Abstract
Theory predicts that altruism is only evolutionarily stable if it is preferentially directed towards relatives, so that any such behaviour towards seemingly unrelated individuals requires scrutiny. Queenless army ant colonies, which have anecdotally been reported to fuse with queenright foreign colonies, are such an enigmatic case. Here we combine experimental queen removal with population genetics and cuticular chemistry analyses to show that colonies of the African army ant Dorylus molestus frequently merge with neighbouring colonies after queen loss. Merging colonies often have no direct co-ancestry, but are on average probably distantly related because of overall population viscosity. The alternative of male production by orphaned workers appears to be so inefficient that residual inclusive fitness of orphaned workers might be maximized by indiscriminately merging with neighbouring colonies to increase their reproductive success. We show that worker chemical recognition profiles remain similar after queen loss, but rapidly change into a mixed colony Gestalt odour after fusion, consistent with indiscriminate acceptance of alien workers that are no longer aggressive. We hypothesize that colony fusion after queen loss might be more widespread, especially in spatially structured populations of social insects where worker reproduction is not profitable.
Keywords: altruism, army ant, Dorylus, inclusive fitness, queen death, worker reproduction
1. Introduction
How evolution by natural selection can produce altruists, individuals that help others at an overall cost to their own fitness, remains one of the central problems in evolutionary biology. Following Hamilton's (1964) seminal paper, an immense research effort has been devoted to solving this puzzle, which has produced the consensus that phenotypic altruism can only evolve and be evolutionarily stable if the behaviour is preferentially directed towards individuals that carry identical alleles, either by common descent or by explicit recognition (‘green beards’) (Lehmann & Keller 2006).
The best known examples of altruism are colonies of eusocial insects in which workers forage and care for the brood of their mother queens. The most complex insect societies are highly integrated entities that have been described as ‘superorganisms’ (Wheeler 1911; Hölldobler & Wilson 2009). Similar to conventional organisms, they have evolved mechanisms to maintain their colony integrity and to guard themselves against intrusion and exploitation by unrelated individuals from outside (Wheeler 1911; Hölldobler & Wilson 2009). Because of kinship ties, a social insect worker would normally maximize inclusive fitness by remaining in her natal colony, rather than helping unrelated individuals in other nests. Analogously to immune systems, social insects therefore use nest-mate recognition cues that allow them to discriminate between self (individuals belonging to their own colony) and non-self (individuals belonging to foreign colonies). These cues are mainly expressed as a blend of hydrocarbons on the cuticle, and form a colony-specific Gestalt odour (Vander Meer & Morel 1998; Lenoir et al. 1999).
The few known cases where social insect workers actively join foreign colonies are therefore highly intriguing. The vast supercolonies of some invasive ants are one example where workers are not related and freely move between nests without being attacked. While their native counterparts have genetically structured populations, it has been argued that invasive supercolonies might not be stable over evolutionary time because kin selection can no longer act on worker traits (see Helanterä et al. 2009 for a recent review). A second example is social insect workers that drift into foreign colonies and are apparently able to evade host colony recognition systems. Although the occasional drifter could merely be a consequence of individual navigation and recognition errors, recent work has shown that nest drifting can often be understood as a form of social parasitism rather than altruism (see Beekman & Oldroyd 2008 for a recent review). Drifting is also adaptive in the paper wasp Polistes canadensis, but here fitness rewards are indirect, because populations are viscous and drifters end up in nests to which they are related (Sumner et al. 2007).
Fusions between the huge societies of army ants after queen loss potentially constitute a third striking example of workers actively joining foreign colonies. However, the sporadic accounts are largely anecdotal and only concern the neotropical genus Eciton. Schneirla (1949) and Schneirla & Brown (1950) reported that while intact colonies of E. burchellii and E. hamatum never mingle when they meet during swarm raids or emigrations, workers of queenless colonies actively trace and sometimes join foreign conspecific colonies. Schneirla (1971) hypothesized that the loss of the queen was quickly followed by pronounced changes in the colony Gestalt odour, which should weaken nest-mate recognition and ultimately allow colonies to merge without aggression. This might be a plausible proximate explanation because ant queens can contribute substantially to the colony Gestalt odour (Breed & Bennett 1987), but it does not offer an ultimate explanation for why colony fusions have evolved.
Assuming that merging colonies are unrelated, Lin & Michener (1972) viewed colony fusions in army ants as direct evidence against inclusive fitness effects being a major driver for the evolution of social behaviour and concluded that, by merging with foreign colonies after queen loss, ‘they (army ants) cannot benefit their own genotype, but instead benefit a competing one’ (Lin & Michener 1972). Bourke & Franks (1995) agreed that fusing army ant colonies are probably not closely related because their nomadic lifestyle will lead to the dispersal of daughter colonies. Assuming that queenless workers have no alternative options to increase their inclusive fitness, they conjectured that, although little was to be gained, selection on orphaned workers to resist mergers should be weak, while queenright colonies should strongly benefit from the increase in labour force (Bourke & Franks 1995). However, this does not explain why queen-deprived colonies appear to actively engage in the fusion process (Schneirla & Brown 1950). On the other hand, if fusing colonies are related, both host colonies and adopted orphaned workers may gain from fusions of this kind (West-Eberhard 1975; Franks 1980). We therefore decided to gather data that can test both the proximate and ultimate aspects of this hypothesis.
2. Material and methods
(a). Study organism and sample collection
The army ant Dorylus (Anomma) molestus is abundant in the forests and secondary growth habitats at Mt Kenya up to an altitude of 3000 m. The ants are general predators, mainly of invertebrates, and form massive swarm raids on the ground and in the low vegetation (Schöning et al. 2008a). Nests are typically constructed in subterranean cavities and may contain several million individuals (Raignier & Van Boven 1955; Leroux 1982). Colonies are headed by a single, highly polyandrous queen, and reproduce by fission (Kronauer et al. 2004b). Our main study plot (2.3 × 1.8 km) was the area around the Chogoria Forest Station at the edge of the Mt Kenya Forest Reserve on the eastern slopes of Mt Kenya (0°10′ S, 37°30′ E) (electronic supplementary material, figure S1). We opened a total of 18 nests with shovels and successfully collected the queen on 10 occasions. Although this procedure strongly perturbs colonies, the impact is likely to be comparable to the most severe naturally occurring attacks by subterranean Dorylus (Typhlopone) army ants (Leroux 1982; Gotwald 1995) or chimpanzees, gorillas and pangolins when they open nest cavities to prey on adult ants and brood (Gotwald 1995; Schöning et al. 2008b). After queen removal, orphaned colonies were checked daily for foraging trails, emigrations, the production of males or colony fusions, without further disturbing them. After a colony fusion was suspected, the nest was opened and a sample of workers and brood was taken for genetic and cuticular chemistry analysis. Voucher specimens from this population have been deposited in the entomology collection of the Zoological Museum Copenhagen (Kronauer et al. 2006).
Two legs of the captured queens were removed and stored for genetic analyses. The queens’ head and thorax were washed in 900 µl pentane as described below for workers to study their cuticular hydrocarbons (CHCs). In a subset of colonies, three to five workers were collected for genetic and chemical analyses immediately before queen removal, one day after queen removal, and subsequently once a week until the colony merged or died/disappeared. If a colony fusion was suspected, 10 workers were collected from the putatively mixed colony. For all individuals whose cuticular profiles were analysed, we removed the left hind leg and stored it for genetic analyses to allow assignment of workers to one of two parental colonies in recently merged nests. To extract CHCs, each worker was washed in 300 µl pentane (HPLC grade, Sigma-Aldrich) for 10 min, after which the solvent was allowed to evaporate and the extract stored for later analysis.
Because colony emigrations of D. molestus are partly subterranean and not easy to follow, we relied on genetic microsatellite analyses to unambiguously demonstrate cases where an orphaned colony had merged with an intact colony. For this purpose, we sampled workers before or during each colony excavation and from the putatively mixed colonies. In most cases, we were also able to analyse worker samples from the adopting colonies that had been collected before the merger. At least one individual from each original colony was also sequenced for a stretch of mitochondrial DNA (see below). All samples for genetic analyses were preserved in 96 per cent ethanol upon collection.
We collected and analysed two further sets of samples to estimate the population-wide allele frequencies and the extent of population viscosity. The first set was used to estimate ‘local’ allele frequencies and consisted of one worker each from all 48 colonies found in the main study plot that were not involved in any colony fusions. The second set was used to estimate overall allele frequencies in a larger section of the entire eastern slope population of Mt Kenya and consisted of one worker each from 30 colonies that were sampled in regularly spaced intervals along an ‘altitudinal’ and a ‘horizontal’ transect of 22 km length each (electronic supplementary material, figure S1). We refer to this sample as the ‘overall’ population below. The highest and lowest points along the altitudinal transect were slightly above the Chogoria entrance gate to Mt Kenya National Park at 2936 m, and at 1663 m altitude about 4 km from the town of Chogoria (inside the main study plot). The altitudinal transect divided the horizontal transect (2026–2127 m altitude) in roughly two equal parts (electronic supplementary material, figure S1). All samples for background allele frequencies were genotyped for both nuclear microsatellites and mitochondrial DNA. Exact nest positions of all sampled colonies were recorded with a GPS. A total of 102 individuals were sequenced for mitochondrial DNA, 500 individuals were genotyped for microsatellites and 70 individuals were analysed for CHCs. An overview of samples analysed for the experimental colonies is given in table 1, and details of all analysed samples are given in the electronic supplementary material.
Table 1.
Overview of experimental colonies. Mitochondrial (mt) haplotypes are given for orphaned and adopting colonies, along with the number (n) of workers (w), queens (q) and males (m) genotyped for microsatellites in the orphaned, adopting and mixed colony, as well as the number of individuals in the mixed colony that were assigned to the orphaned and adopting colony, respectively. The number of individuals (n) analysed for CHCs and the time between queen removal and fusion are given in columns six and seven, respectively. See text for details on the case of JC26. Detailed information on analysed samples and colony behaviour is given in the electronic supplementary material.
| orphaned colony (mt haplotype) | adopting colony (mt haplotype) | n orphaned (microsats) | n adopting (microsats) | n mixed (microsats) (orphaned/adopting) | n CHCs | time to fusion (days) |
|---|---|---|---|---|---|---|
| Q12 (3) | JC2 (2) | 1q | 10w | 30w (9/21) | — | 5 |
| JC26 (2) | JC33 (2) | 10w; 1q | 10w | 30w (n.a.) | — | 5 |
| JC35 (1) | X1 (2) | 1q | — | 50w (17/33) | — | 5 |
| JC18 (2) | JC36 (2) | 1q | 20w | 26w (7/19) | 53w; 1q | 4 |
| Q4 (2) | X2 (3) | 15w; 1q | 15w | 20w (7/13) | — | 7 |
| Q2 (2) | X3 (3) | 15w; 1q | 15w | 20w (1/19) | — | 43 |
| Q1 (3) | X4 (2) | 1q | 2m | — | — | 10 |
| Q13 (2) | — | 30w; 1q; 31m | — | — | 15w; 1q | no fusion |
(b). Molecular protocols
DNA for microsatellite analysis was extracted by heating ant legs to 95°C for 15 min in 100 µl Chelex 100 (Bio-Rad) resin. We genotyped individuals for five microsatellite loci (DmoB, DmoC, DmoD, DmoG and DmoO), which have expected heterozygosities between 0.53 and 0.85 in the study population (for details, see Kronauer et al. 2004a).
Samples used for DNA sequencing were extracted with the DNeasy kit from QIAGEN. We amplified and sequenced parts of the mitochondrial cytochrome oxidase I gene (COI), an intergenic spacer, tRNA–Leu, and parts of the cytochrome oxidase II gene (COII) using PCR primers George and Barbara (Simon et al. 1994). The annealing temperature was 50°C, and we used a 2.5 mM concentration of MgCl2 in the PCR cocktail. PCR products were purified using the MicroSpin kit from Omega Bio-Tek and sent to a commercial facility (MWG-Biotech) for sequencing. Most PCR products were sequenced in both directions. We generated a final concatenated alignment of 781 bp. A single sample from the altitudinal transect yielded an apparently non-functional sequence, probably corresponding to a nuclear insertion of mitochondrial DNA (numt), as we have reported previously (Kronauer et al. 2007a). This sample was amplified and sequenced with primers Barbara and tRNALeu (Kronauer et al. 2007a) to give a functional mitochondrial sequence of 591 bp (haplotype 8 in the electronic supplementary material, table S1). An overview and GenBank accession numbers for all haplotypes are given in the electronic supplementary material (electronic supplementary material, table S1).
(c). Statistical analyses
Overall and local allele frequencies for both mitochondrial and nuclear markers were estimated using the program SpageDi 1.2 (Hardy & Vekemans 2002), based on the samples from the two transects and the study plot colonies that were not involved in fusions, respectively. The same program was used to calculate gene diversities for each dataset (Nei 1978) as well as pairwise Wright's fixation indices (Weir & Cockerham 1984) for both nuclear (FST) and mitochondrial (ΦST) markers between the overall population and the study plot. SPAGeDi 1.2 was also used to calculate regression slopes of pairwise genetic kinship coefficients (Loiselle et al. 1995; Ritland 1996) on the spatial distance between samples. Deviations of these regression slopes from zero were assessed using 20 000 permutations of individual locations and, for microsatellite markers, by jackknifing over loci. Reported p-values for regression slopes are from one-tailed tests. This analysis was conducted separately for mitochondrial and nuclear data for the study plot (using local background allele frequencies) and for the two transects separately and combined (using overall background allele frequencies).
Workers of supposedly fused colonies were assigned to matrilines using the maximum-likelihood approach implemented in the program Colony 1.2 (Wang 2004). Estimated local allele frequencies were used as fixed background allele frequencies. Queens of D. molestus mate with many males (Kronauer et al. 2004b) and were therefore defined as the polygamous sex. Allelic dropouts and other typing errors were accounted for in the analysis and error rates at all loci were set to 0.05 as default. The known genotypes of mother queens from queenless colonies were not included in this analysis, and worker offspring of known maternity (i.e. samples that had been collected from either colony before the fusion) were not a priori assigned to any queen. These data were only used afterwards to confirm the accuracy of deduced queen genotypes and worker assignments by Colony 1.2 (Wang 2004). Details of the separate analyses of all colony fusions are given in the electronic supplementary material.
Estimates of pairwise regression relatedness between the two queens of fused colonies were obtained for both the mitochondrial sequences and the nuclear loci using the program Relatedness v. 5.0.8 (Queller & Goodnight 1989; Goodnight & Queller 1998), once with the overall and once with the local background allele frequencies.
Male brood was considered worker-derived if it carried at least one allele that was not compatible with the genotype of the collected queen. The probability of detecting worker-derived males in a colony was calculated as the mean probability that a worker son carries at least one allele that is distinct from the queen genotype (Foster & Ratnieks 2001)
where n is the number of patrilines in a colony, pi is the proportional representation of each patriline among the sampled workers and li is the number of informative loci for the ith patriline, i.e. the number of loci where the single paternal allele is distinct from the two maternal alleles.
(d). Chemical analyses
The CHCs of individual samples were redissolved in 100 µl pentane, and 2 µl of this extract was injected into an Agilent 6890N gas chromatograph, equipped with an HP-5MS capillary column (30 m × 250 µm × 0.25 µm), a split-splitless injector and a 5975 Agilent mass spectrometer with 70 eV electron impact ionization. The carrier gas was helium at 1 ml min−1. After an initial hold of 1 min at 70°C, the temperature was increased to 180°C at a rate of 30°C min−1, and then to 320°C at 3°C min−1. Injections were performed with an Agilent automatic liquid sampler (7683B ALS).
We selected 50 compounds in the worker cuticular profiles that consistently appeared in all chromatograms. Compounds were identified by inspecting the mass spectra. For each profile, the area of each of the 50 peaks (figure 1a) was integrated. For use in principal component analysis (PCA), the peak areas were normalized using a Z-transformation to reduce the ‘closure’ effect, i.e. the relative contribution of one peak affecting the relative contribution of the rest of the compounds (Christensen & Tomasi 2007). The principal component scores were used for discriminant analysis to determine whether pre-defined groups could be separated by their chemical profiles. Multivariate statistical analyses were done with the program Statistica 7.1 (StatSoft Inc., USA).
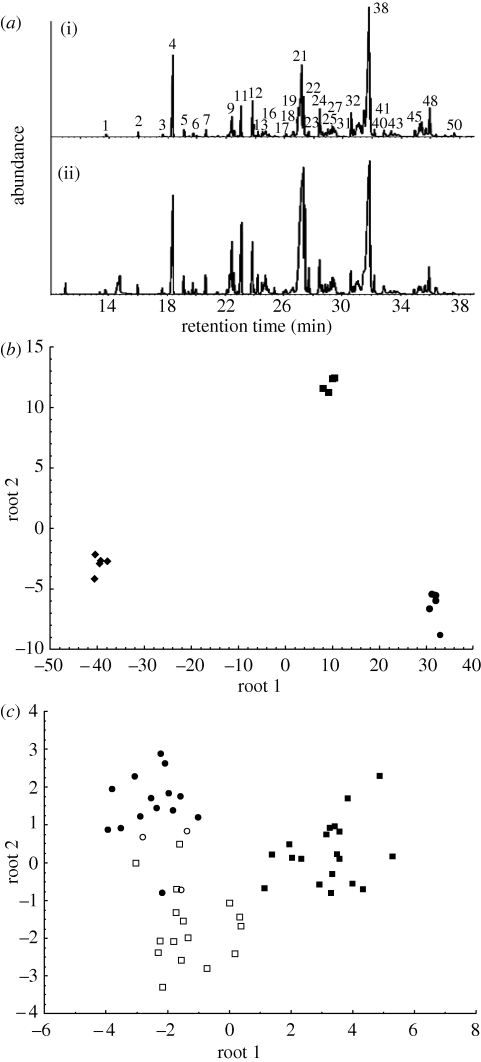
Figure 1.
(a) Representative gas chromatograms of (i) a worker and (ii) a queen of D. molestus. The chemical profile is characterized by 50 regularly occurring peaks, representing different classes of hydrocarbons ranging from a chain length of 21–31 carbon atoms. For identification of the compounds, see the electronic supplementary material, table S2. (b) Discriminant function analysis reveals that the CHC profiles of workers from three unmanipulated D. molestus colonies are clearly colony-specific (Wilks's Lambda = 0.00001, F18,6 = 100.31, p < 0.00001). Filled diamonds = Q13; filled squares = JC36; filled circles = JC18. (c) Discriminant function analysis based on the CHC profiles of workers from two colonies collected at different times before and after fusion. The CHC profiles of workers from colony JC18 before the fusion (filled circles), workers from colony JC36 before the fusion (filled squares) and workers in the new combined colony (open circles, daughter workers of the JC18 queen in the merged colony; open squares, daughter workers of the JC36 queen in the merged colony) are clearly distinct from each other (Wilks's Lambda = 0.054, F26,74 = 9.3424, p < 0.001).
To estimate the relative volatility of entire hydrocarbon profiles, weighted retention times were calculated by multiplying the average retention time of each peak with the relative peak area (i.e. the weight), and taking the sum of these values over the entire profile. Queen and worker profiles were compared by checking for qualitative differences and comparing the mean weighted retention times (van Zweden et al. 2009).
3. Results
We succeeded in removing the queen from 10 colonies. Subsequently, six of the queenless colonies merged with other colonies, one reared worker-produced male larvae, for one we suspected but were not able to unambiguously demonstrate a merger and two were lost during emigrations. Detailed accounts of the fates of the individual queenless colonies are given in the electronic supplementary material and a summary is given in table 1.
(a). Colony fusions—genetic markers
Colony fusions typically took place 4–10 days after queen removal (mean: 6.2 ± 2.39 s.d.; n = 5) and about a third of the workers in the mixed colony samples came from the orphaned colony (mean proportion: 0.32 ± 0.04 s.d.; n = 4). Four of the mixed colonies were sampled within 3 days after the fusion. One was sampled 55 days after the fusion but 35 per cent of the workers still came from the orphaned colony. In one apparently atypical case, colony fusion occurred 43 days after queen removal and only 5 per cent of the workers in the mixed colony sample came from the orphaned colony. If we assume no undetected sampling bias, these observed proportions of adopted workers in mixed colony samples should be a useful proxy for overall proportions of adopted and resident workers.
Of the six observed fusions, five clearly occurred between colonies without a shared maternal pedigree, because they differed in mitochondrial haplotype. In one case (the fusion between colonies JC18 and JC36), both the mitochondrial and microsatellite genotypes of the two queens were consistent with a mother–daughter relationship (identical mitochondrial haplotypes and at least one shared allele at each nuclear locus). In neither colony did we detect a patriline that could have produced the respective potential daughter queen, but precise reconstructions at this level remain somewhat uncertain as we have most likely not detected all patrilines in our limited worker sample (queens of this species typically mate with 10–30 males; Kronauer et al. 2004b). For a seventh colony (JC26) we suspected a fusion, but the evidence remained ambiguous, and we therefore excluded this case from further analysis (see the electronic supplementary material for details).
All estimates of average relatedness between the queens of fused colonies were negative, but the 95 per cent confidence intervals from jackknifing over groups included zero in all cases except for the mitochondrial estimate using the local background allele frequencies (overall background allele frequencies: nuclear (r = −0.01; CI: 0.20), mitochondrial (r = −0.06; CI: 0.56); local background allele frequencies: nuclear (r = −0.05; CI: 0.26), mitochondrial (r = −0.70; CI: 0.45).
(b). Colony fusions—cuticular hydrocarbons
CHCs of workers and queens were examined in the male-producing colony (Q13) and in one colony (JC18) that merged with another (JC36) four days after queen loss. For JC36, we also analysed worker CHC profiles over a period of four weeks leading up to the fusion. The chemical profile of D. molestus is complex and rich, being characterized by at least 50 hydrocarbons belonging to different structural classes (linear, branched and unsaturated hydrocarbons; figure 1a and electronic supplementary material, table S2). The CHC profiles of workers in unmanipulated colonies were clearly colony-specific (figure 1b) and thus provided the necessary variation to be used for nest-mate recognition. CHC profiles seem to change slightly after queen loss, but only over extended periods of time (as shown by the time series of worker CHC profiles over six weeks after queen removal from colony Q13; figure 2). This implies that the change in CHC profiles over the few days between orphanage and fusion (figure 2) is probably negligible. Importantly, the CHC profiles of workers from different colonies were still clearly distinct even several weeks after queen loss (figure 2). However, the CHC profiles of workers in the merged colony (JC36 + JC18) did change and became intermediate between the CHC profiles of the two original colonies in a matter of days (figure 1c). This shift is particularly significant in light of the constant CHC profiles of the adopting colony during the four weeks preceding the merger.
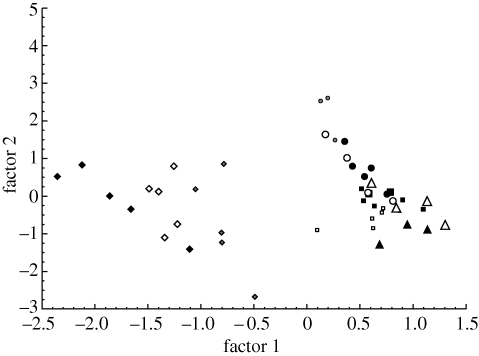
Figure 2.
PCA based on the CHC profiles of workers from experimental colonies before and after queen removal. Factors 1 and 2 explain 33.3 and 12% of the variance respectively. The data for colony JC36, a colony with repeated sampling without queen removal, are shown for comparison. Open triangles, JC36 on day 1; filled triangles, JC36 on day 8; filled squares, JC36 on day 14; open squares, JC36 on day 21; filled diamonds, Q13 with queen still present; open diamonds, Q13 1 day after queen removal; grey diamonds, Q13 six weeks after queen removal; filled circles, JC18 with queen still present; open circles, JC18 1 day after queen removal; grey circles, JC18 4 days after queen removal.
The mean weighted retention time of worker profiles before queen removal was longer than that of the queen (t-test for single means, p < 0.0001 in both colonies), but this was mostly due to quantitative and not qualitative differences between queens and workers. The mean weighted retention times of workers collected immediately before and one day, four days and six weeks after queen removal were not significantly different (ANOVA, p > 0.1 in both colonies). Data were normally distributed (Kolmogorov–Smirnoff test) and the variances homogeneous (Levene test). Since there is no difference in the profile of the queenless workers collected at different times after queen removal, we can assume that the queen does not significantly influence the chemical profile of the workers.
(c). Worker reproduction
One colony (Q13) stopped emigrating 12 days after queen removal and showed no obvious signs of colony fusion. We opened the nest 84 days after queen removal and found a total of 31 large male larvae and no further brood in the nest cavity. If we assume a developmental time of approximately 56 and 38 days for males and workers, respectively (Raignier 1972; estimates are for D. wilverthi, a closely related species), the colony should not have contained queen-derived brood anymore at this time. Of the 31 males, 24 were clearly worker-derived, while seven could potentially have been queen sons. The mean probability to detect a worker-derived male was 0.83, which is very close to the proportion of detected worker sons among all males (24/31 or 0.77), indicating that all collected males were likely worker-derived. All genotyped males had a single allele at each microsatellite locus and therefore appeared to be haploid, rather than being queen-produced diploid males because of matched matings at the sex-determining locus (Kronauer et al. 2007c). The probability of the alternative, a diploid individual being homozygous at all loci, was very low (p = 0.001 based on expected heterozygosities). A minimum of five worker patrilines was necessary to account for the male genotypes, which means that no single worker or single worker patriline had monopolized male production, and that probably many workers had joined in this reproductive effort. It is not clear whether this male brood would have been viable as it was collected at the larval/pupal stage. A brood of 31 males is notably small compared with what is typically observed and represents only about 1 per cent of the largest male broods raised by fully functional colonies (Raignier et al. 1974; Leroux 1982; C. Schöning 2001–2007, personal observation).
(d). Population structure
Strong and significant genetic differentiation between the overall eastern slope population and the study plot was evident for the mitochondrial marker (ΦST = 0.221; p < 0.0001; 20 000 random permutations). Despite the considerably larger sample size, alleles sampled from the study plot (n = 3) represented only a subset of the alleles sampled in the overall population (n = 8). Gene diversity was therefore considerably lower (0.407 in the study plot versus 0.848 in the overall population) (electronic supplementary material, table S1). Taken together, this clearly demonstrates that the alleles sampled from the study plot were not a random sample from the overall eastern slope population. In contrast, the estimate of differentiation at nuclear microsatellite loci was close to zero (FST = 0.007; p > 0.05; 20 000 random permutations), and gene diversity over loci was not significantly different between the two sets of samples (paired t-test: p > 0.05).
Regression analyses between spatial and mitochondrial distances were significant for the overall eastern slope sample and for the two transects when analysed separately (p < 0.05 for all analyses except when using the kinship estimator of Loiselle et al. 1995 for the horizontal transect). The regression analyses between spatial and mitochondrial distances within the study plot and all regression analyses between spatial and nuclear distances were not significant (all p > 0.05).
4. Discussion
Workers in orphaned colonies of many species of eusocial Hymenoptera raise a last batch of queen-derived reproductives and/or worker sons before colonies die (e.g. Forsyth 1981; Wenseleers & Ratnieks 2006; Dijkstra & Boomsma 2007; Smith et al. 2007). In some species, however, functionally monogynous colonies contain additional inseminated queens that can become reproductively active replacement queens after the original queen is lost (e.g. Tschinkel & Howard 1978). In honeybees (Apis mellifera) and some stingless bees, workers can even raise an emergency replacement queen if a suitable diploid brood is present at the time of queen death (Winston 1987; Faustino et al. 2002). Orphaned army ant colonies have never been observed to rear emergency replacement queens (Schneirla 1971; Raignier 1972), but workers possess functional ovaries (Whelden 1963; Gotwald & Schaefer 1982) and worker reproduction might therefore be a feasible option (Raignier 1972). However, we found that most of the investigated D. molestus colonies fused with neighbouring queenright colonies, rather than rearing worker sons.
Our data suggest that rapid changes in worker CHC profiles after queen loss do not occur and that colonies retain their original distinct Gestalt odour. This implies that pre-merger changes in CHC profiles are unlikely to provide the proximate mechanism that allows colonies to merge, as has been suggested by Schneirla (1971). We consider it more likely that worker behaviour changes significantly shortly after queen loss, which would be sufficient to explain colony fusions at a proximate level if all colonies accept non-aggressive workers irrespective of their CHC profiles. Workers in queenless fire ant colonies have reduced octopamine levels and as a consequence fail to discriminate between nest-mates and non-nest-mates (Vander Meer et al. 2008). Future studies will have to show whether similar changes contribute to colony fusions in army ants. The mixing of worker CHC profiles in merged colonies after fusion indicates that the label representing the colony odour is highly dynamic, which allows workers originating from two merging colonies to become chemically similar and thus fully integrated in a relatively short time. The same has recently been shown for Argentine ants (Linepithema humile) under laboratory conditions (Vásquez et al. 2009).
At the ultimate level, the benefits for the adopting colony seem obvious. We estimated that fusions increased colony size by about 50 per cent in our study (typically one-third of the sampled workers in fused colonies stemmed from the orphaned colony). This sudden and substantial increase in a colony's worker force will boost foraging efficiency, competitiveness and reproductive output (Bourke 1999), an advantage that incurs no cost as long as the newly adopted workers will not reproduce (Kikuchi et al. 2007). From the perspective of the orphaned workers, fusions could be explained by potential direct fitness benefits, potential indirect fitness benefits or as a non-selected by-product of another set of traits. In the paragraphs below, we discuss these alternative explanations.
Although ultimate proof is lacking, it seems highly unlikely that orphaned workers fuse with neighbours to gain direct fitness benefits via producing males in the adopting colony. First, males produced by queenright army ant colonies are exclusively sons of the queen and given the polyandrous mating system, it seems likely that, as in honeybees, an efficient worker-policing system is in place (Kronauer et al. 2006, 2007b). Second, army ants raise males only periodically and in discrete batches, so that any attempt by workers to reproduce will fail most of the time even if policing was not effective. The alternative, that they receive indirect fitness benefits by fusing with related colonies, seems more realistic. We will discuss three mechanisms that could account for positive relatedness between fusing colonies: (i) backtracking, (ii) recognition and preferential fusion with relatives, and (iii) population viscosity.
Upon queen loss, army ants develop backtracking columns along the route of previous emigrations (Schneirla 1949, 1971; Schneirla & Brown 1950). This behaviour is probably aimed at finding the queen in case she becomes separated from the colony during an emigration, but it also maximizes the chance for queenless colonies of getting into contact with their queenright sister colony after recent colony fission. This scenario could have applied in the ambiguous fusion of JC26, and this mechanism alone would lead to a significantly positive average relatedness between fusing colonies.
In addition to backtracking behaviour, orphaned workers could in theory seek out and recognize related colonies for fusion (Vásquez et al. 2009). That this is not the case is clearly demonstrated by the fact that most merging colonies had different mitochondrial haplotypes and therefore lacked recent maternal co-ancestry in five out of six unambiguous fusions.
A third alternative mechanism for creating significantly positive but low average relatedness between neighbouring colonies would be population viscosity (West-Eberhard 1975), which by itself can be sufficient to allow for the evolution of indiscriminate altruism towards neighbours (Gardner & West 2006; Lehmann et al. 2008). Because army ant queens are permanently wingless and new colonies originate by colony fission, gene flow is mainly mediated via the winged males that leave their natal colony to mate with young queens inside foreign nests. Populations are therefore expected to be highly genetically structured for mitochondrial markers, but much less so for nuclear markers. However, because the nomadic lifestyle of army ants implies that queens disperse on foot throughout their lives (Schöning et al. 2005), the magnitude of the overall sex-specific effect on gene flow remains to be assessed (e.g. Berghoff et al. 2008). Our data for Dorylus army ants demonstrate that viscosity at mitochondrial loci is substantial, even in continuous army ant populations, while male dispersal minimizes population structure at nuclear loci. Nevertheless, this implies that even by fusing with random neighbouring colonies, orphaned D. molestus workers are likely to gain some indirect fitness benefits, because the adopting colonies will, on average, be significantly related via the maternal side when compared with the larger background population.
Given these considerations, average relatedness between fusing colonies is expected to be small, and large and unrealistic sample sizes would probably have been needed to detect this effect statistically. This probably explains why our average relatedness estimates were not significantly different from zero. Assuming a standard deviation of 0.1 (based on our samples) and r = 0.05 between fusing colonies, a sample size of 45 fusions would have been needed to detect positive relatedness at α = 0.05 in a one sample t-test with a power of 0.95 (27 fusions for a power of 0.8; calculations were done with the program G * Power 3.1.0; Faul et al. 2007).
Alternatively, colony fusions could be a by-product of worker backtracking behaviour. Army ant workers are behaviourally and physiologically highly specialized altruists so that selection on traits allowing raiding parties to locate the colony fragment with the mother queen must have been strong. This implies that occasional errors of merging with an unrelated colony would not be selected against if there were no realistic alternative options to gain fitness benefits via worker male production. At the same time, queenright colonies would be strongly selected to accept such foreign orphaned workers (Bourke & Franks 1995).
The alternative to colony fusion for queenless army ant colonies would be to produce a last worker-derived male brood. In Eciton army ants, this outcome has not been observed, and colonies seem to either merge with other colonies or simply disintegrate and disappear (Schneirla 1949; Schneirla & Brown 1950). In the present study, worker reproduction occurred in only one out of eight cases, and this effort produced very few male larvae whose viability and reproductive options seem ambiguous at best. Raignier (1972) reported on three worker-derived male broods in queen-deprived colonies of the closely related species D. wilverthi, all of which were eventually cannibalized by the workers (see the electronic supplementary material for details). Thus, there seems little doubt that even if such broods could in principle mature, their probability of reaching that stage is extremely low because queenless colonies rapidly loose workers that are no longer replaced. Taken together, the data therefore suggest that worker reproduction in army ants is not a profitable option, even in queenless colonies, so that very small inclusive fitness benefits would suffice to make colony fusions after queen loss adaptive (of the order of r = 0.002–0.01 between orphaned workers and a reproductive brood produced by the adopting colony; see the electronic supplementary material).
We have demonstrated for the first time that orphaned doryline army ant colonies fuse with neighbouring colonies. This outcome appears to be considerably more frequent than raising a last small brood of worker-derived males, although a larger sample size would be needed to fully substantiate this. Documenting colony fusions has implications even beyond army ant life history and evolution. First, owing to fusions, the biomass and colony density of army ants will remain more constant than would otherwise be the case, which probably is important for the stability of predator–prey interactions between army ants and other invertebrates (Franks 1980). Second, colony fusions constitute an alternative mode of horizontal transmission for army ant-specific pathogens and myrmecophiles, which otherwise should either be transmitted strictly vertically during colony fission or disperse via the winged army ant males.
We hypothesize that there may be other eusocial insect species where colony mergers occur after queen death (e.g. Neumann et al. 2001; Vásquez et al. 2009), but that this possibility has remained understudied or has been dismissed because only data on captive colonies were available. Particularly when worker reproduction is not a profitable option (e.g. Dijkstra & Boomsma 2007 and the present study) and populations are spatially structured, there is no compelling reason to exclude the possibility that colony fusion after queen loss could have been selected for. It would therefore be rewarding to conduct similar field studies on other species and to use theoretical modelling to explore the parameter space in which colony fusions are adaptive.
Acknowledgements
We thank Sylvia Mathiasen for assistance with genotyping, Wanja Kinuthia for logistical support in Kenya and Volker Nehring for assistance with chemical data. We are grateful to Washington Njagi and Mwenda Tiraka for help during fieldwork and the Kenya Wildlife Service and the Kenyan Ministry of Education, Science and Technology for granting research permission. Financial support was provided by the Danish National Research Foundation and the Alexander von Humboldt-Foundation. Two anonymous referees and the editor helped to significantly improve the manuscript.
References
- Beekman M., Oldroyd B. P.2008When workers disunite: intraspecific parasitism in eusocial bees. Ann. Rev. Entomol. 53, 19–37 (doi:10.1146/annurev.ento.53.103106.093515) [DOI] [PubMed] [Google Scholar]
- Berghoff S. M., Kronauer D. J. C., Edwards K. J., Franks N. R.2008Dispersal and population structure of a New World predator, the army ant Eciton burchellii. J. Evol. Biol. 21, 1125–1132 (doi:10.1111/j.1420-9101.2008.01531.x) [DOI] [PubMed] [Google Scholar]
- Bourke A. F. G.1999Colony size, social complexity and reproductive conflict in social insects. J. Evol. Biol. 12, 245–257 (doi:10.1046/j.1420-9101.1999.00028.x) [Google Scholar]
- Bourke A. F. G., Franks N. R.1995Social evolution in ants Princeton, NJ: Princeton University Press [Google Scholar]
- Breed M. D., Bennett B.1987Kin recognition in highly eusocial insects. In Kin recognition in animals (eds Fletcher D. J. C., Michener C. D.), pp. 209–242 New York, NY: Wiley [Google Scholar]
- Christensen J. H., Tomasi G.2007Practical aspects of chemometrics for oil spill fingerprinting. J. Chromatogr. A 1169, 1–22 (doi:10.1016/j.chroma.2007.08.077) [DOI] [PubMed] [Google Scholar]
- Dijkstra M. B., Boomsma J. J.2007The economy of worker reproduction in Acromyrmex leafcutter ants. Anim. Behav. 74, 519–529 (doi:10.1016/j.anbehav.2006.11.020) [Google Scholar]
- Faul F., Erdfelder E., Lang A. G., Buchner A.2007G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 [DOI] [PubMed] [Google Scholar]
- Faustino C. D., Silva-Matos E. V., Mateus S., Zucchi R.2002First record of emergency queen rearing in stingless bees (Hymenoptera, Apinae, Meliponini). Insect. Soc. 49, 111–113 (doi:10.1007/s00040-002-8287-x) [Google Scholar]
- Forsyth A.1981Sex ratio and parental investment in an ant population. Evolution 35, 1252–1253 (doi:10.2307/2408139) [DOI] [PubMed] [Google Scholar]
- Foster K. R., Ratnieks F. L. W.2001Convergent evolution of worker policing by egg eating in the honeybee and common wasp. Proc. R. Soc. Lond. B 268, 169–174 (doi:10.1098/rspb.2000.1346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. R. 1980. The evolutionary ecology of the army ant Eciton burchelli on Barro Colorado Island, Panama. PhD thesis, University of Leeds, UK.
- Gardner A., West S. A.2006Demography, altruism, and the benefits of budding. J. Evol. Biol. 19, 1707–1716 (doi:10.1111/j.1420-9101.2006.01104.x) [DOI] [PubMed] [Google Scholar]
- Goodnight K. F., Queller D. C. Relatedness 5.0.8. Goodnight Software. Houston, TX: 1998. See http://www.gsoftnet.us/GSoft.html . [Google Scholar]
- Gotwald W. H., Jr1995Army ants: the biology of social predation Ithaca, NY: Cornell University Press [Google Scholar]
- Gotwald W. H., Jr, Schaefer R. F., Jr1982Taxonomic implications of doryline worker ant morphology: Dorylus subgenus Anomma (Hymenoptera: Formicidae). Sociobiology 7, 187–204 [Google Scholar]
- Hamilton W. D.1964The genetical evolution of social behaviour, I & II. J. Theor. Biol. 7, 1–52 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- Hardy O. J., Vekemans X.2002SpageDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2, 618–620 (doi:10.1046/j.1471-8286.2002.00305.x) [Google Scholar]
- Helanterä H., Strassmann J. E., Carrillo J., Queller D. C.2009Unicolonial ants: where do they come from, what are they and where are they going? Trends Ecol. Evol. 24, 341–349 (doi:10.1016/j.tree.2009.01.013) [DOI] [PubMed] [Google Scholar]
- Hölldobler B., Wilson E. O.2009The superorganism: the beauty, elegance, and strangeness of insect societies London, UK: W. W. Norton & Co [Google Scholar]
- Kikuchi T., Tsuji K., Ohnishi H., Le Breton J.2007Caste-biased acceptance of non-nestmates in a polygynous ponerine ant. Anim. Behav. 73, 559–565 (doi:10.1016/j.anbehav.2006.04.015) [Google Scholar]
- Kronauer D. J. C., Boomsma J. J., Gadau J.2004aMicrosatellite markers for the driver ant Dorylus (Anomma) molestus. Mol. Ecol. Notes 4, 289–290 (doi:10.1111/j.1471-8286.2004.00645.x) [Google Scholar]
- Kronauer D. J. C., Schöning C., Pedersen J. S., Boomsma J. J., Gadau J.2004bExtreme queen-mating frequency and colony fission in African army ants. Mol. Ecol. 13, 2381–2388 (doi:10.1111/j.1365-294X.2004.02262.x) [DOI] [PubMed] [Google Scholar]
- Kronauer D. J. C., Schöning C., Boomsma J. J.2006Male parentage in army ants. Mol. Ecol. 15, 1147–1151 (doi:10.1111/j.1365-294X.2005.02850.x) [DOI] [PubMed] [Google Scholar]
- Kronauer D. J. C., Schöning C., Vilhelmsen L., Boomsma J. J.2007aA molecular phylogeny of Dorylus army ants provides evidence for multiple evolutionary transitions in foraging niche. BMC Evol. Biol. 7, 56 (doi:10.1186/1471-2148-7-56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronauer D. J. C., Rodríguez Ponce E. R., Lattke J. E., Boomsma J. J.2007bSix weeks in the life of a reproducing army ant colony: male parentage and colony behaviour. Insect. Soc. 54, 118–123 (doi:10.1007/s00040-007-0919-8) [Google Scholar]
- Kronauer D. J. C., Johnson R. A., Boomsma J. J.2007cThe evolution of multiple mating in army ants. Evolution 61, 413–422 (doi:10.1111/j.1558-5646.2007.00040.x) [DOI] [PubMed] [Google Scholar]
- Lehmann L., Keller L.2006The evolution of cooperation and altruism—a general framework and classification of models. J. Evol. Biol. 19, 1365–1376 (doi:10.1111/j.1420-9101.2006.01119.x) [DOI] [PubMed] [Google Scholar]
- Lehmann L., Ravigné V., Keller L.2008Population viscosity can promote the evolution of altruistic sterile helpers and eusociality. Proc. R. Soc. B 275, 1887–1895 (doi:10.1098/rspb.2008.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir A., Fresneau D., Errard C., Hefetz A.1999The individuality and the colonial identity in ants: the emergence of the social representation concept. In Information processing in social insects (eds Detrain C., Deneubourg J. L., Pasteels J.), pp. 219–237 Basel, Switzerland: Birkhäuser Verlag [Google Scholar]
- Leroux J. M.1982Ecologie des populations de dorylines Anomma nigricans dans la région de Lamto (Côte d'Ivoire) Publications du Laboratoire de Zoologie, no. 22 Paris, France: Ecole Normale Supérieure [Google Scholar]
- Lin N., Michener C. D.1972Evolution of sociality in insects. Quart. Rev. Biol. 47, 131–159 [Google Scholar]
- Loiselle B. A., Sork V. L., Nason J., Graham C.1995Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). Am. J. Bot. 82, 1420–1425 (doi:10.2307/2445869) [Google Scholar]
- Nei M.1978Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89, 583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P., Pirk C. W. W., Hepburn R., Radloff S. E.2001A scientific note on the natural merger of two honeybee colonies (Apis mellifera capensis). Apidologie 32, 113–114 (doi:10.1051/apido:2001116) [Google Scholar]
- Queller D. C., Goodnight K. F.1989Estimating relatedness using genetic markers. Evolution 43, 258–275 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- Raignier A.1972Sur l'origine des nouvelles sociétés des fourmis voyageuses Africaines (Hymenopteres, Formicidae, Dorylinae). Insect. Soc. 19, 153–170 (doi:10.1007/BF02226624) [Google Scholar]
- Raignier A., van Boven J. K. A.1955Étude taxonomique, biologique et biométrique des Dorylus du sous-genre Anomma (Hymenoptera Formicidae). Annales Musée Royal du Congo Belge Nouvelle Série in Quarto Sciences Zoologiques 2, 1–359 [Google Scholar]
- Raignier A., van Boven J. K. A., Ceusters R.1974Der Polymorphismus der afrikanischen Wanderameisen unter biometrischen und biologischen Gesichtspunkten. In Sozialpolymorphismus bei Insekten. Probleme der Kastenbildung im Tierreich (ed. Schmidt G. H.), pp. 668–693 Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft [Google Scholar]
- Ritland K.1996Estimators for pairwise relatedness and individual inbreeding coefficients. Genet. Res. Camb. 67, 175–185 (doi:10.1017/S0016672300033620) [Google Scholar]
- Schneirla T. C.1949Army-ant life and behavior under dry-season conditions. 3. The course of reproduction and colony behavior. Bull. Am. Mus. Nat. Hist. 94, 1–82 [Google Scholar]
- Schneirla T. C.1971Army ants. A study in social organization San Francisco, CA: W. H. Freeman and Company [Google Scholar]
- Schneirla T. C., Brown R. Z.1950Army-ant life and behavior under dry-season conditions. 4. Further investigation of cycle progresses in behavioral and reproductive functions. Bull. Am. Mus. Nat. Hist. 95, 263–354 [Google Scholar]
- Schöning C., Njagi W. M., Franks N. R.2005Temporal and spatial patterns in the emigrations of the army ant Dorylus (Anomma) molestus in the montane forest of Mt Kenya. Ecol. Entomol. 30, 532–540 (doi:10.1111/j.0307-6946.2005.00720.x) [Google Scholar]
- Schöning C., Njagi W., Kinuthia W.2008aPrey spectra of two swarm-raiding army ant species in East Africa. J. Zool. 274, 85–93 [Google Scholar]
- Schöning C., Humle T., Möbius Y., McGrew W. C.2008bThe nature of culture: technological variation in chimpanzee predation on army ants revisited. J. Hum. Evol. 55, 48–59 (doi:10.1016/j.jhevol.2007.12.002) [DOI] [PubMed] [Google Scholar]
- Simon C., Frati F., Beckenbach A., Crespi B., Liu H., Flook P.1994Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87, 651–701 [Google Scholar]
- Smith C. R., Schoenick C., Anderson K. E., Gadau J., Suarez A. V.2007Potential and realized reproduction by different worker castes in queen-less and queen-right colonies of Pogonomyrmex badius. Insect. Soc. 54, 260–267 (doi:10.1007/s00040-007-0940-y) [Google Scholar]
- Sumner S., Lucas E., Barker J., Isaac N.2007Radio-tagging technology reveals extreme nest-drifting behavior in a eusocial insect. Curr. Biol. 17, 140–145 (doi:10.1016/j.cub.2006.11.064) [DOI] [PubMed] [Google Scholar]
- Tschinkel W. R., Howard D. F.1978Queen replacement in orphaned colonies of the fire ant. Solenopsis invicta. Behav. Ecol. Sociobiol. 3, 297–310 (doi:10.1007/BF00296315) [Google Scholar]
- Vander Meer R. K., Morel L.1998Nestmate recognition in ants. In Pheromone communication in social insects (eds Vander Meer R. K., Breed M. D., Espelie K. E., Winston M. L.), pp. 79–103 Boulder, CO: Westview Press [Google Scholar]
- Vander Meer R. K., Preston C. A., Hefetz A.2008Queen regulates biogenic amine level and nestmate recognition in workers of the fire ant Solenopsis invicta. Naturwissenschaften 95, 1155–1158 (doi:10.1007/s00114-008-0432-6) [DOI] [PubMed] [Google Scholar]
- van Zweden J. S., Heinze J., Boomsma J. J., d'Ettorre P.2009Ant queen egg-marking signals: matching deceptive laboratory simplicity with natural complexity. PLoS One 4, e4718 (doi:10.1371/journal.pone.0004718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez G. M., Schal C., Silverman J.2009Colony fusion in Argentine ants is guided by worker and queen cuticular hydrocarbon profile similarity. J. Chem. Ecol. 35, 922–932 (doi:10.1007/s10886-009-9656-y) [DOI] [PubMed] [Google Scholar]
- Wang J.2004Sibship reconstruction from genetic data with typing errors. Genetics 166, 1963–1979 (doi:10.1534/genetics.166.4.1963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir B. S., Cockerham C. C.1984Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 (doi:10.2307/2408641) [DOI] [PubMed] [Google Scholar]
- Wenseleers T., Ratnieks F. L. W.2006Enforced altruism in insect societies. Nature 444, 50 (doi:10.1038/444050a) [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J.1975The evolution of social behavior by kin selection. Quart. Rev. Biol. 50, 1–33 [Google Scholar]
- Wheeler W. M.1911The ant-colony as an organism. J. Morphol. 22, 307–325 (doi:10.1002/jmor.1050220206) [Google Scholar]
- Whelden R. M.1963The anatomy of the adult queen and workers of the army ants Eciton burchelli Westwood and Eciton hamatum Fabricius. J. NY Entomol. Soc. 71, 158–178 [Google Scholar]
- Winston M. L.1987The biology of the honeybee. Cambridge, MA: Harvard University Press [Google Scholar]