Abstract
Studies made with both entomological and meteorological radars over the last 40 years have frequently reported the occurrence of insect layers, and that the individuals forming these layers often show a considerable degree of uniformity in their headings—behaviour known as ‘common orientation’. The environmental cues used by nocturnal migrants to select and maintain common headings, while flying in low illumination levels at great heights above the ground, and the adaptive benefits of this behaviour have long remained a mystery. Here we show how a wind-mediated mechanism accounts for the common orientation patterns of ‘medium-sized’ nocturnal insects. Our theory posits a mechanism by which migrants are able to align themselves with the direction of the flow using a turbulence cue, thus adding their air speed to the wind speed and significantly increasing their migration distance. Our mechanism also predicts that insects flying in the Northern Hemisphere will typically be offset to the right of the mean wind line when the atmosphere is stably stratified, with the Ekman spiral in full effect. We report on the first evidence for such offsets, and show that they have significant implications for the accurate prediction of the flight trajectories of migrating nocturnal insects.
Keywords: orientation behaviour, windborne insect migration, nocturnal boundary layer, atmospheric dispersion models
1. Introduction
Early authors, if they considered the matter at all, thought that insects flying at high altitude at night would orient at random owing to the presumed lack of environmental cues ‘in what is almost a sensory vacuum’ (Berry & Taylor 1968; see also Taylor 1974; Kennedy 1975). However, when X-band scanning radars were first deployed for entomological purposes, it became clear that large (greater than 40 mg) nocturnally migrating insects (such as acridoid grasshoppers or noctuid moths) often showed a degree of common alignment (Riley 1975; Schaefer 1976), sometimes with quite small angular dispersions (approx. 15°) around the mean. Aerial densities were often far too sparse for the insects to have maintained the alignments by visual reference to one another (Riley 1989), and so the orientation patterns must have been due to individual responses to some environmental cue or cues. Recent observations using Doppler meteorological radars (e.g. Lang et al. 2004; Rennie et al. 2008) have revealed echo patterns symptomatic of common alignment over huge areas, demonstrating that the degree of uniformity in heading is not due to insects following localized linear landmarks, such as roads or rivers.
Entomological scanning radars showed that the observed orientation directions were often close to the downwind direction (Schaefer 1976; Riley & Reynolds 1986) and this would seemingly be adaptive in that large insects could add their self-propelled flight speed (air speed) to the wind speed, thus maximizing their displacement distance in a given time.
Many populations of high-flying nocturnal insect migrants are plainly influenced by wind-related factors when taking up their flight headings. For example, in some radar studies, the mean heading closely followed the downwind direction despite veering of the wind with altitude (Schaefer 1976; Riley & Reynolds 1983). In other cases, insects flying at the same altitude maintained their (relatively large) off-wind orientation angle after a substantial shift in the wind direction (Drake 1983; Riley & Reynolds 1986). Drake (1983), for example, reports a case where orientation was maintained at about 40° to the downwind direction before and after a sudden change in wind direction by approximately 90°. There is also evidence that insects take account of wind velocity by flying preferentially (and thus forming horizontal layers) at altitudes with fast-moving and stable wind streams (see references in Reynolds et al. 2009).
The question thus arises as to how this wind-related orientation is maintained at altitudes of several hundreds of metres often under conditions of severely reduced illumination, and this problem has proved difficult to resolve. The obvious means for a flying insect to determine the wind direction is by the visual perception of the apparent movement of the ground, in a similar way to the ‘optomotor response’ that occurs in flight near the ground (Kennedy 1951; Franceschini et al. 2007; Webb 2007). However, evidence has steadily accumulated which suggests that, in some cases at least, a visually mediated optomotor-type mechanism is unlikely. This evidence includes the following points.
Authors are agreed that the presence and degree of the common orientation do not seem to depend on the intensity of the illumination—it occurs at all illuminance levels between overcast and clear moonlit nights; tight orientation also occurs in situations where there is very little in the way of significant light sources on the ground (e.g. in remote places in Mali, West Africa; Riley & Reynolds 1986; Riley 1989).
Migrants are able to orient in conditions (i.e. flying hundreds of metres above the ground at night, as mentioned above) that would entail the detection of very small angular movements under illumination levels that often seem to be inadequate (Riley et al. 1988), and with very stringent stability constraints on the ‘viewing platform’ (i.e. the insect's head) (Kennedy 1975; Riley & Reynolds 1986).
There appears to be no general indication that angular dispersion increases with altitude as might be expected with an optomotor-based mechanism; in fact, the opposite occurs (Riley 1989 and see results below).
It is not straightforward to account for the frequent offsetting of the orientation direction with respect to the mean wind direction (Riley & Reynolds 1986; Riley 1989). If the off-wind orientations were achieved by maintaining the speed of apparent ground movement transverse to the body axis at some preferred value, one would expect the angle to the wind to increase with height; and if the insects orientated so as to produce a symmetric distribution of transverse angular rates, this would result in a skewed heading distribution (provided that headings are more than 45° from the downwind direction) (Riley & Reynolds 1986). Neither of these features is observed, although in the latter case the skewness may often be negligibly small because observed headings are within 45° to downwind. An alternative hypothesis—that off-wind headings are maintained by the ratio of lateral to backward angular rates—would account for the independence of off-wind angle with altitude, but not the general symmetry of observed off-wind heading distributions.
The height above the surface at which an insect is no longer constrained by the optomotor effect of ground patterns (either because the pattern is not resolvable or the image motion is too slow to produce a compensatory response) is termed the ‘maximum compensatory height’ (Kennedy 1951). The maximum compensatory height, above which an insect is ‘unembarrassed by optomotor responses’ (Johnson 1965) and free to ride fast-moving airstreams, seems to occur at quite low altitudes (a few metres or tens of metres) in day-flying locusts (Kennedy 1951). It thus seems unlikely that nocturnal insects would be able to use this mechanism on dark nights at heights up to and beyond a kilometre above the ground, where common orientation has been habitually observed.
Rather than being due to relative movement of ground features, there are increasing indications that high-altitude orientation may be maintained by some intrinsic feature of the wind itself—perhaps some form of anisotropic turbulence that provides cues for the selection of both orientation direction and flight altitude (Reynolds et al. 2009). In this connection, it is worth noting that increases in the degree of orientation seem to be associated with increases in the extent to which migrants are layered (Drake 1983; Hobbs & Wolf 1989; Drake & Rochester 1994), indicating that the same cues might facilitate both phenomena. In a recent paper, it was suggested that certain insect layers could form if individuals actively climbed or descended in the direction of gusts in the vertical component of wind velocity (Reynolds et al. 2009). This active response would lead to migrants preferentially concentrating into regions where the turbulent kinetic energy has local minima, thereby minimizing the energetic costs of flight. The formation of these layers closely mimics field observations (Drake & Farrow 1988), both in terms of the degree of concentration of the layers and the rate at which they form. In the present paper, we show that such responses also provide a mechanism for orientation, and account for the consistent bias for flight headings to be offset to the right of the mean downwind direction. This phenomenon is revealed through the analysis of a substantial dataset on the migratory flights of ‘medium-sized’ (10–70 mg) nocturnal insects observed by two vertical-looking radars throughout the summer in the UK. These migrants did not show compensatory orientation in preferred seasonal directions, and thus are very different from large nocturnal insects (i.e. noctuid moths, such as Autographa gamma; approx. 100–200 mg), which do show these specialized orientation behaviours (Chapman et al. 2008a,b and J. W. Chapman 2009, unpublished data).
2. A wind-mediated mechanism for layering and orientation
It is instructive to consider first the case of neutrally buoyant particles that are passively advected by a turbulent airstream. Provided that the particle density is much greater than the air density, many terms in the general particle momentum equation can be neglected. These neglected terms include the pressure gradient force, the virtual mass and the Basset history integral (Maxey & Riley 1983). The particle momentum equation then reduces to:
| 2.1 |
where vi is the velocity of the particle, ui is the velocity of the airstream at the particle location and τ is the aerodynamic response time of the particle, which determines how rapidly the particle responds to changes in the air velocity. F is a body force that when present propels the particle and m is the mass of the particle. For small spherical particles, the aerodynamic response time can be approximated by 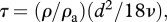 where ρ is the particle density, ρa is the air density, d is the particle diameter and ν is the kinematic viscosity. The subscripts denote Cartesian components (i.e. u1, u2, u3, respectively, denote the streamwise, crosswind and vertical component of the air velocity).
where ρ is the particle density, ρa is the air density, d is the particle diameter and ν is the kinematic viscosity. The subscripts denote Cartesian components (i.e. u1, u2, u3, respectively, denote the streamwise, crosswind and vertical component of the air velocity).
According to equation (2.1), the particle velocity is:
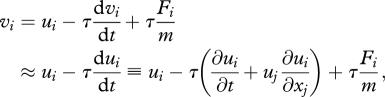 |
2.2 |
where there is summation over repeated indices and where the particle acceleration, 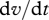 , has been approximated by the acceleration of the surrounding air,
, has been approximated by the acceleration of the surrounding air, 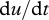 ; an approximation that produces accurate predictions for the velocities of particles where τ is much less than the time scale over which fluctuations in wind speed are correlated (Rani & Balachandar 2003). Results from this approximate but illustrative approach are found to be consistent with data from state-of-the-art simulations, described later, in which the approximation
; an approximation that produces accurate predictions for the velocities of particles where τ is much less than the time scale over which fluctuations in wind speed are correlated (Rani & Balachandar 2003). Results from this approximate but illustrative approach are found to be consistent with data from state-of-the-art simulations, described later, in which the approximation 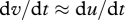 is not made. As a consequence of equation (2.2), the average velocity of a particle in a statistically stationary
is not made. As a consequence of equation (2.2), the average velocity of a particle in a statistically stationary 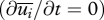 incompressible air flow
incompressible air flow 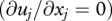 is given by:
is given by:
| 2.3 |
where the overbar denotes an average over particles and air flows through an elemental volume at a given spatial location, and  are the Reynolds stresses owing to turbulence. It is apparent from equation (2.3) that the body forces give rise to an air speed
are the Reynolds stresses owing to turbulence. It is apparent from equation (2.3) that the body forces give rise to an air speed 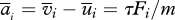 when gradients in the Reynolds stresses,
when gradients in the Reynolds stresses, 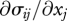 , vanish. It is also apparent from equation (2.3) that even in the absence of a driving force the mean particle velocity,
, vanish. It is also apparent from equation (2.3) that even in the absence of a driving force the mean particle velocity, 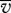 , will in general be different from the mean velocity of the surrounding airstream,
, will in general be different from the mean velocity of the surrounding airstream, 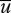 . Consider, for example, the case when the air velocity co-variances vary with height, z, but not with the streamwise and crosswind positions; a scenario corresponding to a homogeneous atmospheric boundary layer. In this case, the mean air velocity
. Consider, for example, the case when the air velocity co-variances vary with height, z, but not with the streamwise and crosswind positions; a scenario corresponding to a homogeneous atmospheric boundary layer. In this case, the mean air velocity 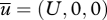 while the mean particle velocity is:
while the mean particle velocity is:
when F = 0. Particles, unlike air parcels, will therefore tend to drift upwards at a rate 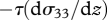 to become preferentially concentrated in regions of the air flow where the turbulent kinetic energy is low; typically corresponding to the regions of fast-moving air. The degree of layering predicted by this model is, however, very much weaker than that observed in the field (Reynolds et al. 2009). Nevertheless, models closely related to equation (2.1) in which organisms actively respond to turbulent gusts by amplifying their effects, do predict layer formation that closely resembles the field observations (Reynolds et al. 2009). A previously overlooked consequence of these mechanisms is the fact that the average streamwise velocity of the particle
to become preferentially concentrated in regions of the air flow where the turbulent kinetic energy is low; typically corresponding to the regions of fast-moving air. The degree of layering predicted by this model is, however, very much weaker than that observed in the field (Reynolds et al. 2009). Nevertheless, models closely related to equation (2.1) in which organisms actively respond to turbulent gusts by amplifying their effects, do predict layer formation that closely resembles the field observations (Reynolds et al. 2009). A previously overlooked consequence of these mechanisms is the fact that the average streamwise velocity of the particle 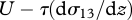 is less than the average streamwise velocity of the air when, as is usually the case,
is less than the average streamwise velocity of the air when, as is usually the case, 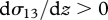 (figure 1). In other words, air is on average flowing past the particle, and this provides a cue for the mean wind direction. We note that this cue is only present in sheared turbulent flows—particles moving within laminar airstreams and within homogeneous turbulence move with an average speed equal to the average speed of the surrounding airstream. Passive particles with an aerodynamic response time
(figure 1). In other words, air is on average flowing past the particle, and this provides a cue for the mean wind direction. We note that this cue is only present in sheared turbulent flows—particles moving within laminar airstreams and within homogeneous turbulence move with an average speed equal to the average speed of the surrounding airstream. Passive particles with an aerodynamic response time 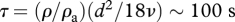 (equating to a 100 mg moth of length 0.01 m) will on average be travelling about 0.05–0.1 m s−1 slower than the average velocity of the surrounding airstream in a nocturnal boundary layer (figure 1e,f). Thus, an insect that is moving faster than
(equating to a 100 mg moth of length 0.01 m) will on average be travelling about 0.05–0.1 m s−1 slower than the average velocity of the surrounding airstream in a nocturnal boundary layer (figure 1e,f). Thus, an insect that is moving faster than 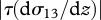 , as would normally be the case, would experience an average air flow over its body directly from front to back only when moving precisely in a streamwise direction. Insects could therefore use this average air flow to determine the mean wind direction. This affect could be enhanced if the effective value of τ were larger than the aerodynamic τ, as would happen if insects try for a while (i.e. for time τ) to deliberately resist any wind changes, as may be the case (Reynolds et al. 2009). Air flows do, however, decrease with decreasing insect size (equation (2.3)) and for sufficiently small insects will be undetectable, so precluding wind-mediated orientation in these species.
, as would normally be the case, would experience an average air flow over its body directly from front to back only when moving precisely in a streamwise direction. Insects could therefore use this average air flow to determine the mean wind direction. This affect could be enhanced if the effective value of τ were larger than the aerodynamic τ, as would happen if insects try for a while (i.e. for time τ) to deliberately resist any wind changes, as may be the case (Reynolds et al. 2009). Air flows do, however, decrease with decreasing insect size (equation (2.3)) and for sufficiently small insects will be undetectable, so precluding wind-mediated orientation in these species.
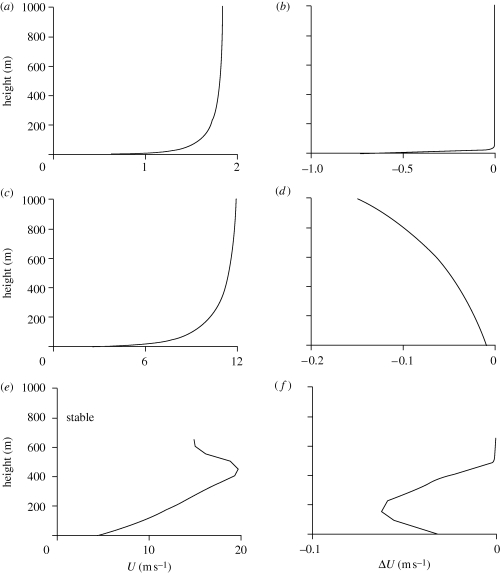
Figure 1.
Mean airstream velocity profiles, U, and mean air speeds, 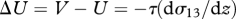 (equation (2.3)), of passively advected particles with aerodynamic response time
(equation (2.3)), of passively advected particles with aerodynamic response time 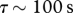 . The mean air speed of a particle is the difference between the mean speed of the particle and the mean speed of the surrounding air. Profiles are shown for: (a,b) a weakly sheared convective boundary layer
. The mean air speed of a particle is the difference between the mean speed of the particle and the mean speed of the surrounding air. Profiles are shown for: (a,b) a weakly sheared convective boundary layer 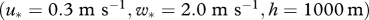 ; (c,d) a more strongly sheared convective boundary layer
; (c,d) a more strongly sheared convective boundary layer 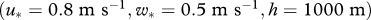 ; (e,f) a stably stratified boundary layer
; (e,f) a stably stratified boundary layer 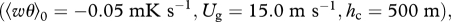 where
where 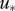 is the friction velocity and
is the friction velocity and 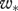 is the convective velocity scale, h is the height of the boundary layer,
is the convective velocity scale, h is the height of the boundary layer, 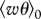 is the temperature flux at the ground, Ug is the speed of the geostrophic wind and hc is the height of the inversion top. Mean velocity profiles and estimates of ΔU are based on the flow parameterizations of Rotach et al. (1996) and Saiki et al. (2000). (a,b) Around sunset following a warm summer day, ΔU ∼0 m s−1. (c–f) For more stable atmosphere conditions that are expected after sunset, predicted ‘signal-to-noise’ ratios
is the temperature flux at the ground, Ug is the speed of the geostrophic wind and hc is the height of the inversion top. Mean velocity profiles and estimates of ΔU are based on the flow parameterizations of Rotach et al. (1996) and Saiki et al. (2000). (a,b) Around sunset following a warm summer day, ΔU ∼0 m s−1. (c–f) For more stable atmosphere conditions that are expected after sunset, predicted ‘signal-to-noise’ ratios 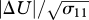 tend to increase with increasing height, peaking at around 0.1 (σ11 is the airstream velocity variance). Consequently, angular dispersion is predicted to decrease with increasing height.
tend to increase with increasing height, peaking at around 0.1 (σ11 is the airstream velocity variance). Consequently, angular dispersion is predicted to decrease with increasing height.
The presence of an Ekman spiral complicates matters slightly. The Ekman spiral is the rotation of the mean wind direction with increasing height owing to the Coriolis effect. Surface winds in the Northern Hemisphere tend to blow to the left of winds aloft while in the Southern Hemisphere they tend to blow to the right of winds aloft. The effect is most apparent in stably stratified boundary layers where turbulent mixing is low. When the mean air velocity 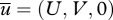 turns with height, so will the mean particle velocity:
turns with height, so will the mean particle velocity:
Continuous stably stratified boundary layers are typically characterized by the presence of a low-level jet with mean speed 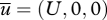 and, for the most part, by turbulent profiles with
and, for the most part, by turbulent profiles with 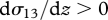 and for the Northern Hemisphere by
and for the Northern Hemisphere by 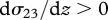 (for the Southern Hemisphere
(for the Southern Hemisphere 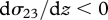 ) (e.g. Saiki et al. 2000). Particles will preferentially concentrate into the jet where the turbulence is a local minimum. Particles within and just below the jet will not be moving in the direction of the jet but instead their displacements will be offset to the right of the mean wind line;
) (e.g. Saiki et al. 2000). Particles will preferentially concentrate into the jet where the turbulence is a local minimum. Particles within and just below the jet will not be moving in the direction of the jet but instead their displacements will be offset to the right of the mean wind line;
Thus, if insects orient at any angle other than
they will experience a net sideways flow to which they could be expected to respond by turning until it disappears. Insects would then be flying to the right of the mean wind line (in the Northern Hemisphere). Left offsets would arise if the mean wind direction turned anticlockwise rather than clockwise with increasing height. This would be a common occurrence in the Southern Hemisphere, but rare in the Northern Hemisphere being associated with baroclinicity (variation of geostrophic wind with height), developing or evolving flows and advective effects (horizontal inhomogeneity).
Predictions from the foregoing analytic analysis are supported by the results of simulation data from Lagrangian stochastic models of particle trajectories within the atmospheric boundary layer with stabilities ranging from purely convective to stably stratified (data not shown). A description of this modelling approach, which is currently the best way to model atmospheric dispersal, can be found in Reynolds et al. (2009). These models take explicit account of the instantaneous turbulent structures, ‘gusts’, and the observed differences between up and down gusts as encapsulated by velocity skewness statistics and turbulent dissipation rates.
3. Orientations to the right of the mean wind direction
To look for evidence of wind-mediated common orientation in nocturnal insect migrants, we used data from two specially developed vertical-looking entomological radars (VLRs) located in the southern UK that provide information on the flight characteristics of individual, high-altitude, insect migrants (Chapman et al. 2003, 2004a; Reynolds et al. 2005). Overflying insects can be simultaneously detected within 15 different height bands (‘range gates’ of 45 m depth), between approximately 150 and 1200 m above the VLRs, and the displacement direction and body alignment of each individual is routinely recorded. This enabled us to examine the orientation behaviour of medium-sized (10–70 mg) nocturnal insects migrating high above the ground, in relation to the wind currents in which they were flying (Chapman et al. 2008a,b). Nocturnal migrants in this size class are likely to be predominantly composed of larger ‘micro’-Lepidoptera (e.g. including migrant pyralids such as Nomophila noctuella and Udea ferrugalis), some small noctuid moths, green lacewings (Neuroptera) and certain families of Diptera and Coleoptera (Chapman et al. 2002, 2004b, 2006; Reynolds et al. 2005, 2008; Wood et al. 2006, 2009). Small insects (less than 10 mg) are only detectable within the lowest sampling ranges of the VLRs, and thus were excluded from our dataset.
We selected 58 occasions during the summer months of 2000–2006 when sufficient individual insects were recorded during a 1 h period sometime after dusk within a small number of range gates (typically 4 or 5, spanning approx. 300 m of the vertical sampling region of the VLRs) to analyse the collective orientation patterns of the migrants. This dataset may include some occasions when the wind was turning anticlockwise with height (rather than clockwise as is typical for the Northern Hemisphere). We calculated the mean displacement direction, and the mean heading direction, of the migrants flying within the 4 or 5 range gates that contained the greatest density of insects during the 1 h period on each occasion (ranging from 16 to 242 individuals on each night, with an overall total of 3530 insects), using the procedure previously described (Chapman et al. 2008a). Two further parameters were calculated for each occasion: the mean resultant length ‘R’ (a measure of angular dispersion, ranging from 0 to 1), and the probability that the distribution of directional data differed from uniform (using the Rayleigh test of uniformity for circular data; Fisher 1993). Occasions where the Rayleigh test of the distribution of headings produced a p-value greater than 0.05 (indicating a uniform distribution) were excluded from further analysis. If the p-value for the distribution of flight headings was less than 0.05, this was taken to be indicative of significant unimodal mutual alignment (i.e. common orientation along a preferred direction). The displacement direction of these small insects is assumed to be very similar to the downwind direction (as wind speeds at migration altitudes are typically between five and 10 times faster than the air speeds of these insects), and so has been used as a proxy for downwind direction at flight height in our analyses. The final stage was to calculate the degree of offset of the mean heading direction from the mean displacement direction (downwind direction), and the direction of this offset (i.e. to the left or right), for each of the occasions with significant common orientation. Left offsets were arbitrarily assigned negative values, while right offsets were assigned positive values. We then analysed the distribution of offsets to see whether there was a significant bias towards one side of the downwind direction or not, by calculating the mean offset and 95 per cent confidence intervals (CI) and comparing these with an expected mean offset of zero.
On 48 of the 58 occasions analysed, the distribution of flight headings exhibited significant collective orientation about a common direction (Rayleigh test, p < 0.05 on all 48 occasions). There was no obvious overall pattern to the mean flight headings (figure 2a), as they were randomly distributed and occurred in most possible compass directions (Rayleigh test, no overall mean direction, n = 48, R = 0.18, p = 0.196). The mean displacement directions on each of the 48 occasions were also randomly distributed (Rayleigh test, no overall mean direction, n = 48, R = 0.17, p = 0.231), indicating that winds blew in all possible compass directions during the occasions analysed (figure 2b). Mean flight headings were typically close to the downwind direction on each occasion, but offset by a reasonable amount (mean offset ± 1 s.e.m. = 33 ± 3°, range 1–78°, n = 48). These results indicate that there were no clear seasonal patterns of bi-directional migrations, as seen in large noctuid moth migrants (Chapman et al. 2008a,b), and that insect orientation was related to the downwind direction, but was not usually closely aligned with it. Considering the direction of the offsets relative to the downwind direction, only nine of the offsets were to the left of the mean flow (assigned negative values), while 39 of the offsets were to the right (positive values). This distribution of offsets was significantly different from zero (mean offset = +22°, 95% CI = 9°, p < 0.00001, figure 3), indicating that offsets were much more likely to occur on the right of the mean flow than on the left.
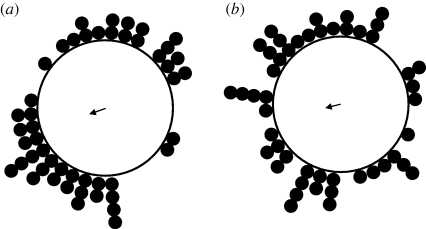
Figure 2.
Circular distributions of directional data from high-altitude migrations (300–900 m above the ground) of medium-sized (10–70 mg) nocturnal insects. The mean direction from each of the 48 migration events with common orientation is plotted (small filled circles at periphery). The bearing of the arrow indicates the overall mean of the mean directions, and the length of the arrow is proportional to the angular dispersion of the dataset around the overall mean direction. (a) The mean flight headings (body orientations) of the 48 migration events (no significant overall mean direction, R = 0.18, p = 0.196). (b) The mean displacement directions (migratory tracks) of the 48 migration events (no significant overall mean direction, R = 0.17, p = 0.231). Neither dataset shows consistent migratory headings or tracks in any particular direction; thus, in these insects, the common orientation phenomenon occurs on winds from all compass directions.
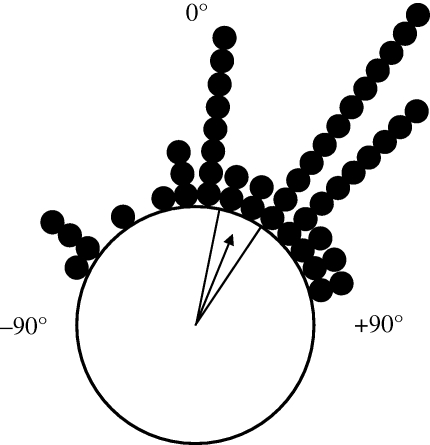
Figure 3.
Mean offset angles (difference between heading direction and displacement direction) for the 48 migration events. An offset of 0° (small filled circles at periphery) indicates that the mean heading direction of the nocturnal insects was exactly aligned with the mean displacement direction (and therefore the downwind direction) on that particular occasion. Offsets to the left of the downwind direction were assigned negative values, while offsets to the right were assigned positive values. The bearing of the arrow shows the mean direction (+22°, i.e. 22° to the right of the mean wind flow), and the solid lines show the upper and lower 95% CI. This distribution is significantly different from zero (p < 0.00001), indicating that offsets to the right of the downwind direction were much more likely to occur than simply by chance, thus fitting the outcome of our theory for wind-mediated orientation in medium-sized nocturnal insects.
These results provide support for our theory that the mechanism underlying the take-up of common flight headings by nocturnal insects along the downwind direction is a wind-mediated mechanism, and that the preponderance of right offsets was a result of the physical action of the Ekman spiral on the flying insects. An additional analysis of variation in orientation patterns with flight altitude also provides further evidence that the insects use wind-related cues to take up their headings, rather than the visual assessment of movement direction relative to the ground. If the insects were able to visually assess the downwind direction, then we would expect their ability to do this to decrease with height above the ground. However, we found the opposite to be the case: angular dispersion of the distribution of headings significantly decreased with altitude (linear regression of R-values against flight height, n = 48, F1,46 = 10.7, r2 = 0.17, p = 0.002; figure 4), indicating that insects were better at orientating with respect to the wind direction the higher they were above the ground, as predicted by our model (see figure 1 and caption). The right offsets exhibited by these medium-sized nocturnal insects will have a substantial effect on their migration trajectories: for example, a migrating insect with an air speed of 3 m s−1 and flying with a mean offset of 20° from an airstream moving at 20 m s−1, in comparison with an insect flying precisely downwind, will have a lateral displacement of 27 km after a single hour's flight (of 74 km distance).
Figure 4.
Regression of R (a measure of angular dispersion around the mean flight heading) against insect flight altitude for the 48 migration events. Higher values of R denote occasions with lower angular dispersion (i.e. ‘tighter’ orientation around the mean). There is a highly significant positive relationship between R and flight altitude (r2 = 0.17; p = 0.002), indicating that nocturnal insects orient their flight headings with respect to the downwind direction better at higher altitudes. This provides strong evidence that visual assessment is not the mechanism used for alignment of headings with the downwind direction.
4. Discussion
The evidence quoted in §1, and our results on the variation of angular dispersion of heading directions with altitude, casts doubt on the proposition that high-altitude nocturnal insect migrants are using visual mechanisms to detect the wind direction. Accordingly, we have presented a new fluid-dynamic theory that accounts for the frequent occurrence of layer concentrations and the considerable degree of common orientation, in a predominantly downwind direction, in these migrants. The theory thus explains two facets of insect migration that have puzzled researchers for more than 40 years in one simple but comprehensive package. Additionally, the theory predicts that insect flights in the Northern Hemisphere should be orientated to the right of the downwind direction. The offsets and the degree of orientation are predicted to increase with increasing stability to become most apparent when the atmosphere is stably stratified (figure 1e,f). We report the first evidence for this effect in data for medium-sized nocturnal insect migrants from two vertical-looking insect-monitoring radars. The theory fills the gap between, on the one hand, some large (greater than 100 mg) high-flying noctuid moths that employ a range of orientation strategies (some of which involve quite complex compass-mediated behaviour) to facilitate long-range movements (Chapman et al. 2008a,b; J. W. Chapman 2009, unpublished data) and, on the other hand, small migrant insects (less than 10 mg). Some of the latter, such as the brown planthopper (Nilaparvata lugens), orient more or less at random (Riley et al. 1991) while other (crepuscular) species appear to make use of sunset (polarized light) patterns (J. W. Chapman 2009, unpublished data).
Our work reveals clearly that there really are asymmetries in the small-scale, accelerative motion of wind flow, and that these motions could, in principle at least, provide directional cues that insects could detect and orient to (as has long been suspected in general terms; Williams et al. 1942; Nisbet 1955). Similar cues could lead to layering (Reynolds et al. 2009). In this case, it is not the (vertical) turbophoretic advective flow itself that produces insect layering, but the active climb/descend response of the insects to the accelerative cues (Reynolds et al. 2009). The weak horizontal advective flow may be similarly enhanced.
Horizontal components of the fluctuating flow (equations (2.1)–(2.3)) tend to be aligned with the mean wind direction, or offset to one side if the Ekman spiral is in full effect. We believe that the orientation patterns analysed here are the result of insects trying to head downwind so as to maximize their displacement distance in a given time, but that they are frequently being ‘misled’ by the action of the Ekman spiral. These angular offsets seem to have no biological significance and are quite distinct from the above-mentioned compensatory flights of large moths, where flying to one or other side of the wind line results in displacements towards a seasonally favourable goal. It would be interesting to test whether medium-sized nocturnal insects in the Southern Hemisphere have a tendency to fly to the left of the mean wind direction, as predicted.
Our findings have clear implications for the accurate prediction of the flight trajectories of migrating nocturnal insects, because over long distances even relatively small but consistent offsets will have quite significant effects. The mechanism(s) by which airborne insects detect turbulent air flows remain to be elucidated. The mechanism could involve, for example, differential measurements of air pressure on either side of the body, linear or angular acceleration, or proprioceptors in the wings (that feed directly into neurons controlling wing beat and angle of attack). If so, then it may not be necessary to compensate or set the ranges of these signals against ‘noise’ due to wing beats. Antennae could also be the mechano-receptors responsible for detecting the weak air flows (through their action on Johnston's organ) (Sane et al. 2007; Kamikouchi et al. 2009; Yorozu et al. 2009). Together, the antennae and Johnston's organ are involved in very sensitive responses to cues that result in changes in flight behaviour. Wind-sensitive setae like those on the face of the desert locust (Camhi 1969) may well be involved. Mechano-receptors on the antennae or on the anterio-dorsal head capsule are, of course, positioned in front of the vortices produced by the flapping wings, which will tend to dampen small lateral differences in the airstream about the insect. Finally, we note that some insects possess sensors that are capable of detecting extremely faint air movements—of the order of µm s−1 (Magal et al. 2006).
Acknowledgements
Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council. We acknowledge the assistance provided by Darcy Ladd at the Science & Technology Facilities Council (STFC) Chilbolton Observatory. A.M.R. thanks David Thomson for constructive communications regarding stably stratified boundary layers. We thank Alistair Drake and Joe Riley for comprehensive comments on an earlier draft, Suzanne Clark for help with circular statistical procedures and Ian Russell and an anonymous referee for suggesting mechanisms by which airborne insects can detect small air flows.
References
- Berry R. E., Taylor L. R.1968High-altitude migration of aphids in maritime and continental climates. J. Anim. Ecol. 37, 713–722 [Google Scholar]
- Camhi J. M.1969Locusts wind receptors. I. Transducer mechanics and sensory response. J. Exp. Biol 50, 335–348 [DOI] [PubMed] [Google Scholar]
- Chapman J. W., Reynolds D. R., Smith A. D., Riley J. R., Pedgley D. E., Woiwod I. P.2002High-altitude migration of the diamondback moth, Plutella xylostella, to the UK: a study using radar, aerial netting and ground trapping. Ecol. Entomol. 27, 641–650 (doi:10.1046/j.1365-2311.2002.00472.x) [Google Scholar]
- Chapman J. W., Reynolds D. R., Smith A. D.2003High-altitude insect migration monitored with vertical-looking radar. BioScience 53, 503–511 (doi:10.1641/0006-3568(2003)053[0503:VRANTF]2.0.CO;2) [Google Scholar]
- Chapman J. W., Reynolds D. R., Smith A. D.2004aMigratory and foraging movements in beneficial insects: a review of radar monitoring and tracking methods. Int. J. Pest Manag. 50, 225–232 (doi:10.1080/09670870410001731961) [Google Scholar]
- Chapman J. W., Reynolds D. R., Smith A. D., Smith E. T., Woiwod I. P.2004bAn aerial netting study of insects migrating at high-altitude over England. Bull. Entomol. Res. 94, 123–136 [DOI] [PubMed] [Google Scholar]
- Chapman J. W., Reynolds D. R., Brooks S. J., Smith A. D., Woiwod I. P.2006Seasonal variation in the migration strategies of the green lacewing Chrysoperla carnea species complex. Ecol. Entomol. 31, 378–388 (doi:10.1111/j.1365-2311.2006.00797.x) [Google Scholar]
- Chapman J. W., Reynolds D. R., Mouritsen H., Hill J. K., Riley J. R., Sivell D., Smith A. D., Woiwod I. P.2008aWind selection and drift compensation optimise migratory pathways in a high-flying moth. Curr. Biol. 18, 514–518 (doi:10.1016/j.cub.2008.02.080) [DOI] [PubMed] [Google Scholar]
- Chapman J. W., Reynolds D. R., Hill J. K., Sivell D., Smith A. D., Woiwod I. P.2008bA seasonal switch in compass orientation in a high-flying migrant moth. Curr. Biol. 18, R908–R909 (doi:10.1016/j.cub.2008.08.014) [DOI] [PubMed] [Google Scholar]
- Drake V. A.1983Collective orientation by nocturnally migrating Australian plague locusts, Chortoicetes terminifera (Walker) (Orthoptera Acrididae): a radar study. Bull. Entomol. Res. 73, 679–692 (doi:10.1017/S0007485300009287) [Google Scholar]
- Drake V. A., Farrow R. A.1988The influence of atmospheric structure and motions on insect migration. Ann. Rev. Entomol. 33, 183–210 (doi:10.1146/annurev.en.33.010188.001151) [Google Scholar]
- Drake V. A., Rochester W. A.1994. The formation of layer concentrations by migrating insects. In Proc. of 21st Conf. on Agricultural and Forest Meteorology—11th Conf. on Biometeorology, San Diego, CA, 7–11 March 1994, pp. 411–414 Boston, MA: American Meteorological Society [Google Scholar]
- Fisher N. I.1993Statistical analysis of circular data Cambridge, UK: Cambridge University Press [Google Scholar]
- Franceschini N., Ruffier F., Serres J.2007A bio-inspired flying robot sheds light on insect piloting abilities. Curr. Biol. 17, 329–335 (doi:10.1016/j.cub.2006.12.032) [DOI] [PubMed] [Google Scholar]
- Hobbs S. E., Wolf W. W.1989An airborne radar technique for studying insect migration. Bull. Entomol. Res. 79, 693–704 (doi:10.1017/S000748530001885X) [Google Scholar]
- Johnson C. G.1965Migration. In The physiology of Insecta, vol. 2 (ed. Rockstein M.), pp. 187–226 New York, NY: Academic Press [Google Scholar]
- Kamikouchi A., Inagaki H. K., Effertz T., Hendrich O., Fiala A., Göpfert M. C., Ito K.2009The neural basis of Drosophila gravity-sensing and hearing. Nature 458, 165–172 (doi:10.1038/nature07810) [DOI] [PubMed] [Google Scholar]
- Kennedy J. S.1951The migration of the desert locust (Schistocerca gregaria). I. The behaviour of swarms. II. A theory of long-range migrations. Phil. Trans. R. Soc. Lond. B 235, 163–290 (doi:10.1098/rstb.1951.0003) [DOI] [PubMed] [Google Scholar]
- Kennedy J. S.1975Insect dispersal. In Insects, science and society (ed. Pimental D.), pp. 103–119 New York, NY: Academic Press [Google Scholar]
- Lang T. J., Rutledge S. A., Stith J. L.2004Observations of quasi-symmetric echo patterns in clear air with the CSU-CHILL polarimetric radar. J. Atmos. Oceanic Technol. 21, 1182–1189 (doi:10.1175/1520-0426(2004)021<1182:OOQEPI>2.0.CO;2) [Google Scholar]
- Magal C., Dangles O., Caparroy P., Casas J.2006Hair canopy of cricket sensory system tuned to predator signals. J. Theor. Biol. 241, 459–466 (doi:10.1016/j.jtbi.2005.12.009) [DOI] [PubMed] [Google Scholar]
- Maxey M. R., Riley J. J.1983Equation of motion for a small rigid sphere in a nonuniform flow. Phys. Fluids 26, 883–889 (doi:10.1063/1.864230) [Google Scholar]
- Nisbet I. C. T.1955Atmospheric turbulence and bird flight. Br. Birds 48, 557–559 [Google Scholar]
- Rani S. L., Balachandar S.2003Evaluation of the equilibrium Eulerian approach for the evolution of particle concentration in isotropic turbulence. Int. J. Multiphase Flow 29, 1793–1816 (doi:10.1016/j.ijmultiphaseflow.2003.09.005) [Google Scholar]
- Rennie S., Illingworth A., Dance S., Ballard S.2008Utilization of Doppler radar wind measurements from insect returns. In ERAD 2008: Fifth European Conf. on Radar in Meteorology and Hydrology, Helsinki, Finland, 30 June–4 July 2008 (Poster P2.6) Helsinki, Finland: Finnish Meteorological Institute; See http://erad2008.fmi.fi/proceedings/extended/erad2008--0123-extended.pdf [Google Scholar]
- Reynolds D. R., Chapman J. W., Edwards A. S., Smith A. D., Wood C. R., Barlow J. F., Woiwod I. P.2005Radar studies of the vertical distribution of insects migrating over southern Britain: the influence of temperature inversions on nocturnal layer concentrations. Bull. Entomol. Res. 95, 259–274 (doi:10.1079/BER2004358) [DOI] [PubMed] [Google Scholar]
- Reynolds D. R., Smith A. D., Chapman J. W.2008A radar study of emigratory flight and layer formation at dawn over southern Britain. Bull. Entomol. Res. 98, 35–52 [DOI] [PubMed] [Google Scholar]
- Reynolds A. M., Reynolds D. R., Riley J. R.2009Does a ‘turbophoretic’ effect account for layer concentrations of insects migrating in the stable night-time atmosphere? J. R. Soc. Interface 6, 87–95 (doi:10.1098/rsif.2008.0173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J. R.1975Collective orientation in night-flying insects. Nature 253, 113–114 (doi:10.1038/253113a0) [Google Scholar]
- Riley J. R.1989Orientation by high-flying insects at night: observations and theories. In Orientation and navigation—birds, humans and other animals, Conf. of the Royal Institute of Navigation, 6–8 April 1989 Cardiff(Paper no. 21) London: The Royal Institute of Navigation [Google Scholar]
- Riley J. R., Reynolds D. R.1983A long-range migration of grasshoppers observed in the Sahelian zone of Mali by two radars. J. Anim. Ecol. 52, 167–183 [Google Scholar]
- Riley J. R., Reynolds D. R.1986Orientation at night by high-flying insects. In Insect flight: dispersal and migration (ed. Danthanarayana W.), pp. 71–87 Berlin, Germany: Springer-Verlag [Google Scholar]
- Riley J. R., Kreuger U., Addison C. M., Gewecke M.1988Visual detection of wind-draft by high-flying insects at night: a laboratory study. J. Comp. Physiol. A 169, 793–798 [Google Scholar]
- Riley J. R., Cheng X. N., Zhang X. X., Reynolds D. R., Xu G. M., Smith A. D., Cheng J. Y., Bao A. D., Zhai B. P.1991The long distance migration of Nilaparvata lugens (Stål) (Delphacidae) in China: radar observations of mass return flight in the autumn. Ecol. Entomol. 16, 471–489 (doi:10.1111/j.1365-2311.1991.tb00240.x) [Google Scholar]
- Rotach M. W., Gryning S.-E., Tassone C.1996A two-dimensional Lagrangian stochastic dispersion model for daytime conditions. Q. J. R. Meteorol. Soc. 122, 367–389 (doi:10.1002/qj.49712253004) [Google Scholar]
- Saiki E., Moeng C.-H., Sullivan P. R.2000Large-eddy simulations of the stably stratified planetary boundary-layer. Bound. Layer Meteorol. 95, 1–30 (doi:10.1023/A:1002428223156) [Google Scholar]
- Sane S. P., Dieudonne A., Willis M. A., Daniel T. L.2007Antennal mechanosensors mediate flight control in moths. Science 315, 863–866 (doi:10.1126/science.1133598) [DOI] [PubMed] [Google Scholar]
- Schaefer G. W.1976. Radar observations of insect flight. In Insect flight, Symposia of the Royal Entomological Society of London (ed. Rainey R. C.), no. 7, pp. 157–197 Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- Taylor L. R.1974Insect migration, flight periodicity and the boundary layer. J. Anim. Ecol. 43, 225–238 [Google Scholar]
- Webb B.2007Insect behaviour: controlling flight altitude with optic flow. Curr. Biol. 17, R124–R125 (doi:10.1016/j.cub.2006.12.008) [DOI] [PubMed] [Google Scholar]
- Williams C. B., Cockbill G. F., Gibbs M. E., Downes J. A.1942Studies in the migration of Lepidoptera. Trans. R. Entomol. Soc. Lond. 92, 101–283 [Google Scholar]
- Wood C. R., Chapman J. W., Reynolds D. R., Barlow J. F., Smith A. D., Woiwod I. P.2006The influence of the atmospheric boundary layer on nocturnal layers of moths migrating over southern Britain. Int. J. Biometeorol. 50, 193–204 (doi:10.1007/s00484-005-0014-7) [DOI] [PubMed] [Google Scholar]
- Wood C. R., Reynolds D. R., Wells P. M., Barlow J. F., Woiwod I. P., Chapman J. W.2009Flight periodicity and the vertical distribution of high-altitude moth migration over southern Britain. Bull. Entomol. Res. 99, 525–535 (doi:10.1017/S0007485308006548) [DOI] [PubMed] [Google Scholar]
- Yorozu S., Wong A., Fischer B. J., Dankert H., Kernan M. J., Kamikouchi A., Ito K., Anderson D. J.2009Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature 458, 201–205 (doi:10.1038/nature07843) [DOI] [PMC free article] [PubMed] [Google Scholar]