Abstract
While insect cold tolerance has been well studied, the vast majority of work has focused on the effects of a single cold exposure. However, many abiotic environmental stresses, including temperature, fluctuate within an organism's lifespan. Given that organisms may trade-off survival at the cost of future reproduction, we investigated the effects of multiple cold exposures on survival and fertility in the model organism Drosophila melanogaster. We found that multiple cold exposures significantly decreased mortality compared with the same length of exposure in a single sustained bout, but significantly decreased fecundity (as measured by r, the intrinsic rate of increase) as well, owing to a shift in sex ratio. This change was reflected in a long-term decrease in glycogen stores in multiply exposed flies, while a brief effect on triglyceride stores was observed, suggesting flies are reallocating energy stores. Given that many environments are not static, this trade-off indicates that investigating the effects of repeated stress exposure is important for understanding and predicting physiological responses in the wild.
Keywords: life-history trade-off, cold tolerance, Drosophila melanogaster, environmental variability, fluctuating thermal regimes
1. Introduction
Abiotic environmental factors such as light availability, precipitation and temperature fluctuate on a broad range of temporal scales (Ruel & Ayers 1999; Sinclair 2001; Ozgul et al. 2009). As a result, many organisms are faced with cycling of environmental conditions within their lifespans. These environmental conditions can produce important physiological stresses upon organisms, and, given limited resources, force trade-offs of survival and maintenance with reproduction (Stearns 1992).
Life-history trade-offs have been intensely investigated with the model species Drosophila melanogaster owing to its tractability in the laboratory, and its easily measured fitness proxies (Zera & Harshman 2001; Hoffmann et al. 2003). Both wild-type and laboratory-selected lines have been used to investigate correlations among life-history traits. For example, stress resistance (desiccation resistance and heat resistance) is positively correlated with longevity (Lin et al. 1998) and negatively correlated with fecundity in D. melanogaster (Luckinbill et al. 1984; Chippindale et al. 1996, 1998; Partridge et al. 1999). While modelling efforts suggest that stress encountered in early life may trade off with performance later in life (Mangel 2008), trade-offs induced by early fluctuating stress remain under active investigation. For example, while both repeated non-lethal high- and low-temperature stress positively impact later longevity and heat resistance, only repeated mild heat stress reduces later fertility (Hercus et al. 2003; LeBourg 2007).
Low temperatures are a significant source of stress for many insect species, and are thought to limit fitness and consequently distribution (Bale 2002; Sinclair et al. 2003). Low temperatures can cause delayed development and death through chilling injuries such as denaturation of proteins, membrane phase transitions, hypoxia and loss of membrane potential in insects (see Storey & Storey 1988; Kostal et al. 2007). Similarly, temperature-induced changes in gene expression and protein production can reduce fitness by decreasing the viability of eggs laid by treated adult D. melanogaster (Silbermann & Tatar 2000). However, while the majority of these studies report on the results of a single stress exposure, it is clear that, in nature, animals are exposed to thermal stress on a regular and repeated basis (Sinclair 2001), and understanding the consequences of these repeated stresses is essential for interpreting and predicting climatic change effects in the natural world (Chown & Terblanche 2007; Helmuth 2009).
Fluctuating thermal regimes (FTR; long-term cold exposure broken by repeated short warm temperature exposures) increase cold survival in many chill-susceptible insects (discussed by Kostal et al. 2007). FTR induce upregulation of metabolic pathways in Aphidius colemani, which is consistent with repair of cold injuries occurring during the warm period of the temperature cycle (Colinet et al. 2007). Similarly, populations of D. melanogaster and Drosophila simulans exposed to fluctuating temperatures maintain development at temperatures that would be lethal under a constant low-temperature regime (Petavy et al. 2001). While multiple cold exposures appear to be beneficial for chill-susceptible insects, freeze tolerant insects appear to incur fitness consequences with repeated cold exposure (Bale et al. 2001; Sinclair & Chown 2005).
This previous work on fluctuating temperatures is limited because, first, the multiple cold exposure groups experienced less net time at the lower temperature, even though the net amount of time spent at low temperatures may drive the accumulation of injuries (Petavy et al. 2001). Second, the binary responses of survival or emergence versus death in the short term do not account for longer term fitness, so there is a need to quantify the sub-lethal effects of multiple cold exposure (Partridge et al. 1991).
Here we address the impacts of repeated stress exposure from a life-history trade-off perspective using a D. melanogaster model system. Drosophila melanogaster is chill-susceptible: while the lower lethal temperature for a 2 h exposure in adults is −5°C, freezing does not occur until −20°C (Czajka & Lee 1990). The sub-lethal costs of multiple cold exposures were quantified through fecundity (both total number of offspring produced and intrinsic rate of population increase—r) and measures of energy reserves (Djawdan et al. 1998; Dillon et al. 2007). We tested three competing hypotheses on the fitness costs of cold exposure as measured by r. First, chill accumulation: fitness cost is based on the total amount of time spent cold, regardless of warming intervals. Under this hypothesis, we predict that multiple and sustained exposure groups will incur similar costs. Second, repair: warming intervals allow for repair to take place. We predict that the sustained exposure group will incur the most cost. Finally, increased cost: the opportunity to repair is costly. In this case, we predict that the multiple exposures group will incur the most cost.
2. Material and Methods
(a). Fly collection and rearing
The experimental population was established by combining 35 isofemale lines of D. melanogaster collected six months previously from London, Ontario, Canada (43°00′ N, 81°15′ W) and Niagara on the Lake, Ontario, Canada (43°04′ N, 79°04′ W). Typical temperatures in London, Ontario, Canada include multiple cold cycles (see the electronic supplementary material, figure S1). Flies were reared on cornmeal-agar medium in 35 ml plastic vials at low density (Markow & O'Grady 2005). For flies reared for experimentation, 60 vials of adult flies (approx. 100 flies each) were emptied into each population cage. Eggs were collected from a Petri dish of food medium that was left in the cage for 8 h, and placed into new 35 ml medium vials at a density of 100 eggs per vial. After 10 days, virgin females were collected under CO2 anaesthesia and placed in new 35 ml medium vials in groups of 15. Virgin males were also collected at this time, also placed in new 35 ml medium vials at a density of approximately 30 per vial, and maintained at 22°C. All experiments started on the third day after sorting to avoid CO2 effects on cold tolerance (Nilson et al. 2006). Any vial that subsequently showed signs of larvae was discarded.
(b). Experimental design
All cold treatments were conducted in inverted food vials placed into an incubator (MIR153, Sanyo, Bensenville, IL, USA) set to −0.5± 0.25°C as measured in medium vials by iButton dataloggers (Maxim Integrated Products, Inc., Sunnyvale, CA, USA). There are potential interactions between age and cold tolerance (Czajka & Lee 1990) and age and fecundity (Dillon et al. 2007), so several controls were required to isolate the effects of multiple cold exposure. Four main experimental groups were created: (i) a control group maintained at 22°C; (ii) a sustained cold group, exposed to −0.5°C for 10 h; (iii) a multiple cold group: two, three, four or five 2 h exposures to −0.5°C, each separated by 22 h at 22°C and all ending at 7 days of age; and (iv) a single short cold group that received a single 2 h exposure to −0.5°C. To control for the potential interaction of age and cold tolerance, sustained and single short treatments were conducted 3, 5, and 7 days after sorting. Control flies were sampled at 3, 5, and 7 days after sorting.
(c). Mortality and fertility assays
Immediately after the final treatment for a group of flies, 50 females per age × treatment combination were narcotized with CO2 and placed individually into new food vials with two untreated virgin males of the same age. After 2 days, the triads were transferred to new vials. Females were examined daily for 4 days following treatment, and were recorded as ‘dead’ if unable to right themselves. After 4 days, all adult flies were removed from their vials and vials were monitored daily for emergence of offspring, which were removed, counted and sexed each day until eclosion ceased.
(d). Energy reserves
Immediately after cold treatment, as well as 24, 48, and 72 h later, groups of 15 flies were snap frozen in liquid nitrogen and stored at −80°C until analysis. Flies in groups of five were homogenized in 0.05 per cent Tween 20 using 1 mm diameter glass beads in a Bullet Blender (Next Advance Inc., Averill Park, NY, USA). Samples were centrifuged at 16 000g for 1 min and the supernatant stored at −20°C (Gefen et al. 2006).
Triglyceride and glycogen content were measured in triplicate according to Gefen et al. (2006), with the following modifications for the glycogen assay: liquid glucose reagent was added to the samples and free glucose determined. Then 10 µL Rhizopus amyloglucosidase was added, and the plate left overnight at 20°C to allow conversion of glycogen to glucose, which was determined as the difference between free glucose and the glucose in the digested sample.
(e). Data analysis
Each treatment group (multiple, control, sustained or single short) was first examined separately for the effects of age. If age was a non-significant factor, data from flies of all ages for a treatment were combined together. Mortality was compared within and then between treatments by a generalized linear model with binary error distributions and a logit link function in SPSS. Fecundity (sex ratio, total number of offspring and intrinsic rate of population increase) was analysed by separate general linear models with treatment and age as factors, and Tukey post hoc tests. Energy reserves (triglyceride and glycogen) assays were compared by separate general linear models with treatment and time after treatment as factors and Tukey post hoc tests. Sex ratio was calculated as the proportion of females out of total offspring per vial. Intrinsic rate of population increase (r) was calculated as per Dillon et al. (2007) with the following modifications: instead of only including surviving females, the probability of survival for each individual each day for each treatment was calculated, and then used as the lx term in the calculation of net reproductive value (R0). Only vials where both males survived and there were no escapees were counted and only the non-iterative technique was used to calculate r (Birch 1948). Net reproductive value was calculated for each treated fly as
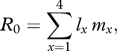 |
2.1 |
where x is the time after treatment in days, lx the probability of being alive at time after treatment x and mx the number of female offspring produced by that female on that day. Generation time (Tg) was then estimated by calculating the mean development time of the female's offspring by
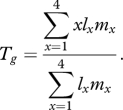 |
2.2 |
These two calculations allowed estimation of intrinsic rate of population increase (r, Birch 1948) by
| 2.3 |
Analyses were conducted in R using the base package (R Development Core Team 2008) and SPSS (v. 17.0). Because of the high power of this study, α was set a priori to 0.01.
3. Results
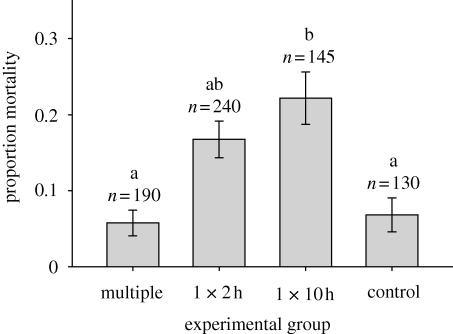
We monitored survival for 4 days post-cold exposure in a total of 50 female flies in each of 15 age × treatment interaction groups. Overall mortality in the experiment was 13 per cent, and number of cold exposures did not affect mortality in multiply exposed flies (Wald χ2 = 1.645, d.f. = 3, p = 0.649). Similarly, age did not significantly affect survival in control (Wald χ2 = 0.603, d.f. = 2, p = 0.740), sustained (10 h; Wald χ2 = 6.257, d.f. = 2, p = 0.044), or single short (2 h) cold exposure flies (Wald χ2 = 10.693, d.f. = 4, p = 0.030). Therefore, all age interaction groups were pooled within each treatment, as were all treatments within the multiple group, and a significant difference was found between groups (Wald χ2 = 23.941, d.f. = 3, p < 0.001). Multiply exposed and single short exposed flies had similar survival to controls, while sustained exposure flies had higher mortality (figure 1).
Figure 1.
Mortality 4 days after cold treatment at −0.5°C in female adult Drosophila melanogaster. Treatments with the same letter are not significantly different (p < 0.01) in a generalized linear model. Error bars are binomial s.e., and sample sizes are indicated in the figure.
(a). Total offspring produced
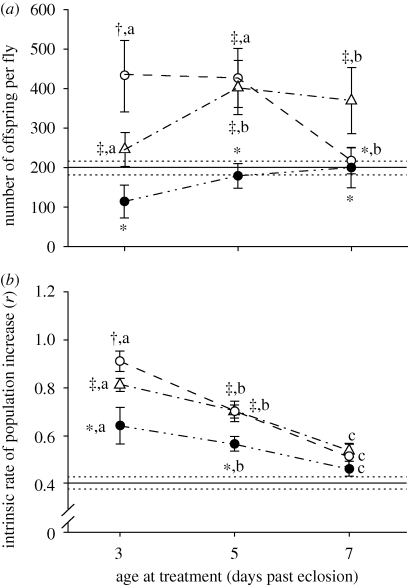
Increased number of cold exposures led to a decreased number of offspring produced in the multiply exposed flies (F3,195 = 10.407, p < 0.001; figure 2a). Age also affected total number of offspring produced by control flies (F2,147 = 10.828, p < 0.001), sustained exposure (1 × 10 h) flies (F2,147 = 7.969, p < 0.001) and single short exposure (1 × 2 h) flies (F4,242 = 12.534, p < 0.001; figure 2a), In general, multiple and sustained (10 h) exposure flies produced significantly fewer offspring than single short (2 h) or control flies (figure 2a; F3,742 = 60.102, p < 0.001).
Figure 2.
Fertility effects of cold exposure at −0.5°C. (a) Total number (mean ± 99% CI) of offspring produced in a 4-day-period after cold treatment at −0.5°C by female adult Drosophila melanogaster. Points with the same superscript symbols are not significantly different between treatments at a given age at p < 0.01. Points with the same lower-case letters are not significantly different within a treatment at different ages at p < 0.01. Solid horizontal line indicates mean number of offspring of multiple treatment group (two to five 2 h treatments), with dotted lines indicating 99% CI, n = 42–50 females per point. (b) Intrinsic rate of population increase (r, mean ± 99% CI) after cold treatment at −0.5°C as a function of treatment and age in female adult Drosophila melanogaster. Points with the same superscript symbols are not significantly different between treatments at a given age at p < 0.01. Points with the same lower-case letters are not significantly different within a treatment at different ages at p < 0.01. Solid horizontal line indicates intrinsic rate of increase of the multiple treatment group that received five 2 h treatments, with dotted lines indicating 99% CI, n = 42–50 females per point (filled circles, 1 × 10 h; open circles, 1 × 2 h; open triangles, control).
(b). Sex ratio
Number of cold exposures did not significantly affect offspring sex ratio in the multiple exposures group, nor did age of exposure (p > 0.07 in all cases, see the electronic supplementary material, table S1). However, when all age interaction groups were pooled within each treatment, as were all treatments within the multiple group, the multiply exposed group had a significantly male-biased sex ratio (mean ± s.e. = 0.457 ± 0.006, F3,705 = 8.615, p < 0.001) compared with other groups (mean ± s.e. = 0.501 ± 0.009).
(c). Intrinsic rate of population increase (r)
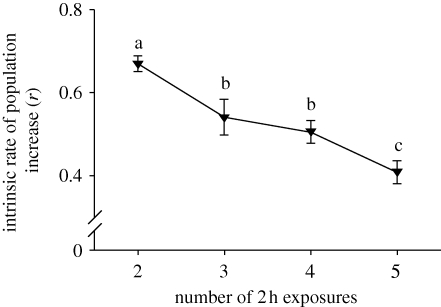
Increasing numbers of short cold exposures significantly decreased r in multiply exposed flies (figure 3, F3,189 = 93.309, p < 0.001). Similarly, younger flies had higher values of r than older flies in control flies (F2,142 = 116.360, p < 0.001), sustained cold (10 h) flies (F2,128 = 25.640, p < 0.001) and single short exposure (2 h) flies (F4,227 = 105.080, p < 0.001; figure 2b). There was a significant effect of treatment when all ages were pooled within each treatment and compared with the five 2 h exposures group (F3,459 = 63.512, p < 0.001). Flies with five 2 h exposures had the lowest r in every case except one: there was no significant difference between the five 2 h exposure group and the oldest sustained (10 h) exposure flies, although the five 2 h exposure group had a lower mean r (figure 2b).
Figure 3.
Intrinsic rate of population increase (mean ± 99% CI) after cold treatment at −0.5°C as a function of number of 2 h treatments in female adult Drosophila melanogaster. Points with the same lower-case letters are not significantly different at p < 0.01.
(d). Energy reserves
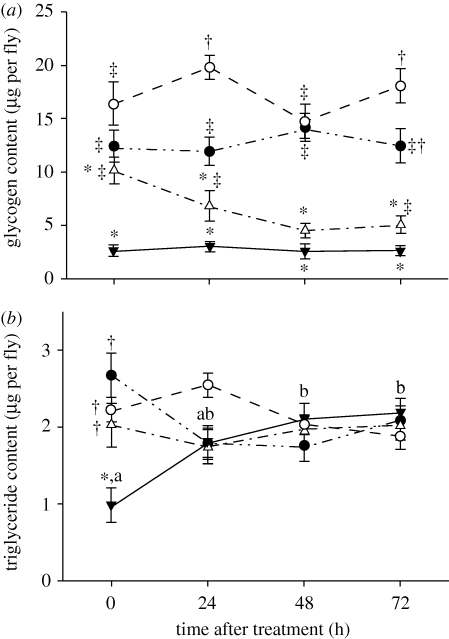
There was no effect of either age cohort or time after treatment on glycogen content in multiply exposed, sustained exposed (10 h) or control flies. In single short (2 h) exposure flies, the youngest age cohort (3 days old at treatment) had significantly less glycogen than 5- or 6-day-old flies (p < 0.001 for each). Multiply exposed flies contained less glycogen than other cold exposure groups but not controls when treatments were compared (F3,252 = 106.654, p < 0.001; figure 4a).
Figure 4.
Effects of cold treatment on energy reserves. (a) Glycogen mass (mean ± s.e.) after cold treatment at −0.5°C as a function of treatment and time after treatment in female adult Drosophila melanogaster. Points with the same superscript symbols are not significantly different between treatments at a given time after treatment at p < 0.01, n = 12–20 samples of five flies per point. (b) Triglyceride mass (mean ± s.e.) after cold treatment at −0.5°C as a function of treatment and time after treatment in female adult Drosophila melanogaster. Points with the same superscript symbols are not significantly different between treatments at a given time after treatment at p < 0.01. Points with the same lower case letters are not significantly different within the multiples group only between time points. n = 13–20 samples of five flies per point (filled circles, 1 × 10 h; open circles, 1 × 2 h; filled inverted triangles, multiple; open triangles, control).
Number of cold treatments did not affect triglyceride content in multiply exposed flies, although there was a significant effect of time after cold exposure in each group where triglyceride content was low immediately after exposure, then climbed following cold exposure (F3,59 = 8.221, p < 0.001). There were significant effects of age, time after treatment, and the interaction between them on triglyceride mass on single short (2 h) cold treatment flies (p < 0.001 in each case). However post hoc comparisons reveal that this was driven by only the significant difference between the 7-day-old and 5-day-old flies at 72 h after exposure (p < 0.001). Similarly, there were significant effects of age, treatment and the interaction between them on triglyceride content in sustained (10 h) exposure flies (p < 0.001 in each case), however the only significant difference between ages was between the 7-day-old and 3-day-old flies immediately after exposure (p < 0.001). Finally, the interaction between age and time after treatment significantly affected control flies (F6,46 = 4.059, p = 0.002), however there were no significant post hoc differences. Therefore all ages were pooled in all treatments. The interaction between treatment and time after treatment had a significant effect on triglyceride mass of flies (F9,271 = 5.8653, p < 0.001). Immediately after cold treatment, multiply exposed flies had significantly lower triglyceride content than any other treatment group (p < 0.001; figure 4b).
4. Discussion
While providing a warming period between cold exposures causes a decrease in mortality in several insect species (Petavy et al. 2001; Colinet et al. 2007; Kostal et al. 2007), our study provides, to our knowledge, the first evidence of a trade-off between immediate survival and future fitness as a result of a multiple stress response. Regardless of the age of the flies, we found that multiply exposed flies had lower mortality than flies exposed to cold for the same amount of time without opportunity for repair. However, we also found that this improvement in survival is costly: flies exposed to cold on multiple occasions had a lower intrinsic rate of population increase than any other cold treatment. Therefore, our increased cost hypothesis was supported: there is a fitness consequence to repeated cold exposure.
Our study is, to our knowledge, the first to quantify a long-term negative fitness impact of repeated cold injury. This response was found only in multiply exposed flies, and not in response to a single cost stress. We therefore believe this response is probably not owing to the rapid cold-hardening (RCH) response (Czajka & Lee 1990) because it is not found in our single 2 h exposure group, which is an equivalent treatment to RCH. While Overgaard et al. (2007) showed a significant reduction in fertility in D. melanogaster for 8 h after an RCH treatment, Kelty & Lee (1999) showed no decrease in egg-laying in a 5-day-period after RCH. Also, RCH only depressed fertility compared with controls, and provided a beneficial effect when compared with an acute cold shock (Overgaard et al. 2007). Similarly, a 1–3 day mild low temperature stress (18°C) caused a decrease in fertility in D. melanogaster during the stress period, but a return to normal temperatures allowed for compensation in egg production, and no overall depression of fitness was detected (Dillon et al. 2007).
According to Trivers & Willard (1973), individuals in poor condition should produce more females because females have a more uniform number of offspring than males. While this hypothesis is well supported in mammals and D. melanogaster appears to follow the assumptions of this hypothesis (Burke & Little 1995), multiply exposed flies in our work had a male-biased offspring sex ratio. Because the cold exposures in our study occurred before mating, this result is not owing to differential retention of sperm. Possibly female offspring are more costly to produce, or are more sensitive during development. There is evidence that D. melanogaster can adaptively bias sex ratios: females mated with older males (9–13 days post-eclosion) are more likely to produce female offspring, which Long & Pischedda (2005) interpreted as possible compensation for the fact that sons of older male flies fair poorly in competitive mating assays. By contrast, we find that after repeated stress, D. melanogaster females bias sex ratio in an apparently maladaptive fashion. There are two possible interpretations for this result: either female offspring are more costly to produce, or the sex ratio change is adaptive. During the experiment, we noted large numbers of offspring of the multiple exposures flies that failed to completely eclose and died in their pupal cases. While we did not sex these flies, it is possible that female offspring of the multiple exposure group were unable to properly complete development. Further work on the performance of the F1 offspring will be necessary to determine the consequences of, and perhaps mechanism, underlying this bias.
One life-history aspect we did not address in this study is the trade-off between offspring size and the number predicted by theory (Stearns 1992). This trade-off assumes that the ‘decision’ to allocate resources to total reproductive output is independent of the decision to allocate resources to individual offspring. However, one of the first mathematical models testing this assumption found a link between the optimal number of offspring and total reproductive output (Winkler & Wallin 1987). This was empirically tested in D. melanogaster selected for egg size by Schwarzkopf et al. (1999) who found that while egg size significantly responded to selection, relative fecundity remained unchanged. This suggests that offspring size and number are related in D. melanogaster and that calculations of intrinsic rate of increase correspond closely to total reproductive allocation in this species.
Triglyceride reserves significantly decline immediately after cold exposure only in multiply exposed flies, and this decline was consistent for all multiple exposure groups (two, three, four and five 2 h exposures). Glycogen stores in multiple exposure flies were also significantly lower than any other cold treatment at every time interval after exposure. Thus, the multiple exposure flies appear to have a different physiological response to injurious cold exposure than either the single short (2 h) or sustained (10 h) exposure flies. Given that there was no effect of number of cold exposures on either triglyceride mass or glycogen mass in flies that experienced between two and five 2 h exposures, this different response appears to be primed by a single 2 h exposure, then activated by a second. Our flies had access to high quality diet following each cold exposure, which may explain the increase in glycogen stores in single short (2 h) and sustained (10 h) exposure flies. While access to the diet was identical for each experimental group, it is possible that cold injury caused a decrease in feeding rates or assimilation efficiency. Investigation of the mechanism of the multiple cold exposure response should include this possibility.
With predicted increases in temperature variability (Easterling et al. 2000), understanding the physiological and ecological effects of fluctuating temperatures becomes more urgent, particularly when attempting to model climate effects (Helmuth 2009). While the impacts of single low temperature exposures on insects are well studied (Bale 2002), only recently have investigators focused on the effects of repeated injurious cold exposure on insect populations (Bale et al. 2001; Petavy et al. 2001; Sinclair & Chown 2005; Colinet et al. 2007; Kostal et al. 2007). Our study points to two important aspects of repeated cold exposures. First, that interpreting the results of FTR studies is far more complex than simply assessing mortality since we have shown that multiple cold exposure induces a trade-off between survival and future reproduction. Second, that multiple cold exposure can produce nonlinear changes in energy reserves that may characterize a new physiological response to fluctuating temperature conditions. These effects can also produce consequences such as changes in offspring sex ratio that can impact fitness. Taken together, these results imply that laboratory studies without fluctuating temperatures may deviate significantly from field situations, reducing the utility of interpreting the results of laboratory studies in a field context (i.e. Kristensen et al. 2008). In addition, we point out that many other stresses such as desiccation, high temperatures and exercise affect organisms on a repeated basis in the field. Investigation of the impacts of repeated exposure to these stressors is vital when interpreting and predicting the physiological responses of organisms in the wild.
Acknowledgements
We are grateful to Tim Hain, Steven Chown and two anonymous referees for comments that improved the manuscript. Diana Balmer, Steven Holterman ten Hove and Amro Mohammad helped with fly rearing and sorting and Dan Marshall and Evan Horn with laboratory analyses. This work was supported by grants to B.J.S. from NSERC and the Canadian Foundation for Innovation.
References
- Bale J. S.2002Insects and low temperatures: from molecular biology to distributions and abundance. Phil. Trans. R. Soc. Lond. B 357, 849–862 (doi:10.1098/rstb.2002.1074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale J. S., Worland M. R., Block W.2001Effects of summer frost exposures on the cold tolerance strategy of a sub-Antarctic beetle. J. Insect Physiol. 47, 1161–1167 (doi:10.1016/S0022-1910(01)00097-X) [DOI] [PubMed] [Google Scholar]
- Birch L. C.1948The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 17, 15–26 (doi:10.2307/1605) [Google Scholar]
- Burke R. L., Little L. E.1995Drosophila do not skew offspring sex ratio as predicted by Trivers and Willard (Diptera: Drosophilidae). J. Insect Behav. 8, 231–239 (doi:10.1007/BF01988907) [Google Scholar]
- Chippindale A. K., Chou T. J. F., Rose M. R.1996Complex trade-offs and the evolution of starvation resistance in Drosophila melanogaster. Evolution 50, 753–766 (doi:10.2307/2410848) [DOI] [PubMed] [Google Scholar]
- Chippindale A. K., Gibbs A. G., Sheik M., Yee K. J., Djawdan M., Bradley T. J., Rose M. R.1998Resource acquisition and the evolution of stress resistance in Drosophila melanogaster. Evolution 52, 1342–1352 (doi:10.2307/2411304) [DOI] [PubMed] [Google Scholar]
- Chown S. L., Terblanche J. S.2007Physiological diversity in insects: ecological and evolutionary contexts. Adv. Insect Physiol. 33, 50–152 (doi:10.1016/S0065-2806(06)33002-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet H., Nguyen T. T. A., Cloutier C., Michaud D., Hance T.2007Proteomic profiling of a parasitic wasp exposed to constant and fluctuating cold exposure. Insect Biochem. Mol. Biol. 37, 1177–1188 (doi:10.1016/j.ibmb.2007.07.004) [DOI] [PubMed] [Google Scholar]
- Czajka M. C., Lee R. E.1990A rapid cold-hardening response protecting against cold shock injury in Drosophila melanogaster. J. Exp. Biol. 148, 245–254 [DOI] [PubMed] [Google Scholar]
- Dillon M. E., Cahn L. R. Y., Huey R. B.2007Life history consequences of temperature transients in Drosophila melanogaster. J. Exp. Biol. 210, 2897–2904 (doi:10.1242/jeb.007591) [DOI] [PubMed] [Google Scholar]
- Djawdan M., Chippindale A. K., Rose M. R., Bradley T. J.1998Metabolic reserves and evolved stress resistance in Drosophila melanogaster. Physiol. Zool. 71, 584–594 [DOI] [PubMed] [Google Scholar]
- Easterling D. R., Meehl G. A., Parmesan C., Changnon S. A., Karl T. R., Mearns L. O.2000Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074 (doi:10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- Gefen E., Marlon A. J., Gibbs A. G.2006Selection for desiccation resistance in adult Drosophila melanogaster affects larval development and metabolite accumulation. J. Exp. Biol. 209, 3293–3300 (doi:10.1242/jeb.02397) [DOI] [PubMed] [Google Scholar]
- Helmuth B.2009From cells to coastlines: how can we use physiology to forecast the impacts of climate change? J. Exp. Biol. 212, 753–760 (doi:10.1242/jeb.023861) [DOI] [PubMed] [Google Scholar]
- Hercus M. J., Loeschcke V., Rattan S. I. S.2003Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology 4, 149–156 (doi:10.1023/A:1024197806855) [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Sorensen J. G., Loeschcke V.2003Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175–216 (doi:10.1016/S0306-4565(02)00057-8) [Google Scholar]
- Kelty J. D., Lee R. E.1999Induction of rapid cold hardening by cooling at ecologically relevant rates in Drosophila melanogaster. J. Insect Physiol. 45, 719–726 (doi:10.1016/S0022-1910(99)00040-2) [DOI] [PubMed] [Google Scholar]
- Kostal V., Renault D., Mehrabianova A., Bastl J.2007Insect cold tolerance and repair of chill-injury at fluctuating thermal regimes: role of ion homeostasis. Comp. Biochem. Physiol. A 147, 231–238 (doi:10.1016/j.cbpa.2006.12.033) [DOI] [PubMed] [Google Scholar]
- Kristensen T. N., Hoffmann A. A., Overgaard J., Sorensen J. G., Hallas R., Loeschcke V.2008Costs and benefits of cold acclimation in field-released Drosophila. Proc. Natl Acad. Sci. USA 105, 216–221 (doi:10.1073/pnas.0708074105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBourg E.2007Hormetic effects of repeated exposures to cold at young age on longevity, aging and resistance to heat or cold shocks in Drosophila melanogaster. Biogerontology 8, 431–444 (doi:10.1007/s10522-007-9086-6) [DOI] [PubMed] [Google Scholar]
- Lin Y. J., Seroude L., Benzer S.1998Extended life-span and stress resistance in the Drosophila mutant methuselah. Science 282, 943–946 (doi:10.1126/science.282.5390.943) [DOI] [PubMed] [Google Scholar]
- Long T. A. F., Pischedda A.2005Do female Drosophila melanogaster adaptively bias offspring sex ratios in relation to the age of their mate? Proc. R. Soc. B 272, 1781–1787 (doi:10.1098/rspb.2005.3165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckinbill L. S., Arking R., Clare M. J., Cirocco W. C., Buck S. A.1984Selection for delayed senescence in Drosophila melanogaster. Evolution 38, 996–1003 (doi:10.2307/2408433) [DOI] [PubMed] [Google Scholar]
- Mangel 2008Environment, damage and senescence: modelling the life-history consequences of variable stress and caloric intake. Funct. Ecol. 22, 422–430 (doi:10.1111/j.1365-2435.2008.01410.x) [Google Scholar]
- Markow T. A., O'Grady P. M.2005Drosophila: a guide to species identification and use New York, NY: Academic Press [Google Scholar]
- Nilson T. L., Sinclair B. J., Roberts S. P.2006The effects of carbon dioxide anesthesia and anoxia on rapid cold-hardening and chill coma recovery in Drosophila melanogaster. J. Insect Physiol. 52, 1027–1033 (doi:10.1016/j.jinsphys.2006.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard J., Malmenda A., Sørensen J. G., Bundy J. G., Loeschcke V., Nielsen N. C., Holmstrup M.2007Metabolomic profiling of rapid cold hardening and cold shock in Drosophila melanogaster. J. Insect Physiol. 53, 1218–1232 (doi:10.1016/j.jinsphys.2007.06.012) [DOI] [PubMed] [Google Scholar]
- Ozgul A., Tuljapurkar S., Benton T. G., Pemberton J. M., Clutton-Brock T. H., Coulson T.2009The dynamics of phenotypic change and the shrinking sheep of St Kilda. Science 325, 464–467 (doi:10.1126/science.1173668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L., Sibly R., Beverton R. J. H., Hill W. G.1991Constraints in the evolution of life histories. Phil. Trans. R. Soc. Lond. B 332, 3–13 (doi:10.1098/rstb.1991.0027) [Google Scholar]
- Partridge L., Prowse N., Pignatelli P.1998Another set of responses and correlated responses to selection on age at reproduction in Drosophila melanogaster. Proc. R. Soc. Lond. B 266, 255–261 (doi:10.1098/rspb.1999.0630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petavy G., David J. R., Gibert P., Moreteau B.2001Viability and rate of development at different temperatures in Drosophila: a comparison of constant and alternating thermal regimes. J. Therm. Biol. 26, 29–39 (doi:10.1016/S0306-4565(00)00022-X) [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2008R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org [Google Scholar]
- Ruel J. J., Ayers M. P.1999Jensen's inequality predicts effects of environmental variation. Trends Ecol. Evol. 14, 361–366 (doi:10.1016/S0169-5347(99)01664-X) [DOI] [PubMed] [Google Scholar]
- Schwarzkopf L., Blows M. W., Caley M. J.1999Life history consequences of divergent selection on egg size in Drosophila melanogaster. Am. Nat. 154, 333–340 (doi:10.1086/303242) [DOI] [PubMed] [Google Scholar]
- Silbermann R., Tatar M.2000Reproductive costs of heat shock protein in transgenic Drosophila melanogaster. Evolution 54, 2038–2045 [DOI] [PubMed] [Google Scholar]
- Sinclair B. J.2001Field ecology of freeze tolerance: interannual variation in cooling rates, freeze-thaw and thermal stress in the microhabitat of the alpine cockroach Celatoblatta quinquemaculata. Oikos 93, 286–293 (doi:10.1034/j.1600-0706.2001.930211.x) [Google Scholar]
- Sinclair B. J., Chown S. L.2005Deleterious effects of repeated cold exposure in a freeze-tolerant sub-Antarctic caterpillar. J. Exp. Biol. 208, 869–879 (doi:10.1242/jeb.01455) [DOI] [PubMed] [Google Scholar]
- Sinclair B. J., Vernon P., Klok C. J., Chown S. L.2003Insects at low temperatures: an ecological perspective. Trends Ecol. Evol. 18, 257–262 (doi:10.1016/S0169-5347(03)00014-4) [Google Scholar]
- Stearns S. C.1992The evolution of life histories Oxford, UK: Oxford University Press [Google Scholar]
- Storey K. B., Storey J. M.1988Freeze tolerance in animals. Physiol. Rev. 68, 27–84 [DOI] [PubMed] [Google Scholar]
- Trivers R. L., Willard D. E.1973Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92 (doi:10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- Winkler D. W., Wallin K.1987Offspring size and number: a life history model linking effort per offspring and total effort. Am. Nat. 129, 708–720 (doi:10.1086/284667) [Google Scholar]
- Zera A. J., Harshman L. G.2001The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126 (doi:10.1146/annurev.ecolsys.32.081501.114006) [Google Scholar]