (Sarcophilus harrisii) within a study population to be interacting within a single contact network with potentially catastrophic consequences, since this would permit devil facial tumour disease (an infectious cancer threatening the species with extinction) to spread throughout the population from any single infected individual (Hamede et al. 2009). For group-living species such as European badgers (Meles meles), variation in the rates of intra- and intergroup associations between individuals is likely to profoundly affect the maintenance and transmission of infectious diseases such as TB (Böhm et al. 2008). Both individual- and group-level movements of badgers have been found to be positively correlated with the likelihood of becoming infected with M. bovis (Vicente et al. 2007). However, limitations in data collection technique—such as a reliance on the trapping of badgers to quantify intergroup movement rates—meant that the roles of specific behaviours in the transmission of M. bovis were not quantified in this study.
Meerkats (Suricata suricatta) are small (<1 kg) mongooses from the arid regions of southern Africa. They live in groups of up to 40 individuals consisting of a dominant female and male and a variable number of helpers of both sexes who aid in rearing the young (Clutton-Brock et al. 2001). Interactions within meerkat groups may be antagonistic, such as aggressive dominance assertions (Kutsukake & Clutton-Brock 2006a) and eviction of subordinate females (Clutton-Brock et al. 1998a), or placatory, such as grooming (Kutsukake & Clutton-Brock 2006b; Madden & Clutton-Brock 2009). Interactions between different groups consist principally of temporary intergroup forays by roving males (Young et al. 2007) and aggressive encounters between entire groups (Drewe et al. 2009c). A population of wild meerkats living in southern Kalahari has been the focus of detailed behavioural ecology studies since 1993 (Clutton-Brock et al. 1998b). Tuberculosis owing to infection with a member of the animal-adapted lineage of the M. tuberculosis complex (probably M. bovis) is endemic within this study population (Drewe 2009). Habituation of these meerkats offers a unique opportunity to study the role of specific social interactions in the transmission of TB in a wild animal population. The results are likely to aid our understanding of TB transmission dynamics in other social mammal species, such as badgers in the UK (Delahay et al. 2000) and possums in New Zealand (Corner et al. 2003b). I predicted that individuals engaging in the greatest amounts of social interaction would be most at risk of contracting TB.
2. Material and methods
(a). Data collection
Data and samples were collected at the Kalahari Meerkat Project in the Northern Cape, South Africa (26°58′ S, 21°49′ E). Further details of the study site and population are given by Clutton-Brock et al. (1998b). Detailed ad libitum observations of up to 300 individually identified meerkats in 14 social groups were made over 24 months from January 2006 to December 2007. Each group was visited on at least 4 days each week, with observation periods lasting for at least 3 h in the morning after the meerkats emerged from their burrows and for at least 1 h before they re-entered their burrow in the evening. To account for a slightly unequal number of visits to each group, data were standardized by multiplying with a correction factor (the number of half-days in the study period divided by actual number of half-day visits made to the group) to ensure that comparisons between individuals and groups were based on similar amounts of observation time. I investigated associations between the following interactions and changes in M. bovis infection status of individuals: grooming (both giving and receiving), aggression (both giving and receiving), eviction of subordinate females, extra-territorial roving of males and aggressive intergroup encounters. In all cases, the identities of the initiator(s) and receiver(s) were recorded. The number of meerkats included in each of the analyses varies because it was not possible to sample every meerkat at all time points.
(b). Definitions of social interactions
Grooming—when one individual groomed another, typically in the morning or evening at their communal burrow (Kutsukake & Clutton-Brock 2006b). Subordinate individuals may groom more dominant individuals to avoid harassment (Kutsukake & Clutton-Brock 2006b) or as a facultative response to antagonism (Madden & Clutton-Brock 2009). When three or more animals were grooming together in a huddle, interactions were recorded as dyads with one record per dyad in the same huddle. A separate grooming bout was considered to have started if the animals resumed grooming after a pause (during which there was no grooming at all) of more than 1 min. Grooming interactions between 94 meerkats (54 males and 40 females) in four social groups were recorded, a total of 11 360 interactions (table 1).
Table 1.
Associations between meerkat grooming and aggression networks and M. bovis infection of initiators (outdegree), receivers (indegree) and individuals acting as connections between others in the network (flow-betweenness). Regression coefficients (r) and associated probabilities (p) based on 30 000 permutations of interactions between 94 meerkats in four social groups are shown. Italic values indicate significant relationships after Bonferroni correction (p < 0.002).
| grooming |
aggression |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| time point | date | n | outdegree | indegree | flow-betweenness | n | outdegree | indegree | flow-betweenness | ||
| 2006 | |||||||||||
| t1 | January–March | 1438 | r | 0.49 | 0.37 | 0.34 | 1688 | r | 0.36 | 0.40 | 0.32 |
| p | <0.001 | 0.001 | 0.002 | p | 0.001 | <0.001 | 0.003 | ||||
| t2 | April–June | 869 | r | 0.08 | −0.03 | 0.13 | 1201 | r | −0.03 | 0.07 | −0.04 |
| p | 0.18 | 0.83 | 0.11 | p | 0.62 | 0.14 | 0.94 | ||||
| t3 | July–September | 2227 | r | 0.21 | −0.02 | 0.14 | 1120 | r | 0.05 | 0.15 | −0.03 |
| p | 0.03 | 0.88 | 0.12 | p | 0.77 | 0.14 | 0.83 | ||||
| t4 | October–December | 852 | r | 0.37 | 0.10 | 0.16 | 948 | r | 0.07 | 0.50 | 0.10 |
| p | 0.001 | 0.34 | 0.09 | p | 0.34 | <0.001 | 0.33 | ||||
| 2007 | |||||||||||
| t5 | January–March | 1085 | r | 0.20 | 0.19 | −0.01 | 823 | r | 0.42 | 0.08 | 0.43 |
| p | 0.05 | 0.06 | 0.94 | p | 0.002 | 0.50 | 0.001 | ||||
| t6 | April–June | 1846 | r | 0.26 | 0.08 | −0.01 | 438 | r | −0.04 | 0.07 | −0.03 |
| p | 0.03 | 0.51 | 0.99 | p | 0.71 | 0.53 | 0.82 | ||||
| t7 | July–September | 2280 | r | 0.35 | 0.24 | 0.28 | 698 | r | 0.06 | 0.22 | −0.08 |
| p | 0.002 | 0.02 | 0.01 | p | 0.62 | 0.04 | 0.49 | ||||
| t8 | October–December | 763 | r | 0 | 0.02 | 0.02 | 458 | r | −0.01 | 0.12 | 0.21 |
| p | 1.00 | 0.90 | 0.93 | p | 0.94 | 0.14 | 0.06 | ||||
| total | 2006–2007 | 11 360 | r | 0.32 | 0.27 | 0.14 | 7374 | r | 0.19 | 0.36 | 0.30 |
| p | 0.001 | 0.005 | 0.18 | p | 0.08 | <0.001 | 0.001 | ||||
Intragroup aggression—recorded if two meerkats competed for dominance or if one meerkat asserted its dominance over another (see also Kutsukake & Clutton-Brock 2006a). Interactions included biting, hitting, slamming, wrestling and chin-marking (Kutsukake & Clutton-Brock 2008). Aggressive interactions between 94 meerkats (54 males and 40 females) in four social groups were recorded, a total of 7374 interactions (table 1).
Eviction of subordinate females—repeated chasing and physical attacking of the subordinates by the dominant females (and sometimes other group members of either sex). Dominant females in the latter stages of pregnancy often forcibly evict subordinate females, as the culmination of escalating aggression over the course of several days (Clutton-Brock et al. 1998b, 2006). Evicted females may live on the group periphery for several days before often being accepted back into the group after the dominant female has given birth (Clutton-Brock et al. 1998a). Eviction of 46 subordinate female meerkats from five social groups was recorded, a total of 239 eviction events (table 2).
Table 2.
Associations between aggressive evictions of subordinate females from meerkat groups (outdegree) and M. bovis infection of the evicted individuals. Regression coefficients (r) and associated probabilities (p) based on 30 000 permutations of 46 female meerkat evictions from five social groups are shown. No relationships are significant after Bonferroni correction (p < 0.006).
| time point | date | number of evictions | outdegree | |
|---|---|---|---|---|
| 2006 | ||||
| t1 | January–March | 51 | r | 0.19 |
| p | 0.22 | |||
| t2 | April–June | 19 | r | 0 |
| p | 1.00 | |||
| t3 | July–September | 54 | r | −0.12 |
| p | 0.80 | |||
| t4 | October–December | 38 | r | −0.14 |
| p | 0.58 | |||
| 2007 | ||||
| t5 | January–March | 14 | r | 0.12 |
| p | 0.32 | |||
| t6 | April–June | 14 | r | −0.09 |
| p | 0.97 | |||
| t7 | July–September | 26 | r | 0.05 |
| p | 0.89 | |||
| t8 | October–December | 23 | r | 0.35 |
| p | 0.04 | |||
| total | 2006–2007 | 239 | r | −0.01 |
| p | 0.96 | |||
Intergroup movements of roving males—when a male meerkat left its original social group, either singly or as part of a coalition of males, and actively sought out and approached another group of meerkats in a non-aggressive manner (Doolan & Macdonald 1996). This usually occurred as males sought breeding opportunities in other groups (Young et al. 2005). Rovers were only recorded if they subsequently returned to their original group, which usually occurred on the same day. The intergroup movements of 64 male meerkats from five social groups visiting up to nine other groups were recorded, a total of 2054 interactions (table 3).
Table 3.
Associations between intergroup movements of roving male meerkats and changes in M. bovis infection status in those males and in members of groups being visited. Outdegree data (‘rovers leaving’) refers to temporary departures of 64 male meerkats from five social groups. Indegree data (‘rovers visiting’) refers to visits to 96 meerkats in five social groups by rovers from up to nine other social groups. Regression coefficients (r) and associated probabilities (p) based on 30 000 permutations are shown. Italic values indicate significant relationships after Bonferroni correction (p < 0.006).
| rovers leaving |
rovers visiting |
||||||
|---|---|---|---|---|---|---|---|
| time point | date | n | outdegree | n | indegree | ||
| 2006 | |||||||
| t1 | January–March | 265 | r | 0.03 | 36 | r | −0.07 |
| p | 0.83 | p | 0.47 | ||||
| t2 | April–June | 190 | r | 0 | 48 | r | 0 |
| p | 1.00 | p | 1.00 | ||||
| t3 | July–September | 292 | r | 0.05 | 151 | r | 0 |
| p | 0.75 | p | 0.98 | ||||
| t4 | October–December | 319 | r | 0.58 | 85 | r | 0.04 |
| p | <0.001 | p | 0.68 | ||||
| 2007 | |||||||
| t5 | January–March | 162 | r | 0.37 | 33 | r | 0.11 |
| p | 0.006 | p | 0.27 | ||||
| t6 | April–June | 74 | r | 0 | 31 | r | −0.01 |
| p | 1.00 | p | 0.91 | ||||
| t7 | July–September | 79 | r | 0.38 | 49 | r | −0.09 |
| p | 0.01 | p | 0.59 | ||||
| t8 | October–December | 173 | r | 0.13 | 67 | r | 0.13 |
| p | 0.05 | p | 0.50 | ||||
| total | 2006–2007 | 1554 | r | 0.42 | 500 | r | −0.07 |
| p | 0.001 | p | 0.48 | ||||
Intergroup encounters—when two or more social groups met and interacted in an aggressive manner. Such encounters are frequently very aggressive and may include chasing, fighting and excavation of burrows to dig out meerkats from another group (Drewe et al. 2009c). The intergroup encounters between five social groups (96 meerkats, 50 males and 46 females) with up to nine other groups were recorded, a total of 604 intergroup interactions (table 4).
Table 4.
Associations between networks of aggressive intergroup interactions and risk of M. bovis infection in meerkats within those groups. Regression coefficients (r) and associated probabilities (p) based on 30 000 permutations of intergroup interactions (degree) between five focal meerkat groups (96 meerkats) and up to 13 other social groups are shown. No relationships are significant after Bonferroni correction (p < 0.006).
| time point | date | number of interactions | degree | |
|---|---|---|---|---|
| 2006 | ||||
| t1 | January–March | 69 | r | −0.09 |
| p | 0.38 | |||
| t2 | April–June | 122 | r | 0 |
| p | 1.00 | |||
| t3 | July–September | 70 | r | 0.03 |
| p | 0.86 | |||
| t4 | October–December | 107 | r | 0.06 |
| p | 0.57 | |||
| 2007 | ||||
| t5 | January–March | 132 | r | 0.23 |
| p | 0.02 | |||
| t6 | April–June | 49 | r | −0.01 |
| p | 0.94 | |||
| t7 | July–September | 24 | r | 0.19 |
| p | 0.03 | |||
| t8 | October–December | 31 | r | 0.18 |
| p | 0.07 | |||
| total | 2006–2007 | 604 | r | 0.16 |
| p | 0.11 | |||
(c). Mycobacterium bovis infection status of individual meerkats
I sampled 110 meerkats in five social groups, each up to eight times, every three months between January 2006 and December 2007 (a total of 362 samples). Median age of meerkats at first sampling was 10 months (range, 3–89 months). Details of the sampling procedure are given elsewhere (Drewe et al. 2009a). Briefly, meerkats were caught by hand and anaesthetized with isoflurane (Isofor; Safe Line Pharmaceuticals, Johannesburg, South Africa) administered by face mask. Blood was collected and subjected to two serological tests to detect presence of mycobacterial antibodies, and a tracheal wash was undertaken for mycobacterial culture (for test details see Drewe et al. 2009a). Test results for serology and culture were interpreted in parallel, meaning tests at each time point were run concurrently with a positive diagnosis requiring that only one test result be positive. This was done to maximize diagnostic sensitivity (the ability of the tests to correctly identify infected animals as test-positive) at 89 per cent (95% CI: 75–97%), while diagnostic specificity (the ability of the tests to correctly identify non-infected animals as test-negative) was 72 per cent (95% CI: 48–82%; figures calculated from data in Drewe et al. 2009a). Each meerkat was classified as test-positive for TB from the first time point at which a positive serological result was obtained or M. bovis was cultured from a tracheal wash sample; otherwise it was considered to be test-negative for TB.
(d). Social network measures
I calculated three measures of social network centralization for each meerkat: outdegree, an indication of the proportion of interactions initiated by a focal animal; indegree, an indication of the proportion of interactions received by a focal animal (Wasserman & Faust 1994); and flow-betweenness, an indication of the prominence or ‘importance’ of each meerkat in the network calculated from the number of direct and indirect connections passing through it as a proportion of the total flow in the network (Hanneman & Riddle 2005). If social interactions are interpreted as representing the potential flow of disease within a network, then flow-betweenness is a measure of the number of paths that pass through a focal meerkat along the shortest path between all other meerkats (Freeman et al. 1991). It has been suggested that an individual lying on the shortest path regulates the flow of information (e.g. disease) between two indirectly linked individuals (Borgatti 2005). The higher the flow-betweenness score, the more influential an individual is as an intermediary for contact between others. If an individual with high flow-betweenness centrality is removed from the network, the speed and certainty of transmission of infectious disease from a random individual within the network to another is more affected than if an individual with a low score is removed (Borgatti 1995).
All networks were constructed using weighted data (that is, I considered relative amounts of each interaction rather than simply recording the presence or absence of an interaction). For infections such as M. bovis that require close contact for transmission to occur, both the regularity of encounters and the weight of interactions are important (Read et al. 2008). For each interaction type, a single network containing all meerkats was constructed and individual network measures for each meerkat were calculated. An exception was aggressive intergroup encounter degree scores, which were calculated on a group-level basis and the same score allocated to all members of that group present at the time point in question.
(e). Data analysis
I analysed data in blocks of three months (i.e. eight time points within the 24-month study period) for three reasons. First, detailed network analysis of the stability of intra- and intergroup social interactions over a variety of time intervals revealed social networks constructed from data collected over three months to be representative of both shorter time intervals (down to one week for intragroup interactions) and longer time intervals (up to 24 months for intergroup interactions; Drewe et al. 2009c). Second, since it is crucial to match data collection for transmission networks to the dynamics of the particular pathogen being studied (Perkins et al. 2009), networks constructed using three months of interactions data are likely to be biologically meaningful given the chronic nature of TB. I made the assumption that a meerkat becoming infected during one three-month time period was unlikely to go on to infect others during the same time period owing to the apparently long incubation period of TB in meerkats (Drewe 2009) but acknowledge that this may be affected by the route of transmission. Third, the inter-sampling interval for TB testing of meerkats was approximately three months and thus relationships between social interactiveness and change in infection status could be examined together over the same time period.
A logistic regression was used to examine for associations between sex, age and dominance status, and the first TB test result of each meerkat to test for bias owing to left censorship of data. This was necessary because meerkats entered the study after birth and the most likely individuals to become infected may have already done so prior to the study. Since not all meerkats were sampled at every time point, a logistic regression was used to examine for an association between monitoring time and TB test outcome for each animal, in case exposure time was a confounding variable in the incidence of testing TB-positive. These analyses were carried out in SPSS v. 15 with sex and dominance status as binary variables, and age (in months) and monitoring time (in days) as continuous variables.
Behavioural correlates of the incidence of testing TB positive were analysed using social network analysis in UCInet (Borgatti et al. 2002). Individual M. bovis infection incidence status was recorded as a dichotomous variable (0 = negative, i.e. all serologic and culture results negative; 1 = incident case of infection, i.e. the first time point at which serology and/or culture results were positive for that animal). Infection status was used as the dependent variable, with outdegree, indegree and flow-betweenness centrality measures as individual-level explanatory variables. I tested for associations between each meerkat's social network scores and change in M. bovis infection status during each three-month period using node-level OLS regression tests (Hanneman & Riddle 2005). This analysis accounts for autocorrelation between data points in the network. The algorithm proceeds by first determining the slope coefficients for a regression. It then recalculates these statistics over a large number (here, 30 000) of repetitions in which covariates are randomly redistributed among nodes (meerkats), while keeping the topology of the network—and any interdependencies therein—fully intact. The p-value for each statistic is the proportion of permutations that yielded a statistic as extreme as the one initially produced. To account for multiple testing of each interaction over nine time periods, I applied a Bonferroni correction and considered relationships significant where p < 0.006 for tests of a single network measure, and p < 0.002 for tests of three network measures.
3. Results
Tuberculosis was detected in the meerkat population throughout the entire study period, with the incidence of new cases testing positive ranging from 4 per cent (3/69 meerkats; time point 5) to 21 per cent (10/48 meerkats; time point 6) (figure 1). Although 22 per cent (24/110) of meerkats tested positive on their first TB test, no relationships were found between the result of an individual's first TB test and its sex (odds ratio (OR) = 0.64, p = 0.35), age (OR = 1.02, p = 0.29) or dominance status (OR = 1.17, p = 0.88). No relationship was found between exposure time and TB test outcome (TB-negative animals: mean (range) exposure time = 380 (37–801) days; TB-positive animals = 304 (45–769) days; OR = 1, p = 0.13).
Figure 1.
Tuberculosis (TB) dynamics over the study period. Meerkats were sampled at eight time points (t1–t8) throughout 2006 and 2007. The number of meerkats sampled at each time point varied owing to births and deaths. Prevalence refers to the total number of meerkats testing positive at each time point; incidence refers to new cases testing positive since the previous time point. Only deaths attributable to TB (confirmed by mycobacterial culture) are shown. Dotted line, sampled; black line, prevalence; grey line, incidence; dashed line, deaths.
(a). Is grooming between meerkats associated with becoming TB-positive by either the groomer or the groomee, or both?
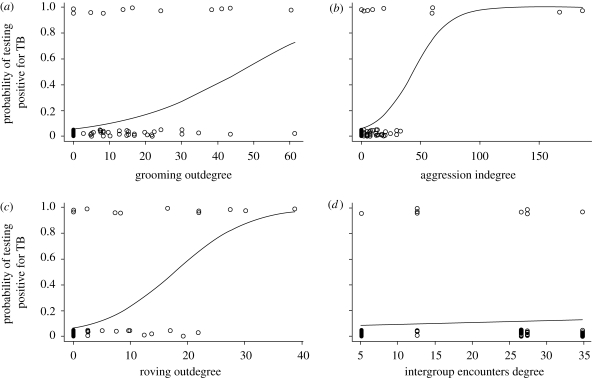
Grooming was associated with the groomer testing positive for TB at three out of the eight time points studied, and this correlation remained when all eight time points were analysed together (p = 0.001, table 1: grooming outdegree). Thus, meerkats that frequently groomed others were more likely to be infected than those that groomed others infrequently or not at all (figures 2a and 3a). Comparative degree distributions for meerkats of different TB test statuses are shown in figure 4a. Being groomed, however, was not generally associated with testing positive for M. bovis infection, although these were correlated at one time point (table 1; grooming indegree). Meerkats with high flow-betweenness scores, that is, individuals acting as links between two or more others not directly linked, generally did not show an increased risk of being infected with M. bovis (table 1; grooming flow-betweenness).
Figure 2.
Social networks and TB transmission in a meerkat group. Comparative networks of (a) grooming and (b) aggressive interactions over a three-month period are shown. Each node (circle) represents a meerkat; node size is proportional to outdegree centrality (an indication of how much interaction each individual initiated). Arrowhead size is proportional to frequency of interactions, thus the sum of arrowheads around each node gives an indication of indegree centrality (the relative amount of interaction received by that meerkat). Asterisks indicate the three individuals that became TB test-positive during the time period for which the interaction data are shown. Meerkats are arranged in descending order of age from top to bottom of each diagram. White nodes, females; grey nodes, males; D, dominant individuals.
Figure 3.
Fitted logistic regressions of probability of individual meerkats testing positive for TB as a function of (a) grooming outdegree (n = 94, r = 0.37, p = 0.001); (b) aggression indegree (n = 94, r = 0.50, p < 0.001); (c) roving male outdegree (n = 64, r = 0.58, p < 0.001); (d) intergroup encounters degree (n = 96 meerkats in five groups, r = 0.06, p = 0.57). Regression coefficients and their statistical significance were assessed using network permutation tests. Data shown are from time point 4 (October–December 2006).
Figure 4.
Degree distributions for (a) grooming interactions initiated and (b) aggressive interactions received over a three-month period (t4, October–December 2006) by meerkats testing negative (white bars) or positive (black bars) for TB at the end of this period. Both interactions were positively correlated with risk of TB infection (grooming outdegree, r = 0.37, p = 0.001; aggression indegree, r = 0.50, p < 0.001; network permutation tests with n = 94 meerkats in both cases).
(b). Is aggression between meerkats within a social group associated with acquisition of Mycobacterium bovis by either the aggressor or the receiver, or both?
Aggression within meerkat social groups showed an opposite, although less clear, relationship with M. bovis infection status than did grooming interactions. Meerkats that initiated aggression did not show a consistent or overall increased risk of being infected with M. bovis although correlations existed at two time points (table 1; aggression outdegree). Meerkats that were on the receiving end of aggression showed a significant likelihood of being infected with M. bovis at two of the eight time points studied, and this correlation remained when all eight time points were analysed together (p < 0.001, table 1: aggression indegree; figures 2b and 3b). Comparative degree distributions for meerkats of different TB test statuses are shown in figure 4b. Although meerkats' aggression flow-betweenness scores were not consistently associated with being infected with M. bovis, a significant relationship was seen when all eight time points were analysed together (p = 0.001, table 1; aggression flow-betweenness), suggesting that individuals acting as intermediates between others in a chain of aggressive interactions are at risk of infection with M. bovis.
(c). Is temporary eviction of subordinate female meerkats from a social group associated with acquisition of Mycobacterium bovis by the evicted meerkat?
No association was found between the eviction of subordinate female meerkats from a group and any change in M. bovis infection status of the evicted meerkats (table 2).
(d). Is intergroup roving by male meerkats associated with a greater risk of becoming infected with Mycobacterium bovis by either the roving male or members of the group being visited, or both?
Intergroup roving by male meerkats was significantly correlated with the rovers themselves being infected in two out of eight time points, and this correlation remained when all eight time points were analysed together (p = 0.001, table 3: outdegree; figure 3c). Members of either sex in groups frequently visited by rovers from elsewhere were not, however, at increased risk of being infected (table 3; indegree). This suggests that, for individual meerkats, being a rover carries more risk of TB infection than does being visited by rovers from other groups.
(e). Is involvement in aggressive intergroup encounters associated with tuberculosis risk?
No association was found between involvement in aggressive intergroup encounters and any change in M. bovis infection status of group members (table 4; figure 3d).
4. Discussion
Exposure time was less important than involvement in specific social interactions in influencing TB risk. Infection in meerkats was more frequently associated with groomers than groomees, suggesting that meerkats which groom others are at higher risk of infection than are those that receive grooming. An uninfected meerkat that grooms an infected individual is likely to be at risk of infection by at least three routes: from inhaling infectious aerosols, from bite wounding (injection of infection) and by ingesting infectious bacteria from draining sinus tracts. While all three of these transmission routes appear to occur in meerkats (Drewe et al. 2009b), the greatest risk is likely to result from inhalation owing to the low minimum infective dose of this route: five bacilli or fewer are enough to establish pulmonary infection in cattle (Chausse 1913, cited by Phillips et al. 2003) compared with the oral route where several million bacilli are required to establish infection owing to the mycobactericidal effects of gastric secretions (Gaudier and Gernez-Rieux 1962, cited by Corner 2006). Thus, despite the high prevalence of discharging lymph node abscesses in infected meerkats (Drewe et al. 2009b), meerkats that ingest small amounts of infectious pus during grooming of such individuals may actually be at a low risk of establishing infection owing to the requirement for a very high oral dose (Corner 2006). Risk is likely to increase with duration of grooming but this was not measured in the present study.
Meerkats on the receiving end of aggression (those with a high aggression indegree centrality score in the preceding three months) were more likely to become infected with M. bovis than those that received less aggression. Although this finding was not consistent over all the eight time periods studied, a significant overall correlation was seen when all time periods were analysed together. Intragroup aggression in meerkats may result in severe bites, suggesting that direct inoculation of M. bovis via bite wounding may occur. Injection of infected saliva via bite wounds is thought to be an important means of TB transmission in badgers (Clifton-Hadley et al. 1993) and this has been linked to subsequent haematogenous spread of M. bovis infection (Jenkins et al. 2008). Meerkat saliva may sometimes be infectious (probably because of contamination with respiratory secretions) and infected skin wounds are common in tuberculous meerkats (Drewe et al. 2009b). Based on the correlation between aggression indegree centrality, high infection rates of skin wounds (Drewe et al. 2009b) and the similarities with patterns of disease seen in badgers, it seems likely that M. bovis may be transmitted via bite wounding in meerkats. Meerkats that initiated aggression were overall no more likely to become infected with M. bovis than those that did not initiate aggression. Thus, biting others does not appear to be a significant risk factor for gaining TB by the aggressor in meerkat societies. This seems intuitive, since unless a meerkat happens to bite into an abscess on an infected individual, transmission of infection is unlikely. This goes some way to explaining why some very socially interactive dominant meerkats do not become infected. Dominant females are more likely to be groomed than to groom others (Kutsukake & Clutton-Brock 2006b) and are more likely to be aggressive than receive aggression (Kutsukake & Clutton-Brock 2006a). The present study has shown that neither of these specific behaviours (receiving grooming and initiating aggression) is related to a change in TB infection status.
While being on the receiving end of intragroup aggression was associated with becoming infected with M. bovis, being evicted from the group as a subordinate female was not. This is perhaps surprising, since eviction of meerkats is mediated by aggression (Stephens et al. 2005). However, it may be explained by the fact that during eviction events intragroup aggression originates mainly from the dominant female, who, as described above, may actually be at low risk of carrying infection. It is possible that the type or duration of aggression preceding eviction differs from that occurring within the group generally although no differences were observed in this study. Finally, the lack of association may be erroneous and simply related to the small sample size (239 eviction events in total over the 24-month period) and loss to follow-up of evictees who died or disappeared. More subordinate female meerkats should be sampled in future studies to clarify this.
Intergroup roving by male meerkats was associated with these individuals subsequently testing TB-positive, but not with any change in TB status of group members being visited. It is not possible to deduce from the study methodology whether it is the act of visiting other groups that carries infection risk or whether there is something else about being a rover that puts these individuals at risk of infection. Since TB status was not found to be affected by sex, age or dominance status, an individual's infection risk must be mediated by other factors. One possibility is that immunosuppressive stress hormones such as cortisol may play a role in disease susceptibility. Levels of glucocorticoid metabolites in faeces are significantly elevated in subordinate female meerkats when evicted from the safety of their group (Young et al. 2006). A similar increase in stress hormones in male meerkats away from their group would offer a possible explanation for the increased TB risk in roving males shown in the present study.
An important limitation of testing live animals of many species for TB is the suboptimal accuracy of diagnostic tests (Woodroffe et al. 1999). In particular, test sensitivity is usually low meaning early stages of infection are likely to be missed, resulting in an underestimation of the infectious proportion of the population (Chambers et al. 2002). To minimize the impact of false-negative test results in the present study, I used a parallel system of interpretation of three tests and considered an animal positive for TB from the time of its first positive test result onwards, resulting in an overall diagnostic sensitivity of up to 89 per cent. While this is likely to have increased the chances of correctly identifying individuals in the later stages of infection, it would not have improved detection of animals in the early stages of infection since the tests used were more likely to detect established cases of disease rather than indicate the timing of infection (Chambers et al. 2008). Misclassification of the TB status of some individuals means that infection may have preceded the timing of social interactions included in this study. While no changes in behaviour were observed in infected individuals until just before death (J. A. Drewe 2007, personal observation), it is possible that other meerkats may alter their behaviour towards infected individuals, perhaps targeting them with more (or less) aggression or grooming. The application of other tests that may detect early stages of infection, such as the gamma interferon test (Dalley et al. 2008), would be one possible solution. Logistical limitations such as the remoteness of the study site precluded the use of this test in the meerkat population.
In conclusion, transmission of M. bovis within meerkat groups appears to be associated with grooming and aggression, but not eviction of subordinate females. Intergroup transmission seems to be associated with roving males but not antagonistic intergroup encounters involving entire groups. These social interactions appear to be more important than the amount of exposure time in influencing the risk of testing TB-positive. Directionality of interaction appears to be important in the spread of infection, explaining why the most socially interactive individuals—the dominant male and female—are not necessarily at highest risk of infection. A similar finding was found in a study of contact networks and pathogen transmission in bumble-bees (Bombus impatiens) where a bee's sociality (degree centrality) did not influence its risk of infection (Otterstatter & Thomson 2007). Thus an individual's unique position in the social network (who is connected to whom) would seem to be more important than the total amount of social interaction an individual engages in, and frequent social contact (normally equated with ‘high-risk’ behaviour) does not necessarily increase the likelihood of infection. While not definitively proving causation, the temporal correlation between meerkats engaging in specific social interactions and testing positive for M. bovis, together with consideration of the pathology of this disease and the routes of infection and excretion, indicate the likely importance of grooming, biting and roving in the transmission of M. bovis within this meerkat population. This extends our knowledge of the mechanisms of social transmission of TB in wild mammal populations.
Acknowledgements
This research was conducted under ethical permits from the University of Pretoria.
The Northern Cape Conservation Service gave permission to work at the study site. I thank Rob Sutcliffe, Tom Flower, Dave Bell and the volunteers at Kuruman River Reserve for their help with sampling and data collection. Anita Michel and Gilly Dean facilitated TB testing of the meerkat samples. I am grateful to Tim Clutton-Brock and Gareth Pearce for advice and discussion, and to two anonymous referees whose comments vastly improved the manuscript.
Funding. Main funding for work was provided by the Cambridge Infectious Diseases Consortium. Subsidiary funding came from the Jowett Fund and the Northern Cape Department of Agriculture and Land Reform (South Africa).
References
- Altizer S., et al. 2003Social organization and parasite risk in mammals: integrating theory and empirical studies. Ann. Rev. Ecol. Evol. Syst. 34, 517–547 (doi:10.1146/annurev.ecolsys.34.030102.151725) [Google Scholar]
- Böhm M., Palphramand K. L., Newton-Cross G., Hutchings M. R., White P. C. L.2008Dynamic interactions among badgers: implications for sociality and disease transmission. J. Anim. Ecol. 77, 735–745 (doi:10.1111/j.1365-2656.2008.01377.x) [DOI] [PubMed] [Google Scholar]
- Borgatti S. P.1995Centrality and AIDS. Connections 18, 112–115 [Google Scholar]
- Borgatti S. P.2005Centrality and network flow. Social Netw. 27, 55–71 (doi:10.1016/j.socnet.2004.11.008) [Google Scholar]
- Borgatti S. P., Everett M. G., Freeman L. C.2002UCInet for Windows: software for social network analysis. Harvard, MA: Analytic Technologies; Available at http://www.analytictech.com/ucinet6/ucinet.htm [Google Scholar]
- Chambers M. A., Pressling W. A., Cheeseman C. L., Clifton-Hadley R. S., Hewinson R. G.2002Value of existing serological tests for identifying badgers that shed Mycobacterium bovis. Vet. Microbiol. 86, 183–189 [DOI] [PubMed] [Google Scholar]
- Chambers M. A., Crawshaw T., Waterhouse S., Delahay R., Hewinson R. G., Lyashchenko K. P.2008Validation of the BrockTB Stat-Pak assay for detection of tuberculosis in Eurasian badgers (Meles meles) and influence of disease severity on diagnostic accuracy. J. Clin. Microbiol. 46, 1498–1500 (doi:10.1128/JCM.02117-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton-Hadley R. S., Wilesmith J. W., Stuart F. A.1993Mycobacterium bovis in the European badger (Meles meles): epidemiological findings in tuberculous badgers from a naturally infected population. Epidemiol. Infect. 111, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Brotherton P. N. M., Smith R., McIlrath G. M., Kansky R., Gaynor D., O'Riain M. J., Skinner J. D.1998aInfanticide and expulsion of females in a cooperative mammal. Proc. R. Soc. Lond. B 265, 2291–2295 (doi:10.1098/rspb.1998.0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., et al. 1998bCosts of cooperative behaviour in suricates, Suricata suricatta. Proc. R. Soc. Lond. B 265, 185–190 (doi:10.1098/rspb.1998.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., et al. 2001Cooperation, control, and concession in meerkat groups. Science 291, 478–481 (doi:10.1126/science.291.5503.478) [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Hodge S. J., Spong G., Russell A. F., Jordan N. R., Bennett N. C., Sharpe L. L., Manser M. B.2006Intrasexual competition and sexual selection in cooperative mammals. Nature 444, 1065–1068 (doi:10.1038/nature05386) [DOI] [PubMed] [Google Scholar]
- Corner L. A.2006The role of wild animal populations in the epidemiology of tuberculosis in domestic animals: how to assess the risk. Vet. Microbiol. 112, 303–312 (doi:10.1016/j.vetmic.2005.11.015) [DOI] [PubMed] [Google Scholar]
- Corner L. A., Pfeiffer D. U., Morris R. S.2003aSocial-network analysis of Mycobacterium bovis transmission among captive brushtail possums (Trichosurus vulpecula). Prev. Vet. Med. 59, 147–167 (doi:10.1016/S0167-5877(03)00075-8) [DOI] [PubMed] [Google Scholar]
- Corner L. A., Stevenson M. A., Collins D. M., Morris R. S.2003bThe re-emergence of Mycobacterium bovis infection in brushtail possums (Trichosurus vulpecula) after localised possum eradication. NZ Vet. J. 51, 73–80 [DOI] [PubMed] [Google Scholar]
- Cross P. C., Lloyd-Smith J. O., Johnson P. L. F., Getz W. M.2005Duelling timescales of host movement and disease recovery determine invasion of disease in structured populations. Ecol. Lett. 8, 587–595 (doi:10.1111/j.1461-0248.2005.00760.x) [Google Scholar]
- Cross P. C., Drewe J. A., Patrek V., Pearce G. P., Samuel M. D., Delahay R. J.2009Wildlife population structure and parasite transmission: implications for disease management. In Management of disease in wild mammals (eds Delahay R. J., Hutchings M. R., Smith G. C.), pp. 9–29 Tokyo, Japan: Springer Publishing; (doi:10.1007/978-4-431-77134-0_2) [Google Scholar]
- Dalley D., Dave D., Lesellier S., Palmer S., Crawshaw T., Hewinson G. H., Chambers M. A.2008Development and evaluation of a gamma interferon assay for tuberculosis in badgers (Meles meles). Tuberculosis 88, 235–243 (doi:10.1016/j.tube.2007.11.001) [DOI] [PubMed] [Google Scholar]
- Delahay R. J., Langton S., Smith G. C., Clifton-Hadley R. S., Cheeseman C. L.2000The spatio-temporal distribution of Mycobacterium bovis (bovine tuberculosis) infection in a high-density badger population. J. Anim. Ecol. 69, 428–441 [Google Scholar]
- Doolan S. P., Macdonald D. W.1996Dispersal and extra-territorial prospecting by slender-tailed meerkats (Suricata suricatta) in the south-western Kalahari. J. Zool. 240, 59–73 [Google Scholar]
- Drewe J. A.2009Social networks and infectious disease transmission: epidemiology of tuberculosis in wild meerkats. PhD thesis, University of Cambridge, Cambridge, UK
- Drewe J. A., Dean G. S., Michel A. L., Lyashchenko K. P., Greenwald R., Pearce G. P.2009aAccuracy of three diagnostic tests for determining Mycobacterium bovis infection status in live-sampled wild meerkats (Suricata suricatta). J. Vet. Diagn. Invest. 21, 31–39 [DOI] [PubMed] [Google Scholar]
- Drewe J. A., Foote A. K., Sutcliffe R. L., Pearce G. P.2009bPathology of Mycobacterium bovis infection in wild meerkats (Suricata suricatta). J. Comp. Pathol. 140, 12–24 (doi:10.1016/j.jcpa.2008.09.004) [DOI] [PubMed] [Google Scholar]
- Drewe J. A., Madden J. R., Pearce G. P.2009cThe social network structure of a wild meerkat population: 1. Inter-group interactions. Behav. Ecol. Sociobiol. 63, 1295–1306 (doi:10.1007/s00265-009-0782-x) [Google Scholar]
- Freeman L. C., Borgatti S. P., White D. R.1991Centrality in valued graphs: a measure of betweenness based on network flow. Social Netw. 13, 141–154 [Google Scholar]
- Hamede R. K., Bashford J., McCallum H., Jones M.2009Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecol. Lett. 12, 1147–1157 (doi:10.1111/j.1461-0248.2009.01370.x) [DOI] [PubMed] [Google Scholar]
- Hanneman R. A., Riddle M.2005Introduction to social network methods Riverside, CA: University of California; Published in digital form at http://www.faculty.ucr.edu/~hanneman/nettext/ [Google Scholar]
- Jenkins H. E., et al. 2008The prevalence, distribution and severity of detectable pathological lesions in badgers naturally infected with Mycobacterium bovis. Epidemiol. Infect. 136, 1350–1361 (doi:10.1017/S0950268807009909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling M. J.2005The implications of network structure for epidemic dynamics. Theoret. Popul. Biol. 67, 1–8 (doi:10.1016/j.tpb.2004.08.002) [DOI] [PubMed] [Google Scholar]
- Kutsukake N., Clutton-Brock T. H.2006aAggression and submission reflect reproductive conflict between females in cooperatively breeding meerkats Suricata suricatta. Behav. Ecol. Sociobiol. 59, 541–548 (doi:10.1007/s00265-005-0079-7) [Google Scholar]
- Kutsukake N., Clutton-Brock T. H.2006bSocial functions of allogrooming in cooperatively breeding meerkats. Anim. Behav. 72, 1059–1068 (doi:10.1016/j.anbehav.2006.02.016) [Google Scholar]
- Kutsukake N., Clutton-Brock T. H.2008Do meerkats engage in conflict management following aggression? Reconciliation, submission and avoidance. Anim. Behav. 75, 1441–1453 (doi:10.1016/j.anbehav.2007.09.018) [Google Scholar]
- Lloyd-Smith J. O., Schreiber S. J., Kopp P. E., Getz W. M.2005Superspreading and the effect of individual variation on disease emergence. Nature 438, 355–359 (doi:10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden J. R., Clutton-Brock T. H.2009Manipulating grooming by decreasing ectoparasite load causes unpredicted changes in antagonism. Proc. R. Soc. B 276, 1263–1268 (doi:10.1098/rspb.2008.1661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden J. R., Drewe J. A., Pearce G. P., Clutton-Brock T. H.2009The social network structure of a wild meerkat population: 2. Intragroup interactions. Behav. Ecol. Sociobiol. 64, 81–95 (doi:10.1007/s00265-009-0820-8) [Google Scholar]
- Otterstatter M. C., Thomson J. D.2007Contact networks and transmission of an intestinal pathogen in bumble bee (Bombus impatiens) colonies. Oecologia 154, 411–421 (doi:10.1007/s00442-007-0834-8) [DOI] [PubMed] [Google Scholar]
- Perkins S. E., Ferrari M. F., Hudson P. J.2008The effect of social structure and sex-biased transmission on macroparasite infection. Parasitology 135, 1561–1569 (doi:10.1017/S0031182008000449) [DOI] [PubMed] [Google Scholar]
- Perkins S. E., Cagnacci F., Stradiotto A., Arnoldi D., Hudson P. J.2009Comparison of social networks derived from ecological data: implications for inferring infectious disease dynamics. J. Anim. Ecol. 78, 1015–1022 (doi:10.1111/j.1365-2656.2009.01557.x) [DOI] [PubMed] [Google Scholar]
- Phillips C. J. C., Foster C. R., Morris P. A., Teverson R.2003The transmission of Mycobacterium bovis infection to cattle. Res. Vet. Sci. 74, 1–15 [DOI] [PubMed] [Google Scholar]
- Read J. M., Eames K. T. D., Edmunds W. J.2008Dynamic social networks and the implications for the spread of infectious disease. J. R. Soc. Interface 5, 1001–1007 (doi:10.1098/rsif.2008.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P. A., Russell A. F., Young A. J., Sutherland W. J., Clutton-Brock T. H.2005Dispersal, eviction, and conflict in meerkats (Suricata suricatta): an evolutionarily stable strategy model. Am. Nat. 165, 120–135 [DOI] [PubMed] [Google Scholar]
- Vicente J., Delahay R. J., Walker N. J., Cheeseman C. L.2007Social organization and movement influence the incidence of bovine tuberculosis in an undisturbed high-density badger Meles meles population. J. Anim. Ecol. 76, 348–360 (doi:10.1111/j.1365-2656.2006.01199.x) [DOI] [PubMed] [Google Scholar]
- Wasserman S., Faust K.1994Social network analysis: methods and applications Cambridge, UK: Cambridge University Press [Google Scholar]
- Woodroffe R., Frost S. D. W., Clifton-Hadley R. S.1999Attempts to control tuberculosis in cattle by removing infected badgers: constraints imposed by live test sensitivity. J. Appl. Ecol. 36, 494–501 [Google Scholar]
- Woodroffe R., et al. 2009Social group size affects Mycobacterium bovis infection in European badgers (Meles meles). J. Anim. Ecol. 78, 818–827 (doi:10.1111/j.1365-2656.2009.01545.x) [DOI] [PubMed] [Google Scholar]
- Young A. J., Carlson A. A., Clutton-Brock T.2005Trade-offs between extraterritorial prospecting and helping in a cooperative mammal. Anim. Behav. 70, 829–837 (doi:10.1016/j.anbehav.2005.01.019) [Google Scholar]
- Young A. J., Carlson A. A., Monfort S. L., Russell A. F., Bennett N. C., Clutton-Brock T.2006Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA 103, 12 005–12 010 (doi:10.1073/pnas.0510038103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. J., Spong G., Clutton-Brock T.2007Subordinate male meerkats prospect for extra-group paternity: alternative reproductive tactics in a cooperative mammal. Proc. R. Soc. B 274, 1603–1609 (doi:10.1098/rspb.2007.0316) [DOI] [PMC free article] [PubMed] [Google Scholar]