Abstract
In Europe, rotavirus gastroenteritis peaks in late winter or early spring suggesting a role for weather factors in transmission of the virus. In this study, multivariate regression models adapted for time-series data were used to investigate effects of temperature, humidity and rainfall on reported rotavirus infections and the infection-rate parameter, a derived measure of infection transmission that takes into account population immunity, in England, Wales, Scotland and The Netherlands. Delayed effects of weather were investigated by introducing lagged weather terms into the model. Meta-regression was used to pool together country-specific estimates. There was a 13 per cent (95% confidence interval (CI), 11–15%) decrease in reported infections per 1°C increase in temperature above a threshold of 5°C and a 4 per cent (95% CI, 3–5%) decrease in the infection-rate parameter per 1°C increase in temperature across the whole temperature range. The effect of temperature was immediate for the infection-rate parameter but delayed by up to four weeks for reported infections. There was no overall effect of humidity or rainfall. There is a direct and simple relationship between cold weather and rotavirus transmission in Great Britain and The Netherlands. The more complex and delayed temperature effect on disease incidence is likely to be mediated through the effects of weather on transmission.
Keywords: rotavirus, weather, temperature, relative humidity, rainfall, transmission
1. Introduction
Every year, an estimated 610 000 children under 5 years of age die from rotavirus gastroenteritis globally, with the great majority occurring in developing countries (Parashar et al. 2006). In developed countries, rotavirus gastroenteritis remains a common cause of childhood hospitalization at great cost to health services (Giaquinto et al. 2007).
In temperate regions, rotavirus gastroenteritis is highly seasonal, and typically peaks during the winter or early spring (Cook et al. 1990). In the tropics, rotavirus gastroenteritis is more common during the dry season, but the seasonality is less marked (Cunliffe et al. 1998). The fading of the seasonal pattern with proximity to the equator and the difference of approximately six months between the peak incidence in the Northern and Southern Hemispheres (Cook et al. 1990) suggests that climatic factors in part underlie the seasonality of rotavirus infections. Under laboratory conditions, rotavirus survival is optimal at low temperature (Moe & Shirley 1982; Moe & Harper 1983; Ijaz et al. 1985, 1994) but the relationship with relative humidity is less clear. Most survival studies of aerosolized rotavirus (Ijaz et al. 1985; Ansari et al. 1991) show that medium relative humidity is optimal for rotavirus survival, while survival tests of rotavirus on various surfaces have found optimal survival at low- and high-relative humidity (Moe & Shirley 1982) or best survival at low-relative humidity alone (Sattar et al. 1986).
Observational studies of human rotavirus disease have suggested that lower temperature, lower relative humidity and lower levels of rainfall are associated with increased risk of rotavirus infections (Brandt et al. 1982; Paul & Erinle 1982; Ram et al. 1990; Gomwalk et al. 1993; Armah et al. 1994; Hashizume et al. 2007; D'Souza et al. 2008). A recent meta-analysis of the seasonality of rotavirus disease in the tropics concluded that higher numbers of rotavirus infections were found at colder and drier times of the year (Levy et al. 2008). However, only two of these studies (Hashizume et al. 2007; D'Souza et al. 2008) have used statistical methods that control for underlying trends and seasonal confounders. With crude methods, coincidental occurrences (e.g. in winter, low-temperature and high-rotavirus case counts) may be incorrectly posited as causal.
Rotavirus is transmitted by the faeco-oral route, from person to person directly or via contaminated fomites, food or water (Ansari et al. 1991). Some evidence also exists for airborne droplet spread (Zhaori et al. 1991). We hypothesize that weather factors influence the incidence of rotavirus disease by affecting transmission of the virus and its survival in the environment. Rotavirus infection confers partial immunity (Velazquez et al. 1996), and thus the level of population immunity will also affect patterns of disease. As a result, climatic effects on the epidemiology of rotavirus disease are likely to be complex. Observed patterns of disease result from the dynamic interaction of seasonal patterns of viral transmissibility with levels of population immunity. Indeed, the delay and reduction in intensity of the rotavirus season in the USA following the introduction of vaccination (Tate et al. 2009) points to the role of population immunity in mediating seasonal patterns. A corollary of our hypothesis is that there is a simpler relationship (a more parsimonious model) between weather factors and transmissibility than between weather and disease patterns. The infection-rate parameter, which we estimate from a time series of laboratory-based surveillance data, is a measure of rotavirus transmissibility that accounts for changing levels of population immunity.
In this study, we fitted multivariate regression models adapted for time-series data to investigate the relationship between short-term variations in weather factors (ambient temperature, relative (and absolute) humidity and rainfall), and (i) the number of laboratory-confirmed rotavirus infections and (ii) the calculated rotavirus infection-rate parameter in Great Britain (England, Wales and Scotland) and The Netherlands.
2. Methodology
(a). Rotavirus surveillance data
The Health Protection Agency collects reports of laboratory-confirmed rotavirus infections from medical microbiology laboratories across England and Wales. Health Protection Scotland and the National Institute for Public Health and the Environment (RIVM) collect such reports in Scotland and The Netherlands, respectively. In all four countries, reporting by diagnostic laboratories is voluntary, but a recent survey in England and Wales indicated that in these two countries, laboratory testing practices are generally consistent year round (Atchison et al. 2009a). Weekly counts of laboratory-confirmed rotavirus infections between 1993 and 2007, based on illness onset date of the individual from whom the specimen was collected, were extracted from the national surveillance databases. Cases in older age groups are less frequent and are mainly restricted to overseas travellers, the immunocompromised and elderly individuals involved in institutional outbreaks (Bernstein 2009). Therefore, cases aged older than 5 years were excluded from the study, as infections in these groups are less likely to be associated with local weather factors.
(b). Quantifying the transmission of rotavirus infections in the population
The rotavirus surveillance data were used to quantify the transmission of rotavirus infections in the population, on the simplifying assumption that they reflect a ‘mass-action’ process. We estimated the transmission of rotavirus in the population from the relationship between the number of newly infected individuals in a given time period and the number of infectious and susceptible individuals in the same time period. We assumed that cases mix randomly with susceptibles in the population, that successive cases appear as chains of transmission, that susceptible individuals become cases after one serial interval of the infection (i.e. interval between identical stages of an infection in two successive cases) and that cases in one-time period become immune by the next time period.
The rate at which susceptibles become infected is described as follows (Stegeman et al. 1999):
| 2.1 |
where Ct is defined as the number of newly infected individuals per t unit time and βt is the infection-rate parameter which is defined as the number of susceptibles (St) infected by one infectious individual (It) during a given time period t. When the infection-rate parameter is above one, the number of new cases will tend to rise, and cases fall when it drops below one. By definition, the population Nt = Ct + St + It. The infection-rate parameter βt is calculated by simple re-arrangement of equation (2.1).
For calculating the infection-rate parameter, we assumed that rotavirus follows a simple susceptible–infectious–recovered/immune (S–I–R) pattern of infection. This is a simplification of the natural history of rotavirus, as children often experience multiple infections (Velazquez et al. 1996). However, moderate-to-severe diarrhoea predominantly occurs in primary infections (28% of primary infections), and disease severity is the primary determinant of laboratory confirmation of childhood diarrhoea in England (Tam et al. 2003). Primary infections are responsible for 86 per cent of cases of moderate-to-severe diarrhoea with second and subsequent infections responsible for 14 per cent and 0 per cent, respectively (Velazquez et al. 1996). For these reasons, we assumed that children are only susceptible to one moderate-to-severe disease episode and that only these disease episodes are captured in the laboratory reports, thus reflecting the pattern of an S–I–R disease.
Based on these characteristics and assumptions, we estimated the initial number of susceptibles at the start of the study period (S0) and the degree of under-ascertainment in the surveillance data for all rotavirus infections as well as for moderate-to-severe infections only. We used methods similar to those used by Fine & Clarkson (1982, 1984) to study the distribution of immunity in the population to pertussis and measles. These methods are described in detail in the electronic supplementary material, S1. The initial number of susceptibles estimated was 601 209, 33 757, 62 334 and 215 032 for England, Wales, Scotland and The Netherlands, respectively. Susceptibles made up approximately 20–22% of the under 5-year-old population in each study setting. The estimated overall under-ascertainment ratio for England of one reported case for every 44 community cases was broadly similar to that obtained in a population-based cohort study in England which estimated a ratio of 1 in 35 (Wheeler et al. 1999).
The number of infectious individuals in a given time period (It) was assumed to be the number of moderate-to-severe cases of rotavirus disease in the population in time period t. This estimate was obtained by multiplying the number of reported laboratory-confirmed rotavirus infections in time period t by an under-ascertainment factor for moderate-to-severe cases. The number of newly infected individuals during time t (Ct) was assumed to be It + 1. Therefore, this estimate was obtained by multiplying the number of reported laboratory-confirmed rotavirus infections in time period t + 1 by an under-ascertainment factor for moderate-to-severe cases. Methods for estimating the under-ascertainment of moderate-to-severe rotavirus cases in the surveillance data are described in detail in the electronic supplementary material, S1.
In the initial analyses, we assumed that the serial interval (t) was one week. Infectiousness begins with the onset of symptoms 24–72 h after infection, and the period of transmission lasts as long as the duration of symptoms (normal range 4–6 days) (Heymann 2004). Therefore, the serial interval can be as short as 1 day and as long as 9 days. A one-week interval was used because daily surveillance data are likely to suffer from reporting bias for certain days of the week. The infection-rate parameter calculated using a 4-day interval between successive cases closely resembled the pattern seen using a one-week interval.
We constructed a series of time periods by re-iterating equation (2.1), calculating the number susceptible at each time point as follows:
| 2.2 |
where the number of susceptibles remaining in the next time period (St + 1) is a function of the number of susceptibles in the current time period (St), minus the number of these that become immune (Kt), plus the number of susceptibles introduced or born into the population (Bt). Assuming an S–I–R pattern of disease, the number immune (Kt) is equivalent to the number of individuals with a history of infection. Moderate-to-severe disease (It) represents only 28 per cent of these infections. Therefore, It is adjusted accordingly to represent 100 per cent infections to obtain an estimate of the number immune (Kt). Bt is the number of births per time period. We took into account annual and seasonal variation in birth rates over the surveillance period because birth rates (Pitzer et al. 2009) and season of birth (Atchison et al. 2009b) have been shown to affect timing and risk of rotavirus infections, and could in part explain the seasonal pattern of rotavirus disease. We assume deaths from rotavirus infections to be negligible as they are rare in developed countries (Jit et al. 2007) relative to the frequency of births or incidence of rotavirus infections.
(c). Meteorological data
Data on weather variables were obtained from the MIDAS Land Surface Observation database available from http://badc.nerc.ac.uk/data/ukmo-midas. For each region of England and Wales, Scotland and The Netherlands, and for each weather variable, weather stations were selected if they provided data for at least 75 per cent of the days from 1993 to 2007. At least 10 weather stations were used for each weather series. These stations were scattered throughout the geographical area that they represented.
Daily mean values for ambient temperature, relative humidity and total rainfall across the weather stations were calculated and these measurements were used to construct population-weighted mean weekly series for the regions of England (the Northeast, Northwest, Yorkshire and Humberside, East Midlands, West Midlands, East of England, London, the Southeast and Southwest) and Wales, Scotland and The Netherlands. Weighting of weather measurements from each station contributing to the weather series was relative to the population density in immediate vicinity of the station. We used population-weighted weather series to better represent weather conditions in populated areas and improve our ability to detect the influence of weather factors on rotavirus infections. For temperature and relative humidity, the average daily measurement for the week was used. Total rainfall for the week was used to reflect the amount of excess water present in the environment and hence give a measure of exposure to water sources.
(d). Statistical analysis
Regression techniques adapted for analysis of time-series data (by incorporating lag effects, accounting for background seasonality, auto-correlation, over-dispersion) were used to model the relationship between temperature, relative humidity and total rainfall on rotavirus reports and the infection-rate parameter. We used an analogous approach for building up Poisson and simple linear multivariate regression models for weekly laboratory reports and the infection-rate parameter, respectively. In the final multivariate models, we controlled for confounding between weather factors and adjusted for long-term trends and background seasonality. This allowed us to estimate short-term effects of variables of interest (weather). The equations for the final models are described in detail in the electronic supplementary material, S2.
Long-term trends and background seasonal patterns were accounted for as part of the confounder model so that regular patterns (i.e. both cold weather and high-rotavirus incidence occur in winter) were not inferred to be causal. To control for long-term trends, an indicator variable for a year was included in the model. Seasonal patterns not directly due to weather factors were accounted for by using an indicator variable for a month. Artificial drops in reporting during weeks that included public holidays were also accounted for by including an indicator variable for these weeks. To account for over-dispersion in the data, standard errors were scaled using the square root of Pearson-χ2-goodness-of-fit statistic (Schwartz et al. 1996).
To investigate the delayed effects of weather on the outcome variables, lagged weather factors were introduced in the models (Armstrong 2006), e.g. the effect of lag one would be the effect of a weather variable measured in the week before, on the current week's number of reported cases or infection-rate parameter. Detailed analyses demonstrated no delay in the effect of weather factors on the infection-rate parameter. A delay in weather effect of up to four weeks was observed for reported cases; therefore the mean of each weather factor between a given week and the four preceding weeks was used in the models to represent the combined effects of exposure over a zero to four week period on subsequent rotavirus reports.
To determine the shape of the relationship between exposures and outcomes, natural cubic splines were fitted to the data, allowing the exposure–outcome relationship to be modelled by a smooth, nonlinear function (Durrleman & Simon 1989). All three sets of splines, one set for each weather variable, were included in the final model to control for confounding between weather factors. Where there was evidence of a linear association between a weather variable and the outcome (likelihood-ratio test p-value < 0.05), a simple linear term for the weather variable was included in the multivariate model to estimate the effect of the weather variable on the outcome. Where indicated by visual inspection of a plot of the spline model, a linear threshold term for the weather variable was included in the multivariate model. This model then assumed a linear relationship between the outcome and the weather variable up to a certain threshold, beyond which there was no association. The identification of the optimum threshold value was based on repeated regressions, varying the threshold by one exposure unit (e.g. 1°C) to find the best-fitting model as determined by Akaike's information criterion (AIC) (Armstrong 2006). Confidence intervals (CI) for thresholds were estimated from the profile of likelihoods generated from these repeated regressions (Armstrong 2006).
The type of regression model selected for the two different outcome variables was based on the distribution of the outcome data and on respecting model assumptions. Counts of reported rotavirus cases were Poisson-distributed and therefore Poisson regression models were used. The effect measure was the risk ratio (RR), representing the relative increase (or decrease) in the number of rotavirus reports per unit change in temperature (°C), relative humidity (%) or total rainfall (mm). The infection-rate parameter was normally distributed and therefore linear regression models were used. The effect measure was the regression coefficient, representing the absolute increase (or decrease) in the infection-rate parameter (the number of susceptibles infected by one infectious individual during one week) per unit change in temperature (°C), relative humidity (%) or total rainfall (mm).
We investigated if weather effects were modified by season by introducing interaction terms but found that the effects were consistent between seasons. The seasons were defined as winter (December, January and February), spring (March, April and May), summer (June, July and August) and autumn (September, October and November).
Finally, for each outcome variable, we identified the optimum single threshold value common to all countries, as determined by AIC (Armstrong 2006). Measures of effect (relative risk/regression coefficient) were then re-estimated for all countries using these single common thresholds, to allow valid comparisons between the measures of weather effect (slope of regression line) across study settings without the added complexity of varying thresholds. Meta-analysis using fixed effect models was used to pool together the setting-specific estimates, providing a mean-effect estimate for each weather variable.
A range of sensitivity analyses were performed to assess the robustness of the results to different methods of adjusting for seasonal trends. These models included using 3, 9 or 12 pairs of Fourier terms instead of the month indicator term, as well as models with an indicator term for week of the year instead of month. Fourier terms are combinations of sine and cosine waves that are fitted to the data to capture regular seasonal cycles of varying wavelengths. The number of harmonics reflects the level of adjustment required, with 12 harmonics corresponding to a seasonal pattern which cycles every four weeks (Stolwijk et al. 1999). None of the results were sensitive to the way background seasonality was modelled.
STATA v. 10.0 (Stata Corporation, College Station, TX, USA) was used for all statistical analyses.
Relative humidity is dependant on the temperature of the air whereas absolute humidity (actual amount of water vapour in the air) is irrespective of temperature and has been suggested to have a greater biological effect on certain infectious diseases than relative humidity (Shaman & Kohn 2009). We built up a multivariate regression model, by methods similar to those described, to include absolute humidity in place of relative humidity, to determine the relationship between absolute humidity and our two outcome variables controlling for temperature and rainfall. No associations were found (methods and results in the electronic supplementary material, S5).
3. Results
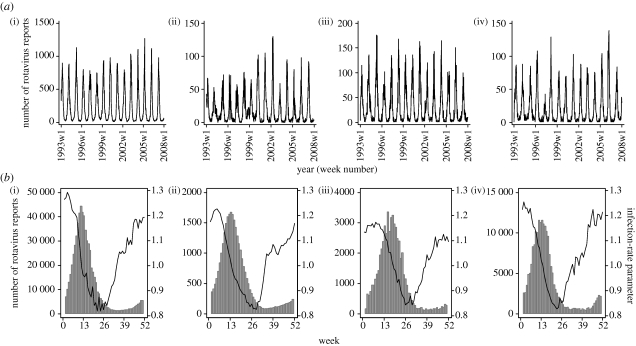
There was a distinct and highly consistent late winter or early spring peak in laboratory reports in all populations studied (figure 1). The infection-rate parameter showed a different pattern: it began to rise in weeks 28–32 and peaked at the beginning of each year in all settings, thus preceding the increase and peak in case reports by 10–12 weeks (figure 1). In every setting, when the infection-rate parameter climbed above one, the number of reported cases tended to rise and cases fell when it dropped below one (figure 1).
Figure 1.
(a) Weekly reported rotavirus infections in children under 5 years of age. (b) Average weekly infection-rate parameter (black line) and reported rotavirus infections adjusted for under-ascertainment (grey bars) over the years 1993–2007. (i) England (aggregated across the nine regions), (ii) Wales, (iii) Scotland and (iv) The Netherlands, 1993–2007.
(a). Ambient temperature and reported rotavirus infections
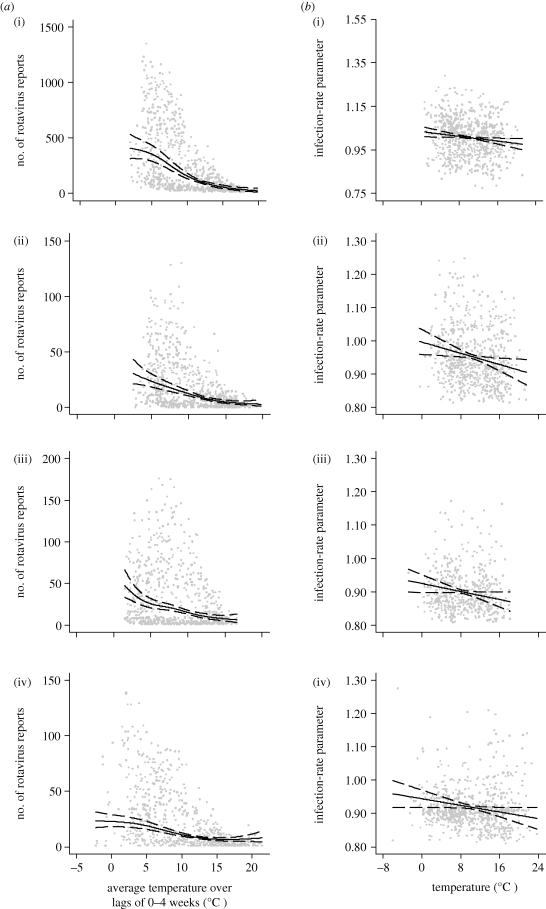
In all nine regions of England, and in Wales, Scotland and The Netherlands, there was an inverse relationship between mean temperature in the previous four weeks and rotavirus reports after controlling for trend, seasonality, public holidays, relative humidity and rainfall (figure 2 and table 1). For six regions of England and in The Netherlands, a temperature threshold was identified below which there was no longer an association. The effect of temperature was broadly similar in all countries, ranging from a 7 per cent (London, England) to a 16 per cent (the Northeast, West Midlands and the Southeast, England) decrease in number of cases per 1°C rise in mean weekly temperature either above a threshold or across the whole temperature range.
Figure 2.
Scatter plot of (a) reported rotavirus infections and average weekly temperatures over lags of 0–4 weeks and (b) the infection-rate parameter and average weekly temperature. The centre line is the estimated natural cubic spline (4 d.f), and the upper and lower lines represent the 95% CI. (i) England (aggregated across the nine regions), (ii) Wales, (iii) Scotland and (iv) The Netherlands, 1993–2007.
Table 1.
Risk ratio (RR), 95% confidence interval (CI) and p-values for reported rotavirus infections per 1°C increase in mean weekly temperature over lags of 0–4 weeks. Regression coefficient, 95% CI and p-values for the infection-rate parameter per 1°C increase in mean weekly temperature. Temperature thresholds were estimated where there was statistical evidence at the 5% level of a nonlinear relationship.
| rotavirus reports |
infection-rate parameter |
|||||
|---|---|---|---|---|---|---|
| country | temperature range (°C) | temperature threshold (°C) | RR (95% CI) | p-value | regression coefficient (×10−2) (95% CI) | p-value |
| England | ||||||
| the Northeast | −2.1–19.9 | — | 0.88 (0.83–0.93) | <0.001 | −5.77 (−10.93 to −0.60) | 0.03 |
| the Northwest | −1.4–21.1 | — | 0.84 (0.80–0.88) | <0.001 | −2.68 (−5.24 to −0.12) | 0.04 |
| Yorkshire & Humberside | −2.1–20.9 | 6 (6–7) | 0.87 (0.84–0.91) | <0.001 | −1.69 (−2.91 to −0.47) | 0.01 |
| East Midlands | −2.1–21.8 | 4 (4–5) | 0.85 (0.82–0.89) | <0.001 | −1.43 (−3.74–0.87) | 0.22 |
| West Midlands | −1.9–22.1 | 5 (4–5) | 0.84 (0.81–0.86) | <0.001 | −1.48 (−2.33 to −0.63) | 0.001 |
| East of England | −1.8–23.4 | 5 (5–6) | 0.85 (0.82–0.87) | <0.001 | −1.67 (−2.87 to −0.48) | 0.01 |
| London | −0.9–25.9 | — | 0.93 (0.90–0.96) | <0.001 | −1.09 (−2.37–0.19) | 0.09 |
| the Southeast | −1.9–23.9 | 5 (4–5) | 0.84 (0.82–0.87) | <0.001 | −1.51 (−2.94 to −0.09) | 0.04 |
| the Southwest | −1.6–22.2 | 6 (6–7) | 0.88 (0.85–0.91) | <0.001 | −2.38 (−4.53 to −0.22) | 0.03 |
| Wales | −0.5–21.5 | — | 0.88 (0.84–0.91) | <0.001 | −2.53 (−4.61 to −0.44) | 0.02 |
| Scotland | −4.6–18.1 | — | 0.87 (0.83–0.92) | <0.001 | −2.30 (−4.30 to −0.29) | 0.03 |
| The Netherlands | −7.4–24.1 | 4 (3–4) | 0.91 (0.89–0.94) | <0.001 | −2.00 (−3.85 to −0.16) | 0.03 |
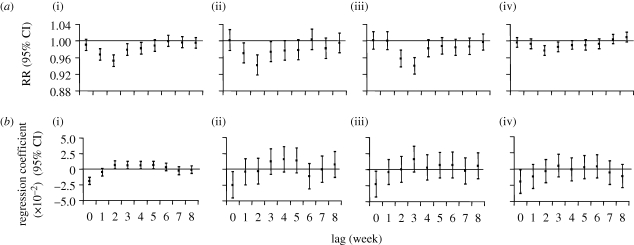
Distributed lag models estimating individual lag effects demonstrated that temperature had the greatest effect two to three weeks preceding an increase in cases with diminishing but positive effects up to four weeks (figure 3). Including further lag weeks in the model had negligible impact on the effect estimates.
Figure 3.
(a) The effect of temperature for each lag week on reported rotavirus infections and (b) the infection-rate parameter. (i) England (aggregated across the nine regions), (ii) Wales, (iii) Scotland and (iv) The Netherlands, 1993–2007.
(b). Relative humidity, rainfall and reported rotavirus infections
Overall, there was a weak and inconsistent association between relative humidity or total rainfall and reported rotavirus infections (electronic supplementary material, S3 and S4).
(c). Ambient temperature and the infection-rate parameter
In all nine regions of England and in Wales, Scotland and The Netherlands, there was an inverse relationship between temperature and the infection-rate parameter in the same week after controlling for trend, seasonality, public holidays, relative humidity and rainfall (figure 2 and table 1). For East Midlands and London, this did not reach statistical significance at the 95 per cent level. The decrease in the infection-rate parameter with increasing temperature was broadly similar in all countries, ranging from a 1.09 × 10−2 (London, England) to a 5.77 × 10−2 absolute (the Northeast and England) decrease in the infection-rate parameter per 1°C rise in mean weekly temperature across the whole temperature range.
Distributed lag models demonstrated that temperature in the same week had the greatest effect on the infection-rate parameter (figure 3).
(d). Relative humidity, rainfall and the infection-rate parameter
Overall, there was a weak and inconsistent association between relative humidity or total rainfall and the infection-rate parameter (electronic supplementary material, S3 and S4).
(e). Meta-analysis
The mean effect of temperature on reports of laboratory-confirmed rotavirus infections for England, Wales, Scotland and The Netherlands was a 13 per cent (95% CI, 11–15%) fall in number of reported infections per 1°C increase in temperature above a threshold of 5°C, and below which it was assumed that there was no effect of temperature on rotavirus reports (figure 4).
Figure 4.
Forrest plot with fixed effects summary estimate for (a) a 1°C increase in mean weekly temperature above a threshold of 5°C on reported rotavirus infections and (b) a 1°C increase in mean weekly temperature on the infection-rate parameter across the whole temperature range. England, Wales, Scotland and The Netherlands, 1993–2007.
The mean effect of temperature on the rotavirus infection-rate parameter for England, Wales, Scotland and The Netherlands was a 1.71 × 10−2 unit (95% CI, −2.14 × 10−2 to −1.27 × 10−2) absolute decrease in the infection-rate parameter per 1°C increase in temperature across the whole temperature range (figure 4). This corresponds to a 4 per cent (95% CI, 3–5%) decrease in the infection-rate parameter per 1°C increase in temperature across the whole temperature range when the absolute decrease is expressed as a percentage of the range of values for the infection-rate parameter in each country.
4. Discussion
Our study has demonstrated a seasonal pattern of rotavirus transmission which is consistent across Great Britain and The Netherlands. It is likely that this pattern of transmission underlies the seasonality of rotavirus disease incidence in these countries. Of the weather factors studied, only ambient temperature was consistently associated with changes in rotavirus transmission and disease. Indeed, this effect was consistently significant, universally in the same direction and of similar magnitude across the 12 study settings, suggesting strongly that these are not chance findings as a result of multiple testing. A rise in ambient temperature was associated with a drop in the infection-rate parameter, a measure of viral transmissibility and a reduction in the number of reported rotavirus infections. The nature of the relationship between temperature and the two outcome variables is revealing. Our original hypothesis was that weather factors influence the incidence of rotavirus infections by affecting the likelihood of transmission between infectious and susceptible individuals. Our findings support this hypothesis. Firstly, we demonstrated that the number of reported rotavirus infections was affected by temperatures in the preceding weeks, while the infection-rate parameter was affected only by temperatures in the same week. Secondly, the linear association between temperature and rotavirus reports was only apparent above a certain temperature threshold, while there was evidence of a linear relationship between temperature and the infection-rate parameter over the whole temperature range. The lagged association between temperature and rotavirus reports may reflect the fact that temperature affects case counts primarily through its influence on transmission. The relationship between transmission and the resulting number of clinical cases is a complex, nonlinear process that depends additionally on contact rates between infectious and susceptible individuals, virus transmissibility and host immunity. The final models for the infection-rate parameter fitted the data better and were more parsimonious than the models for case counts, as evidenced by plots of the fitted splines against the observed data (figure 2), plots of the model residuals and the much lower values of AIC. This also supports our hypothesis that temperature has a more direct effect on transmission than on rotavirus incidence per se.
We are aware of no other studies that have examined the relationship between a measure of rotavirus transmission and weather factors. Some studies have attempted to identify an association between the incidence of rotavirus cases and weather factors. However, only two studies, one set in Australia (D'Souza et al. 2008) and the other in Bangladesh (Hashizume et al. 2007), have used statistical methods to adjust for the potential confounding owing to other seasonal factors. In Australia (D'Souza et al. 2008), they found an increase in rotavirus diarrhoeal admissions in Brisbane, Melbourne and Canberra with lower temperatures and lower relative humidity in the previous week. In Bangladesh (Hashizume et al. 2007), they found that high temperatures, low-relative humidity and high-river level in the previous four weeks were associated with an increase in hospital visits for rotavirus diarrhoea. Our finding of a strong negative association between reported rotavirus cases and temperature is consistent with the results from Australia and with laboratory evidence (Moe & Shirley 1982; Moe & Harper 1983). Our findings do not agree with the temperature results from the Bangladesh study, which also contrast with findings from the majority of laboratory and observational studies. As most of the high-temperature weeks in Bangladesh occurred during the monsoon season and there was evidence for temperature-effect modification across seasons in this study, the temperature effect may be exerted through complex temperature-dependent pathways in the monsoon season. Our findings for relative humidity are in contrast with those from Australia and Bangladesh. This could be explained by the fact that the range of relative humidity was greater in Australia and Bangladesh. Weather factors are likely to interact in complex ways specific to the setting and may be influenced by other unmeasured factors, including the degree of hygiene and sanitation in an area and specific human behaviours. These setting-specific factors may determine the extent to which weather factors influence the incidence of rotavirus cases and could account for the discrepancies in results seen in different study settings. In addition, laboratory studies that have looked at rotavirus survival at different relative humidity levels have produced contradictory findings which suggest that the association may be complex.
We found no evidence of an association between number of rotavirus reports and rainfall. Studies that have looked at the relationship between rainfall and rotavirus have produced conflicting results (Brandt et al. 1982; Paul & Erinle 1982; Armah et al. 1994; Hashizume et al. 2007), and it may be that the effects of rainfall are complex, indirect and/or setting-specific.
Pitzer et al. (2009) recently investigated the relative contribution of climate factors and secular changes in birth rates on the timing of rotavirus epidemics in the US states. They found that environmental factors, including solar radiation, precipitation, vapour pressure and temperature, did not explain the observed variability in the peak week of rotavirus activity, either across states or over time. Our analysis differs from that of Pitzer et al. (2009), in that we focused on short-term effects of weather rather than on the time of year during which epidemics occur. Importantly, our analysis indicates that the effect of weather on rotavirus incidence is likely to be mediated through its effects on transmissibility. The difference between our results and those of Pitzer et al. (2009) is thus not so surprising; although weather influences rotavirus transmissibility, once a certain level of transmissibility is exceeded, population transmission dynamics are likely to determine the size and duration of the epidemic, and the influence of weather factors may be much less pronounced.
To estimate the infection-rate parameter, we had to make a number of simplifying assumptions. The assumption of a single homogeneous mixing population is not realistic for the populations included in this study, as social mixing patterns determine a limited and non-random contact set for each individual. Therefore, in our study, we have calculated an average infection-rate parameter for the entire population and it is likely that the actual transmission rate is underestimated. In addition, we did not include asymptomatic and mild cases in our calculation of the infection-rate parameter. The majority of household transmission studies have suggested that symptomatic individuals are more infectious and important in transmission than incubating or asymptomatically infected individuals (Haug et al. 1978; Rodriguez et al. 1979; Grimwood et al. 1983; Banerjee et al. 2008). Therefore, we feel that not accounting for asymptomatic and mild cases in our calculation of the infection-rate parameter was justified. However, if asymptomatic and mild cases do contribute significantly to transmission, then it is likely that the actual transmission rate is underestimated, but the seasonal pattern of the infection-rate parameter will remain unchanged as asymptomatic and mild cases exhibit the same seasonal incidence as moderate-to-severe cases in our study settings (Walther et al. 1983; Iturriza-Gomara et al. 2009).
The quality of the rotavirus surveillance data may be a limitation of our study. Only a proportion of cases in the community are reported in national surveillance data, and those cases reported are not necessarily representative of all cases, as reported cases are likely to be more severe (Tam et al. 2003). This is unlikely to affect the validity of the comparisons made over time, provided that the nature and degree of under-ascertainment remained constant over the study period. A recent survey of clinical laboratory practices for rotavirus detection (Atchison et al. 2009a) found that most laboratories in England and Wales test for rotavirus all year round in all cases of gastroenteritis under 5 years of age and that the degree of under-ascertainment and the testing criteria for rotavirus did not vary significantly over time. The under-ascertainment estimates for England were internally consistent and similar to a previous empirical estimate (Wheeler et al. 1999).
Another potential limitation of our study is the high variability of the data, apparent from the considerable scatter of the data points above and below the fitted curve (figure 2). The reason for this is uncertain, but might depend on a number of factors. The use of regional and country weather series could have diluted the weather effects and resulted in the high variability. More detailed city-specific analysis might have addressed this problem albeit at the loss of statistical power. We used population-weighted weather series to better reflect weather conditions in more populated areas and to improve our ability to detect the influence of weather variables on rotavirus infections.
It is not known where (outdoors versus indoors) children are being infected with rotavirus, although it is known from transmission studies that infants and young children are commonly infected outside of the household (Koopman et al. 1989). In England and Wales, significant risk factors for rotavirus infection include contact with persons with rotavirus infection, living in rented public housing and accommodation with fewer rooms (Sethi et al. 2001). If infection is transmitted indoors by individuals living in close proximity to each other (i.e. households with fewer rooms and overcrowding), then one possible explanation for the cold-weather effect could be that low ambient temperatures encourage these individuals to stay indoors in tightly closed households, increasing their exposure to contaminated air, surfaces or individuals. If infection is transmitted outdoors, an important effect of low temperature might be to prolong survival of the virus in the environment (Moe & Shirley 1982; Ansari et al. 1991), increasing the likelihood of exposure to infectious virus.
In summary, our study shows that colder weather has a direct and immediate effect on the transmission of rotavirus infections, and a more complex and delayed effect on the incidence of rotavirus disease that is likely to be mediated by increases in transmission potential. The transmission of rotavirus is complex and it is unlikely that weather factors alone can fully explain the seasonality of rotavirus infections. Understanding the effects of weather factors on rotavirus disease could improve the accuracy of estimates for diarrhoeal burden of disease attributable to climate change. On a more practical level, knowing that there is a lag of at least one week between low temperature and an increase in reported rotavirus infections could help predict demands on health services and assist appropriate rationalization of healthcare resources.
References
- Ansari S., Springthorpe V., Sattar S.1991Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev. Infect. Dis. 13, 448–461 [DOI] [PubMed] [Google Scholar]
- Armah G. E., Mingle J. A., Dodoo A. K., Anyanful A., Antwi R., Commey J., Nkrumah F. K.1994Seasonality of rotavirus infection in Ghana. Ann. Trop. Paediatr. 14, 223–229 [DOI] [PubMed] [Google Scholar]
- Armstrong B.2006Models for the relationship between ambient temperature and daily mortality. Epidemiology 17, 624–631 (doi:10.1097/01.ede.0000239732.50999.8f) [DOI] [PubMed] [Google Scholar]
- Atchison C., Lopman B., Harris C., Tam C., Iturriza Gomara M., Gray J. Clinical laboratory practices for the detection of rotavirus in England and Wales: can surveillance based on routine laboratory testing data be used to evaluate the impact of vaccination? Euro. Surveill. 2009a;14 doi: 10.2807/ese.14.20.19217-en. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19217 . [DOI] [PubMed] [Google Scholar]
- Atchison C. J., Tam C. C., Lopman B. A.2009bSeason of birth and risk of rotavirus diarrhoea in children aged <5 years. Epidemiol. Infect. 137, 957–960 [DOI] [PubMed] [Google Scholar]
- Banerjee I., Primrose Gladstone B., Iturriza-Gomara M., Gray J. J., Brown D. W., Kang G.2008Evidence of intrafamilial transmission of rotavirus in a birth cohort in South India. J. Med. Virol. 80, 1858–1863 (doi:10.1002/jmv.21263) [DOI] [PubMed] [Google Scholar]
- Bernstein D. I.2009Rotavirus overview. Pediatr. Infect. Dis. J. 28, S50–S53 (doi:10.1097/INF.0b013e3181967bee) [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Parrott R. H.1982Rotavirus gastroenteritis and weather. J. Clin. Microbiol. 16, 478–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. M., Glass R. I., LeBaron C. W., Ho M. S.1990Global seasonality of rotavirus infections. Bull. World Health Organ. 68, 171–177 [PMC free article] [PubMed] [Google Scholar]
- Cunliffe N. A., Kilgore P. E., Bresee J. S., Steele A. D., Luo N., Hart C. A., Glass R. I.1998Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bull. World Health Organ. 76, 525–537 [PMC free article] [PubMed] [Google Scholar]
- D'Souza R. M., Hall G., Becker N. G.2008Climatic factors associated with hospitalizations for rotavirus diarrhoea in children under 5 years of age. Epidemiol. Infect. 136, 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrleman S., Simon R.1989Flexible regression models with cubic splines. Stat. Med. 8, 551–561 (doi:10.1002/sim.4780080504) [DOI] [PubMed] [Google Scholar]
- Fine P. E., Clarkson J. A.1982Measles in England and Wales—I: an analysis of factors underlying seasonal patterns. Int. J. Epidemiol. 11, 5–14 [DOI] [PubMed] [Google Scholar]
- Fine P. E., Clarkson J. A.1984Distribution of immunity to pertussis in the population of England and Wales. J. Hyg. (Lond.) 92, 21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinto C., van Damme P., Huet F., Gothefors L., van der Wielen M.2007Costs of community-acquired pediatric rotavirus gastroenteritis in 7 European countries: the REVEAL Study. J. Infect. Dis. 195(Suppl. 1), S36–S44 (doi:10.1086/516716) [DOI] [PubMed] [Google Scholar]
- Gomwalk N. E., Umoh U. J., Gosham L. T., Ahmad A. A.1993Influence of climatic factors on rotavirus infection among children with acute gastroenteritis in Zaria, northern Nigeria. J. Trop. Pediatr. 39, 293–297 [DOI] [PubMed] [Google Scholar]
- Grimwood K., Abbott G. D., Fergusson D. M., Jennings L. C., Allan J. M.1983Spread of rotavirus within families: a community based study. Br. Med. J. (Clin. Res. Ed.) 287, 575–577 (doi:10.1136/bmj.287.6392.575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume M., Armstrong B., Wagatsuma Y., Faruque A. S., Hayashi T., Sack D. A.2007Rotavirus infections and climate variability in Dhaka, Bangladesh: a time-series analysis. Epidemiol. Infect. 136, 1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug K. W., Orstavik I., Kvelstad G.1978Rotavirus infections in families: a clinical and virological study. Scand. J. Infect. Dis. 10, 265–269 [DOI] [PubMed] [Google Scholar]
- Heymann D. L.2004Gastroenteritis, acute viral. In Control of communicable diseases manual (ed. Heymann D. L.), pp. 224–227 Washington, DC: American Public Health Association [Google Scholar]
- Ijaz M. K., Sattar S. A., Johnson-Lussenburg C. M., Springthorpe V. S., Nair R. C.1985Effect of relative humidity, atmospheric temperature, and suspending medium on the airborne survival of human rotavirus. Can. J. Microbiol. 31, 681–685 [DOI] [PubMed] [Google Scholar]
- Ijaz M. K., Sattar S. A., Alkarmi T., Dar F. K., Bhatti A. R., Elhag K. M.1994Studies on the survival of aerosolized bovine rotavirus (UK) and a murine rotavirus. Comp. Immunol. Microbiol. Infect. Dis. 17, 91–98 (doi:10.1016/0147-9571(94)90034-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriza-Gomara M., Elliot A. J., Dockery C., Fleming D. M., Gray J. J.2009Structured surveillance of infectious intestinal disease in pre-school children in the community: ‘the nappy study'. Epidemiol. Infect. 137, 922–931 (doi:10.1017/S0950268808001556) [DOI] [PubMed] [Google Scholar]
- Jit M., Pebody R., Chen M., Andrews N., Edmunds W. J.2007Estimating the number of deaths with rotavirus as a cause in England and Wales. Hum. Vaccin. 3, 23–26 [DOI] [PubMed] [Google Scholar]
- Koopman J. S., Monto A. S., Longini I. M., Jr1989The Tecumseh study. XVI: family and community sources of rotavirus infection. Am. J. Epidemiol. 130, 760–768 [DOI] [PubMed] [Google Scholar]
- Levy K., Hubbard A. E., Eisenberg J. N.2008Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int. J. Epidemiol. Dec 4 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe K., Harper G. J.1983The effect of relative humidity and temperature on the survival of bovine rotavirus in aerosol. Arch. Virol. 76, 211–216 (doi:10.1007/BF01311105) [DOI] [PubMed] [Google Scholar]
- Moe K., Shirley J. A.1982The effects of relative humidity and temperature on the survival of human rotavirus in faeces. Arch. Virol. 72, 179–186 (doi:10.1007/BF01348963) [DOI] [PubMed] [Google Scholar]
- Parashar U., Gibson C., Bresee J., Glass R.2006Rotavirus and severe childhood diarrhoea. Emerg. Infect. Dis. 12, 304–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M. O., Erinle E. A.1982Influence of humidity on rotavirus prevalence among Nigerian infants and young children with gastroenteritis. J. Clin. Microbiol. 15, 212–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer V. E., et al. 2009Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science 325, 290–294 (doi:10.1126/science.1172330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram S., Khurana S., Khurana S. B., Sharma S., Vadehra D. V., Broor S.1990Bioecological factors and rotavirus diarrhoea. Indian J. Med. Res. 91, 167–170 [PubMed] [Google Scholar]
- Rodriguez W. J., et al. 1979Common exposure outbreak of gastroenteritis due to type 2 rotavirus with high secondary attack rate within families. J. Infect. Dis. 140, 353–357 [DOI] [PubMed] [Google Scholar]
- Sattar S. A., Lloyd-Evans N., Springthorpe V. S., Nair R. C.1986Institutional outbreaks of rotavirus diarrhoea: potential role of fomites and environmental surfaces as vehicles for virus transmission. J. Hyg. (Lond.) 96, 277–289 (doi:10.1017/S0022172400066055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., et al. 1996Methodological issues in studies of air pollution and daily counts of deaths or hospital admissions. J. Epidemiol. Commun. Health 50(Suppl. 1), S3–S11 (doi:10.1136/jech.50.Suppl_1.S3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi D., Cumberland P., Hudson M. J., Rodrigues L. C., Wheeler J. G., Roberts J. A., Tompkins D. S., Cowden J. M., Roderick P. J.2001A study of infectious intestinal disease in England: risk factors associated with group A rotavirus in children. Epidemiol. Infect. 126, 63–70 (doi:10.1017/S0950268801005088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J., Kohn M.2009Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl Acad. Sci. USA 106, 3243–3248 (doi:10.1073/pnas.0806852106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegeman A., Elbers A. R., Smak J., de Jong M. C.1999Quantification of the transmission of classical swine fever virus between herds during the 1997–1998 epidemic in The Netherlands. Prev. Vet. Med. 42, 219–234 (doi:10.1016/S0167-5877(99)00077-X) [DOI] [PubMed] [Google Scholar]
- Stolwijk A. M., Straatman H., Zielhuis G. A.1999Studying seasonality by using sine and cosine functions in regression analysis. J. Epidemiol. Commun. Health 53, 235–238 (doi:10.1136/jech.53.4.235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C. C., Rodrigues L. C., O'Brien S. J.2003The study of infectious intestinal disease in England: what risk factors for presentation to general practice tell us about potential for selection bias in case–control studies of reported cases of diarrhoea. Int. J. Epidemiol. 32, 99–105 (doi:10.1093/ije/dyg007) [DOI] [PubMed] [Google Scholar]
- Tate J. E., Panozzo C. A., Payne D. C., Patel M. M., Cortese M. M., Fowlkes A. L., Parashar U. D.2009Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics 124, 465–471 (doi:10.1542/peds.2008-3528) [DOI] [PubMed] [Google Scholar]
- Velazquez F. R., et al. 1996Rotavirus infections in infants as protection against subsequent infections. N. Engl. J. Med. 335, 1022–1028 (doi:10.1056/NEJM199610033351404) [DOI] [PubMed] [Google Scholar]
- Walther F. J., Bruggeman C., Daniels-Bosman M. S., Pourier S., Grauls G., Stals F., Bogaard A. V.1983Symptomatic and asymptomatic rotavirus infections in hospitalized children. Acta Paediatr. Scand. 72, 659–663 (doi:10.1111/j.1651-2227.1983.tb09790.x) [DOI] [PubMed] [Google Scholar]
- Wheeler J. G., Sethi D., Cowden J. M., Wall P. G., Rodrigues L. C., Tompkins D. S., Hudson M. J., Roderick P. J.1999Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The infectious intestinal disease study executive. BMJ 318, 1046–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhaori G. T., Fu L. T., Xu Y. H., Guo Y. R., Peng Z. J., Shan W. S.1991Detection of rotavirus antigen in tracheal aspirates of infants and children with pneumonia. Chin. Med. J. (Engl.) 104, 830–833 [PubMed] [Google Scholar]