Abstract
Male Club-winged Manakins, Machaeropterus deliciosus (Aves: Pipridae), produce a sustained tonal sound with specialized wing feathers. The fundamental frequency of the sound produced in nature is approximately 1500 Hz and is hypothesized to result from excitation of resonance in the feathers' hypertrophied shafts. We used laser Doppler vibrometry to determine the resonant properties of male Club-winged Manakin's wing feathers, as well as those of two unspecialized manakin species. The modified wing feathers exhibit a response peak near 1500 Hz, and unusually high Q-values (a measure of resonant tuning) for biological objects (Q up to 27). The unmodified wing feathers of the Club-winged Manakin do not exhibit strong resonant properties when measured in isolation. However, when measured still attached to the modified feathers (nine feathers held adjacent by an intact ligament), they resonate together as a unit near 1500 Hz, and the wing produces a second harmonic of similar or greater amplitude than the fundamental. The feathers of the control species also exhibit resonant peaks around 1500 Hz, but these are significantly weaker, the wing does not resonate as a unit and no harmonics are produced. These results lend critical support to the resonant stridulation hypothesis of sound production in M. deliciosus.
Keywords: feather, resonance, sonation, laser vibrometry, sexual selection, morphological novelty
1. Introduction
Courting male Club-winged Manakins, Machaeropterus deliciosus, are hypothesized to use a mechanism of sonation, or non-vocal sound production (sensu Bostwick & Prum 2003), unique to vertebrates: resonant stridulation of modified feathers to produce a briefly sustained, unwavering, tonal sound (Bostwick & Prum 2005). The resonant stridulation hypothesis was inferred from: (i) description of the two modified feathers which show enlarged and ridged shafts; (ii) analysis of high-speed videos which show the males actively drive approximately 107 Hz medio-lateral oscillations of the modified feathers, and thereby repeatedly knock the modified feather shafts together across their midline; and (iii) analyses of the acoustic structure of the sound which show it is a sustained (approx. 0.33 s), unmodulated fundamental frequency of approximately 1500 Hz with integer harmonics. The authors proposed that the repeated oscillations of the modified feathers allowed for stridulation—multiplication of the frequency of motion-generating events—induced when each of the seven ridges on the surface of the modified feathers' shafts are rubbed twice (once as the feathers converge medially, and once as they diverge laterally) for each cycle of wing oscillation. In order for the stridulation mechanism to function as proposed and produce the seamless tone, however, the authors predicted that the modified feathers should also exhibit distinctive resonant properties at or near the fundamental frequency of the sound produced in nature, such that each percussive event excites resonance in the thickened shafts, and that this resonance is sustained by the stridulatory impulses (Bostwick & Prum 2005).
Feathers constituting the wing either insert along the length of the manus (wing hand) or the ulna (fore-arm), called primaries and secondaries, respectively. The nine secondary feathers of M. deliciosus include typical avian secondaries with long, slender, evenly tapering rachi (shafts), and several highly derived feathers (figure 1). Secondaries 1–5 deviate from typical secondaries by exhibiting increasingly wider rachi and an increasingly pronounced transition from continuously tapering to an abrupt taper around the distal two-third, three-fourth and then four-fifth for the third, fourth and fifth secondaries, respectively. Colocated with the abrupt taper on the fifth secondary, the rachis also suddenly bends medially. When at rest, this ‘kink’ in the rachis of the fifth secondary causes it to overlap and contact the rachis of the adjacent sixth secondary feather. The sixth and seventh secondaries are the most obviously and distinctively modified: the rachis is thick at the base, and at approximately one-half of its length, the feather rachis more than doubles its width and twists along its long axis so that the dorsal feather surface is oriented medially. The average length of the sixth and seventh secondary feathers is approximately 39 mm and approximately 35 mm, respectively.
Figure 1.
(a) Ventral view of secondary feathers of M. deliciosus (UMMZ 255 055): secondaries 1–9 ‘attached’ and labelled. Note enlarged rachi of modified sixth and seventh secondaries. (b) Dorsal surface of the fifth secondary, (c) anatomically ‘medial’ surface of the sixth secondary twisted to orient ventrally. (a) Scale bar, 1 cm.
A male Club-winged Manakin produces his courtship sonation when he flips the dorsal surface of his wings cranio-dorsally, so the secondaries form an upright wall in a plane roughly perpendicular to the male's back. As the wings are being positioned, the male adducts the medial edges of the wings together such that the innermost pair of the modified feathers (seventh secondaries) collides across the back. Immediately following this collision, the wings are shivered laterally and medially, pulling these feathers just millimetres apart, only to be adducted approximately 9 ms later to produce another collision. The pair of feathers knock and rebound as the wing shivers, and the sonation tone is produced continuously throughout this process (see electronic supplementary material, movie S2, Bostwick & Prum 2005).
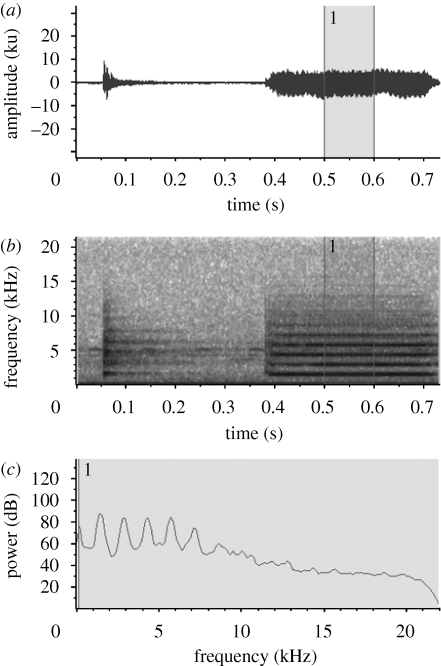
Two syllable types are frequently produced together during courtship, ticks and tings. The tick is a brief beat, produced singly or doubly for each one of the second syllable produced, the more sustained ting (figure 2; Bostwick 2000). These two syllables differ primarily in their duration, and but also in their peak frequencies. Measured from 10 tick–ting calls in one male, the peak frequency of the tick is 1.59 ± 0.05 kHz, and the duration is 0.014 ± 0.007 ms (n = 17), while the peak frequency during the sustained ting is 1.49 ± 0.01 kHz, and the duration is 0.335 ± 0.015 ms (n = 10; unpublished details from Bostwick & Prum 2005). Variation within and between individuals in this species is believed to be relatively low, with tings measured in two other individuals measured at 1.44 ± 0.08 kHz and 1.48 ± 0.04 kHz (n = 20 for each of two individuals; Bostwick 2000).
Figure 2.
(a) Waveform, (b) spectrogram and (c) power spectrum of tick and ting of M. deliciosus showing sustained fundamental tone and harmonics. Power spectrum was calculated from segment of sound highlighted in (a,b) using Raven Pro 1.4 (www.birds.cornell.edu/raven).
This functional system represents a striking novelty among vertebrates requiring further study. Unfortunately, Club-winged Manakins cannot be studied in captivity; they are endemic to a small region of northwestern Ecuador and southwestern Columbia (Ridgely & Tudor 1994), both countries from which export of birds is exceptionally difficult. Further, this species is extremely sensitive to handling (K. S. Bostwick 2008, personal observation), probably owing to specialized physiology that allows them to shiver their wings at exceedingly high rates.
In order to further understand the details of the mechanism by which male M. deliciosus produce sound with modified feathers, we here use materials extracted from museum specimens. We characterize the vibratory properties of M. deliciosus secondary feathers compared with homologous, unmodified feathers of two close relatives to test the critical prediction of the resonant stridulation hypothesis, that the modified feathers resonate at or near the sonation frequency of approximately 1500 Hz.
2. Material and methods
(a). Materials
Secondary feathers of three species of Pipridae (Aves) were examined: M. deliciosus (n = 4, the ‘treatment’), Lepidothrix coronata and Pipra fascicauda (n = 1, respectively, the ‘controls’) (table 1). For one individual of each species, the resonance of each of the secondaries (1–8) was measured while that feather was physically attached in a life-like configuration to all of the other secondaries. ‘Attached’ feathers have been removed as a unit from the ulna of the bird, but still have the inter-remigial ligament in place holding the feathers aligned and adjacent in their natural orientations. Individual feathers were also measured in isolation for three other specimens of M. deliciosus (‘isolated’).
Table 1.
Species and secondary feathers measured. (Feather numbers refer to secondary feathers counted from wrist along ulna distally to proximally, how feathers were measured (isolated or attached to other secondaries), and lengths of isolated feathers are included parenthetically. UMMZ, University of Michigan Museum of Zoology; AMNH, American Museum of Natural History; KU, University of Kansas Natural History Museum.)
| species | specimen | feathers measured (length in mm) |
|---|---|---|
| Lepidothix coronata | KU 66 851 | 1–8 (attached) |
| Pipra fascicauda | KU 73 336 | 1–8 (attached) |
| Machaeropterus deliciosus | AMNH 493 121 | 6 (39.62), 7 (34.93) (isolated) |
| M. deliciosus | AMNH 430 058 | 6 (38.10), 7 (34.92) (isolated) |
| M. deliciosus | UMMZ 255 054 | 1 (50.8), 5 (44.45), 6 (38.10), 7 (34.83) (isolated) |
| M. deliciosus | UMMZ 255 055 | 1–9 (attached) |
We were forced to work with small sample sizes owing to the extreme rarity of M. deliciosus specimens in collections, and because measuring feather properties required permanently removing these wing feathers from the sampled specimens. Specimens, and special permission for destructive use thereof, were obtained from the American Museum of Natural History (AMNH), the University of Michigan Museum of Zoology (UMMZ) and the University of Kansas Natural History Museum (KUNHM).
(b). Stimulus and response experiments
In order to stimulate the feathers, each was mounted firmly by its base on a vibration mini-shaker (B&K Type 4810 Mini-shaker, B&K Type 2706 Power Amplifier) using a custom-built holder. The holder was composed of two parallel, rubber-lined plastic plates that were held together and tightened by a pair of adjustable screws. The holder device itself screwed directly onto the surface of the mini-shaker. The proximal ends, or bases, of the feathers, whether isolated or attached, were sandwiched between the holder plates by their bases. An anatomical feature at the base of the feather shaft was used to guide the depth of clamping, attempting to mimick the proportion of the calamus that was ‘fixed’ by soft tissue to the ulna in life by clamping up to the superior umbilicus (Lucas & Stettenheim 1972). The feathers were orientated so that their tips were perpendicular to the up-and-down plane of motion of the mini-shaker. The behaviour of the feather rachi was measured by a laser Doppler vibrometer (LDV, see below) while it was stimulated by the mini-shaker's motion: a broad, continuous sweep of frequencies (10Hz–4.5 kHz) lasting a total of 400 ms and generated using a custom Matlab (The Mathworks) script.
The velocity (the indicator of resonant response) of each point on the feather was recorded using an LDV (Polytec OFV 3001 controller, OFV 511 sensor head; Waldbronn, Germany) (Michelsen et al. 1982; Elias et al. 2003), digitized (National Instruments PCI-6023E, 25 kHz sampling rate) and analysed using Matlab (The Mathworks). Laser Doppler vibrometry is a non-contact method of recording vibration that measures the velocity of a moving surface by detecting the Doppler shift of a reflected laser beam. Small (less than 1 mm2) pieces of reflective tape (Scotchlite 7610 and 8850 retro-reflective tape manufactured by 3M and distributed by Motion Lab Systems Inc.) were attached to points on the rachis (shaft) of individual feathers to serve as measurement points for the LDV. For isolated feathers, we measured four different points determined by the feather morphology (roughly feather ‘base’ (approx. one-fifth length of feather), midpoint 1 (approx. one-third length), midpoint 2 (approx. two-third length) and ‘tip’(as close as possible to true tip)). For attached feathers, we measured a single point near the base of the feathers, at approximately one-fifth the total length of the feather (tapering rachi of unmodified feathers made more distal measurements difficult). The LDV sensor head was mounted on a micromanipulator and orientated perpendicular to the plane of stimulation.
Responses were calculated as transfer functions with the input signal being the measured response at the holder and the output signal being the vibration of the feathers at each measurement point. Peak frequencies were measured from vibration spectra. All recordings were made on a vibration-isolated table in a sound-attenuated chamber.
(c). Resonant systems and the quality factor
Resonance is the tendency of a system to oscillate at maximum amplitude at certain frequencies (system resonance, f0). If a structure is excited at f0 by a periodic driving force, it will vibrate with a relatively larger amplitude because the system stores vibrational energy (Rossing & Fletcher 1995). When damping (resistance to motion) is small, f0 is approximately equal to the natural frequency of the system, which is the frequency of freely decaying vibrations (Fletcher 1992).
The quality factor Q measures a resonant system's damping as well as the rate at which such a system reaches the maximum amplitude (Prestwich & O'Sullivan 2005). Q, derived from the frequency spectrum of the vibrational response of the system, is defined to be the ratio of the frequency of the peak response divided by the spectral width at the two points above and below f0 with amplitudes 0.707 times the peak value (the spectral method; Fletcher 1992). Q can be estimated using different methods:
where BW − 3 dB,SPL is the bandwidth at 3 dB SPL (re. 20 µPa) below the peak, loge (decrement) is the natural logarithm of the rate of decrease of the amplitude of an undriven oscillator, M is the mass of the system and R is the damping (specific acoustic) resistance (Prestwich et al. 2000). Because we used a continuous FM sweep, rather than an impulsive stimulus, to excite feathers, we calculated Q using the spectral method.
3. Results
(a). The treatment feathers, Machaeropterus deliciosus
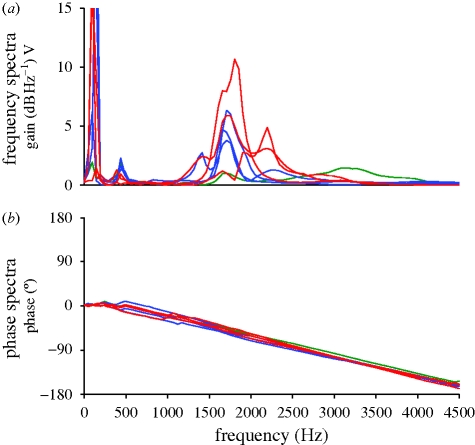
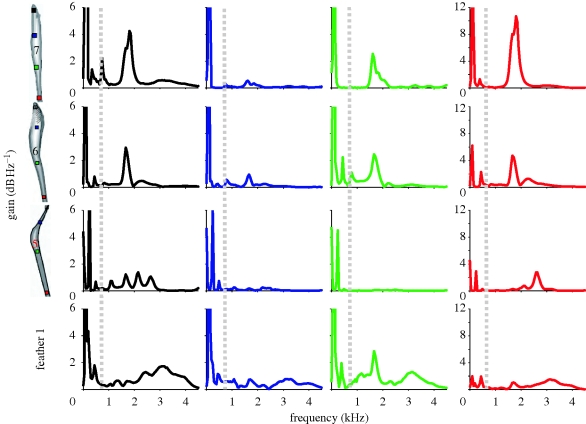
Measured in isolation, M. deliciosus secondaries 6 and 7 (modified) and 1 (unmodified) show multiple peaks in vibration spectra when stimulated with a frequency sweep (figure 3). There was a strong low-frequency peak near 100 Hz, a weak peak between 400 and 500 Hz and other higher frequency peaks between 1.4 and 2.3 kHz. The first strong (greater than 2 dB above background) peak above 1000 Hz will hereafter be referred to as the ‘fundamental’ peak, as it is in the range of frequencies that could potentially relate to the production of the known sound. Individual feathers varied in the tuning and magnitude of peaks. The relative amplitudes of the fundamental peak also varied among the measurements from different locations along the feather shafts (figure 4). In feathers 6 and 7, the high-frequency peak was more pronounced at the most proximal measurement points along the feather shafts (notice the y-axis compressed for the most proximal point graphs in red), and nearly absent at two-third length (this measurement point was nearly centred in the thickened part of the modified feather shafts). Feathers 1 and 5 showed weaker resonant qualities, with 5 only exhibiting a single distinct higher frequency peak at the most proximal measurement point. A strong fundamental peak in feather 1 (which is not modified) was only observed at one point along the rachis.
Figure 3.
Transfer functions and phase spectra for multiple individual isolated secondary feathers 1 (green line) (unmodified, n = 1), 6 (red lines) and 7 (blue lines) (modified, n = 3) from M. deliciosus.
Figure 4.
Transfer functions for multiple points on rachi of isolated secondary feathers 1 (unmodified), 5, 6 and 7 (modified) from M. deliciosus UMMZ 255 054. (Set of graphs show one representative of three sixth and seventh secondaries, as averaging among feathers obscures structure of resonant tuning in individual feathers). Vibration responses of each individual feather (rows) was measured at four different locations along the shaft (columns) where the colour of the plot corresponds to the point on the feather (red being the most proximal, green being one-third length, blue being two-third length and black being the most distal). Dashed lines indicate the fundamental frequency of the ting sound produced during M. deliciosus sonation.
Q factors of all the modified M. deliciosus feathers studied here were above 10 (the only Q below 10 was found in the control feather, secondary 1) and as high as 27 (table 2), which suggests these structures have characteristics of good to excellent biological resonators.
Table 2.
Mean Q-values calculated for different feathers.
| feather from individual | f0 | s.d. | Q | s.d. |
|---|---|---|---|---|
| wing 225 055 | 1.52 | 0 | 17.48 | 2.39 |
| sixth 493 121 | 1.71 | 0.01 | 15 | 1.71 |
| seventh 493 121 | 1.57 | 0.03 | 20.14 | 1.55 |
| sixth 430 058 | 1.57 | 0.05 | 14.20 | 1.84 |
| seventh 430 058 | 1.43 | 0.02 | 10.33 | 1.16 |
| fifth 255 054 | 2.58 | 0.21 | 20.98 | 2.06 |
| sixth 255 054 | 1.41 | 0.01 | 24.62 | 3.58 |
| seventh 255 054 | 2.20 | 0 | 27.42 | 1.72 |
| first 255 054 | 1.4 | 0.22 | 9.03 | 2.73 |
Vibration spectra peaks were more consistent between feathers when feathers were measured as part of an ensemble held in natural relative positions. Under these conditions, all eight feathers showed a similar pattern of vibration in response to stimulation with a frequency sweep—a strong low-frequency peak near 100 Hz, a weak low-frequency peak near 440 Hz and a high-frequency peak at 1.5 kHz (1503.75 ± 1.83 Hz, n = 8). Tuning of the high-frequency peak, therefore, consistently matched the dominant frequency of sonation signals found in nature. Feather 5, in addition to exhibiting a distinct vibration peak at 1.5 kHz that had been absent when the feather was measured in isolation, showed a greater response at 3.0 kHz (the first harmonic of the sonation frequency), as did three of the four unmodified feathers (figure 5a(i)). Phase spectra for attached feathers showed little variation, with the exception of feather 1, which showed progressively greater deviation at higher frequencies (figure 5a(ii)).
Figure 5.
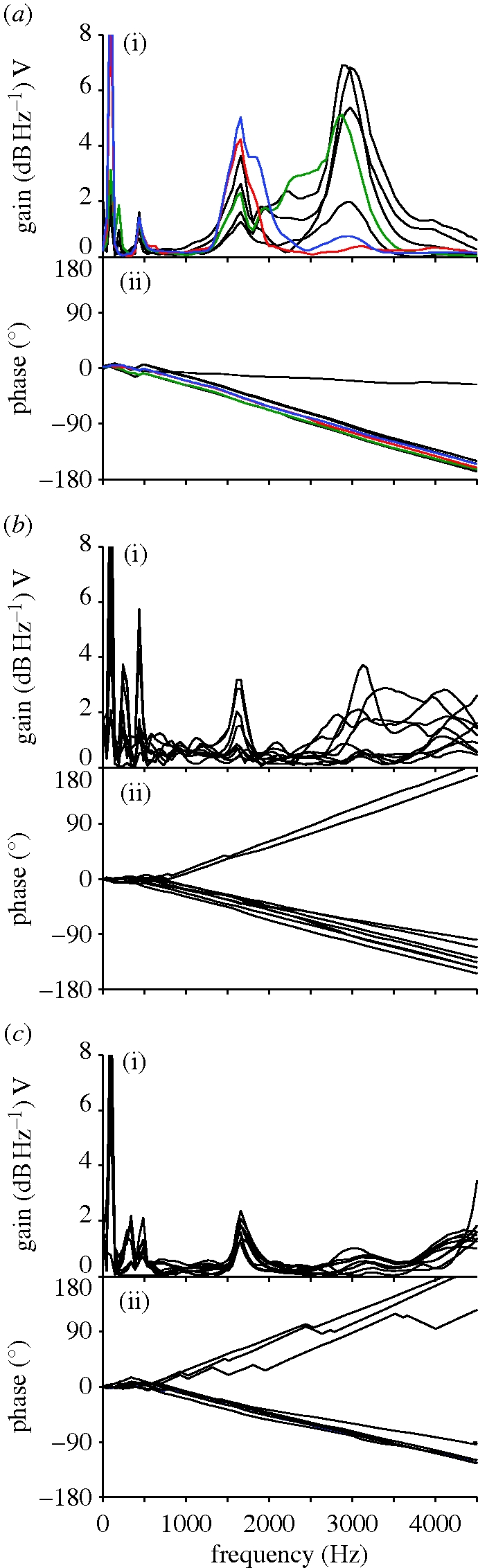
Transfer functions (i) and phase spectra (ii) for attached secondaries 1–8 of (a) M. deliciosus, (b) P. fascicauda and (c), L. coronata. All species show multiple frequency peaks between 100 and 500 Hz, and their first higher, or fundamental, frequency peaks between 1500 and 1700 Hz. Machaeropterus deliciosus exhibits stronger peaks, and equally strong or stronger harmonics. Phase spectra in lower panels show M. deliciosus feathers oscillate more coherently (a(ii)) than controls (b(ii),c(ii)). Green lines, feather 5; red lines, feather 6; blue lines, feather 7.
(b). The control feathers, Pipra fascicauda and Lepidothrix coronata
We measured vibration responses of attached feather ensembles from P. fascicauda and L. coronata. In both of these species, as in M. deliciosus, individual feather responses within ensembles were quite consistent. Furthermore, feather vibration responses of both control species showed some general similarities with those of M. deliciosus, with most feathers showing a strong low-frequency peak near 100 Hz, peaks between 250 and 500 Hz (in these cases two such peaks) and a fundamental peak near 1.5 kHz (figure 5b(i),c(i)). However, the amplitudes of the fundamental peaks were significantly smaller in the controls: the highest peak measured on the transfer function spectra in the range of 500–4000 Hz was 5.16 dB ± 1.56 s.d. (n = 8) for M. deliciosus versus 1.78 dB ± 1.26 s.d. (n = 8) for P. fascicauda and 1.60 dB ± 0.43 s.d. (n = 8) for L. coronata, respectively. An overall ANOVA: F2,21=22.911, p < 0.000, and a post hoc test yielded significant differences between M. deliciosus and P. fascicauda (p < 0.0001), and M. deliciosus and L. coronata (p < 0.0001).
Finally, variance in the phase (relative timing) of vibration among individual feathers was significantly greater in P. fascicauda and L. coronata than in M. deliciosus (Rao's test = 10.94, p = 0.004), whereas P. fascicauda and L. coronata had similar phase variance among feathers (Rao's test = 0.1622, p = 0.69) (figure 5b(ii),c(ii)).
4. Discussion
The resonant frequency of a bar is determined by structural attributes such as the bar's length, the second moment of inertia, Young's modulus and density of the constituent materials (Fletcher 1992). Whether measured in isolation or attached to the other secondaries, the structural properties of the sixth and seventh secondary feathers of M. deliciosus cause them to resonate near the fundamental frequency of the tone produced in nature. Thus, the modified sixth and seventh secondary feathers of male Club-winged Manakins exhibit resonant properties consistent with the prediction of the resonant stridulation hypothesis of sound production in this species.
When measured in isolation, there was variation among feathers, and measurement points within feathers, in the frequency tuning and amplitudes of resonant peaks. The varying pattern of response along the length of the modified feathers is particularly interesting, especially the strong response near the base of the feather where the clamping occurs, and the near absence of the resonant frequency at the two-third measuring point near where the ridges for stridulation on the sixth secondary are located and the sixth and seventh secondaries collide (as evidenced by holes worn in the feather vanes in this area). We can only hypothesize that it is the highly irregular shape of the modified feathers that is causing them to behave in non-intuitive ways; that is, the distribution of mass down the feathers' length may cause these feathers to behave differently from typical vibrating bars. The less modified fifth secondary and the unmodified first secondary feathers did not show strong or consistent resonant peaks near the sonation frequency, indicating that they probably play secondary roles in the production of the sound.
When attached M. deliciosus feathers were stimulated, all showed vibration peaks matching the sonation frequency and most also showed peaks at the second harmonic. Furthermore, the attached M. deliciosus feathers vibrated in-phase, indicating that the wing vibrates as a coherent unit, consistent with efficient radiation of sound. These results suggest that coupling between feathers, when they are excited into vibration as an ensemble as they would be in nature, is critical to the precise tuning and efficiency of sound radiation during sonation.
Interestingly, feathers of non-sonating species L. coronata and P. fascicauda also showed vibration peaks at a similar frequency to those of M. deliciosus, albeit of lower amplitude. Feathers in these species also showed significantly greater variation in phase, indicating that in these species wings do not vibrate coherently. Nevertheless, the tendency of unmodified feathers in non-sonating species to vibrate near the same frequency as those of M. deliciosus suggests the possibility that sonation-related adaptations exploit common mechanical characteristics of feathers, which in turn influence spectral characteristics of sonations. Anatomical specializations of secondaries 6 and 7 in M. deliciosus may therefore be primarily associated with quality of the resonance in terms of the Q-value, rather than the location of the tuning in terms of frequency.
The Q-values for biological structures typically range from about 1 to 30 (Fletcher 1992), with resonance being broadly tuned for Q-values near 1, more sharply tuned for higher Q-values, and reasonably good resonators exhibiting at least Q > 5. Several of the Q-values reported here are relatively high within this range and provide evidence that these feathers constitute ‘sharp’ or well-tuned biological resonators.
One intriguing aspect of this particular case of sound production is that what appears to generate the excitation of the feather resonance—the periodic beating of the resonant feathers together at 107 Hz—does not consistently or strongly affect the amplitude of sound. The high Q-values discovered here are probably a critical property of the feathers for minimizing amplitude modulation during sound production, as the temporal patterning of the amplitude of a sound depends on the rate of energy input and the rate of decay of that energy (the Q-values; Ewing 1989).
While the high Q-values undoubtably contribute to the low-amplitude modulation of the tone, there are nonetheless still two ways of modelling the functions of the interactions among the modified feathers. In one model, the periodic knocking of feathers across the back directly excites sound-radiating oscillations of the wing, but the nature of the periodic knocking (timing and high frequency) is such that it leaves only the slightest trace of amplitude modulation. If this was the case, stridulatory input could be unnecessary, as sufficiently high Q-values have been argued to explain the production of a continuous tone using repeated percussive energy input (Young & Josephson 1983). Alternatively, the knocking may serve to generate relative movement between modified feathers that results in stridulation of the sixth secondary feather that stimulates the resonance (Bostwick & Prum 2005). These hypotheses need not be mutually exclusive, but instead may represent two ends of a complementary continuum of excitatory input, such that relatively frequent knocking together with stridulatory excitation of a high Q-value object could reinforce excitatory inputs and smooth the sound envelope.
Our results support the hypothesis that sonation in M. deliciosus depends on the resonant vibration of modified wing feathers and further suggest additional details of the model of sound production in Club-winged Manakin males. The ‘instrument’ is composed of a pair of two coupled resonators (each wing having one pair of the modified sixth and seventh secondaries). These resonating structures lie medial to a series of up to five other secondary feathers that themselves oscillate in phase with the sixth and seventh feathers. High-speed video has shown that at least one of the two pairs of modified feathers, number 7, percusses repeatedly across the midline at a rate 1/14 of the frequency of sound produced in nature, presumably creating impulsive stimulation of these highly tuned feathers (Bostwick & Prum 2005). However, the secondary wing feathers seem to act as a unit transmitting the 1.5 kHz vibration and its harmonics, providing evidence of mechanical coupling among feathers, perhaps through the feathers' common attachment to the ulna, and/or the presence of the inter-remigial ligament, and/or through contact between the feather vanes, or alternatively through sympathetic vibrations.
5. Conclusions
As predicted by Bostwick & Prum (2005), the modified sixth and seventh secondary feathers of male Club-winged Manakins exhibit properties necessary to produce unmodulated tonal sounds, and further, appear to drive the other feathers of the wing to act together to radiate sound efficiently. This functional system would be less surprising in insects, which employ diverse, often scleratized, body parts in sound production and have evolved multiple mechanisms of frequency multiplication and a wide variety of resonant structures (Dumortier 1963; Bennet-Clark 1975; Ewing 1989; Conner 1999). However, given that the vast majority of vertebrate communication is performed using an internal ‘vocal’ apparatus (Simmons et al. 2003), this system represents an unprecedented novelty.
The mechanism employed by male Club-wing Manakins crosses taxonomic boundaries and places these birds squarely among more arthropod-typical mechanisms of sound production. Particularly intriguing is the convergence of this mechanism of sound production to the Castanet moth, Hecatesia exultans (Bailey 1978). In H. exultans, a brief tonal sound pulse is generated by percussive contact between paired castanet-like structures on the wings. Further, as with many arthropods, there is anatomical evidence that the functioning instrument of M. deliciosus also includes the use of a mechanism of frequency multiplication via stridulation. The presence of these extremely rare traits in a bird highlights an arthropod-vertebrate convergence enacted by choosy females, with the structural features of the modified secondary feathers appearing to have been exaggerated from resonant characteristics that probably existed in the ancestral feathers. This in turn highlights the power of sexual selection to modify not just behaviours, or to form simple ornaments, but also to fundamentally alter physiological and morphological aspects of the phenotype that directly relate to important naturally selected functions, in this case flight, to generate complex instruments.
Acknowledgements
Thanks to the extensive comments of two anonymous reviewers whose input significantly improved this manuscript. This project was funded by a grant from the National Science Foundation (IOB-0547709) to K.S.B., and by the National Institute of Health NRSA (1F32GM076091-01A1) to D.O.E. Thanks to the museums and collectors who contributed the rare anatomical materials that enabled this research.
References
- Bailey W. J.1978Resonant wing systems in the Australian whistling moth Hecatesia (Agaristidae, Lepidoptera). Nature 272, 444–446 (doi:10.1038/272444a0) [Google Scholar]
- Bennet-Clark H. C.1975Sound production in insects. Sci. Prog. 62, 263–283 [PubMed] [Google Scholar]
- Bostwick K. S.2000Display behaviors, mechanical sounds, and evolutionary relationships of the Club-winged Manakin (Machaeropterus deliciosus). Auk 117, 465–478 (doi:10.1642/0004-8038(2000)117[0465:DBMSAE]2.0.CO;2) [Google Scholar]
- Bostwick K. S., Prum R. O.2003High-speed video analysis of wing-snapping in two manakin clades (Pipridae: Aves). J. Exp. Biol. 206, 3693–3706 (doi:10.1242/jeb.00598) [DOI] [PubMed] [Google Scholar]
- Bostwick K. S., Prum R. O.2005Courting bird sings with stridulating wing feathers. Science 309, 736 (doi:10.1126/science.1111701) [DOI] [PubMed] [Google Scholar]
- Conner W. E.1999‘Un chant d’appel amoureux': acoustic communication in moths. J. Exp. Biol. 202, 1711–1723 [DOI] [PubMed] [Google Scholar]
- Dumortier B.1963Morphology of sound emission apparatus in Arthropoda. In Acoustic behaviour of animals (ed. Busnel R.-G.), pp. 277–345 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- Elias D. O., Mason A. C., Maddison W. P., Hoy R. R.2003Seismic signals in a courting male jumping spider (Araneae: Salticidae). J. Exp. Biol. 206, 4029–4039 (doi:10.1242/jeb.00634) [DOI] [PubMed] [Google Scholar]
- Ewing A. W.1989Arthropod bioacoustics: neurobiology and behavior Ithaca, NY: Cornell University Press [Google Scholar]
- Fletcher N. H.1992Acoustic systems in biology Oxford, UK: Oxford University Press [Google Scholar]
- Lucas A. M., Stettenheim P. R.1972Avian anatomy: integument: Part I, p. 236 Washington, DC: Department of Agriculture [Google Scholar]
- Michelsen A., Fink F., Gogala M.1982Plants as transmission channels for insect vibrational songs. Behav. Ecol. Sociobiol. 11, 269–281 (doi:10.1007/BF00299304) [Google Scholar]
- Prestwich K. N., O'Sullivan K.2005Simultaneous measurement of metabolic and acoustic power and the efficiency of sound production in two mole cricket species (Orthoptera: Gryllotalpidae). J. Exp. Biol. 208, 1495–1512 (doi:10.1242/jeb.01550) [DOI] [PubMed] [Google Scholar]
- Prestwich K. N., Lenihan K. M., Martin D. M.2000The control of carrier frequency in cricket calls: a refutation of the subalar-tegminal resonance/auditory feedback model. J. Exp. Biol. 203, 585–596 [DOI] [PubMed] [Google Scholar]
- Ridgely R. S., Tudor G.1994The birds of South America, volume II: the suboscine passerines Austin, TX: University of Texas Press [Google Scholar]
- Rossing T. D., Fletcher N. H.1995Principles of vibration and sound New York, NY: Springer-Verlag [Google Scholar]
- Simmons A. M., Popper A. N., Fay R. R.(eds)2003Acoustic communication. Springer Handbook of Auditory Research, vol. 16(56 illus.) Berlin, Germany: Springer [Google Scholar]
- Young D., Josephson R. K.1983Pure-tone songs in cicadas with special reference to the genus Magicicada. J. Comp. Physiol. 152, 197–207 (doi:10.1007/BF00611184) [Google Scholar]