Abstract
Plant surfaces covered with crystalline epicuticular waxes are known to be anti-adhesive, hardly wettable and preventing insect attachment. But there are insects that are capable of gluing their eggs to these surfaces by means of proteinaceous secretions. In this study, we analysed the bonding region between the eggs of Crioceris asparagi and the plant surface of Asparagus officinalis using light and cryo-scanning electron microscopy. The wettability of the plant surface by egg secretion was compared with that by Aqua Millipore water, aqueous sugar solution and chicken egg white. Furthermore, the force required to remove C. asparagi eggs from the plant surface was measured, in order to evaluate the egg's bonding strength. Mean pull-off force was 14.7 mN, which is about 8650 times higher than the egg weight. Egg glue was observed spreading over the wax crystal arrays on the plant cladophyll and wetting them. Similar wetting behaviour on the A. officinalis surface was observed for chicken egg white. Our results support the hypothesis that the mechanism of insect egg adhesion on micro- and nanostructured hydrophobic plant surfaces is related to the proteinaceous nature of adhesive secretions of insect eggs. The secretion wets superhydrophobic surfaces and after solidifying builds up a composite, consisting of the solidified glue and wax crystals, at the interface between the egg and plant cuticle.
Keywords: adhesion, adhesive strength, epicuticular waxes, hydrophobic surfaces, insect egg, plant–insect interaction
1. Introduction
Plants have a great diversity of surfaces. Many of them are densely covered with crystalline epicuticular waxes serving different functions, such as, for example, self-cleaning owing to water repellence (Barthlott & Wollenweber 1981; Barthlott & Neinhuis 1997). Such plants are known to reduce and even prevent insect attachment (e.g. Knoll 1914; Gorb 2001). This has been shown experimentally, for instance, in leaf beetles Phaedon cochleriae Fbr. (Stork 1980) and Chrysolina fastuosa Scop. (Coleoptera, Chrysomelidae) (Gorb & Gorb 2002). Plant surfaces become more hydrophobic through an increase in surface roughness owing to crystalline waxy microstructures, resulting in a decrease in the solid–liquid contact area (reviewed by Koch et al. 2008). Effects of the decreased contact area have also been discussed in studies on the attachment forces of leaf beetles on surfaces with various roughnesses (Peressadko & Gorb 2004; Voigt et al. 2008), ranging in their root mean square from 90.0 to 238.4 nm. This range of roughness corresponds to the dimension of plant epicuticular wax crystals.
Attachment forces are generally reduced on micro- and nanostructured surfaces. Because of the reduction of the real contact area between two solids and owing to the low wettability by water, it is a challenge for insects to attach their eggs to such surfaces. Interestingly, there are some insects specialized for egg adhesion to these surfaces. For example, the butterfly Pieris brassicae L. (Lepidoptera, Pieridae) and aphids Brevicoryne brassicae L. (Hemiptera, Aphididae) attach their eggs to plant species from the family Brassicaceae (Beament & Lal 1957; Ahman 1990), Hessian flies Mayetiola destructor Say (Diptera, Cecidomyiidae) to wheat leaves Triticum aestivum L. (Poaceae) (Kanno & Harris 2000), Opodiphthera sp. moths (Lepidoptera, Saturniidae) and leaf beetle species belonging to the genus Chrysophtharta (Coleoptera, Chrysomelidae) to leaves of Eucalyptus spp. (Myrtaceae) (Ramsden & Elek 1998; Howlett & Clarke 2003; Li et al. 2008). Lily beetles Lilioceris merdigera L. (Coleoptera, Chrysomelidae) prefer to lay their eggs on leaves of Polygonatum multiflorum (L.) All. (Ruscaceae) (Schmitt 1988). Elongated, oval-shaped, 1.28 mm long and 0.53 mm wide eggs of the asparagus beetles Crioceris asparagi L. (Coleoptera, Chrysomelidae) are glued perpendicularly to the substrate with only the posterior pole attached. They are located in groups of three to eight on the filiform, elongated cladophylls of mainly apical, wax crystal-covered shoots of Asparagus officinalis L. (Asparagaceae) (Dingler 1934; Schmitt 1982; Wold-Burkness et al. 2006). Since these eggs have a relatively small contact area with the plant surface, they are quite considerably exposed to weathering and have to withstand rainfall, wind and plant vibration. Thus, they must adhere strongly to the substrate as shown by earlier experiments of Dingler (1934), who tried unsuccessfully to mechanically remove the eggs from the plant surface without damaging the plant tissue. Gupta & Riley (1967) observed an eggshell adhesive secreted by the ovariole pedicel epithelium of C. asparagi, which has been described to be tenacious, initially yellow, changing its colour to brownish green and finally appearing shiny black with a greenish shimmer (Dingler 1934). Adhesive fluids have been repeatedly reported to surround insect eggs and glue them to substrates (e.g. Adiyodi & Adiyodi 1976; Hinton 1981; Hilker et al. 2005). Chemical analyses suggest a largely proteinaceous nature of insect egg adhesives (Beament & Lal 1957; Hilker et al. 2005; Li et al. 2008).
The interface between the plant surface and chrysomelid eggs has been previously studied in detail in relation to insect egg-induced plant defence (Hilker & Meiners 2006) as well as to the protection of eggs against environmental harm (Müller & Rosenberger 2006). The degree of association between insect eggs and plants varies greatly and may also depend on the physico-chemical properties of the plant (Hilker & Meiners 2006; Müller & Rosenberger 2006). The secretion surrounding the egg of Lilioceris lilii (L.) has been observed to be released simultaneously with the egg, being very sticky for some time, but slowly turning more viscous, allowing the egg to remain attached to the non-polar hydrophobic plant surface, whereas the substances of the secretion are absorbed into the plant cuticle (Müller & Rosenberger 2006). However, it remains unclear what the egg–plant surface boundary looks like, especially in the case of plant surfaces covered with epicuticular wax crystals. What is the mechanism of egg attachment to hardly wettable crystalline waxy plant surfaces?
The aim of the present study was to gather structural and experimental data on the secretion-mediated egg adhesion to a plant surface covered with wax crystals. We analysed the bonding region between the egg of the asparagus beetle C. asparagi and the plant surface of A. officinalis, using cryo-scanning electron microscopy (cryo-SEM), which is a suitable technique for visualization of fluids and interfaces in situ (Gorb 2006; Gorb et al. 2007). Light and cryo-SEM images were used to compare the wettability of the plant surface by egg secretion with that of Aqua Millipore water, aqueous sugar solution and chicken egg white. Furthermore, we estimated the adhesive strength of the bond between the egg of C. asparagi and the cladophyll surface A. officinalis. The data presented shed light on the adhesion mechanism of C. asparagi eggs on its host plant surface, which is hardly wettable by water.
2. Material and methods
(a). Plants and insects
Shoots of A. officinalis (phenological stage: 10% of fruits have reached final size, corresponding to BBCH71 according to Hack et al. 1992) with attached eggs of the common Asparagus beetle, C. asparagi, were collected in the field of the Botanical Garden of the University of Hohenheim (Stuttgart-Hohenheim, Germany) and kept in Petri dishes with food plant and moist filter paper. Experiments were carried out at 23.7 ± 1.7°C temperature, 47.3 ± 10.0% relative humidity and a 16 h photoperiod.
(b). Microscopy
Light microscopic studies have been carried out using a stereomicroscope Olympus SZX12 with a DF PLAPO 1×PF objective (Olympus Corporation, Tokyo, Japan). Images of plant and beetles were taken using a Nikon Coolpix E995 digital camera adapted to the stereomicroscope with a C-Mount adapter and an MDC2 relay lens MXA29005 (Nikon Corporation, Tokyo, Japan).
The plant surface and its interface with the chrysomelid eggs were visualized at high resolutions using a cryo-SEM Hitachi S-4800 (Hitachi High-Technologies Corp., Tokyo, Japan) equipped with a Gatan ALTO2500 cryo-preparation system (Gatan Inc., Abingdon, UK). Fresh cladophyll samples of A. officinalis covered with eggs or fluids were mounted on metal holders using polyvinyl alcohol Tissue-Tek OCT compound (Sakura Finetek Europe, Zoeterwoude, The Netherlands), frozen in the preparation chamber at −140°C, sputter-coated with gold–palladium (6 nm) and examined in a frozen state in the cryo-SEM at −5 kV accelerating voltage and −120°C temperature. Dimensions of plant wax crystals were measured from cryo-SEM micrographs using the software SigmaScan Pro 5 (SPSS Inc.).
Following the described cryo-SEM procedure, the interface between the cladophyll surface and (i) beetle eggs, (ii) Aqua Millipore water, (iii) aqueous sugar solution, and (iv) pure chicken egg white was comparatively studied (a) in contact and (b) dried and detached after contact formation before they were frozen in cryo-SEM. For the aqueous sugar solution (20%), commercial crystal saccharose was dissolved in Aqua Millipore water at room temperature for 30 min using a magnetic stirrer.
(c). Contact angle measurements
Contact angles of the different fluids mentioned above on the surface of A. officinalis cladophylls were estimated from binocular (Aqua Millipore water) and cryo-SEM (sugar solution, chicken egg white) images.
For comparison, static contact angles of these fluids to normal and hydrophobic silanized glass surfaces (microscope slides ISO 8037/I, Carl Roth GmbH & Co. KG, Karlsruhe, Germany) were measured using the sessile drop method (drop volume 1 µl). The ellipse fitting was applied to the droplet images obtained with the high-speed optical contact angle measuring device OCAH200 and SCA20 3.7.4 software (Data-Physics Instruments GmbH, Filderstadt, Germany). Glass was cleaned prior to experiments by successive immersions in Piranha solution (mixture of sulphuric acid H2SO4 and hydrogen peroxide H2O2, 3 : 1), rinsed with Aqua Millipore water and dried immediately by means of compressed air. To hydrophobize the glass surface, it was silanized with 1H,1H,2H,2H-perfluorodecyltrichlorosilane 97 per cent (C10H4Cl3F17Si, SIH5841.0, ABCR GmbH & Co. KG, Karlsruhe, Germany). For evaluation of contact angles with Aqua Millipore water, the OCAH 200 automatic drop dispenser was used. For other liquids, drops were manually positioned on the glass surface with an Eppendorf Research pipette (Eppendorf AG, Hamburg, Germany). Statistical differences of contact angle values (i) between fluids on the same surface and (ii) between surfaces for the same fluid were evaluated using SigmaStat 3.1.1 software (Systat Software, Inc., Richmond, CA, USA).
(d). Adhesion force measurements
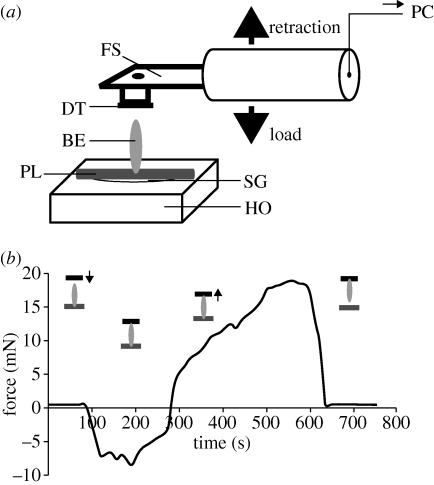
To measure egg adhesion to the plant surface, a force transducer (10 g capacity, Biopac Systems Ltd, Santa Barbara, CA, USA) combined with a motorized micromanipulator DC3314R and a controller MS314 (World Precision Instruments Inc., Sarasota, FL, USA) was used (figure 1a). A piece of fresh cladophyll with an egg adhering perpendicularly facing up was attached to a holder with super glue Loctite (Henkel Loctite Deutschland GmbH, Munich, Germany). Double-sided adhesive carbon tape, firmly adhering to the force transducer, was moved up and down with a velocity of 200 µm s−1. First, it was brought into contact with the free terminal of the egg at a load of approximately 15 mN and then retracted perpendicularly from the plant surface. The pull-off force during the retraction was measured (figure 1b). Force–time curves were recorded using AcqKnowledge 3.7.0 software (Biopac Systems, Inc., Goleta, CA, USA). The pull-off forces of a total of 20 single eggs were measured. SigmaStat 3.1.1 software was applied for statistical data evaluation.
Figure 1.
(a) Experimental setup for measuring adhesion force of single eggs of C. asparagi attached to the plant surface of A. officinalis. The fresh plant sample (PL) with an intact beetle egg (BE) was mounted on a horizontal holder (HO) with superglue (SG). A force sensor (FS) with a firmly adhering piece of double-sided carbon tape (DT) was moved down, using a motorized micromanipulator, until contact between the DT and the free egg terminal was made. Then the sensor with the egg adhering to the DT was pulled up. The time–force sensor signal was recorded and processed further in a computer (PC). (b) Representative force–time curve obtained in the pulling-force experiment. Schematic drawings indicate the position of double-sided tape (black) and the detachment of insect egg (grey) from the plant surface (dark grey).
Detached eggs were individually weighed using an Ultra Microbalance UMX2 (Mettler Toledo GmbH, Greifensee, Schweiz).
The contact area of the plant surface covered with the egg glue of three individual eggs was estimated from cryo-SEM micrographs using the SigmaScan Pro 5 software.
3. Results
(a). The plant–egg interface
Eggs of C. asparagi, attached to the host plant, are surrounded by a shiny, dark olive-green to brownish secretion (figure 2). The contact area on the plant surface is a circular patch around the egg base. The free terminal of the egg is slightly tapered, whereas the base, adhering to the plant surface, is slightly flattened. The egg secretion is clearly visible spreading over the egg and plant surface (figure 3c–e).
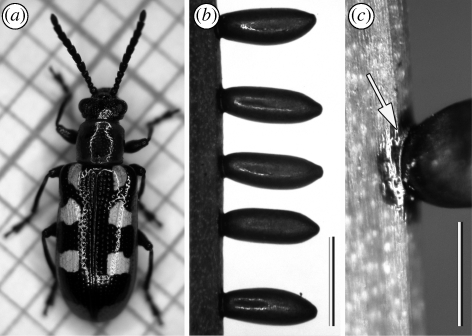
Figure 2.
(a–c) Digital images of the asparagus beetle C. asparagi. Females (a) attach elongated oval-shaped eggs perpendicularly to the plant surface of asparagus' cladophylls (b) by means of a dark-brownish secretion forming a circular patch around the contact area (c, arrow). Scale bar, 1 mm.
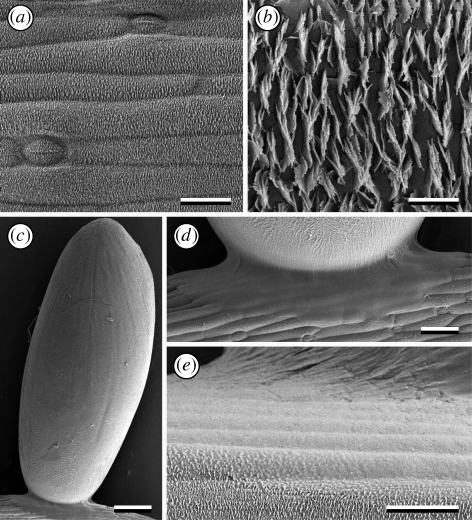
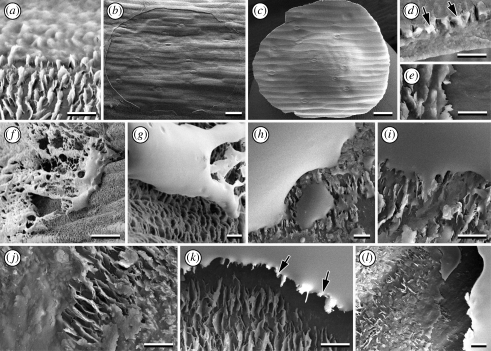
Figure 3.
Cryo-SEM micrographs of the plant A. officinalis and an adhering egg of the beetle C. asparagi. (a) The surface of a young cladophyll is densely covered with wax crystals. (b) Detail of crystalline wax platelets, roughly arranged in approximately parallel rows transversely to the longitudinal axis of the cladophyll. (c) Elongated oval-shaped egg attached by one terminal, perpendicular to the plant surface, owing to the egg adhesive forming a circular patch around the egg base. (d,e) Detail of the bonding region of the egg, showing egg adhesive spread over the plant surface and wax crystals wetted by the secretion. Scale bars: (a,e) 20 µm; (b) 2 µm; (c) 200 µm; (d) 50 µm.
The surface of young asparagus cladophylls is densely covered with irregular crystalline wax platelets (classification according to Barthlott et al. 1998) (figure 3a,b). The arrangement of wax crystals resembles the liliiflorous-characteristic ‘Convallaria-type’ (classification of Barthlott & Frölich 1983), where platelets are arranged transversely to the cladophyll longitudinal axis in parallel rows. On older cladophylls and particularly on stems, sites of apparently smeared or abraded wax crystals appeared frequently (figure 4c,d). Platelets are 1.27 ± 0.133 µm wide, 0.40 ± 0.059 µm long and 0.04 ± 0.013 µm thick, and the distance between crystals is 0.47 ± 0.206 µm (for each, mean ± s.d., n = 10). The aspect ratio (length-to-width) of wax platelets is approximately 0.3.
Figure 4.
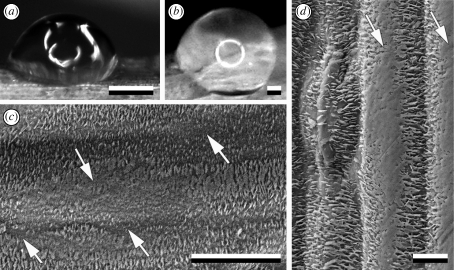
(a,b) Digital images of different drop shapes of Aqua Millipore water on the cladophyll surface of A. officinalis plant. (c) Cryo-SEM micrograph of the surface of A. officinalis cladophyll and (d) stem with damage in the wax coverage (arrows). The presence of such sites explains the strong variability in contact angles of Aqua Millipore water depending on the site. Scale bars: (a,b) 1 mm; (c) 20 µm; (d) 50 µm.
Aqua Millipore water was found to form roughly hemispherical drops on the plant surface with contact angles ranging between 77.8 and 145.1° and corresponding to that of silanized glass (figure 4a,b; table 1). Even if drops evaporated in the cryo-SEM under vacuum conditions, close-up views demonstrated some residues of water on the tips of wax crystals. The water did not wet them completely but was seen just on their tips (figure 5f,g). Similar behaviour was found for the aqueous sugar solution having a mean contact angle of 84.7°. By contrast, beetle egg secretion and egg white showing zero contact angles, completely wetted and embedded wax crystals (figures 3e and 5a,j; table 1).
Table 1.
Contact angles (°) of different fluids (droplet volume 1 µl) on glass surfaces compared with those on the cladophyll surface of A. officinalis, means (minimum–maximum). (Small letters (a, b, c) indicate differences in fluid between different surfaces (one-way ANOVA on ranks and all pairwise multiple comparison procedures, Tukey test, p < 0.05; for Aqua Millipore water: H2,28 = 19.613, p ≤ 0.001; for sugar solution: H2,28 = 26.038, p ≤ 0.001; for chicken egg white: H2,28 = 26.796, p ≤ 0.001). Capital letters (A, B) show differences for one surface between different fluids (for normal glass one-way ANOVA and Tukey test, F2,28 = 117.206, p ≤ 0.001; one-way ANOVA on ranks and all pairwise multiple comparison procedures, Tukey test, p < 0.05; for silanized glass: H2,28 = 22.704, p ≤ 0.001; for plant surface: H2,28 = 24.606, p ≤ 0.001).)
| surface |
|||
|---|---|---|---|
| fluid | normal glass | silanized glass | A. officinalis cladophyll |
| Aqua Millipore water | 39.7a,A (34.2–44.7) | 108.9b,A (105.5–113.5) | 107.4b,A (77.8–145.1) |
| sugar solution | 37.4a,A (28.8–41.6) | 105.6b,A (102.0–108.0) | 84.7c,A (83.5–85.8) |
| egg white | 16.8a,B (12.4–21.2) | 34.3b,B (22.2–50.8) | 0c,B |
Figure 5.
Cryo-SEM micrographs of the wetting behaviour of different fluids on the plant surface of A. officinalis. (a–e) Egg adhesive of C. asparagi spread over the plant surface, completely wetting and embedding wax crystals (a). (b) A wax-free circular area is visible (detail of the border is seen in (e)). When the egg is removed, wax crystals are peeled off and remain glued to the egg adhesive circular plate (plate margin is visible in (d)), which remains on the detached egg and shows a distinct imprint of the cladophyll microtexture (c). (f,g) Residues of Aqua Millipore water do not wet wax crystals. (h,i) A sugar solution adheres to the wax crystals, but does not embed them. (j–l) Egg white spreads over the plant surface wetting and embedding wax crystals. (l) When it is removed after drying, a wax-free area remains on the plant surface, and (k) peeled-off wax crystals remain attached to the dry egg white. Scale bars: (a,d,e,g,i–l) 2 µm; (b,c) 50 µm; (f,h) 20 µm. Arrows point to wax crystals peeled off the plant surface and remaining attached to detached adhesive residues after egg detachment.
Epicuticular wax crystals can be peeled off by removing dehydrated beetle egg adhesive or egg white patches from the plant surface after contact (figure 5b,e,k,l). Wax crystals remain firmly adhered to the detached material of the dried adhesive (figure 5c,d). Wax-free sites remain on the plant surface after such a peeling-off procedure (figure 5b,l). However, the glue layer is not totally removed from the plant surface during the pull-off of the egg: a circular residue is left at the margin of the peeled-off zone (figure 5b).
No cases of detachment could be seen with drops of either water or sugar solution (figure 5h, i). Water disappeared during desiccation without leaving a residue, and the sugar solution left insignificant residues.
(b). Egg adhesion
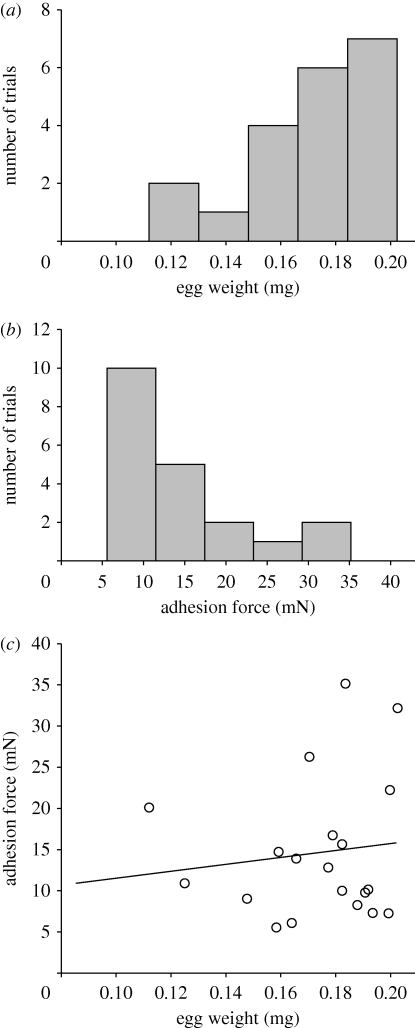
Egg weight averaged 173 ± 24.2 µg (n = 20) (figure 6a). The maximum pull-off force required to pull the egg from the plant surface was 35 mN (figure 6b). However, adhesion force in the majority of trials ranged between 5 and 15 mN. Considering that an egg weighs ca 0.17 mg, the bond could hold up to 20 588 times the weight of the egg.
Figure 6.
Histograms showing the range of (a) egg weight and (b) egg adhesion force measured, number of columns according to Sturges rule (Sturges 1926) for normal distributed data (Kolmogorov–Smirnov test for (a), p = 0.181, and for (b), p = 0.106, each with n = 20). (c) Adhesion force versus weight of beetle eggs, linear regression. (a) Mean ± s.d.: 0.17 ± 0.024 mg. (b) Mean ± s.d.: 14.67 ± 8.514 mg. (c) r2 = 0.0149; y = 7.243 + 42.895x; F1,18 = 0.272; p = 0.608 (one-way ANOVA).
Since the contact area of the removed glue was estimated to be ca 0.13 mm2, the adhesive strength (tenacity) was calculated to reach values from 38.8 to 271.3 kPa, considering minimum and maximum adhesion forces. No correlation was found between force values and egg weight (figure 6c).
4. Discussion
(a). Wettability of plant surface and insect adhesion
The surface of A. officinalis bears a dense coverage of epicuticular wax platelets, the composition of which comprises fractions of predominantly alkanes, also aldehydes and methanol (ketone and wax ester included) (Scora et al. 1986). Since alkanes belong to least wettable plant wax fractions known (Holloway 1969), the asparagus surface is expected to possess distinct hydrophobic properties that, if combined with crystalline waxy microstructures, could result in surface hydrophobicity. Measured contact angles of Aqua Millipore water had a very strong variability, indicating some heterogeneity of the asparagus surface at the micrometer scale. This phenomenon may be explained by the fact that fragile wax structures can be easily altered by mechanic abrasion, degradation, erosion or ageing process, and form amorphous crusts (e.g. Barthlott & Wollenweber 1981; Juniper & Jeffree 1983) (see also figure 7c); or they may be contaminated by biological substances. Cryo-SEM images obtained in the present study for A. officinalis are similar to those published for Pinus sylvestris L. (Pinaceae), where characteristic flattening of epicuticular wax crystals occurred owing to physical damage and rubbing processes. Such damages can result in a heterogeneous wetting of the surface (Crossley & Fowler 1986; Van Gardingen et al. 1991). The incomplete wax crystal coverage allows droplets to come into contact with the more hydrophilic cuticle components (Holloway 1969). Apart from the wax alteration, the low aspect ratio of A. officinalis wax crystals in combination with the relatively large distances between single crystals (one crystal height) offers better wetting conditions for water in contrast to superhydrophobic plant surfaces (figure 7a,b), where crystal packing density is much higher and crystals are longer. Such effects have also been recently shown for artificial hydrophobic surfaces covered with cylindrical microstructures (Reyssat & Quéré 2009). Additionally, true superhydrophobic plant surfaces are characterized by certain hierarchical organization of the surface, for example, convex or papillose shape of epidermal cells and a very dense arrangement of three-dimensional epicuticular waxes 0.5–20 µm long (Barthlott & Neinhuis 1997).
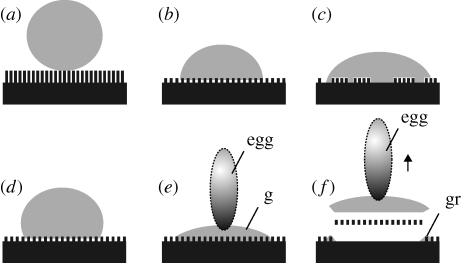
Figure 7.
Schemes of hydrophobic plant surfaces, covered with wax crystals and wetted by different fluid drops. (a) Water drop on a superhydrophobic surface bearing a very dense coverage of epicuticular wax crystals with a high aspect ratio. (b) Water drop on a hydrophobic surface less densely covered with wax crystals having a lower aspect ratio and larger distances between them. This surface exhibits no total wetting because of air pockets remaining between wax crystals. (c) Water drop on a surface with larger wax-less sites. This surface has better wettability with water. No total wetting was observed owing to the presence of air pockets remaining between some wax crystals. (d) Drop of sugar solution on the surface shown in (b) does not embed wax crystals. This surface exhibits no total wetting because of air pockets remaining between wax crystals. (e) Drop of insect egg adhesive (or chicken egg white), fully wetting the surface shown in (b) and also embedding wax crystals. (f) Removing dried egg secretion from the plant surface, wax crystals are peeled off. They remain attached to the egg adhesive. Shadowed vertical ovals indicate eggs. Abbreviations: g, insect glue; gr, glue residuals at the circumference of the glue spot.
However, female C. asparagi preferably oviposit on the apical cladophylls, which are densely covered with intact crystalline wax platelets (Dingler 1934; Schmitt 1982). Egg deposition and their proper adherence to plant surfaces are crucial to phytophagous insects for successful settlement on their host plants. In order to attach their eggs, insects must overcome the wettability problem on hydrophobic and superhydrophobic surfaces.
Why are plant surfaces, covered with wax crystals, anti-adhesive for many insects? Four hypotheses have been previously formulated by Gorb & Gorb (2002) to explain attachment failure in insects' feet: roughness hypothesis, contamination hypothesis, wax-dissolving hypothesis and fluid-absorption hypothesis. For the case dealt with in our study, only the roughness hypothesis is applicable. With respect to insect egg attachment, we can formulate a fifth, the non-wettability hypothesis: adhesion failure could be caused by plant surfaces that are non-wettable by insect adhesives.
(b). Composite nature of the egg–plant interface
Our study shows that the egg adhesive of C. asparagi is able to wet the hydrophobic plant surface of A. officinalis and solidify itself in contact, indicated by the dark staining of the adhesive. Through the combination of wettability and solidifying, it overcomes adhesion reduction. Unlike water and aqueous sugar solution (figure 7a–d), both beetle egg adhesive and chicken egg white spread over the plant surface and wet the wax crystals (figure 7e). After drying, the adhesive can be removed from the plant surface mainly together with wax crystals embedded in the adhesive (figure 7f). Thus, our results indicate the surfactant-like nature of insect egg adhesives, which form a kind of a composite material at the interface between the egg and plant cuticle after solidifying, incorporating wax crystals into the adhesive matrix (Visser 1976).
Though consisting of various components, the main compounds of insect egg adhesives are proteins and lipids (Riley & Forgash 1967; Hinton 1981). Chicken egg white is by nature an aqueous solution containing proteins, small amounts of lipids, carbohydrates and vitamins (Burley & Vadehra 1989). We have demonstrated here that it even wets hydrophobic glass, showing contact angles similar to those of Aqua Millipore water on hydrophilic glass. Proteins, protein–carbohydrate complexes and lipopeptides have been previously reported as biosurfactants exhibiting a high surface activity (e.g. Vogler 1993). Thus, the combination of proteins and lipids may serve as a surfactant wetting non-polar, hydrophobic, microstructured surfaces, such as the plant surface of A. officinalis. The spreading of egg adhesive on the plant surface, with an almost zero contact angle and low initial viscosity, facilitates its mechanical engagement with the surface irregularities. Such properties of adhesives have been previously attributed to polymers optimized for adhesion enhancement (Scherge & Gorb 2001; Graham 2008).
(c). Egg adhesive strength
The pull-off force required to detach eggs of C. asparagi from the cladophylls of A. officinalis ranged from 5 to 35 mN. Such a wide range of values might be possibly caused by (i) difference in the egg age, (ii) desiccation of the egg adhesive and (iii) various egg positions on the plant surface, which might be locally abraded (however, only eggs deposited on cladophyll apexes were used in this study). Further experiments, taking the age and different positions of eggs on the plant into account, may help to clarify the role of different factors in the beetle oviposition and variability of egg attachment in C. asparagi.
The egg weight (mean, 196.1 µg) did not significantly correlate with the measured pull-off force of the egg. Possibly, the egg adhesive forms a similar contact area around the small posterior egg pole, independently of the egg's dimension. Otherwise, the variability in egg size and its contact area would probably be a less critical factor of natural selection in comparison to the extreme reliability of the egg adhesive itself, generating maximum forces (on an available area) comparable to 20 588 times the weight of one egg.
Interestingly, the adhesive strength of the egg glue in C. asparagi (38.8–271.3 kPa) is four to 20 times lower than those reported for several adhesives (cements) of some aquatic organisms. For example, the strength of the adhesive to a smooth glass surface was found to be between 316 and 750 kPa in the plaque of the mussel Mytilus edulis L. (Mollusca, Mytiloida, Mytilidae), and about 930 kPa in an adult barnacle Balanus balanoides L. (Arthropoda, Cirripedia) adhering to a slate surface (DeVore & Gruebel 1978; Yule & Walker 1984; Maruyama et al. 1991). However, we have to take into account that the beetle egg adhesive demonstrates such a strong performance on rough hydrophobic surfaces, whereas marine adhesives do not (Crisp et al. 1985; Yule & Walker 1987).
Marine biological adhesives are adapted to permanent adhesion that is based on proteins and polyphenols, whereby polyphenols play a role in tanning proteinaceous elastic components of the secretion (Pardo et al. 1990; Maruyama et al. 1991). Also, in the water-soluble yellow compound of the egg cement of the large white butterfly P. brassicae, a phenolic, apparently strong reducing agent attached to a protein has been suggested (Beament & Lal 1957). The P. brassicae's egg adhesive therefore conforms to the pattern of permanently adhesive, viscous fluid, gluing eggs to the surface of the plant species from the family Brassicaceae, which often bear a very dense coverage of wax crystals. When butterflies release their egg adhesive, the pale yellow matrix rapidly loses water, becoming more rubbery and somewhat darker. Aside from the same egg alignment, similar colour change and hardening have been observed for the egg adhesive of C. asparagi, whose eggs also permanently adhere to the plant surface (Dingler 1934). Their shells remain attached even after larval hatching. Considering their remarkable adhesive strength, one may conjecture that eggs of the asparagus beetle should be able to well withstand environmental influences and moist weather conditions. It has been repeatedly shown in the literature that attachment structures of eggs could withstand environmental influences (e.g. Beament 1946; Hinton 1981, Gaino & Rebora 2001). However, further studies are required to ensure this hypothesis for C. asparagi.
Only a few quantitative data on the adhesion of other insect eggs to substrates are available in the literature. In comparison with eggs of the silkmoth Bombyx mori L. (Lepidoptera, Bombycidae), the adhesive of C. asparagi eggs appears relatively strong. The tensile shear strength of silkmoth glue substance between two aluminium plates (25 mm−2) has been reported to be 1.18 MPa (Yoshida & Nagata 1997). However, no information is provided at which sites samples were detached. Probably, the authors estimated cohesive forces within the glue layer, whereas pull-off forces measured in the present paper relate to the composite material, consisting of the egg glue and wax crystals, at the interface between eggs of the asparagus beetles and A. officinalis cuticle surface. Additionally, the surface of A. officinalis is waxy, but values cited in the literature are for non-waxy substrata. Thus, the nature of the beetle egg detachment (with fracture of wax crystals and/or their separation from the plant epidermis) means that the observed strength at failure (39–271 kPa) is almost certainly an underestimate of the true adhesive/cohesive strength of C. asparagi's egg secretion. In a recent study, 29 proteinaceous adhesive secretions of insects have been comparatively analysed chemically and six types with substantial protein content identified (Li et al. 2008). Some secretions dry by evaporative loss of a solvent, bonding their adherents as tough flexible dried films. The strongest egg attachment glue was found to be produced by gum moths of the genus Opodiphthera, who lay their eggs on the bloom-like, crystalline waxy surface of eucalyptus plants (Li et al. 2008). The shear strength was about 1–2 MPa, calculated from a destructive test using a layer of isolated dried moth egg adhesive between two wooden medical tongue depressors, which were then pulled apart (Li et al. 2008). Although different experimental setups are hardly comparable, the data indicate strong bonds of specialized insect eggs to plant surfaces covered with crystalline waxes.
5. Conclusion
The egg adhesive of C. asparagi is able to wet hydrophobic, microstructured plant surfaces and thereby increase the strength of the adhesive bond. Since similar wetting behaviour was observed for chicken egg white, our results suggest the surfactant-like nature of adhesive secretions of insect eggs and provide an explanation for the mechanism of egg adhesion on micro- and nanostructured hydrophobic plant surfaces. Such a specialization for a life on plant surfaces covered with crystalline wax provides some selective advantages for an insect, such as escaping from less specialized predators and parasites that may fail to adhere to such plant surfaces.
The results obtained may offer further approaches for research on pest management, targeting egg stages to control phytophagous insects. On the one hand, considering egg adhesive properties, applied ovicides could be modified in such a way that they could dissolve adhesives and detach eggs from plant surfaces. On the other hand, one could try to breed plants with surfaces that prevent egg attachment owing to the particular shape, density and chemistry of wax crystals.
Acknowledgements
The first author acknowledges motivating discussion by Nataly Matushkina (Department of Zoology, Biological Faculty, Kiev National University, Ukraine) and Michael Varenberg (Technion—Israel Institute of Technology, Haifa, Israel). Victoria Kastner (Max Planck Institute for Metals Research, Stuttgart, Germany) kindly helped with linguistic corrections. The study was supported by the Federal Ministry of Education and Research, Germany (BMBF, project BIONA 01RB0802A and project InspiRat 01RI0633D).
References
- Adiyodi K. G., Adiyodi R. G.1976Morphology and cytology of the accessory sex glands in invertebrates. Int. Rev. Cytol. 43, 353–398 (doi:10.1016/S0074-7696(08)60072-8) [Google Scholar]
- Ahman I.1990Plant-surface characteristics and movements of two Brassica-feeding aphids, Lipaphis erysimi and Brevicoryne brassicae. Symp. Biol. Hung. 39, 119–125 [Google Scholar]
- Barthlott W., Frölich D.1983Mikromorphologie und Orientierungsmuster epicuticularer Wachs-Kristalloide, ein neues systematisches Merkmal bei Monokotylen. Plant Syst. Evol. 142, 171–185 (doi:10.1007/BF00985897) [Google Scholar]
- Barthlott W., Neinhuis C.1997Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 2002, 1–8 [Google Scholar]
- Barthlott W., Wollenweber E.1981Zur Feinstruktur, Chemie und Taxonomischen Signifikanz Epikutikularer Wachse und Ähnliche Sekrete. In Tropische und Subtropische Pflanzenwelt (ed. Rau W.), pp. 35–97 Wiesbaden, Germany: Akademie der Wissenschaften und der Literatur, Franz Steiner Verlag GmbH [Google Scholar]
- Barthlott W., Neinhuis C., Cutler D., Ditsch F., Meusel I., Theisen I., Wilhelmi H.1998Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 126, 237–260 (doi:10.1111/j.1095-8339.1998.tb02529.x) [Google Scholar]
- Beament J. W. L.1946Waterproofing mechanism of an insect egg. Nature 157, 370 (doi:10.1038/157370c0) [DOI] [PubMed] [Google Scholar]
- Beament J. W. L., Lal R.1957Penetration through the egg-shell of Pieris brassicae (L.). Bull. Entomol. Res. 48, 109–125 (doi:10.1017/S0007485300054134) [Google Scholar]
- Burley R. W., Vadehra D. V.1989The avian egg, chemistry and biology New York, NY: John Wiley & Sons Inc [Google Scholar]
- Crisp D. J., Walker G., Young G. A., Yule A. B.1985Adhesion and substrate choice in mussels and barnacles. J. Colloid Interface Sci. 104, 40–50 (doi:10.1016/0021-9797(85)90007-4) [Google Scholar]
- Crossley A., Fowler D.1986The weathering of Scots pine epicuticular wax in polluted and clean air. New Phytol. 103, 207–218 (doi:10.1111/j.1469-8137.1986.tb00609.x) [Google Scholar]
- DeVore D. P., Gruebel R. J.1978Dityrosine in adhesive formed by the sea mussel Mytilus edulis. Biochim. Biophys. Res. Comm. 80, 993–999 (doi:10.1016/0006-291X(78)91343-8) [DOI] [PubMed] [Google Scholar]
- Dingler M.1934Über unsere beiden Spargelkäfer (Crioceris duodecimpunctata L. und C. asparagi L.). Ztschr. Angew. Entomol. 21, 415–442 [Google Scholar]
- Gaino E., Rebora M.2001Synthesis and function of the fibrous layers covering the eggs of Siphlonurus lacustris (Ephemeroptera, Siphlonuridae). Acta Zoologica 82, 41–48 (doi:10.1046/j.1463-6395.2001.00067.x) [Google Scholar]
- Gorb S.2001Attachment devices of insect cuticle, p. 305 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- Gorb S.2006Fly microdroplets viewed big, a cryo-SEM approach. Microsc. Today 14, 38–39 [Google Scholar]
- Gorb S., Gorb E.2002Attachment ability of the beetle Chrysolina fastuosa on various plant surfaces. Entomol. Exp. Appl. 105, 13–28 (doi:10.1023/A:1021777517981) [Google Scholar]
- Gorb S. N., Voigt D., Gorb E.2007Visualisation of small fluid droplets on biological and artificial surfaces using the cryo-SEM approach. In Modern research and educational topics in microscopy (eds Méndez-Vilas A., Diaz J.), pp. 812–819 Badajoz, Spain: Formatex [Google Scholar]
- Graham L. D.2008Biological adhesives. In Encyclopedia of biomaterials and biomedical engineering, vol. 1, 2nd edn (eds Wnek G. E., Bowlin G. L.), pp. 236–253 New York, NY: Informa Healthcare [Google Scholar]
- Gupta A. P., Riley R. C.1967Female reproductive system and histology of the ovariole of asparagus beetle Crioceris asparagi (Coleoptera: Chrysomelidae). Ann. Entomol. Soc. Am. 60, 980–988 [Google Scholar]
- Hack H., Bleiholder H., Buhr L., Meier U., Schnock-Fricke U., Weber E., Witzenberger H.1992Einheitliche Codierung der phänologischen Entwicklungsstadien mono- und dikotyler Pflanzen-Erweiterte BBCH-Skala, Allgemein. Nachrichtenbl. Deut. Pflanzenschutz 44, 265–270 [Google Scholar]
- Hilker M., Meiners T.2006Early herbivore alert, insect eggs induce plant defense. J. Chem. Ecol. 32, 1379–1396 (doi:10.1007/s10886-006-9057-4) [DOI] [PubMed] [Google Scholar]
- Hilker M., Stein C., Schroeder R., Varama M., Mumm R.2005Insect egg deposition induces defence responses in Pinus sylvestris: characterisation of the elicitor. J. Exp. Biol. 208, 1849–1854 (doi:10.1242/jeb.01578) [DOI] [PubMed] [Google Scholar]
- Hinton H. E.1981Biology of insect eggs, vol. I–III Oxford, UK: Pergamon Press [Google Scholar]
- Holloway P. J.1969Chemistry of leaf waxes in relation to wetting. J. Sci. Food Agric. 20, 124–128 (doi:10.1002/jsfa.2740200214) [Google Scholar]
- Howlett B. G., Clarke A. R.2003Role of foliar chemistry versus leaf-tip morphology in egg-batch placement by Chrysophtharta bimaculata (Olivier) (Coleoptera, Chrysomelidae). Aust. J. Entomol. 42, 144–148 (doi:10.1046/j.1440-6055.2003.00331.x) [Google Scholar]
- Juniper B. E., Jeffree C. E.1983Plant surfaces London, UK: Edward Arnold Publishers [Google Scholar]
- Kanno H., Harris M. O.2000Physical features of grass leaves influence the placement of eggs within the plant by the Hessian fly. Entomol. Exp. Appl. 96, 69–80 (doi:10.1023/A:1004098214872) [Google Scholar]
- Knoll F.1914Ueber die Ursache des Ausgleitens der Insektenbeine an wachsbedeckten Pflanzenteilen. Jahrb. Wiss. Bot. 54, 448–497 [Google Scholar]
- Koch K., Bhushan B., Barthlott W.2008Diversity of structure, morphology and wetting of plant surfaces. Soft Matter 4, 1943–1963 (doi:10.1039/b804854a) [Google Scholar]
- Li D., Huson M. G., Graham L. D.2008Proteinaceous adhesive secretions from insects, and in particular the egg attachment glue of Opodiphthera sp. moths. Arch. Insect Biochem. Physiol. 69, 85–105 (doi:10.1002/arch.20267) [DOI] [PubMed] [Google Scholar]
- Maruyama N., Etoh H., Sakata K., Ina K.1991Studies on phenoloxidase from Mytilus edulis associated with adhesion. Agric. Biol. Chem. 55, 2887–2889 [Google Scholar]
- Müller C., Rosenberger C.2006Different oviposition behaviour in chrysomelid beetles, characterisation of the interface between oviposition secretion and the plant surface. Arthropod Struct. Dev. 35, 197–205 (doi:10.1016/j.asd.2006.06.001) [DOI] [PubMed] [Google Scholar]
- Pardo J., Gutierrez E., Sáez C., Brito M., Burzio L. O.1990Purification of adhesive proteins from mussels. Protein Expr. Purif. 1, 147–150 (doi:10.1016/1046-5928(90)90008-M) [DOI] [PubMed] [Google Scholar]
- Peressadko A., Gorb S. N.2004Surface profile and friction force generated by insects. In First International Industrial Conference Bionik 2004 (eds Boblan I., Bannasch R.), Fortschr.-Ber, VDI Reihe 15, pp. 257–261 Duesseldorf, Germany: VDI-Verlag [Google Scholar]
- Ramsden N., Elek J.1998Life cycle and development rates of the leaf beetle Chrysophtharta agricola (Chapuis) (Coleoptera, Chrysomelidae) on Eucalyptus nitens at two temperature regimes. Aust. J. Entomol. 37, 238–242 (doi:10.1111/j.1440-6055.1998.tb01577.x) [Google Scholar]
- Reyssat M., Quéré D.2009Contact angle hysteresis generated by strong dilute defects. J. Phys. Chem. B 113, 3906–3909 (doi:10.1021/jp8066876) [DOI] [PubMed] [Google Scholar]
- Riley R. C., Forgash A. J.1967Drosophila melongaster eggshell adhesive. J. Insect Physiol. 13, 509–517 (doi:10.1016/0022-1910(67)90062-5) [DOI] [PubMed] [Google Scholar]
- Scherge M., Gorb S. N.2001Biological micro- and nanotribology. Nature's solutions Berlin, Germany: Springer [Google Scholar]
- Schmitt M.1982Über die Evolution der Zirpkäfer (Criocerinae, Chrysomelidae, Coleoptera). Dissertation, Freie Universität Berlin, Berlin, Germany. [Google Scholar]
- Schmitt M.1988The Criocerinae, biology, phylogeny and evolution. In Biology of Chrysomelidae (eds Jolivet P., Petitpierre E., Hsiao T. H.), pp. 475–495 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- Scora R. W., Mueller E., Guelz P. G.1986Wax components of Asparagus officinalis L. (Liliaceae). J. Agric. Food Chem. 34, 1024–1026 (doi:10.1021/jf00072a023) [Google Scholar]
- Stork N. E.1980Role of waxblooms in preventing attachment to brassicas by the mustard beetle Phaedon cochleariae. Entomol. Exp. Appl. 28, 100–107 (doi:10.1007/BF00422029) [Google Scholar]
- Sturges H. A.1926The choice of a class-interval. J. Am. Statist. Assoc. 21, 65–66 [Google Scholar]
- Van Gardingen P. R., Grace J., Jeffree C. E.1991Abrasive damage by wind to the needle surfaces of Picea sitchensis (Bong.) Carr. and Pinus sylvestris L. Plant Cell Environ. 14, 185–193 [Google Scholar]
- Visser J.1976Adhesion of colloid particles. In Surface and colloid science (ed. Matijevic E.), pp. 3–84 New York, NY: John Wiley and Sons [Google Scholar]
- Vogler E. A.1993Interfacial chemistry in biomaterials science. In Wettability, Surfactant Series, no. 49 (ed. Berg J. C.), pp. 183–250 New York, NY: Marcel Dekker, Inc. [Google Scholar]
- Voigt D., Schuppert J. M., Dattinger S., Gorb S. N.2008Sexual dimorphism in the attachment ability of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera, Chrysomelidae) to rough substrates. J. Insect Physiol. 54, 765–776 (doi:10.1016/j.jinsphys.2008.02.006) [DOI] [PubMed] [Google Scholar]
- Wold-Burkness S. J., Bolin P. C., Hutchison W. D.2006Early-season phenology and temporal dynamics of the common asparagus beetle, Crioceris asparagi (Coleoptera, Chrysomelidae), in southern Minnesota. Great Lakes Entomol. 39, 72–79 [Google Scholar]
- Yoshida K., Nagata M.1997Adhesive strength of the glue substances in the colleterial glands of the silkmoth Bombyx mori. J. Seric. Sci. Jpn 66, 453–456 [Google Scholar]
- Yule A. B., Walker G.1984The adhesion of the barnacle, Balanus balanoides, to slate surfaces. J. Mar. Biol. Assoc. UK 64, 147–156 [Google Scholar]
- Yule A. B., Walker G.1987Adhesion in barnacles. In Crustacean issues: Biology of barnacles (ed. Southward A. J.), pp. 389–402 Rotterdam, The Netherlands: Balkema [Google Scholar]