Abstract
Most corals in tropical localities broadcast their gametes into the water column, yet we have a poor understanding of what forces reproductive schedules. Moreover, recent studies show considerable geographical variation in the duration of the coral spawning season. For example, on the Great Barrier Reef, corals display tight coupling, while corals in Kenya spawn over seven months. This study reconciles the regional variance by testing the hypothesis that regional wind fields are the corals' ultimate reproductive proxy. Regions with short calm periods should be more tightly coupled than regions with calm periods extending for several months. Regional wind fields were assessed at seven localities, between 1997 and 2006, using the 11 GHz channel radiometer tropical microwave imager (TMI) onboard the tropical rainfall measuring mission (TRMM). There was a direct positive relationship between the duration of regional calm periods and the coupling of mass coral spawning. Ultimate long-term evolutionary advantages of releasing gametes during calm periods ensure fertilization and facilitate larval retention and local recruitment. Coupling mass spawning with seasonally calm periods agrees strongly with recent genetic evidence of local dispersal and high local retention.
Keywords: corals, spawning, evolution, reproduction
1. Introduction
(a). Rationale
Rapid climate change is causing high-frequency return periods of anomalous sea surface temperatures (Hoegh-Guldberg 1999). In the last two decades, it has been common to witness considerable thermal stress and differential survival of shallow coral populations (Loya et al. 2001). However, asexual propagation of coral clones alone will produce no further thermal tolerance. Selective processes only weigh heavily when those surviving individuals reproduce. Coral populations will depend almost solely on the formation of sex cells (gametes) and recombination to form more thermally tolerant genotypes and adapt to warming oceans.
Indeed, the loss of individuals from the gene pool, in its strictest sense, is not adaptation, because that involves genome adjustment through differential reproductive rates of individuals in the populations (Lewontin 1970). Therefore, coral survival through rapid climate change is largely dependent on sustained reproduction. In this context, it becomes imperative to understand coral reproductive schedules globally, and ask when, how and why they occur, and what fundamental drivers influence these schedules. Although proximate factors that drive mass spawning are poorly understood, we know even less about the long-term evolutionary advantages of synchronizing gamete release (Harrison & Wallace 1990; Richmond & Hunter 1990).
Over the last several years the tropical literature on coral reproduction schedules has expanded to include several new localities, such as the Solomon Islands (Baird et al. 2001), Singapore (Guest et al. 2002), Palau (Penland et al. 2004), the Philippines (Bermas et al. 1992; Vicentuan et al. 2008) and Kenya (Mangubhai & Harrison 2008). Surprisingly, the duration of the coral spawning season varies considerably among regions. For example, the spawning season in the Galápagos Islands shows a protracted eight- to nine-month spawning season (Glynn et al. 1991, 1994, 1996), in Kenya a seven-month season (Mangubhai & Harrison 2008) and a two-month season on the Great Barrier Reef (Harrison et al. 1984; Willis et al. 1985; Babcock et al 1986). Therefore, there are a multitude of questions that still need answers. For example: why does the coral spawning season vary regionally? Is the duration of the spawning season driven by an environmental factor? Is an extended spawning season the default system, suggesting that gamete release is subjected to strong selective pressures? Or is there a natural drift towards asynchrony in tropical systems?
Coupling gamete release with environmental conditions has been assessed on at least three temporal scales: (i) the time of night (Levitan et al. 2004), (ii) the time of month (Willis et al. 1985; Babcock et al. 1986; Levy et al. 2007), and (iii) the time of year (Harrison et al. 1984; Hayashibara et al. 1993). Night-time is required for gamete release for the majority of broadcast spawning species, although Porites and Pocillopora may spawn in daytime (R. Kinzie 2007, personal communication). During mass spawning, many species stagger their release times through the evening; for example, in southern Japan and Palau, most mussids (Lobophyllia spp.) spawn early, at 18:30, followed by Montipora and Acropora species around 19:00–20:00, faviids at 20:00–21:00; and fungiids and Porites spawn later at 21:00–22:30. Such staggering may facilitate the maintenance of species boundaries (Fukami et al. 2003; Levitan et al. 2004).
On a monthly scale, there is no globally consistent relationship between lunar phase and the timing of coral spawning (Willis et al. 1985; Babcock et al. 1986), because some coral species spawn at full moon in one locality and at quarter moon in an adjacent locality (van Woesik 1995). While the light of the moon may play an important role in the release of gametes, the present study is most interested in the time of the year of coral spawning and elucidating the proximate and ultimate evolutionary cues that drive gamete release.
(b). Proximate cues
The literature contains abundant general statements and possibilities relating marine spawning to seasonal, proximate cues, including temperature (Glynn et al. 1991; Hayashibara et al. 1993), the lunar cycle (Guest et al. 2002), the amount of rainfall (Mendes & Woodley 2002) and solar insolation (van Woesik et al. 2006). Orton (1920) suggested that all marine organisms spawn gametes in synchrony with seasonal increases in water temperatures. Coupling coral reproductive schedules to ‘optimal’ temperature was unquestioned for years, if not decades. Most convincing was the strong evidence from the Great Barrier Reef that showed spawning on near-shore reefs in October, when the water temperatures were approximately 28°C, and spawning on mid-shelf reefs one month later, when the water temperatures were the same (Willis et al. 1985). Most subsequent literature showed the maturation of gametes alongside seasonal temperature profiles, and authors consistently noted the relationship between increasing temperature and gamete maturation and release (Babcock et al. 1986; Szmant-Froelich 1986; Glynn et al. 1991; Hayashibara et al. 1993). An extension of the argument was that because seasonal temperature differences drive gametogenesis, and because localities near the equator have a narrow temperature range, it is therefore highly unlikely that corals in the tropics have synchronized spawning patterns (Oliver et al. 1988; Kenyon 1995; Mangubhai & Harrison 2008). Still, the central premise of the argument is that temperature is driving gametogenesis.
Babcock et al. (1994) showed that coral spawning in Western Australia did not coincide with maximum annual water temperatures. They suggested that the spawning patterns in Western Australia were genetic legacies of upstream Indonesia. Penland et al. (2004) also questioned the relationship between coral spawning in tropical Palau (7° N) and water temperatures; they found two annual spawning periods that coincided with the equinoxes (when the sun crosses the equator). They also showed that broadcast spawning correlated poorly with temperature patterns and pointed to solar insolation as a more reasonable driver of coral reproductive cycles.
In a meta-analysis, van Woesik et al. (2006) formally tested to what extent temperature and solar insolation accounted for synchronous gamete release in the Caribbean. Again, broadcast spawning was positively related to solar insolation cycles, and the rate of temperature change was a poor predictor of spawning throughout the Caribbean, although gamete release coincided with temperatures between 28 and 30°C. A negative rate of change in solar insolation (i.e. the concave-down derivatives) was the best predictor of gamete release (van Woesik et al. 2006). Their work agrees with a flowering study of the ubiquitous rubber tree (Hevea brasiliensis), which spans 20° in latitude, from the tropics to the subtropics. These trees flower bimodally in the tropics during the equinoxes, and unimodally in the subtropics when solar intensity is highest (Yeang 2007). Similarly, in a 10-year study on hundreds of neotropical forest trees, reproductive activity was greatest during highest seasonal irradiance (Zimmerman et al. 2007).
However, did we merely switch proximate cues, from temperature to solar insolation, without gaining any evolutionary insight? Possibly, although most studies describing seasonal increases in temperatures considered proximate and ultimate cues in combination. Typically, certain temperatures are considered optimal for gametogenesis and planulae survival (Harrison et al. 1984), although these temperatures have never been tested formally. Other studies argue that coupling gamete release with seasonally high light fields may increase carbon sequestration, which facilitates gamete production (van Woesik et al. 2006).
But is coral reproduction really carbon-limited in the tropics? Surely releasing gametes at a time of near-maximum solar insolation would also have disadvantages, because high irradiance leads to chronic photoinhibition that compromises the state of the holobiont (the coral animal and its endosymbionts; Jones & Hoegh-Guldberg 2001), which in turn reduces gamete production (Baird & Marshall 2002). Furthermore, what advantage is there for corals to release gametes at a time when fertilized planulae are subjected to harsh irradiance conditions at the water's surface? Recently, Yakovleva et al. (2009) showed that planulae with endosymbionts suffered reactive oxygen species damage at the water's surface. Their research suggests that there are clear evolutionary advantages to not having symbionts through the planulae phase, and acquiring them may be best left until after settlement. Moreover, how do peak solar insolation patterns reconcile a burgeoning literature emphasizing local retention and reef connectivity at a scale of tens of kilometres (Ayre & Hughes 2000, 2004; Palumbi 2003)? Again, a critical question is posed: what are the (ultimate) evolutionary advantages of coupling gamete release with a specific environmental parameter?
(c). Ultimate cues
Some earlier evolutionary thoughts on why spawning synchronization occurred on the Great Barrier Reef, where over 100 coral species spawn over just a few nights every year, was predator satiation (Harrison et al. 1984; Babcock et al. 1986; Pratchett et al. 2001). Oliver et al. (1988) logically refuted this hypothesis by arguing that if the predator satiation hypothesis was correct, spawning would vary randomly because fishes eat all the time. Besides, coral spawning season is regionally predictable. Another hypothesis was the genetic legacy hypothesis, considered for corals spawning outside the thermal optimum in Western Australia (Simpson 1991; Babcock et al. 1994). Coral spawning in Western Australia has now also been observed both in the Austral autumn (Simpson 1991; Babcock et al. 1994) and more recently in the Austral spring (Rosser & Gilmour 2008; Rosser & Baird in press). These biannual spawning modes, for Western Australia, correspond with the solar equinoxes, and are no different than biannual, multi-species spawning in Palau (Penland et al. 2004) and biannual spawning of three Montipora species on the Great Barrier Reef (Stobart et al. 1992). Therefore, the genetic legacy hypothesis may play a minor role, since insolation cycles explain most of the forcing.
A third scenario involves environmental drivers, constraining the optimal time of gamete release (Oliver et al. 1988). Oliver et al. (1988, p. 808) stated that ‘ … other factors also show significant latitudinal variations (e.g. day length, solar angle, etc.) but it is difficult to conceive of a plausible mechanism by which they could act as ultimate factors controlling spawning synchrony’. If environmental drivers can be both a proximate cue and have some evolutionary validity, then seasonal mass spawning patterns should vary regionally in response to variations in the constraining parameter(s).
(d). A novel hypothesis
Seasonal mass spawning does vary regionally, both in timing and in duration. Here, I propose that corals may couple gamete release when winds are light. I argue that regional wind fields are the corals’ ultimate reproductive proxy, and test the hypothesis that regions that experience consistently long periods of calm weather (>20% of the year) have longer coral spawning periods than regions with short periods of calm weather (≤20% of the year). Coral spawning outside calm periods may have been selected against because gametes would be lost from the reef systems, especially in rather isolated locations throughout the Indo-Pacific. Indeed, tightly coupling gamete release around calm periods would be particularly advantageous on isolated reefs and in locations where the wind fields are rarely calm. Likewise, regions with long calm periods should see (on average) extended reproductive seasons. I argue that these conditions have considerable selective advantages, facilitating fertilization, larval retention and local recruitment, which strongly agrees with recent genetic evidence arguing for local reef connection at the scale of tens of kilometres (Ayre & Hughes 2000; Palumbi 2003).
2. Material and Methods
Wind fields were examined at seven localities, for which broadcasting coral spawning records are well known: (i) the Great Barrier Reef (19° S); (ii) Okinawa (26° N); (iii) Palau (7° N); (iv) Kenya (3° S); (v) the Galápagos (0°); (vi) Ningaloo, Western Australia (21° S); and (vii) the Florida Keys (24° S). Monthly averaged wind-speed data (m s−1) were derived from 1996 to 2006 using the 11 GHz channel radiometer (tropical microwave imager, TMI) onboard the tropical rainfall measuring mission (TRMM). Note that the residual mean square retrieval accuracy (or error) is 0.9 m s−1. The TMI radiometer captures 25 km pixel images. The uncompressed TMI data files were downloaded from http://www.remss.com/tmi/tmi_browse.html and loaded into Matlab 6.5 for analysis. Because of side-lobe contamination, microwave instruments do not measure wind fields immediately adjacent to land. Therefore, wind-field data pixels were extracted 35 km immediately offshore from where the reproductive studies were conducted.
A robust least-squares regression was used to test the hypothesis that the duration of calm periods, here taken as the duration when the mean wind speeds were less than 6 m s−1, is a useful predictor of the duration of the spawning period. The threshold of 6 m s−1 was chosen because of the physical principle that significant waves begin to form when the winds are greater than 6 m s−1, resulting in the formation of white caps (Pierson & Moskowitz 1964). Waves disperse larvae across the water's surface (Wolanski 1994). Wave height increases in proportion to the square of the wind speed. Indeed, significant wave height (Hs) at the peak of the spectrum of a fully developed sea, with the wind measured at 19.5 m above the sea surface, follows the Pierson–Moskowitz spectrum equation (Pierson & Moskowitz 1964): Hs = 0.21 U2 g−1, where g is gravity at 9.8 m s−1 and U is the wind speed. Therefore, larval dispersal will increase to the square of wind speed. In words, doubling the wind speed from 4 to 8 m s−1 increases the wave height and dispersal potential fourfold. Moreover, to use only one predictor data point with every response data point and avoid pseudo-replication, a particular month was included in the analysis only when over 30 per cent of the wind-speed data for the 10-year period were less than 6 m s−1. For example, if the month of February had winds greater than or equal to 6 m s−1 for 80 per cent of the time, February was assumed to be, in general, a windy month and not part of the predictor variable.
3. Results
(a). Global patterns
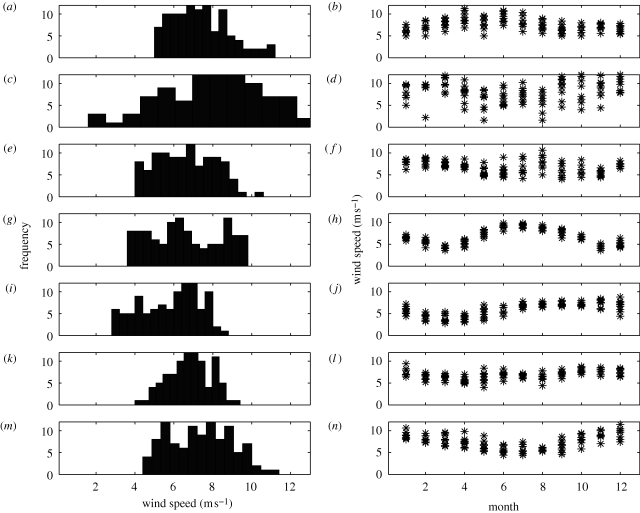
As expected, there were clear regional differences in the global wind-field patterns (figure 1). The Great Barrier Reef data showed that the winds were calm (<6 m s−1) for less than 17 per cent of each year (figure 2a). Calm periods were between September and January, at least at latitude 19° S on the Great Barrier Reef, with the lowest range, or the most consistent conditions, occurring in November (figure 2b). These results agree with spawning times (Harrison et al. 1984; Wallace 1985; Willis et al. 1985; Babcock et al. 1986; Baird et al. 2009), but they also show that spawning may occur outside the October–November window in December and January (figure 2b), and may not be as tightly constrained as previously thought for the Great Barrier Reef (see also Baird et al. 1996; Guest et al. 2008).
Figure 1.
Global wind fields (in m s−1) using the 11 GHz channel radiometer tropical microwave imager (TMI), onboard the tropical rainfall measuring mission (TRMM), for January 2001, where blue is on average less than 5 m s−1.
Figure 2.
Wind-speed frequency distributions and monthly averaged wind-speed data (m s−1) extracted from 1996 to 2006 for (a,b) the central Great Barrier Reef (19° S), (c,d) Okinawa (26° N), (e,f) Palau (7° N), (g,h) Kenya (3° S), (i,j) the Galápagos (0°), (k,l) Ningaloo, Western Australia (21° S) and (m,n) the Florida Keys (24° S). Note that white caps form when the winds are greater than or equal to 6 m s−1.
Wind fields for Okinawa (26° N), Japan, showed a longer calm period than the Great Barrier Reef: just over 20 per cent of the year had winds less than 6 m s−1 (figure 2c). The calmest period was between May and August (figure 2d), with the lowest range (or the most consistent conditions) occurring in June. These months are the reported spawning months, with most species spawning in June and July (Hayashibara et al. 1993; van Woesik 1995).
Palau (7° N) showed that on average, the winds were less than 6 m s−1 for 35 per cent of the year (figure 2e), with two peaks, one in May/June and the other in September, coinciding with gamete release—although the former period had more intense coral spawning than the latter period (Penland et al. 2004). May had the lowest wind-speed range, which suggests that May is the most predictable month (figure 2f); this also coincides with the most species releasing gametes (Penland et al. 2004).
On average, the calm period (with winds <6 m s−1) in Kenya (3° S) lasted from November to April, greater than 40 per cent of the year (figure 2g), with a brief increase in wind strength around January (figure 2h). Both March and November had the lowest wind-speed range, and were therefore the most predictable months for coral spawning. The spawning period for Acropora species, recently reported by Mangubhai & Harrison (2008), extends for seven months, from October to April, which is directly coincidental with the low wind-field season.
The wind fields in the Galápagos Islands (0°) are calm for nearly half the year (figure 2i), extending from January to June, with April showing the most consistent, lowest wind-speed range (figure 2j). The coral reproductive period in the Galápagos Islands is longer than reported for anywhere else in the world. The season extends from February to October (Glynn et al. 1991), with peaks in March for the intensively studied Pocillopora elegans, February to March and again in August/September for Porites lobata (Glynn et al. 1994), and February to May for Pavona gigantea (Glynn et al. 1996).
The calm wind period in Western Australia (21° S) occurs for about 23 per cent of the year, between March and May (figure 2k,l). March has the most predictable wind speeds, showing the lowest wind-speed range. Coral spawning in Western Australia has long been known to occur in Austral autumn, in April (Babcock et al. 1994), although more recently there have also been reports of some sporadic coral spawning in the Austral spring (Rosser & Gilmour 2008; Rosser & Baird in press). The latter reports do not coincide with calm periods; in fact, they coincide with 10-year highs, when winds on average were greater than 7.5 m s−1.
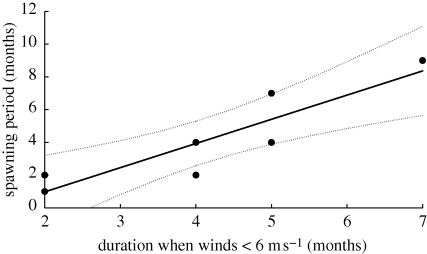
Wind fields less than 6 m s−1 in the Florida Keys (24° S) typically occurred for 28 per cent of the year (figure 2m), and usually in June–August (figure 2n). The lowest wind-speed range, or the most predictable month, was recorded for the month of August. Coral spawning in Florida extends from June to August, with most spawning occurring in July and August (van Woesik et al. 2006). The robust regression analysis on all seven localities showed a strong (R2 = 0.778), significantly (p = 0.008) positive relationship between the amount of time the winds were calm (or <6 m s−1) and the duration of the spawning period (figure 3). The shorter the duration of calm winds, the shorter the spawning season.
Figure 3.
The relationship, using a robust least-squares regression analysis (R2 = 0.778, p = 0.008), between the duration of time (in months) when regional wind fields are on average less than 6 m s−1 (i.e. the predictor variable) and the duration of (broadcast) coral spawning (i.e. the response variable).
4. Discussion
Previously, asynchronous coral spawning was referred to as a tropical feature, with simultaneous multiple-species spawning more typical of the subtropics (Oliver et al. 1988; Kenyon 1995; Mangubhai & Harrison 2008). Yet in the last 10 years, multi-species coral spawning has been widely reported in the tropics (Baird et al. 2001; Guest et al. 2002; Penland et al. 2004). Therefore, instead of asking whether there are any major latitudinal spawning trends (because the answer is no) it seems more pertinent to return to the question: why does the length of the coral spawning season vary regionally? Here I show a consistent relationship between the duration of calm periods and the duration of the spawning period. For example, localities such as Kenya, with long calm periods, also showed long spawning periods, and localities with short calm periods, such as the Great Barrier Reef, showed short spawning periods.
(a). Reconciling proximate and ultimate cues
There is no reported evidence of corals directly detecting calm periods. But corals can clearly detect change in light fields (Gorbunov & Falkowski 2002; Levy et al. 2007; Brady et al. 2009). Previously, van Woesik et al. (2006) showed a strong positive relationship between mass spawning in the Caribbean and maximum insolation, or a near-zero solar insolation derivative (i.e. when the rate of change in solar insolation is near zero). Regional calm periods also occur when the zenithal sun is most intense, directly overlying and heating the underlying land masses, water and atmosphere. But the strong monsoonal wind shifts lag behind the zenithal sun by 1–2 months because of atmospheric inertia (McGregor & Nieuwolt 1998). Therefore, while incoming irradiance may directly affect gametogenesis and spawning cycles, the release of gametes near maximum insolation, when rates of change are near zero, coincides with seasonal calm periods.
This study clearly shows that the duration of the calm wind-field period is directly related to the duration of the spawning period (figure 3). Fertilization is less likely under high winds because gametes are rapidly dispersed by surface currents and waves, and lost from the regional gene pool. In contrast, gamete release during calm periods ensures high fertilization and local retention of offspring.
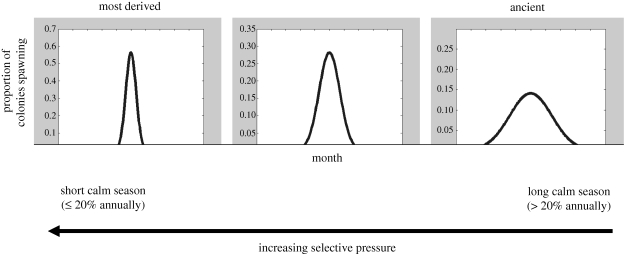
A corollary is that an extended spawning season is the default system, from which more tightly coupled spawning schedules are derived through strong selective pressures (figure 4). Therefore, the evolutionary advantage of coupling spawning with calm periods might be to optimize the windows of opportunity to increase fertilization success, which in turn leads to local retention of gametes. Spawning during regionally calm periods may also be applicable to other marine organisms, such as fishes (Johannes 1981). Therefore, it is not necessarily the local wind conditions during spawning that explain gamete release patterns, but rather wind-field patterns integrated over extended time periods that adjust coral reproductive schedules through selective pressure. Coupling mass spawning with seasonally calm periods agrees strongly with recent genetic evidence of local dispersal and high local retention (Palumbi 2003; Ayre & Hughes 2004).
Figure 4.
Schematic of coral spawning schedules explained by regional wind fields; the asynchronous mode is proposed as the most ancient reproductive state, with strong selective pressure forcing short-seasonal spawning in localities with short-calm periods (for ≤20% of the year).
This argument may also shed some light on the recent pervasive discussions on mass versus multi-species spawning (Harrison & Booth 2007; Guest et al. 2008; Mangubhai & Harrison 2008; Baird & Guest 2009). The dilemma may be simply a gradient response to selective pressure. In other words, corals in localities that consistently have long periods of calm conditions would have weak selective pressure to compress spawning schedules into one or two nights a year. In contrast, selective pressure would be considerable if winds are calm for only a short period, resulting in a short spawning period. For example, the extreme selective pressure on the Great Barrier Reef, which forces spawning through the short season of opportunity, when the winds are calm for up to 17 per cent of the year, contrasts with long calm periods that are less selective for example, in Kenya, with multi-species spawning episodes that are less tightly coupled.
A contemporary example of strong selective pressure may be occurring in Western Australia, where the recently discovered spring spawning is less intense than the autumnal spawning (Rosser & Gilmour 2008; Rosser & Baird in press). Coral spawning takes place on the east coast, along the Great Barrier Reef, in spring. Most interestingly, spring in Western Australia coincides with (10-year) high wind fields, averaging over 7.5 m s−1. Selective pressure to spawn outside this window may be considerable in Western Australia, because the consistently high wind fields in spring probably select against gamete release, and corals that do release gametes at this time of the year are rapidly removed from the gene pool. Moreover, any migrant coral planulae that enter a given regional gene pool would be expected to adapt to those regional weather patterns through the process of natural selection.
(b). Adaptation
In this time of rapid climate change, understanding reproductive schedules is critical (Visser 2008), so that science may influence policy to reduce local disturbances and ensure protection of marine organisms during reproductive periods. Without sexual recombination, there is little chance of coral populations adapting to changing environmental conditions, and indeed adapting to warming oceans. Strong selection forced by a series of thermal stress events effectively selects for holobionts (which include corals and their symbionts) that are more resistant to those stresses (Loya et al. 2001). Asexually reproducing corals may preserve that variation, but will not be able to increase tolerance any further in a warming ocean. Sex and recombination of subsequent generations could increase that tolerance through selective pressure that acts upon offspring, further increasing the frequency of alleles bestowing high temperature tolerance. Within this scenario, however, we assume that the coral populations are still able to reproduce; however, under extreme thermal stresses corals' reproductive capacities are compromised for at least two years (Baird & Marshall 2002). Because the chance event of some adaptive mutation occurring is negligible, at least on an ecological time scale, sexual reproduction and recombination is the most likely chance corals have to adapt to climate change and avoid evolutionary dead ends.
In conclusion, it seems that maximum solar insolation may be the proximate cue that triggers mass coral spawning, although the direct physical consequence of releasing gametes during this period has strong evolutionary connotations because it coincides with seasonally calm periods. Coral spawning during times when regional wind fields are low may be the corals' ultimate reproductive proxy. Releasing gametes through calm periods facilitates maximum fertilization and local reproductive success. Indeed, coral spawning during calm periods may have considerable selective advantages, especially on sparsely distributed reefs, where spawning outside the seasonally calm periods would remove offspring from the gene pool.
Acknowledgements
Thanks to Sandra van Woesik and the three reviewers for many constructive comments on this manuscript. Research is supported by the World Bank and the Global Environmental Facility through the Coral Reef Targeted Research and Capacity Building for Management programme. This is Contribution Number 9 from the Institute for Adaptation to Global Change at the Florida Institute of Technology.
References
- Ayre D. J., Hughes T. P.2000Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution 54, 1590–1605 [DOI] [PubMed] [Google Scholar]
- Ayre D. J., Hughes T. P.2004Climate change, genotypic diversity and gene flow in reef-building corals. Ecol. Lett. 7, 273–278 (doi:10.1111/j.1461-0248.2004.00585.x) [Google Scholar]
- Babcock R. C., Bull G. D., Harrison P. L., Heyward A. J., Oliver J. K., Wallace C. C., Willis B. L.1986Sychronous spawning of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90, 379–394 (doi:10.1007/BF00428562) [Google Scholar]
- Babcock R. C., Willis B. L., Simpson C. J.1994Mass spawning of corals on a high latitude coral reef. Coral Reefs 13, 161–169 [Google Scholar]
- Baird A. H., Guest J. R.2009Spawning synchrony in scleractinian corals: comment on Mangubhai and Harrison. Mar. Ecol. Prog. Ser. 374, 301–304 (doi:10.3354/meps07838) [Google Scholar]
- Baird A. H., Marshall P. A.2002Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 237, 133–141 (doi:10.3354/meps237133) [Google Scholar]
- Baird A. H., Marshall P. A., Wolstenholme J.1996Latitudinal variation in the reproduction of Acropora in the Coral Sea. In Proc. 9th Int. Coral Reef Symp. vol. 1, pp. 385–389 [Google Scholar]
- Baird A. H., Saddler C., Pitt M.2001Synchronous spawning of Acropora in the Solomon Islands. Coral Reefs 19, 286 [Google Scholar]
- Baird A. H., Guest J. R., Willis B. L.2009Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Ann. Rev. Ecol. Evol. Syst. 40, 551–571 [Google Scholar]
- Bermas N. A., Alino P. M., Atrigeno M. P., Uychiaoco A.1992. Observations on the reproduction of scleractinian and soft corals in the Philippines. In Proc. 7th Int. Coral Reef Symp.Guam, vol. 1, pp. 443–447 [Google Scholar]
- Brady A. K., Hilton J. D., Vize P. D.2009Coral spawn timing is a direct response to solar light cycles and is not an entrained circadian response. Coral Reefs 28, pp. 677–680 (doi:10.1007/s00338-009-0498-4) [Google Scholar]
- Fukami H., Omori M., Shimoike K., Hayashibara T., Hatta M.2003Ecological and genetic aspects of reproductive isolation by different spawning times in Acropora corals. Mar. Biol. 142, 679–684 [Google Scholar]
- Glynn P. W., Gassman N. J., Eakin C. M., Cortes J., Smith D. B., Guzman H. M.1991Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). I. Pocilloporidae. Mar. Biol. 109, 355–368 (doi:10.1007/BF01313501) [Google Scholar]
- Glynn P. W., Colley S. B., Eakin C. M., Smith D. B., Cortes J., Gassman N. J., Guzman H. M., Del Rosario J. B., Feingold J. S.1994Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). II. Poritidae. Mar. Biol. 118, 191–208 (doi:10.1007/BF00349785) [Google Scholar]
- Glynn P. W., Colley S. B., Gassman N. J., Black K., Cortes J., Mate J. M.1996Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). III. Agariciidae (Pavona gigantea and Gardineroseris planulata). Mar. Biol. 125, 579–601 [Google Scholar]
- Gorbunov M. Y., Falkowski P. G.2002Photoreceptors in the cnidarian hosts allow symbiotic corals to sense blue moonlight. Limno. Oceanogr. 47, 309–315 [Google Scholar]
- Guest J. R., Chou L. M., Baird A. H., Goh B. P. L.2002Multispecific, synchronous coral spawning in Singapore. Coral Reefs 21, 422–423 [Google Scholar]
- Guest J. R., Baird A. H., Clifton K. E., Heyward A. J.2008From molecules to moonbeams: spawning synchrony in coral reef organisms. Invert. Rep. Dev. 51, 145–149 [Google Scholar]
- Harrison P. L., Booth D. J.2007Coral reefs: naturally dynamic and increasingly disturbed ecosystems. In Marine ecology (eds Connell S. D., Gillanders B. M.), pp. 316–377 Oxford, UK: Oxford University Press [Google Scholar]
- Harrison P. L., Wallace C. C.1990Reproduction, dispersal and recruitment of scleractinian corals. In Ecosystems of the world, volume 25: coral reefs (ed. Dubinsky Z.), pp. 550 Amsterdam, The Netherlands: Elsevier Science Publications [Google Scholar]
- Harrison P. L., Babcock R. C., Bull G. D., Oliver J. K., Wallace C. C., Willis B. L.1984Mass spawning in tropical reef corals. Science 223, 1186–1189 (doi:10.1126/science.223.4641.1186) [DOI] [PubMed] [Google Scholar]
- Hayashibara T., Shimoike K., Kimura T., Hosaka S., Heyward A. J., Harrison P. L., Kudo K., Omori M.1993Patterns of coral spawning at Akajima Island, Okinawa, Japan. Mar. Ecol. Prog. Ser. 101, 253–262 (doi:10.3354/meps101253) [Google Scholar]
- Hoegh-Guldberg O.1999Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshwater Res. 50, 839–866 (doi:10.1071/MF99078) [Google Scholar]
- Johannes R. E.1981Words of the lagoon: fishing and marine lore in the Palau district of Micronesia Los Angeles, CA: University of California Press [Google Scholar]
- Jones R. J., Hoegh-Guldberg O.2001Diurnal changes in the photochemical efficiency of the symbiotic dinoflagellates (Dinophyceae) of corals: photoprotection, photoinactivation and the relationship to coral bleaching. Plant Cell Env. 24, 89–99 (doi:10.1046/j.1365-3040.2001.00648.x) [Google Scholar]
- Kenyon J. C.1995Latitudinal differences between Palau and Yap in coral reproduction synchrony. Pac. Sci. 49, 156–164 [Google Scholar]
- Levitan D. R., Fukami H., Jara J., Kline D., McGovern T. M., McGhee K. E., Swanson C. A., Knowlton N.2004Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the Montastrea annularis complex. Evolution 58, 308–323 [PubMed] [Google Scholar]
- Levy O., Appelbaum L., Leggat W., Gothlif Y., Hayward D. C., Miller D. J.2007Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora. Science 318, 467–470 (doi:10.1126/science.1145432) [DOI] [PubMed] [Google Scholar]
- Lewontin R. C.1970The units of selection. Ann. Rev. Ecol. Syst. 1, 1–18 (doi:10.1146/annurev.es.01.110170.000245) [Google Scholar]
- Loya Y., Sakai K., Yamazato K., Nakano Y., Sambali H., van Woesik R.2001Coral bleaching: the winners and the losers. Ecol. Lett. 4, 122–131 (doi:10.1046/j.1461-0248.2001.00203.x) [Google Scholar]
- Mangubhai S., Harrison P. L.2008Asynchronous coral spawning patterns on equatorial reefs in Kenya. Mar. Ecol. Prog. Ser. 360, 85–96 (doi:10.3354/meps07385) [Google Scholar]
- McGregor G. R., Nieuwolt S.1998Tropical climatology: an introduction to the climates of low latitudes Chichester, UK: John Wiley & Sons [Google Scholar]
- Mendes J. M., Woodley J. D.2002Timing of reproduction in Montastraea annularis: relationship to environmental variables. Mar. Ecol. Prog. Ser. 227, 241–251 (doi:10.3354/meps227241) [Google Scholar]
- Oliver J. K., Babcock R. C., Harrison P. L., Willis B. L.1988Geographic extent of mass coral spawning: clues to ultimate causal factors. In Proc. 6th Int. Coral Reef Symp. vol. 2, pp. 803–810 [Google Scholar]
- Orton J. H.1920Sea temperature, breeding and distribution in marine animals. J. Mar. Biol. Assoc. (UK) 12, 339–366 (doi:10.1017/S0025315400000102) [Google Scholar]
- Palumbi S. R.2003Population genetics, demographic connectivity and the design of marine reserves. Ecol. Appl. 13, S146–S158 (doi:10.1890/1051-0761(2003)013[0146:PGDCAT]2.0.CO;2) [Google Scholar]
- Penland L., Kloulechad J., Idip D., van Woesik R.2004Coral spawning in the western Pacific Ocean is related to solar radiation: evidence of multiple spawning events in Palau. Coral Reefs 23, 133–140 (doi:10.1007/s00338-003-0362-x) [Google Scholar]
- Pierson W. J., Moskowitz L. A.1964Proposed spectral form for fully developed wind seas based on the similarity theory of S. A. Kitaigorodskii. J. Geophys. Res. 69, 5181–5190 (doi:10.1029/JZ069i024p05181) [Google Scholar]
- Pratchett M. S., Gust N., Goby G., Klanten S. O.2001Consumption of coral propagules represents a significant trophic link between corals and reef fish. Coral Reefs 20, 13–17 (doi:10.1007/s003380000113) [Google Scholar]
- Richmond R. H., Hunter C. L.1990Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Mar. Ecol. Prog. Ser. 60, 185–203 (doi:10.3354/meps060185) [Google Scholar]
- Rosser N. L., Baird A. H.In press Multi-specific coral spawning in spring and autumn in far north-western Australia. In Proc. 11th Int. Coral Reef Symp. [Google Scholar]
- Rosser N. L., Gilmour J. P.2008New insights into patterns of coral spawning on Western Australian reefs. Coral Reefs 27, 345–349 (doi:10.1007/s00338-007-0335-6) [Google Scholar]
- Simpson C. J.1991Mass spawning of corals on Western Australian reefs and comparisons with the Great Barrier Reef. J. R. Soc. West. Aust. 74, 85–92 [Google Scholar]
- Stobart B., Babcock R. C., Willis B. L.1992. Biannual spawning of three species of scleractinian coral from the Great Barrier Reef. In Proc. 7th Int. Coral Reef Symp.Guam, vol. 1, pp. 494–499 [Google Scholar]
- Szmant-Froelich A. M.1986Reproductive ecology of Caribbean reef corals. Coral Reefs 5, 43–53 (doi:10.1007/BF00302170) [Google Scholar]
- van Woesik R.1995Coral communities at high latitude are not pseudopopulations: evidence of spawning at 32° N, Japan. Coral Reefs 14, 119–120 (doi:10.1007/BF00303433) [Google Scholar]
- van Woesik R., Lacharmoise F., Koksal S.2006Annual cycles of solar insolation predict spawning times of Caribbean corals. Ecol. Lett. 9, 390–398 (doi:10.1111/j.1461-0248.2006.00886.x) [DOI] [PubMed] [Google Scholar]
- Vicentuan K. C., et al. 2008. Multi-species spawning of corals in north-western Philippines. Coral Reefs 27, 83 (doi:10.1007/s00338-007-0325-8) [Google Scholar]
- Visser M. E.2008Keeping up with a warming world: assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C. C.1985Reproduction, recruitment and fragmentation in nine sympatric species of the coral genus Acropora. Marine Biol. 88, 217–233 (doi:10.1007/BF00392585) [Google Scholar]
- Willis B. L., Babcock R. C., Harrison P. L., Oliver J. K.1985Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. In Proc. 5th Int. Coral Reef Symp., vol. 4, pp. 343–348 [Google Scholar]
- Wolanski E.1994Physical oceanographic processes of the Great Barrier Reef Boca Raton, FL: CRC Press [Google Scholar]
- Yakovleva I. M., Baird A. H., Yamamoto H. Y., Bhagooli R., Nonaka M., Hikaka M.2009Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Mar. Ecol. Prog. Ser. 378, 105–112 (doi:10.3354/meps07857) [Google Scholar]
- Yeang Y.2007The sunshine-mediated trigger of synchronous flowering in the tropics: the rubber tree as a study model. New Phytol. 176, 730–735 (doi:10.1111/j.1469-8137.2007.02258.x) [DOI] [PubMed] [Google Scholar]
- Zimmerman J. K., Wright S. J., Calderon O., Pagan M. A., Paton S.2007Flowering and fruiting of seasonal and aseasonal neotropical forests: the role of annual changes in irradiance. J. Trop. Ecol. 23, 231–251 (doi:10.1017/S0266467406003890) [Google Scholar]