Abstract
Juvenile salmonid fish readily form dominance hierarchies when faced with limited resources. While these social interactions may result in profound behavioural and physiological stress, it is unknown if this social stress is evident at the level of the cellular stress response—specifically, the induction of stress or heat shock proteins (Hsps). Thus, the goal of our study was to determine if Hsps are induced during hierarchy formation in juvenile rainbow trout (Oncorhynchus mykiss). To this end, we measured levels of three Hsps, Hsp70, Hsc (heat shock cognate)70 and Hsp90 in the white muscle, liver and brain of trout that had been interacting for 36 h, 72 h or 6 days. Our data indicate that Hsps are induced in both dominant and subordinate fish in a time- and tissue-specific manner. In further mechanistic experiments on fasted and cortisol-treated fish, we demonstrated that high plasma cortisol does not affect Hsp induction in trout white muscle or liver, but both conditions may be part of the mechanism for Hsp induction with social stress in the brain. We conclude that the behavioural and physiological stress experienced by juvenile rainbow trout in dominance hierarchies can be extended to the induction of Hsps.
Keywords: dominance hierarchies, social stress, juvenile fish, heat shock proteins
1. Introduction
Social interactions in many animals are structured around dominance relationships or hierarchies where the position in the hierarchy governs access to resources and ultimately individual fitness and/or reproductive success. Juvenile salmonid fish are highly aggressive, readily forming dominance hierarchies when faced with limited resources such as food (Metcalfe 1989), and as such are excellent models for the study of social stress. These fish form dominance hierarchies in laboratory situations, comparable to those formed in semi-natural or natural situations (Kalleberg 1958; Bachman 1984). The dominance hierarchies are ‘pecking order’ social structures that are established through agonistic interactions, with each fish being ranked according to its ability to outcompete other individuals within the group (Metcalfe et al. 1989; Adams et al. 1998). Dominant and subordinate individuals can be distinguished by their behaviour, with subordinate fish exhibiting behavioural inhibition including reduced activity, feeding and aggression (Sloman & Armstrong 2002; Gilmour et al. 2005).
Dominant–subordinate relationships can also have profound consequences on the physiological status and responsiveness of an animal. For example, subordinate salmonids experience reduced growth rates, higher metabolic costs, chronic elevation of circulating cortisol concentrations (Sloman & Armstrong 2002; Johnsson et al. 2006) and brain serotonergic activity (Winberg & Nilsson 1993; Johnsson et al. 2006), and immunosuppression (Maule & Vanderkooi 1999). However, social hierarchies are complex in that physiological contributors to social status versus physiological effects of social status are not easily distinguishable. Notably, changes in brain serotonergic activity appear to be both a response to behavioural interactions and a physiological mechanism underlying these behaviours (see Gilmour et al. 2005 for review). Similarly, salmon fry with a higher metabolic scope are more likely to be dominant (Metcalfe et al. 1995) but an increase in metabolic rate is also a consequence of social hierarchy formation for subordinate fish (Sloman et al. 2000). Furthermore, the costs and benefits of social rank are not always consistent. In salmonid fish, social interactions are an acute stressor for all individuals involved as catecholamines are mobilized in a transient fashion in both dominant and subordinate fish (Thomas & Gilmour 2006), and while subordinate fish experience prolonged elevation of cortisol, even dominant fish exhibit a transient increase in circulating cortisol levels as the hierarchy is established (Øverli et al. 1999). Despite this, it is clear that socially subordinate animals experience the more significant, chronic physiological disturbance (Gilmour et al. 2005; Johnsson et al. 2006).
There is now convincing evidence that socially directed changes in behaviour are reflected at the level of the genome. Indeed, the genetic responses to social stimuli can be massive, involving hundreds or thousands of genes and perhaps many different brain/body regions at once (Robinson 2008). Recently, Cummings et al. (2008) used a cDNA microarray analysis to identify several neuronal gene responses that may be an important part of the molecular mechanisms underpinning mate choice behaviour in swordtail fish. In the African cichlid, Astatotilapia burtoni, approximately 5 per cent of genes studied were differentially regulated in the brain according to male dominance phenotype (Renn et al. 2008). Furthermore, distinct brain gene expression profiles were observed in male Atlantic salmon Salmo salar with alternative reproductive strategies (Aubin-Horth et al. 2005a). Preliminary microarray analysis on the effects of social status on gene expression in rainbow trout Oncorhynchus mykiss observed more than 1000 gene differences in dominant, subdominant and subordinate fish including changes in stress-responsive genes (Sneddon et al. 2005). These responses are not limited to fish; for example Whitfield et al. (2003) report a molecular mRNA signature in the honeybee (Apis mellifera) brain that is strongly associated with behaviour. Molecular-level changes in stress response elements may not be surprising given the physiological and behavioural stress responses observed during social interactions in trout. Indeed, changes in corticosteroid signalling significantly impact behaviour, and specifically anxiety, in adult rats (Mitra et al. 2009); however, it is unclear if social stress results in a cellular stress response.
The molecular or ‘cellular stress response’ is a transient defence reaction resulting from damage to proteins, DNA or membranes (Kültz 2005). A main component of this response is the induction of heat shock or stress proteins (Hsps), a conserved family of proteins that prevent and/or repair stress-induced cellular damage. It has been suggested that these Hsps are more important for the survival of ectothermic animals with variable body temperatures, like fishes, than in endotherms with stable body temperatures and a reduced threat of temperature-induced cellular damage (Shabtay & Arad 2005). Moreover, there is increasing evidence linking changes in stress hormones to changes in stress proteins (e.g. Paroo & Noble 1999; Basu et al. 2001; Currie et al. 2008), demonstrating an integration of the physiological and cellular stress responses. Given the profound effect of behaviour on both physiological and molecular responses, our goal was to determine the effects of social stress in rainbow trout by examining the expression of Hsps.
Because subordinate trout experience more behavioural, physiological and molecular disturbances, we hypothesized that Hsp levels would be greater in subordinate fish. To take into account the temporal component of the endocrine stress responses to social interactions, Hsp responses were examined at different times following hierarchy formation. Finally, we sought to determine if certain characteristics of the subordinate state, such as the intensity of social interaction, fasting or high cortisol levels, could explain enhanced levels of Hsps in the tissues of subordinate fish.
2. Material and methods
(a). Experimental animals
Juvenile rainbow trout (O. mykiss; experimental n = 57, mass = 97.0 ± 4.5 g; mean ± s.e.m.) were obtained from Linwood Acres Trout Farm (Campbellcroft, ON, Canada). Fish were maintained in large fibreglass aquaria supplied with flowing, dechlorinated city-of-Ottawa tap water at 13°C. A 12 h : 12 h light : dark photoperiod was used. Trout were fed to satiation with commercial trout pellets on alternate days and were acclimated to the holding conditions for at least two weeks before experiments commenced. We conducted two experimental series as follows.
(i). Series 1
Social hierarchies were established within pairs of size-matched rainbow trout to examine in a time-dependent fashion the impact of social interactions on Hsp expression. Because comparisons of dominant and subordinate fish reveal similar behavioural and physiological traits regardless of whether fish have been held in pairs or small groups (Pottinger & Pickering 1992), we used paired fish, an experimental approach that facilitates the detection of physiological correlates of social status. Fish were lightly anaesthetized (i.e. to the point of losing equilibrium) in a solution of ethyl-p-aminobenzoate (0.065 g l−1), fork length was measured (21.8 ± 0.3 cm; n = 34), and fish were allocated to pairs according to comparable fork lengths (fork length difference averaged 0.3 ± 0.1 cm or 1.2 per cent of fork length). With the exception of control fish that were sampled directly from the holding tanks (see below), fish were confined in pairs in 40 l flow-through Plexiglas observation tanks. Fish were separated by a perforated opaque Plexiglas divider for a 24 h recovery period, and the dividers were then removed to allow pairs of fish to interact; a small piece of PVC tubing was placed within each tank to provide shelter. Social status was assigned through twice-daily observations of behaviour. The control fish were sampled directly from the holding tanks to minimize hierarchy formation and stress hormone levels. Hierarchy formation is reduced by holding conditions that include high fish numbers, large volume tanks and scatter feeding. We opted for controls sampled directly from the holding tank over an isolated fish control as we were concerned that handling stress and the associated transient elevation of blood cortisol in establishing isolation conditions would be a confounding factor here.
After 36 h, 72 h or 6 days of confinement in pairs, fish were euthanized by immersion in a solution of ethyl-p-aminobenzoate (0.15 g l−1). First, blood was withdrawn via caudal puncture (approx. 1 ml) and then white muscle, liver and brain tissues were quickly dissected and immediately freeze-clamped in liquid nitrogen for storage at −80°C until further processing and Hsp analysis. Whole blood was centrifuged at 10 000g and plasma was removed and also frozen at −80°C for later analysis of plasma cortisol concentration using a commercial RIA kit (ICN).
(ii). Series 2
Low social status in rainbow trout is associated with fasting and elevation of circulating cortisol concentrations (Gilmour et al. 2005). The goal of series 2 was to examine the impact of these factors individually on Hsp expression. Control fish were compared with trout that were fasted or exposed to elevated cortisol for 48 h; a 48 h treatment period was selected on the basis of series 1, in which most responses were observed at 36–72 h of social interactions. To elevate cortisol concentrations, fish received a cocoa butter pellet (0.005 ml g−1 fish cocoa butter; Now Personal Care 100% pure cocoa butter) containing dissolved cortisol (110 mg kg−1 fish hydrocortisone 21-hemisuccinate; Sigma-Aldrich). The cocoa butter was injected as a liquid into the peritoneal cavity but solidified rapidly within the fish, acting as a slow-release cortisol implant. After 48 h, during which time fasted or cortisol-treated fish were held in groups of 12 in tanks of approximately 780 l (DiBattista et al. (2005) found little evidence of hierarchy formation under these conditions), fish were terminally sampled as in series 1.
(b). Behavioural observations
Social status was determined by assigning points to each fish based on observations of selected behaviours, including position in the tank, food acquisition, aggressive behaviour and fin damage (an index of aggression received). Behavioural observations were carried out twice daily for 10 min each time. The scoring scheme was similar to that used previously for assigning social status to pairs of rainbow trout (e.g. DiBattista et al. 2005, 2006) and assigns higher scores to more dominant behaviours. A single behaviour score was calculated from all observations with a principal components analysis, and the fish with the higher overall behaviour score within each pair was assigned dominant social status.
(c). Hsp analysis
Soluble protein was extracted from frozen tissue and prepared for SDS–PAGE with equal protein loads of 15 µg as in Fowler et al. (2009). To compare protein levels among gels, a red blood cell sample from a heat-shocked trout was selected as an internal standard for the Hsp70 gels. Recombinant human Hsp90 (Stressgen, SPP-770) and recombinant bovine Hsc (heat shock cognate)70 (Stressgen, SPP-751) protein standards were loaded at 75 ng. Immunodetection proceeded as in Rendell et al. (2006). Rabbit anti-salmonid Hsp90 (AS05063), inducible Hsp70 (AS05061) and constitutive Hsc70 (AS05062) polyclonal antibodies (Agrisera, Sweden) were used at final concentrations of 1 : 50 000. The Hsp70 antibody is specific for the inducible isoform of Hsp70 and does not detect constitutive isoforms; the Hsc70 antibody detects the constitutive isoform and does not cross-react with the inducible Hsp70 (Rendell et al. 2006). Hsp90 also has constitutive (Hsp90βa, Hsp90βb; Ojima et al. 2005) and inducible (Hsp90α) family members in fish; however, our antibody does not distinguish among these isoforms.
Secondary antibody was detected using the ECL Advance Western Blotting Detection Kit (GE Healthcare). Experimental band densities were quantified using Quantity One software (Bio-Rad) and divided by the standard sample band density (red blood cells from heat-shocked fish or commercial standard) to give relative band density. Data are presented relative to the mean control value on each graph.
(d). Statistics
All analyses were conducted using SPSS v. 15, except where indicated. Prior to analysis, data were checked for violation of assumptions of parametric tests. Normality was confirmed using the Kolmogorov–Smirnov test and homogeneity of variance using Levene's test. When variance heterogeneity was detected, data were log10 + 1 transformed. In all cases, results were evaluated for significance at α = 0.05.
In series 1, differences between dominant and subordinate fish in each interaction period were determined with unpaired Student's t-tests. To evaluate whether or not each pair was significantly different from the control group, we examined the 95 per cent confidence interval surrounding the mean. This analysis was chosen over an analysis of variance (ANOVA) randomized block because the control fish did not interact with the paired fish and therefore these groups could not be statistically analysed together. Values were considered significantly different if the mean values of the dominant or subordinate fish did not overlap with the control mean.
To determine the importance of hierarchy formation on Hsp levels, we performed linear regressions on log-transformed data with aggregate behavioural score (+2) as the independent variable and Hsp level as the dependent variable (SigmaPlot v. 11; see electronic supplementary material). We also attempted to correlate Hsp levels and cortisol; however, the raw or transformed data did not fit a linear model. After examination of the plots (Hsp versus cortisol), we concluded that there was no clear relationship between these two variables.
In series 2, we ran a one-way ANOVA to determine significant differences in plasma cortisol and Hsps among control, fasted and cortisol-treated fish. When significance in the ANOVA was detected, a posteriori comparisons were conducted using Tukey's test.
3. Results
(a). Series 1
Social hierarchies were formed for the three interaction periods as indicated by the strongly polarized behaviour scores determined for dominant (0.93 ± 0.09) versus subordinate fish (−0.90 ± 0.05). Plasma cortisol levels in control fish were typical of ‘unstressed’ fish (i.e. less than 10 ng ml−1; Gamperl et al. 1994) and remained near these control values in dominant fish at 36 h, 72 h and 6 days of social interaction. Cortisol values in subordinate fish (78.1 ± 26.4, n = 17) were significantly (Student's t-test, p = 0.002) higher than those in dominant fish (3.7 ± 1.9, n = 17) but because of the variability in these data, the differences were not significant when comparisons were made as a function of interaction time.
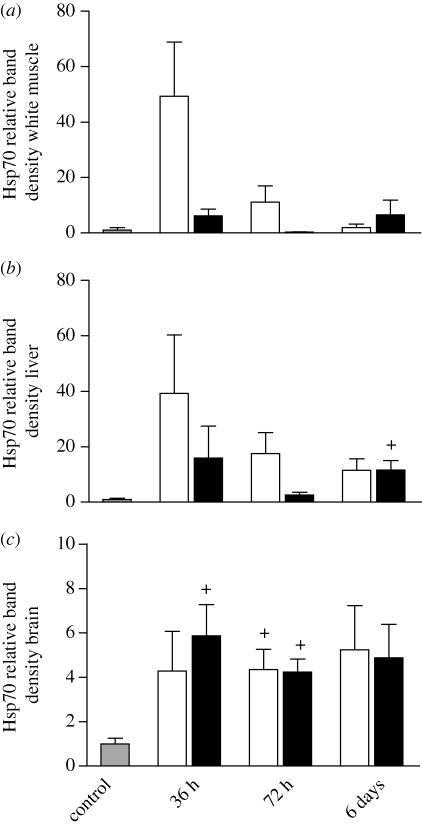
Hsp70 (inducible) levels were low in white muscle, liver and brain of control fish (figure 1). We observed a 40–50-fold increase in Hsp70 in subordinate fish at 36 h in white muscle and liver, but the data were variable and these increases were not statistically significant. Correspondingly, Hsp70 levels after 36 h were not significantly related to behaviour score. In fish interacting for 72 h, however, we observed higher levels of Hsp70 in white muscle and liver in more subordinate fish (white muscle: R2 = 0.621, p = 0.007; liver: R2 = 0.525, p = 0.008). Only liver tissue of dominant fish paired for 6 days showed a significant induction of Hsp70, and this increase was not dramatic (figure 1b).
Figure 1.
Relative levels of Hsp70 (inducible) in (a) white muscle, (b) liver, and (c) brain of subordinate (unfilled bar) and dominant (black bar) rainbow trout following 36 h, 72 h or 6 days of social interaction (n = 6 for each social status at 36 and 72 h; n = 5 for each social status at 6 days). The control (grey bar) represents the unpaired control fish sampled directly from the holding tank (n = 9). Values are means ± 1 s.e.m. A plus sign indicates a value that is not within the 95% confidence interval of the control mean.
Although the magnitude of induction was less, social stress appeared to have a more consistent effect in brain tissue as we observed a significant induction of Hsp70 from control in dominant fish paired for 36 h and in both dominant and subordinate fish interacting for 72 h (figure 1c). We did not observe any differences in Hsp70 levels between dominant and subordinate fish in any of the three interaction periods (figure 1) and the intensity of the social interaction did not affect brain Hsp70.
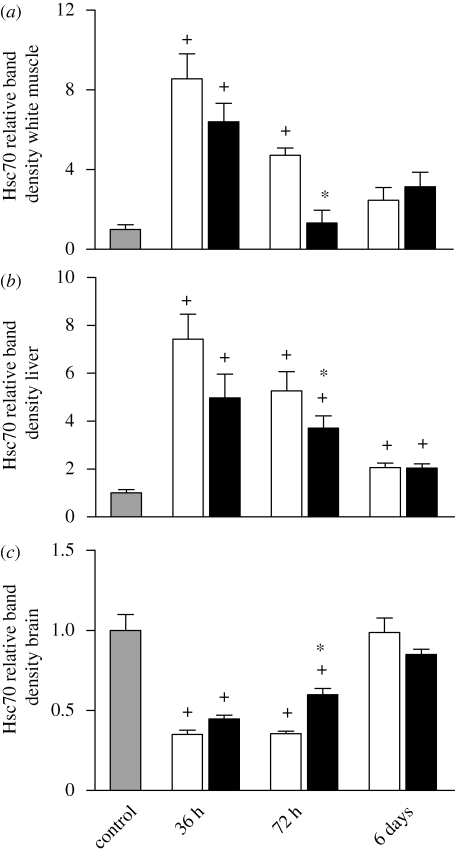
Interestingly, social stress appeared to have more of an effect on the constitutive isoform of Hsp70, Hsc70, in white muscle and liver of both dominant and subordinate trout (figure 2a,b) than it did for inducible Hsp70. After 36 h of interaction, in both white muscle and liver tissues, Hsc70 levels were significantly induced by approximately eightfold in dominant and subordinate fish compared with the control group. After 72 h of interaction, white muscle Hsc70 levels remained significantly elevated in subordinate fish only, and after interactions of 6 days, Hsc70 had returned to control levels. Liver Hsc70 levels were significantly greater than control levels at all pairing times in both dominant and subordinate fish. For both white muscle and liver, subordinate fish had significantly greater levels of Hsc70 than their dominant counterparts following 72 h of interacting (figure 2a,b); however, only in white muscle was the degree of subordination predictive of higher Hsc70 levels (R2 = 0.546; p = 0.015).
Figure 2.
Relative levels of Hsc70 (constitutive) in (a) white muscle, (b) liver, and (c) brain of subordinate (unfilled bar) and dominant (black bar) rainbow trout following 36 h, 72 h or 6 days of social interaction (n = 6 for each social status at 36 and 72 h; n = 5 for each social status at 6 days). The control (grey bar) represents the unpaired control fish sampled directly from the holding tank (n = 9). A plus sign indicates a value that is outside the 95% confidence interval of the control mean. An asterisk denotes a significant difference between dominant and subordinate fish within an interaction period (paired Student's t-test, p < 0.05).
The situation for brain tissue was markedly different than for white muscle and liver, in that there was no induction of Hsc70 with social stress. In fact, Hsc70 levels were significantly lower in fish in dominance hierarchies for 36 or 72 h (figure 2c). After 6 days of interactions, brain Hsc70 levels were no longer significantly different from the control value. As was the case with white muscle and liver, we observed significant differences in dominant and subordinate brain Hsc70 levels after 72 h of interaction (figure 2c). Furthermore, in both the 36 and 72 h groups, the more dominant fish, as determined by the higher behavioural score, were more likely to have higher levels of brain Hsc70 (36 h: R2 = 0.503, p = 0.010; 72 h: R2 = 0.863, p = 0).
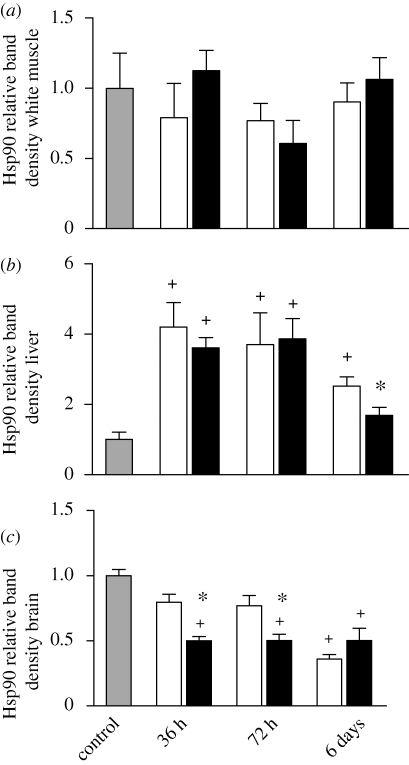
Hsp90 was significantly induced only in liver tissue from socially stressed fish (figure 3). After both 36 and 72 h of interaction, liver Hsp90 levels were significantly greater in both dominant and subordinate fish compared with control animals, increasing by fourfold (figure 3b). Hsp90 remained significantly elevated only in subordinate fish after 6 days. This group was also the only point where we observed significant differences between dominant and subordinate fish liver Hsp90 and notably, the degree of subordination here could predict Hsp90 levels (R2 = 0.466; p = 0.030).
Figure 3.
Relative levels of Hsp90 in (a) white muscle, (b) liver, and (c) brain of subordinate (unfilled bar) and dominant (black bar) rainbow trout following 36 h, 72 h and 6 days of social interaction. The control (grey bar) represents the unpaired control fish sampled directly from the holding tank. A plus sign indicates a value that is outside the 95% confidence interval of the control mean. An asterisk denotes a significant difference between dominant and subordinate fish within an interaction period (paired Student's t-test, p < 0.05).
Similar to brain Hsc70, brain Hsp90 levels were significantly lower in socially stressed animals than in control fish; however, in contrast to the Hsc70 results, only the dominant fish had significantly lower brain Hsp90 levels after 36 and 72 h of pairing (figure 3c). After 6 days, Hsp90 levels were significantly lower in both dominant and subordinate fish. There were differences in brain Hsp90 levels between dominant and subordinate fish after interactions of 36 or 72 h where the higher degree of subordination could predict higher Hsp90 levels (36 h: R2 = 0.594, p = 0.003; 72 h: R2 = 0.405, p = 0.026).
(b). Series 2
In an effort to understand the mechanism of the induction of Hsps in socially stressed fish, we measured Hsp levels in fish that had been fasted for 5 days or that had been fitted with cortisol implants (110 mg kg−1) to simulate specific components of subordinate status. The control fish had low resting plasma cortisol (2.67 ± 0.5 ng ml−1). As expected, plasma cortisol was significantly elevated in the cortisol-treated fish (122.2 ± 26.7 ng ml−1). We did not observe any significant change in plasma cortisol from control in fasted fish (16.7 ± 6.8 ng ml−1).
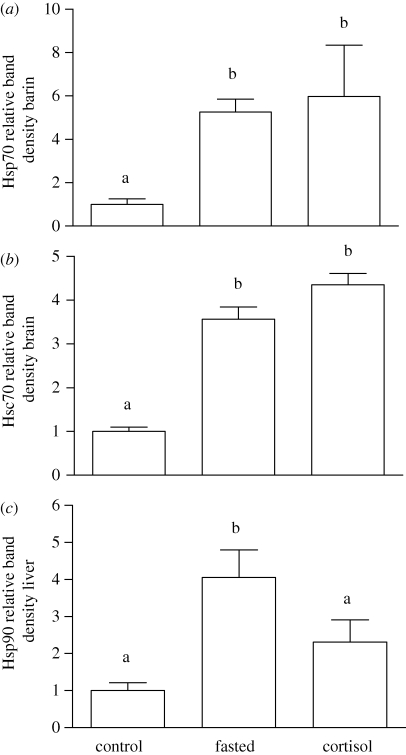
Neither fasting nor cortisol treatment had an effect on Hsp70 or Hsc70 levels in white muscle or liver. However, both treatments resulted in a significant induction of brain Hsp70 (figure 4a) and Hsc70 (figure 4b).
Figure 4.
Relative levels of brain (a) Hsp70, (b) Hsc70, and (c) liver Hsp90 in rainbow trout under control conditions (n = 9), or following fasting (n = 8) or treatment with a cortisol implant (110 mg kg−1; n = 6) for 5 days. Values are means ± 1 s.e.m. Bars that do not share a letter are significantly different from one another (one-way ANOVA and Tukey's post hoc test; p < 0.05).
Hsp90 levels were not affected by fasting or cortisol treatment in white muscle or brain (electronic supplementary material, figure S3), but fasting caused a significant induction of Hsp90 in liver tissue (figure 4c).
4. Discussion
Our data indicate that the formation of dominance hierarchies in juvenile rainbow trout, a phenomenon that results in both physiological and behavioural stress, also has cellular-level consequences in the form of induction of Hsps. Hsp induction in fish occurs in response to a variety of physico-chemical stressors including temperature changes (e.g. Fowler et al. 2009), osmotic stress (Smith et al. 1999) and contaminants (e.g. Deane & Woo 2007). However, only a handful of studies has examined whether Hsp induction occurs in response to psychological or psychosocial stress in any vertebrate (humans, Lewthwaite et al. 2002; mice, Andrews et al. 2000; cats, Fleshner et al. 2004; birds, Hoekstra et al. 1998 and fishes, Kagawa et al. 1999; Kagawa & Mugiya 2002) and to our knowledge, the present study represents the first information on Hsp responses to social stress, specifically. Induction of Hsps by social interactions might be regarded as surprising. Recognition, however, of the physiological consequences of social interactions, such as the high cortisol levels experienced by subordinate trout (see also Øverli et al. 1999; Sloman et al. 2001), provides a context in which the occurrence of a cellular stress response can be considered.
Hsp70 is one of the most stress inducible of the Hsps. For example, the inducible isoform of this protein increases approximately 10-fold in recovery from heat shock in rainbow trout tissues (Fowler et al. 2009), with gene expression increasing by 300-fold (Currie et al. 2008). If social interactions represent a significant cellular stress, we would expect to see clear induction of this Hsp resulting from hierarchy formation and/or prolonged dominant and subordinate interaction. Although there is no information on whether dominance hierarchies induce Hsps, there is some indication that psychological stress caused by the presence of a predator induces Hsp70 mRNA in the brains of goldfish (Carassius auratus; Kagawa et al. 1999; Kagawa & Mugiya 2002) and induces both brain Hsp genes and protein in cats exposed to rats (Fleshner et al. 2004). In these cases, Hsp induction is related to/enhanced by elevated plasma cortisol levels. Although we observed a 40–50-fold increase in Hsp70 in white muscle and liver after 36 h of social interactions, these increases were not statistically significant (figure 1), perhaps at least partly because the nature of the behavioural interaction at this point was also variable. We saw only a modest induction after 6 days of interaction in the liver tissue in dominant fish and did not see any significant differences between dominant and subordinate fish. Despite the lack of differences, after 72 h of interaction, the greater degree of subordinance was predictive of higher levels of white muscle and liver Hsp70. Thus, we conclude from these data that while the physiological and psychological disturbances associated with social stress in trout, such as elevated plasma cortisol, do not consistently induce Hsp70 in liver or muscle, the magnitude of subordinate status may have a modest role in predicting Hsp70 induction in these tissues.
On the other hand, we did observe a statistically significant induction of Hsp70 in brain after 36 and 72 h of interaction, although the magnitude of the increase was not as great (i.e. eightfold in brain versus approx. 40-fold in white muscle and liver). Similarly, the predator stress that resulted in an increase in Hsp70 mRNA in goldfish brains did not have an effect on the hepatopancreas tissue (Kagawa & Mugiya 2002). Why would social stress have a greater effect on brain Hsp70? We know that brain monoaminergic activity increases in salmonid dominance hierarchies (see Johnsson et al. 2006 for review) and that genes involved in neuronal remodelling are upregulated in cichlid fish (Renn et al. 2008) and Atlantic salmon (Aubin-Horth et al. 2005a,b) during social interactions, collectively indicating socially directed plasticity in the brain. Furthermore, upregulation of the brain neurotrophic factor, ependymin, was recently linked to social status in juvenile rainbow trout (L. U. Sneddon, R. Schmidt & A. R. Cossins 2009, unpublished). We speculate that an increase in brain Hsp70 could be involved in neural remodelling and possibly in protecting and/or chaperoning dopaminergic and serotonergic receptors in the brain. The latter idea is compelling given that Hsp70 has been shown to protect dopaminergic neurons in Drosophila (Auluck et al. 2002).
Under ‘unstressed’ conditions, rainbow trout usually have very low, often undetectable levels of Hsp70 in their tissues with relatively high levels of Hsc70 (Zafarullah et al. 1992; Ojima et al. 2005; Rendell et al. 2006). In fishes, Hsp70 genes and protein are dramatically induced with heat shock (Fangue et al. 2006; Currie et al. 2008; Fowler et al. 2009), whereas Hsc70 tends to be weakly induced (Ojima et al. 2005) if at all (Zafarullah et al. 1992). Thus, our observation that white muscle and liver Hsc70 was significantly induced in both dominant and subordinate fish while Hsp70 was not is somewhat surprising. Despite the similarity in molecular structure of these two Hsp70 family members (Welch 1992), they are probably regulated and triggered differently. Generally, stress-inducible Hsp expression is regulated by the heat shock transcription factors (HSFs) of which four are known in vertebrates—HSF1 being the principal transcription factor activated in response to heat stress (reviewed in Wu 1995). In response to stress signals, widely accepted to be the presence of unfolded proteins (Morimoto et al. 1994), most HSFs acquire DNA-binding activity to the heat shock promoter element (HSE) and mediate the rapid transcription of hsp genes resulting in the accumulation of Hsps (Morimoto 1998). The induction of the primarily constitutive Hsc70 with heat shock is likely due to the presence of HSEs in the fish hsc70 gene (Ojima et al. 2005). The weakness of the Hsc70 induction signal compared with Hsp70 can be attributed, at least in trout, to an Hsc70 coding sequence that contains introns, unlike the case for Hsp70 (Zafarullah et al. 1992). Furthermore, differential regulation of these two genes could be the result of differences in HSFs controlling the stress response. Rainbow trout possess at least two HSF1 isoforms (Ojima & Yamashita 2004), thus the preferential induction of Hsc70 over Hsp70 observed here may be explained at the level of the specific HSF(s) activated as a result of social stress. This possibility awaits further experiments.
It is noteworthy that in tissues where Hsc70 levels were high, Hsp70 levels were low and vice versa (figures 2c and 3c) suggesting that Hsp/c70 activate a coordinated cellular response in salmonid dominance hierarchies, possibly protecting or chaperoning tissue-specific targets. That said, similar to white muscle Hsp70 after 72 h of interaction, white muscle Hsc70 levels were higher in the more subordinate fish. In contrast, brain Hsc70 levels were higher in the more dominant fish at both 36 and 72 h of interaction. Our data suggest that not only is this Hsp/c70 cellular response dependent on the extent of agonistic interactions in a tissue-specific manner, it is also probably more important early in hierarchy formation because the Hsp response decreases with interaction time.
The proximate trigger for Hsp/c70 induction during social interactions is unknown. As noted above, the universal signal for Hsp induction is accepted to be the formation of unfolded proteins and/or damaged proteins; this is the case, for example, after heat stress, where protein folding and aggregation is known to be a problem (Kelly & Schlesinger 1978), but it is not known if social stress causes a similar proteotoxic response. It is possible that the mechanism of Hsp70 induction with behavioural stress may be unique and unrelated to the presence of denatured or unfolded proteins. Whatever the actual trigger for Hsp induction with social stress, it is likely that it involves both physical and hormonal elements and our results indicate that induction is also tissue specific. For example, it is clear from the data of series 2 that fasting and exogenous cortisol turn on Hsp/c70 in the brain; however, neither treatment resulted in significant induction in white muscle or liver. This latter finding is consistent with our data in series 1 where there was no relationship between Hsp levels and blood cortisol. Our current research is now attempting to tease apart the mechanisms for Hsp induction with social stress and to understand possible reasons for this tissue specificity.
Hsp90 has broad chaperone functions (Buchner 1999) and is the most highly expressed Hsp in eukaryotic cells (Csermely et al. 1998). It has roles in many signalling pathways and in fishes, it is necessary for both glucocorticoid and estrogen receptor activation in rainbow trout hepatocytes (Sathiyaa & Vijayan 2003; Osborne et al. 2007, respectively). In addition to its constitutive functions, like Hsc70, Hsp90 is somewhat inducible with heat stress (Fowler et al. 2009). Here, social stress did result in a significant induction of Hsp90 but only in liver tissue. Because cortisol increases, at least transiently, in both dominant and subordinate fish when hierarchies are established (Øverli et al. 1999), this liver-specific induction may be linked to Hsp90's role in chaperoning the glucocorticoid receptor (Boone & Vijayan 2002). Higher levels of Hsp90 during social stress could be a result of increased glucocorticoid receptor activity. However, this is probably not the only explanation for the Hsp90 induction observed here as juvenile trout treated with cortisol implants did not induce liver Hsp90. This may not be surprising as cortisol results in a blunted heat shock response in fish cells (e.g. Basu et al. 2001) possibly as a way to limit the negative consequences of a prolonged and/or elevated stress response (see Sapolsky et al. 2000 for review). Fasting, also a hallmark of the subordinate state, did result in significantly higher levels of Hsp90 as has also been shown previously in food-deprived rainbow trout larvae (Cara et al. 2005). Furthermore, DiBattista et al. (2006) demonstrated significant changes in liver metabolism with social status in trout, related to both fasting and increases in plasma cortisol. Finally, the extent of social interactions has a limited capacity to predict liver Hsp90 levels in that only in the 6-day group did the degree of subordinance reflect higher Hsp90. Thus, the induction of Hsp90 in liver with social stress in juvenile fish may be somewhat associated with the characteristics and extent of subordinate status, but is more likely related to the metabolic remodelling that occurs in the liver during social stress.
In conclusion, we have shown that the formation of dominance hierarchies in juvenile rainbow trout induces Hsps in a time- and tissue-specific manner. Overall, it appears that it is the establishment of the hierarchy itself, rather than differences between dominant and subordinate status that is most important in turning on the cellular stress response. Indeed, in the brain, particularly, the intensity of the behavioural interaction predicts the induction of constitutive Hsps, Hsc70 and Hsp90. The rise in plasma cortisol, transient in dominant fish, prolonged in subordinates, may be part of the mechanism for Hsp induction with social stress in the brain, but this stress hormone does not appear to be important for Hsp induction in white muscle or liver. Our results show that the behavioural and physiological stress experienced by juvenile trout when dominance hierarchies are formed is also reflected at the level of the cellular stress response.
Acknowledgements
All experimental procedures described here comply with the requirements outlined by the Canadian Council of Animal Care and were approved by the University of Ottawa Animal Care Committee.
We (SC, KMG) would like to thank the Natural Sciences and Engineering Research Council (NSERC) of Canada for financial support of this work (through the DG, RTI and USRA programmes), our undergraduate students Stephanie Fowler, Tom McConnell and Anusha Jahagirdar for help with the experiments, Bill Fletcher for help with animal husbandry and Dr Diana Hamilton for her statistical advice. We thank Dr L. Sneddon for generous access to her unpublished work.
References
- Adams C. E., Huntingford F. A., Turnbull J. F., Beattie C.1998Alternative competitive strategies and the cost of food acquisition in juvenile Atlantic salmon (Salmo salar). Aquaculture 167, 17–26 (doi:10.1016/S0044-8486(98)00302-0) [Google Scholar]
- Andrews H. N., Kerr L. R., Strange K. S., Emerman J. T., Weinberg J.2000Effect of social housing condition on heat shock protein (HSP) expression in the Shionogi mouse mammary carcinoma (SC115). Breast Cancer Res. Treat. 59, 199–209 (doi:10.1023/A:1006314010958) [DOI] [PubMed] [Google Scholar]
- Aubin-Horth N., Landry C. R., Letcher B. H., Hofmann H. A.2005aAlternative life histories shape brain gene expression profiles in males of the same population. Proc. R. Soc. B 272, 1655–1662 (doi:10.1098/rspb.2005.3125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin-Horth N., Letcher B. H., Hofmann H. A.2005bInteraction of rearing environment and reproductive tactic on gene expression profiles in Atlantic salmon. J. Hered. 96, 261–278 (doi:10.1093/jhered/esi030) [DOI] [PubMed] [Google Scholar]
- Auluck P. K., Chan H. Y. E., Trojanowski J. Q., Lee V. M.-Y., Bonini N. M.2002Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295, 865–868 (doi:10.1126/science.1067389) [DOI] [PubMed] [Google Scholar]
- Bachman R. A.1984Foraging behavior of free-ranging wild and hatchery brown trout in a stream. Trans. Am. Fish. Soc. 113, 1–32 (doi:10.1577/1548-8659(1984)113<1:FBOFWA>2.0.CO;2) [Google Scholar]
- Basu N., Nakano T., Grau E. G., Iwama G. K.2001The effects of cortisol on heat shock protein 70 levels in two fish species. Gen. Comp. Endocrinol. 124, 97–105 (doi:10.1006/gcen.2001.7688) [DOI] [PubMed] [Google Scholar]
- Boone A. N., Vijayan M. M.2002Glucocorticoid-mediated attenuation of the hsp70 response in trout hepatocytes involves the proteasome. Am. J. Physiol. 283, R680–R687 [DOI] [PubMed] [Google Scholar]
- Buchner J.1999Hsp90 and co.—a holding for folding. Trends Biochem. Sci. 24, 136–141 (doi:10.1016/S0968-0004(99)01373-0) [DOI] [PubMed] [Google Scholar]
- Cara J. B., Aluru N., Moyano F. J., Vijayan M. M.2005Food-deprivation induces HSP70 and HSP90 protein expression in larval sea bream and rainbow trout. Comp. Biochem. Physiol. B 142, 426–431 [DOI] [PubMed] [Google Scholar]
- Csermely P., Schnaider T., Söti C., Prohászka Z., Nardai G.1998The 90-kDa molecular chaperone family: structure, function and clinical applications. A comprehensive review. Pharmacol. Therap. 79, 129–168 (doi:10.1016/S0163-7258(98)00013-8) [DOI] [PubMed] [Google Scholar]
- Cummings M. E., Larkins-Ford J., Reilly C. R. L., Wong R. Y., Ramsey M., Hofmann H. A.2008Sexual and social stimuli elicit rapid and contrasting genomic responses. Proc. R. Soc. B 275, 393–342 (doi:10.1098/rspb.2007.1454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie S., Reddin K., McGinn P., McConnell T., Perry S. F.2008β-adrenergic stimulation enhances the heat shock response in fish. Physiol. Biochem. Zool. 81, 414–425 (doi:10.1086/589095) [DOI] [PubMed] [Google Scholar]
- Deane E. E., Woo N. Y.2007Impact of nitrite exposure on endocrine, osmoregulatory and cytoprotective functions in the marine teleost Sparus sarba. Aquat. Toxicol. 82, 85–93 (doi:10.1016/j.aquatox.2007.02.004) [DOI] [PubMed] [Google Scholar]
- DiBattista J., Anisman H., Whitehead M., Gilmour K. M.2005The effects of cortisol administration on social status and brain monoaminergic activity in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 208, 2707–2718 (doi:10.1242/jeb.01690) [DOI] [PubMed] [Google Scholar]
- DiBattista J., Levesque H. M., Moon T. W., Gilmour K. M.2006Growth depression in social subordinate rainbow trout Oncorhynchus mykiss: more than a fasting effect. Physiol. Biochem. Zool. 79, 675–687 (doi:10.1086/504612) [DOI] [PubMed] [Google Scholar]
- Fangue N. A., Hofmeister M., Schulte P. M.2006Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J. Exp. Biol. 209, 2859–2872 (doi:10.1242/jeb.02260) [DOI] [PubMed] [Google Scholar]
- Fleshner M., Campisi J., Amiri L., Diamond D. M.2004Cat exposure induces both intra- and extracellular Hsp72: the role of adrenal hormones. Psychoneuroendocrinology 29, 1142–1152 (doi:10.1016/j.psyneuen.2004.01.007) [DOI] [PubMed] [Google Scholar]
- Fowler S. L., Hamilton D., Currie S.2009A comparison of the heat shock response in juvenile and adult rainbow trout (Oncorhynchus mykiss)—implications for increased thermal sensitivity with age. Can. J. Fish. Aquat. Sci. 66, 91–100 (doi:10.1139/F08-192) [Google Scholar]
- Gamperl A. K., Vijayan M. M., Boutilier R. G.1994Experimental control of stress hormone levels in fishes: techniques and applications. Rev. Fish Biol. Fish. 4, 215–255 (doi:10.1007/BF00044129) [Google Scholar]
- Gilmour K. M., DiBattista J., Thomas J.2005Physiological causes and consequences of social status in salmonid fish. Integr. Comp. Biol. 45, 263–273 (doi:10.1093/icb/45.2.263) [DOI] [PubMed] [Google Scholar]
- Hoekstra K. A., Iwama G. K., Nichols C. R., Godin D. V., Cheng K. M.1998Increased heat shock protein expression after stress in Japanese quail. Stress 2, 265–272 [DOI] [PubMed] [Google Scholar]
- Johnsson J. I., Winberg S., Sloman K. A.2006Social interactions. In Fish physiology. Vol. 24. Behaviour and physiology of fish (eds Sloman K. A., Wilson R. W., Balshine S.), pp. 151–196 San Diego, CA: Academic Press [Google Scholar]
- Kagawa N., Mugiya Y.2002Brain hsp70 mRNA expression is linked with plasma cortisol levels in goldfish (Carassius auratus) exposed to a potential predator. Zool. Sci. 19, 735–740 (doi:10.2108/zsj.19.735) [DOI] [PubMed] [Google Scholar]
- Kagawa N., Ryo K., Mugiya Y.1999Enhanced expression of stress protein 70 in the brains of goldfish, Carassius auratus, reared with bluegills, Lepomis macrochirus. Fish Physiol. Biochem. 21, 103–110 (doi:10.1023/A:1007785707501) [Google Scholar]
- Kalleberg H.1958Observations in a stream tank of territoriality and competition in juvenile salmon and trout. Rep. Inst. Freshw. Res. Drottningholm 39, 55–98 [Google Scholar]
- Kelly P. M., Schlesinger M. J.1978The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell 15, 1277–1286 (doi:10.1016/0092-8674(78)90053-3) [DOI] [PubMed] [Google Scholar]
- Kültz D.2005Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 67, 225–257 (doi:10.1146/annurev.physiol.67.040403.103635) [DOI] [PubMed] [Google Scholar]
- Lewthwaite J., Owen N., Coates A., Henderson B., Steptoe A.2002Circulating human heat shock protein 60 in the plasma of British civil servants: relationship to physiological and psychosocial stress. Circulation 106, 196–201 (doi:10.1161/01.CIR.0000021121.26290.2C) [DOI] [PubMed] [Google Scholar]
- Maule A. G., Vanderkooi S. P.1999Stress-induced immune–endocrine interaction. In Stress physiology in animals (ed. Balm P. H. M.), pp. 205–245 Sheffield, UK: Sheffield Academic Press [Google Scholar]
- Metcalfe N. B.1989A link between competitive ability and life history strategies in Atlantic salmon. Proc. R. Soc. Lond. B 236, 21–27 (doi:10.1098/rspb.1989.0010) [DOI] [PubMed] [Google Scholar]
- Metcalfe N. B., Huntingford F. A., Graham W. D., Thorpe J. E.1989Early social status and the development of life-history strategies in Atlantic salmon. Proc. R. Soc. Lond. B 236, 7–19 (doi:10.1098/rspb.1989.0009) [DOI] [PubMed] [Google Scholar]
- Metcalfe N. B., Taylor A. C., Thorpe J. E.1995Metabolic rate, social status and life-history strategies in Atlantic salmon. Anim. Behav. 49, 431–436 (doi:10.1006/anbe.1995.0056) [Google Scholar]
- Mitra R., Ferguson D., Sapolsky R. M.2009Mineralocorticoid receptor overexpression in basolateral amygdala reduced corticosterone excretion and anxiety. Biol. Psychiatry 66, 686–690 (doi:10.1016/j.biopsych.2009.04.016) [DOI] [PubMed] [Google Scholar]
- Morimoto R. I.1998Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones and negative regulators. Genes Dev. 12, 3788–3796 (doi:10.1101/gad.12.24.3788) [DOI] [PubMed] [Google Scholar]
- Morimoto R. I., Jurivich D. A., Kroger P. E., Mathur S. K., Murphy S. P., Nakai A., Sarge A. K., Abravaya K., Sistonen L. T.1994Regulation of heat shock gene transcription by a family of heat shock factors. In The biology of heat shock proteins and molecular chaperones (eds Morimoto R. I., Tissieres A., Georgopoulos C.), pp. 417–455 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Ojima N., Yamashita M.2004Cloning and characterization of two distinct isoforms of rainbow trout heat shock factor 1. Eur. J. Biochem. 271, 703–712 (doi:10.1111/j.1432-1033.2003.03972.x) [DOI] [PubMed] [Google Scholar]
- Ojima N., Yamashita M., Watabe S.2005Quantitative mRNA expression profiling of heat-shock protein families in rainbow trout cells. Biochem. Biophys. Res. Comm. 329, 51–57 (doi:10.1016/j.bbrc.2005.01.097) [DOI] [PubMed] [Google Scholar]
- Osborne N., Sherry J., Rendell J. L., Currie S.2007The role of hsp90 in 17α-ethynylestradiol-induced endocrine disruption in rainbow trout hepatocytes. Ecotoxicol. Environ. Saf. 68, 13–19 (doi:10.1016/j.ecoenv.2006.12.002) [DOI] [PubMed] [Google Scholar]
- Øverli Ø., Harris C. A., Winberg S.1999Short-term effects of fights for social dominance and the establishment of dominant–subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav. Evol. 54, 263–275 (doi:10.1159/000006627) [DOI] [PubMed] [Google Scholar]
- Paroo Z., Noble E. G.1999Isoproterenol potentiates exercise-induction of Hsp70 in cardiac and skeletal muscle. Cell Stress Chaperones 4, 199–204 [PMC free article] [PubMed] [Google Scholar]
- Pottinger T. G., Pickering A. D.1992The influence of social interaction on the acclimation of rainbow trout, Oncorhynchus mykiss (Walbaum) to chronic stress. J. Fish Biol. 41, 435–447 (doi:10.1111/j.1095-8649.1992.tb02672.x) [Google Scholar]
- Rendell J. L., Fowler S., Cockshutt A., Currie S.2006Development-dependent differences in intracellular localization of stress proteins (hsps) in rainbow trout, Oncorhynchus mykiss, following heat shock. Comp. Biochem. Physiol. D 1, 238–252 [DOI] [PubMed] [Google Scholar]
- Renn S. C. P., Aubin-Horth N., Hofmann H. A.2008Fish and chips: functional genomics of social plasticity in an African cichlid fish. J. Exp. Biol. 211, 3041–3056 (doi:10.1242/jeb.018242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G. E.2008Genes and social behavior. Science 322, 896–900 (doi:10.1126/science.1159277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R. M., Romero M., Munck A. U.2000How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- Sathiyaa R., Vijayan M. M.2003Autoregulation of glucocorticoid receptor by cortisol in rainbow trout hepatocytes. Am. J. Physiol. 284, C1508–C1515 [DOI] [PubMed] [Google Scholar]
- Shabtay A., Arad Z.2005Ectothermy and endothermy: evolutionary perspectives of thermoprotection by HSPs. J. Exp. Biol. 208, 2773–2781 (doi:10.1242/jeb.01705) [DOI] [PubMed] [Google Scholar]
- Sloman K. A., Armstrong J. D.2002Physiological effects of dominance hierarchies: laboratory artefacts or natural phenomena? J. Fish Biol. 61, 1–23 (doi:10.1111/j.1095-8649.2002.tb01733.x) [Google Scholar]
- Sloman K. A., Motherwell G., O'Connor K. I., Taylor A. C.2000The effect of social stress on the standard metabolic rate (SMR) of brown trout, Salmo trutta. Fish Physiol. Biochem. 23, 49–53 (doi:10.1023/A:1007855100185) [Google Scholar]
- Sloman K. A., Metcalfe N. B., Taylor A. C., Gilmour K. M.2001Plasma cortisol concentrations before and after social stress in rainbow trout and brown trout. Physiol. Biochem. Zool. 74, 383–389 (doi:10.1086/320426) [DOI] [PubMed] [Google Scholar]
- Smith T. R., Tremblay G. C., Bradley T. M.1999Hsp70 and a 54kDa protein (Osp54) are induced in salmon (Salmo salar) in response to hyperosmotic stress. J. Exp. Zool. 284, 286–298 (doi:10.1002/(SICI)1097-010X(19990801)284:3<286::AID-JEZ6>3.0.CO;2-J) [DOI] [PubMed] [Google Scholar]
- Sneddon L. U., Margareto J., Cossins A. R.2005The use of transcriptomics to address questions in behavior of a suppression subtractive hybridization library from dominance hierarchies of rainbow trout. Physiol. Biochem. Zool. 78, 695–705 (doi:10.1086/432141) [DOI] [PubMed] [Google Scholar]
- Thomas J., Gilmour K. M.2006The impact of social status on the erythrocyte ß-adrenergic response in rainbow trout, Oncorhynchus mykiss. Comp. Biochem. Physiol. A 143, 162–172 (doi:10.1016/j.cbpa.2005.11.008) [DOI] [PubMed] [Google Scholar]
- Welch W. J.1992Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol. Rev. 72, 1063–1081 [DOI] [PubMed] [Google Scholar]
- Whitfield C. W., Cziko A.-M., Robinson G. E.2003Gene expression profiles in the brain predict behavior in individual honeybees. Science 302, 296–299 (doi:10.1126/science.1086807) [DOI] [PubMed] [Google Scholar]
- Winberg S., Nilsson G. E.1993Roles of brain monoamine neurotransmitters in agonistic behaviour and stress reactions, with particular reference to fish. Comp. Biochem. Physiol. 106C, 597–614 [Google Scholar]
- Wu C.1995Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol. 11, 441–469 (doi:10.1146/annurev.cb.11.110195.002301) [DOI] [PubMed] [Google Scholar]
- Zafarullah M., Wisniewski J., Shworak N. W., Schieman S., Misra S., Gedamu L.1992Molecular cloning and characterization of a constitutively expressed heat-shock-cognate hsc71 gene from rainbow trout. Eur. J. Biochem. 204, 8983–8900 (doi:10.1111/j.1432-1033.1992.tb16709.x) [DOI] [PubMed] [Google Scholar]