Abstract
The hypothesis that plants supplied with organic fertilizers are better defended against insect herbivores than those supplied with synthetic fertilizers was tested over two field seasons. Organic and synthetic fertilizer treatments at two nitrogen concentrations were supplied to Brassica plants, and their effects on the abundance of herbivore species and plant chemistry were assessed. The organic treatments also differed in fertilizer type: a green manure was used for the low-nitrogen treatment, while the high-nitrogen treatment contained green and animal manures. Two aphid species showed different responses to fertilizers: the Brassica specialist Brevicoryne brassicae was more abundant on organically fertilized plants, while the generalist Myzus persicae had higher populations on synthetically fertilized plants. The diamondback moth Plutella xylostella (a crucifer specialist) was more abundant on synthetically fertilized plants and preferred to oviposit on these plants. Glucosinolate concentrations were up to three times greater on plants grown in the organic treatments, while foliar nitrogen was maximized on plants under the higher of the synthetic fertilizer treatments. The varying response of herbivore species to these strong differences in plant chemistry demonstrates that hypotheses on defence in organically grown crops have over-simplified the response of phytophagous insects.
Keywords: agro-ecology, Brassica oleracea var. capitata, glucobrassicin; nitrogen, sinigrin, sustainable agriculture
1. Introduction
Advocates of organic farming have suggested that the use of alternatives to synthetic fertilizers results in lower populations of herbivorous pest species. Organic fertilizers such as animal manures contain nitrogen sources that are released over a longer time scale than the nitrogen in synthetic fertilizers and thus are less readily available to the host plant (Eigenbrode & Pimentel 1988; Phelan et al. 1995, 1996; Hsu et al. 2009). Results from field trials and farm comparisons testing this hypothesis are inconsistent: some studies show a reduction of herbivore populations or performance on plants fertilized with organic compared with synthetic fertilizers (Alyokhin et al. 2005; Ponti et al. 2007), and others show no significant fertilizer effect (Costello & Altieri 1995; Letourneau et al. 1996; Bengtsson et al. 2005) or lower populations under synthetic fertilizer treatments (Culliney & Pimentel 1986). A meta-analysis found no significant effect of crop production system on herbivore species abundance, though the trend was towards higher populations under conventional production (Bengtsson et al. 2005). Apart from Costello & Altieri (1995), the studies above assessed the abundance, preference or performance of just one herbivore species or presented data from a single year of a field trial, so the hypothesis of lower herbivore abundance under organic fertilizers had not been rigorously tested.
Primary metabolites such as nitrogen (Mattson 1980) and secondary metabolites (e.g. glucosinolates; Hopkins et al. 2009) are important attributes of plant quality, which often mediate interactions between insect herbivores and plants. Eigenbrode & Pimentel (1988) found no effect of manure and synthetic fertilizers on concentration of total glucosinolates, while a study that measured a single glucosinolate, sinigrin, found an increase in Brassicas grown in pots in organic compared with synthetic fertilizer treatments (Hsu et al. 2009). As few studies have conducted chemical analyses in addition to assessments of insect abundance under organic and conventional fertilizer treatments, it is not possible to generalize about the possible chemical mechanisms that might explain effects on insect herbivore species.
Glucosinolates are widely considered to play a defensive role in Brassica–herbivore relationships (Bradburne & Mithen 2000) and to have a negative effect on generalist insect herbivores (Hopkins et al. 2009), though some generalist species are able to tolerate or detoxify some glucosinolates (Poelman et al. 2008a). Brassica specialists have very specific interactions with glucosinolates; some compounds act as oviposition and feeding stimulants (Renwick et al. 2006) while others delay larval growth (Hopkins et al. 2009). Some specialist Brassica feeders have evolved detoxification mechanisms (Ratzka et al. 2002; Wittstock et al. 2004).
In the current study, we hypothesized that herbivore populations and foliar nitrogen concentration would be greater on synthetically fertilized plants (Phelan et al. 1995), which would have lower glucosinolate concentrations than organically fertilized plants (Hsu et al. 2009). Organic and synthetic fertilizer treatments were applied at two equivalent levels of total nitrogen to Brassica field plots. This allowed us to differentiate between effects of fertilizer quantity and fertilizer type, as many previous studies have compared synthetic fertilizers that have high total nitrogen concentration with an organic fertilizer that has low nitrogen concentration. The abundance of the dominant herbivorous insect species was assessed over two field seasons, and compared with foliar nitrogen and glucosinolate concentrations. The above evidence on the importance of nitrogen and glucosinolates in Brassica–herbivore interactions meant that these chemical factors were highly likely to be causal in effect, though the influence of other components of plant chemistry (e.g. proteinase inhibitors) cannot be discounted.
2. Material and Methods
(a). Field trial
(i). Experimental design
The field trial was conducted on an experimental farm at the University of Reading, UK (51°24′ N, 0°57′ W) in 2007 and 2008, on a grassland site not cultivated for 20 years. The soil was rotovated to 30 cm three times during the summer preceding the experiment. Sixteen 6 × 6 m plots were laid out in 4 rows × 4 columns separated by 1-m-wide paths. Fertilizer treatments were applied in a randomized four block design. Trifolium repens var. Milvus seeds (108 g) were sown on the organic fertilizer treatment plots each preceding September, to provide a green manure crop. Green manure samples were collected from these plots in mid-April each year by clipping all the vegetation within three 20 × 20 cm quadrats at ground level. Samples were dried at 80°C for 72 h and total nitrogen analysed by a Kjeldahl analysis (AOAC 1995), enabling us to calculate the amount of total nitrogen being provided by the green manure crop.
Fertilizer treatments were added in early May each year: a conventional high fertilizer treatment (ammonium nitrate (Nitram) at 200 kg nitrogen per hectare); a conventional low fertilizer treatment (ammonium nitrate at 100 kg nitrogen per hectare); an organic high-input treatment (green manure plus Greenvale (Yorkshire, UK) organic chicken manure pellets to provide approx. 200 kg nitrogen per hectare in total); and an organic low-input treatment (green manure only, at approx. 100 kg nitrogen per hectare). After fertilizer addition the plots were rotovated again to 30 cm. A drip irrigation system (T Tape, San Diego, USA) supplemented rainfall.
(ii). Plant cultivation
Brassica oleracea var. capitata cv. Derby Day seeds (Tozer Seeds, Sussex, UK) were sown in low-nutrient coir seed compost (Fertile Fibre, Herefordshire, UK) in mid-April each year and grown in an unheated polytunnel. Four weeks later seedlings were transplanted to experimental plots at nine plants per square metre (324 per plot). Plots were hand-weeded each week throughout each field season.
(iii). Insect abundance
Herbivorous insects were counted on 10 randomly selected plants from each plot every week from mid-May to mid-August. Insects were counted on the stem, shoot and both surfaces of each leaf. The majority of aphid and Lepidoptera species were identified in the field. Where necessary, a minority of Lepidoptera larvae were collected and reared through to adults on Brassica foliage in the laboratory, to confirm identification.
(iv). Plant biomass
Three plants from each plot were cut at soil level in mid-June, mid-July and mid-August of each year. Each plant was weighed and dried at 80°C for 72 h to obtain fresh and dry biomass.
(v). Foliar nitrogen content
Foliage from the three plants per plot used for biomass measurements was combined to give one sample per plot on each occasion for foliar nitrogen concentration. Samples were milled through a 1-mm-diameter mesh. Total nitrogen concentration was determined as above.
(vi). Foliar glucosinolate content
The fourth oldest leaf was collected from each of five plants per plot in mid-June 2007, and mid-June, mid-July and mid-August 2008. Due to technical problems we were unable to process foliar samples from later in 2007. The five leaves from each plot formed one sample on each occasion. Samples were placed on ice packs and were frozen in liquid nitrogen within 2 h, prior to being stored at −20°C. Leaves were freeze-dried and milled as above. Glucosinolates were separated and individual compounds identified and quantified (Heaney et al. 1986). Desulphoglucosinolates were extracted as detailed by Kazana et al. (2007). Samples were analysed by high-performance liquid chromatography (HPLC; Agilent 1200 series with a Phenomenex Luna 3 µm C18(2) (150 × 2 mm) reverse phase column). Desulphoglucosinolates were separated using a water–acetonitrile gradient. Retention times of standards were used to identify desulphoglucosinolates and identification confirmed by liquid chromatography-mass spectrometry (LC-MS).
(b). Plutella xylostella oviposition preference (caged field experiments)
Two experiments were conducted: (i) oviposition choice between plants grown in three types of fertilizer; and (ii) oviposition choice between plants grown in four concentrations of ammonium nitrate. Each experiment was replicated 10 times. Brassica oleracea seeds were sown in seed compost as above. Two weeks later, seedlings were transplanted to pots (13 × 12 cm) in compost consisting of 33 per cent peat, 33 per cent loam, 22 per cent sand and 12 per cent grit by volume (Monro Horticulture, Chichester, UK). The three fertilizer type treatments consisted of the addition of 9.28 g ammonium nitrate, 62.8 g John Innes fertilizer (Monro Horticulture, Kent, UK) or 74.5 g organic chicken manure (Greenvale) to 10 l of compost prior to transplanting. This provided 0.32 g total nitrogen per litre of potting compost per treatment. The fertilizer concentration treatments involved the addition of 13.92, 9.28, 4.64 or 0 g of ammonium nitrate to 10 l of compost. The total nitrogen added to each fertilizer treatment in oviposition experiment (i) and the intermediate (9.28 g) ammonium nitrate treatment in experiment (ii) was approximately equivalent to the nitrogen applied to the high conventional and high organic treatments in the field trial. The quantity added to the low (4.64 g) ammonium nitrate treatment in experiment (ii) was equivalent to the nitrogen applied to the two low fertilizer field trial treatments. Plants were grown for four weeks before being used for bioassays.
For each experimental replicate, three (experiment (i)) or four (experiment (ii)) plants were placed in a cage made of a stainless steel frame (KeeKlamp, Berkshire, UK) with Tygan netting sides (2 × 2 × 2 m; mesh size 1 mm) at the field site above, which allowed P. xylostella to receive natural light and temperature cues. Plants were randomized within the cages. Ten unmated female and ten male P. xylostella, both groups emerged in the previous 24 h, were released in the centre of the cage equidistant from the plants on 16th July 2007 for experiment (i) and on 21st August 2007 experiment (ii). Cotton wool soaked in 20 per cent honey solution was placed in the cage to provide a food source. The number of eggs laid on each plant was recorded 72 h later.
(i). Statistical analyses
Values for individual plants were summed to provide a value per treatment plot for each date, as plot was the level of replication. Mixed effects linear models (Crawley 2007) were used to test the effects of the field experiment fertilizer treatments on herbivore abundance, plant biomass, foliar nitrogen and foliar glucosinolate concentrations within each year of the field trial, with the exception of glucosinolate data from 2007, where ANOVA was used. Time-points were included as a random repeated measures factor, and block also included as a random factor (Crawley 2007). Chemical and biomass data were natural-log-transformed prior to analysis approximating a normal distribution and plant herbivore (count) data were analysed using a Poisson distribution. Spearman's rank correlation tests were conducted to test whether there was a relationship between the number of alate B. brassicae and M. persicae on each plant, to investigate potential competitive effects on aphid colonization of plants. Goodness-of-fit (G) tests were used to test the effect of fertilizer type and fertilizer quantity on egg frequency for the oviposition preference experiments (Sokal & Rohlf 1995). Analyses were conducted using R v. 2.7.2 (R Team 2006).
3. Results
(a). Field trial
(i). Insect abundance
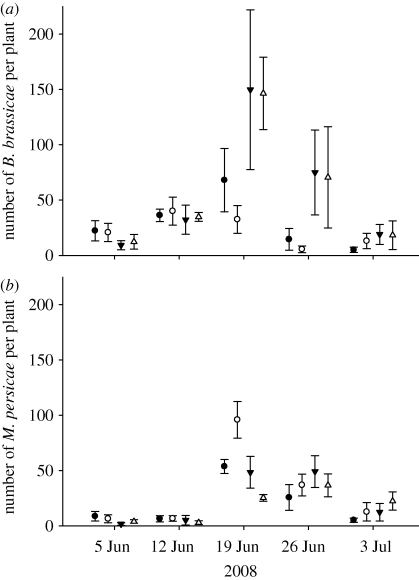
The most common insect herbivore species found were the aphids Brevicoryne brassicae L. (Homoptera: Aphididae) and Myzus persicae Sulzer (Homoptera: Aphididae), and the moth P. xylostella. Brevicoryne brassicae was more abundant in the high fertilizer treatments in both years (2007: fertilizer level z = 2.36, p < 0.05; 2008: fertilizer level z = 10.86, p < 0.001; figure 1a). In 2008, B. brassicae were also more abundant on the organically fertilized plants (fertilizer type: z = 6.64, p < 0.001). There was a significant interaction between fertilizer type and fertilizer level in 2008, as under conventional treatments there were fewer B. brassicae on the low conventional fertilizer treatment compared with the high conventional treatment, while fertilizer level did not affect abundance under the two organic treatments (fertilizer type * fertilizer level: z = 8.68, p < 0.001; figure 1a). Myzus persicae was more abundant on the high fertilizer treatments in 2007 (fertilizer level z = 3.80, p < 0.001). In 2008, the strongest effect on M. persicae abundance was an interaction between fertilizer type and level, as M. persicae were most abundant on the conventional low fertilizer treatment, and least abundant on the organic high treatment (fertilizer type * fertilizer level: z = 8.09, p < 0.001; figure 1b). There was a very weak significant correlation between the occurrence of B. brassicae and M. persicae alates on plants in 2007 (Spearman's correlation coefficient = 0.071, p = 0.002), but this is unlikely to have biological significance due to the small correlation coefficient. In 2008, there was no correlation between the number of B. brassicae and M. persicae alates (Spearman's correlation coefficient = 0.016, p = 0.520).
Figure 1.
Mean (± standard error) number of (a) B. brassicae and (b) M. persicae per B. oleracea plant growing in conventional high nitrogen (filled circle), conventional low nitrogen (open circle), organic high fertilizer (filled down triangle) and organic low input (open triangle) field trial treatments in 2008. Results from 2007 are not shown as aphid populations were very low (maximum M. persicae per plant = 39), and there were no significant differences between the organic and conventional treatments for either species in 2007.
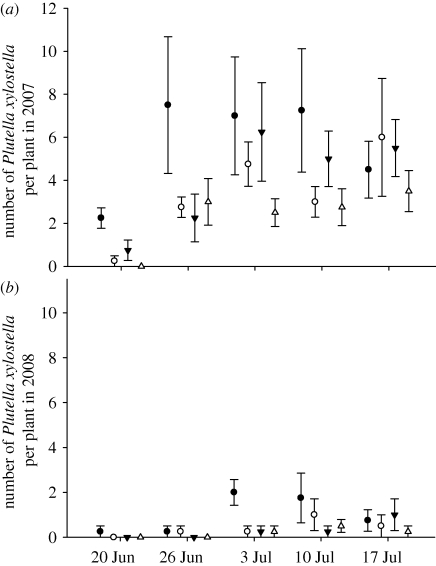
Plutella xylostella were more abundant on conventionally than organically fertilized plants in both years (2007: fertilizer type z = 2.50, p < 0.05; 2008: fertilizer type z = 2.34, p < 0.05; figure 2) and in 2007 P. xylostella were less abundant on plants in the low fertilizer treatments (z = 3.45, p < 0.001). Other Lepidoptera (Mamestra brassicae, Pieris rapae and Pieris brassicae) were present in low numbers in one or both years of the field trial, but were too scarce to analyse abundance statistically.
Figure 2.
Number (mean ± standard error) of P. xylostella per B. oleracea var capitata plant in conventional high nitrogen (filled circle), conventional low nitrogen (open circle), organic high fertilizer (filled down triangle) and organic low input (open triangle) field trial treatments in (a) 2007 and (b) 2008.
(ii). Plant biomass
No fertilizer treatment had a significant effect on plant dry biomass in either year (table 1).
Table 1.
Generalized linear mixed model results for the effects of fertilizer type (conventional versus organic) and fertilizer level (high or low nitrogen) on B. oleracea var capitata above-ground dry biomass and foliar nitrogen and glucosinolate content.
| plant biomass | nitrogen | glucoiberin (3-methyl sulfinylpropyl) | sinigrin (2-propenyl) | glucobrassicin (3-indolyl methyl) | 4-methoxy glucobrassicin (4-methoxy-3-indolylmethyl) | neoglucobrassicin (1-methoxy-3-indolylmethyl) | ||
|---|---|---|---|---|---|---|---|---|
| 2007 | fertilizer type | t9 = 1.28 | t9 = 6.21*** | F1,9 = 15.73** | F1,9 = 24.95*** | F1,9 = 85.72*** | F1,9 = 57.72*** | F1,9 = 12.47** |
| fertilizer level | t9 = 1.47 | t9 = 4.47** | F1,9 = 0.03 | F1,9 = 0.004 | F1,9 = 2.95 | F1,9 = 0.27 | F1,9 = 0.30 | |
| fertilizer type * level | t9 = 0.46 | t9 = 3.72** | F1,9 = 0.54 | F1,9 = 0.002 | F1,9 = 0.73 | F1,9 = 3.81 | F1,9 = 1.40 | |
| 2008 | fertilizer type | t9 = 1.01 | t9 = 6.21*** | t9 = 4.64** | t9 = 4.08** | t9 = 4.04** | t9 = 2.98* | t9 = 1.07 |
| fertilizer level | t9 = 0.76 | t9 = 4.47** | t9 = 2.62* | t9 = 1.13 | t9 = 0.12 | t9 = 2.54* | t9 = 1.14 | |
| fertilizer type * level | t9 = 0.86 | t9 = 3.72** | t9 = 2.01 | t9 = 1.31 | t9 = 0.24 | t9 = 2.81* | t9 = 1.10 |
* p < 0.05.
** p < 0.01.
*** p < 0.001.
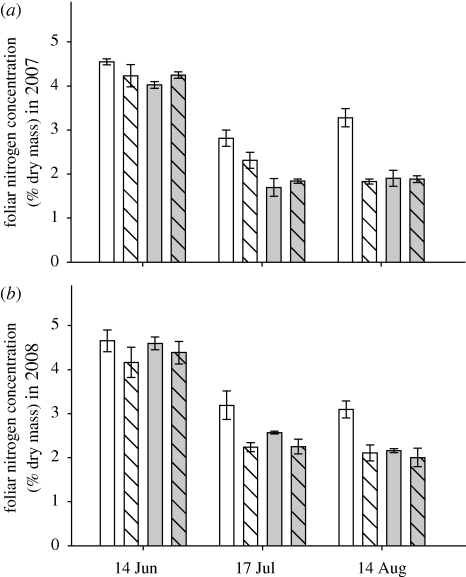
(iii). Foliar nitrogen content
Nitrogen concentration was greater for conventionally fertilized than organically fertilized B. oleracea in both years (figure 3, table 1). There was a significant interaction between fertilizer type and level in both years, as among conventionally fertilized plants there were higher concentrations of nitrogen in the high fertilizer treatments compared with the conventional low fertilizer treatment, while it did not differ between the two organic treatments (figure 3).
Figure 3.
Foliar nitrogen concentration (mean ± standard error, % dry mass) of B. oleracea growing in conventional high nitrogen (white bar), conventional low nitrogen (hatched white bar), organic high fertilizer (grey bar) and organic low input (hatched grey bar) treatments in (a) 2007 and (b) 2008.
(iv). Foliar glucosinolate content
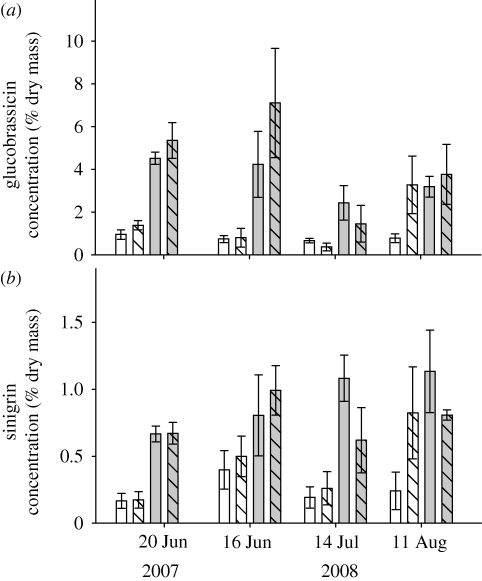
Five glucosinolate compounds were identified: glucoiberin, sinigrin, glucobrassicin, 1-methoxyglucobrassicin and 4-methoxyglucobrassicin. All glucosinolates were more abundant in the organically fertilized plants than the conventionally fertilized plants in both years, except for 1-methoxyglucobrassicin in 2008 (table 1). Differences in glucosinolate concentrations were substantial, with up to three times greater concentrations in organically fertilized plants (figure 4). In addition to effects of fertilizer type, in 2008 glucoiberin was present in greater concentrations in plant foliage under the low fertilizer treatments (table 1).
Figure 4.
Foliar glucosinolate concentration (mean ± standard error, % dry mass) of B. oleracea growing in conventional high nitrogen (white bar), conventional low nitrogen (hatched white bar), organic high fertilizer (grey bar) and organic low input (hatched grey bar) treatments. (a) Glucobrassicin (3-indolylmethyl) and (b) sinigrin.
(b). Plutella xylostella oviposition preference (caged field experiments)
Plutella xylostella laid significantly more eggs on plants fertilized with ammonium nitrate, compared with those fertilized with chicken manure or John Innes fertilizer (table 2). In experiment (ii) significantly more eggs were laid on those fertilized with an intermediate concentration of ammonium nitrate than those that were unfertilized or fertilized with a high or low concentration of ammonium nitrate (table 2).
Table 2.
Number of eggs laid when a P. xylostella female was given the choice between B. oleracea plants grown in (i) different types of fertilizer and (ii) different concentrations of ammonium nitrate fertilizer. GP measures treatment (pooled) difference between all replicates and GH measures heterogeneity between replicates within one experiment.
| replicated goodness-of-fit test |
||||||
|---|---|---|---|---|---|---|
| mean number of eggs per plant |
GP | GH | ||||
| (i) fertilizer type | ammonium nitrate | John Innes | chicken manure | |||
| 40.3 | 26.8 | 23.7 | 49.6*** | 174*** | ||
| (ii) ammonium nitrate concentration | high | medium | low | zero | ||
| 32.4 | 50.4 | 35.9 | 42.5 | 46.2*** | 456*** | |
*** p < 0.001.
4. Discussion
Phelan et al. (1996) suggest that organic fertilizers may result in a decrease in pest insect populations due to a ‘biological buffering effect of organic soils’ in which ‘mineral relationships are optimized’ (the Mineral Balance Hypothesis). Our study demonstrates that herbivore species differ in their responses to organic fertilizer treatments. The organic and conventional fertilizer treatments altered the abundance of each of the dominant herbivore species, but the direction of the response differed across the three species.
Within one feeding guild, the two aphid species showed opposing responses to the organic fertilizer treatments. More B. brassicae were found on the organically fertilized plants compared with the conventional plants, while M. persicae was more abundant on the conventional low fertilizer treatment. The performance of the Brassica specialist B. brassicae has been shown to be more closely related to the glucosinolate content of its host plant than the performance of M. persicae (Cole 1997), which may explain the higher fecundity and populations of B. brassicae on our organically fertilized plants. Myzus persicae can be deterred from feeding by the presence of indole glucosinolates (Kim et al. 2008), which we found in higher concentrations in the organically fertilized plants than the synthetically fertilized plants. However, this does not fully explain the response of M. persicae, as within the synthetic fertilizer treatments they were more abundant on the low compared with the high fertilizer treatment, but overall glucosinolate concentrations were consistently low on both.
Apterous B. brassicae are known to sequester sinigrin from their host plants, which reduces the survival of predatory ladybird larvae (Adalia bipunctata; Francis et al. 2001; Kazana et al. 2007). In contrast, the mortality of A. bipunctata larvae feeding on M. persicae was unaffected by the glucosinolate content of the aphid's diet (Francis et al. 2001). The greater concentration of glucosinolates (including sinigrin) found in organically fertilized plants may explain the larger populations of B. brassicae found on these plants in our study, through higher fecundity, reduced predation or both.
Ponti et al. (2007) used treatments that were very similar to our two low fertilizer treatments, but found higher B. brassicae populations on broccoli plants growing in synthetic fertilizer compared with organic composts. Plant chemistry was not assessed in their study (Ponti et al. 2007). Broccoli foliage contains gluconasturtiin (2-phenylethylglucosinolate; Rodrigues & Rosa 1999), which decreases the survival of B. brassicae in several Brassica varieties, but is not present in cabbages (Cole 1996). If the concentration of all glucosinolate compounds was greater under their organic fertilizer treatment as under our organic fertilizer treatments, an increase in gluconasturtiin concentration may have resulted in reduced B. brassicae survival. This could explain the difference between Ponti et al.'s (2007) results and ours. These specific effects of glucosinolates on specialist herbivore performance suggest that fertilizer effects on aphid abundance can differ both between species and between plant cultivars.
Plutella xylostella was more abundant on the conventionally fertilized plants in both years, and on the high fertilizer treatments in 2007. Plutella xylostella were also generally less abundant in 2008 compared with 2007. This may be due to differences in overwintering survival or in the number of adults migrating in the UK between the 2 years. The abundance of Lepidoptera larvae was greater on plants growing in a synthetic fertilizer compared with cow manure or sewage sludge (Culliney & Pimentel 1986), and P. xylostella larval abundance was correlated with increased nitrogen supply (Jansson et al. 1991). In our field cage experiments, P. xylostella laid more eggs on plants growing in the synthetic fertilizer compared with those in organic chicken manure, and oviposition was maximized on plants in an intermediate synthetic fertilizer treatment, equivalent in concentration to our field trial conventional high treatment (200 kg nitrogen per hectare). Early instar P. xylostella rarely move away from their original host plant (Renwick 1989), so their performance is dependent on female oviposition choice.
Plutella xylostella oviposition is stimulated by a range of glucosinolates (Reed et al. 1989; Poelman et al. 2008b) and their derivatives (Renwick et al. 2006). In our study, P. xylostella oviposition preference did not appear to relate to the greater glucosinolate concentrations found under organic fertilizer treatments in the field trial. Specific compounds may be oviposition stimulants up to a threshold concentration, beyond which oviposition may be unaffected or deterred by increasing concentrations. Poelman et al. (2008a) found that glucosinolate profile did not explain the performance of P. xylostella on a range of B. oleracea cultivars. Plutella xylostella larvae are capable of detoxifying glucosinolates (Ratzka et al. 2002), so foliar nitrogen content may be more important for larval performance than glucosinolate concentration.
Our two organic treatments differed not only in the quantity of fertilizer supplied, but also in the type of fertilizer. Despite these qualitative differences there was little variation in nitrogen or glucosinolate concentrations between the two organic treatments. Nitrogen concentration was greatest in the conventional high fertilizer treatment in both years, in line with a recent review that found consistently higher nitrogen content in conventionally grown produce compared with that produced under organic management (Dangour et al. 2009). Nitrogen concentration remained high throughout the field season in the conventional high fertilizer treatment but decreased over time in the other three treatments, which may suggest that plants in the latter were nitrogen-limited later in the growing season. Although the total nitrogen applied was the same in the two ‘high fertilizer’ (200 kg nitrogen per hectare) and the two ‘low fertilizer’ (100 kg nitrogen per hectare) treatments in our study, the type of fertilizer will have strongly affected the availability of nitrogen to the plants (Alyokhin et al. 2005; Kramer et al. 2006).
Our two conventional fertilizer treatments only supplied nitrogen, while the organic ones supplied a wider range of nutrients for the plants, which may have affected the concentration of primary and secondary metabolites. For example, sulphur is an important prerequisite for the production of glucosinolates in Brassica, and the amount of sulphur supplied can alter the oviposition and performance of insect herbivores (Marazzi & Stadler 2005). Four of the five glucosinolates identified were present in substantially higher concentrations in organically fertilized plants in both years, and the same effect was shown by the fifth compound (neoglucobrassicin) in 2007. Hsu et al. (2009) also found an increase in a single glucosinolate (sinigrin) in Brassica grown in pots with an organic soybean meal treatment compared with those in synthetic fertilizer. Brassica glucosinolate concentrations often decrease in response to increasing fertilizer or nitrogen supply (Fischer 1992; Chen et al. 2004; Aires et al. 2006; Schonhof et al. 2007), but, with the exception of glucoiberin, we found no effect of fertilizer quantity on glucosinolate concentration. The differences in glucosinolate concentration found in the current study were probably constitutive rather than induced (Siemens & Mitchell-Olds 1998), as the dominant chewing herbivore (P. xylostella) was more abundant on the conventional plants, which had a lower concentration of glucosinolates.
For the first time, we connect the response of naturally occurring populations of three herbivore species to controlled organic and conventional fertilizer treatments applied in a field trial over 2 years with detailed chemical analyses of potential causative compounds. Our results demonstrate the importance of assessing plant chemistry to investigate mechanistic links between fertilizer treatments and herbivore responses. The complexity and specificity of these interactions between plant chemistry and herbivores suggest that we cannot currently generalize across species about the effects of organic and conventional fertilizer treatments on populations of insect herbivores.
Acknowledgements
Dr Irene Mueller-Harvey (University of Reading) conducted the foliar nitrogen analysis. We are grateful to Bruce Main and David Mclay for technical assistance. Two anonymous reviewers helped us to improve the manuscript. Funding was provided by BBSRC grant BB/D01154x/1.
References
- Aires A., Rosa E., Carvalho R.2006Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts (B. oleracea var. italica). J. Sci. Food Agric. 86, 1512–1516 (doi:10.1002/jsfa.2535) [Google Scholar]
- Alyokhin A., Porter G., Groden E., Drummond F.2005Colorado potato beetle response to soil amendments: a case in support of the mineral balance hypothesis? Agric. Ecosyst. Environ. 109, 234–244 (doi:10.1016/j.agee.2005.03.005) [Google Scholar]
- AOAC 1995Official methods of analysis of Association of Analytical Communities International, 16th edn. section 4.2.02, method 954.01. Gaithersburg, MD: Association of Analytical Communities International [Google Scholar]
- Bengtsson J., Ahnstrom J., Weibull A. C.2005The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J. Appl. Ecol. 42, 261–269 (doi:10.1111/j.1365-2664.2005.01005.x) [Google Scholar]
- Bradburne R. P., Mithen R.2000Glucosinolate genetics and the attraction of the aphid parasitoid Diaeretiella rapae to Brassica. Proc. R. Soc. Lond. B 267, 89–95 (doi:10.1098/rspb.2000.0971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Z., Lin L., Wang C. W., Yeh C. C., Hwang S. Y.2004Response of two Pieris (Lepidoptera: Pieridae) species to fertilization of a host plant. Zool. Stud. 43, 778–786 [Google Scholar]
- Cole R.1996Abiotic induction of changes to glucosinolate profiles in Brassica species and increased resistance to the specialist aphid Brevicoryne brassicae. Entomol. Exp. Appl. 80, 228–230 (doi:10.1007/BF00194763) [Google Scholar]
- Cole R. A.1997The relative importance of glucosinolates and amino acids to the development of two aphid pests Brevicoryne brassicae and Myzus persicae on wild and cultivated brassica species. Entomol. Exp. Appl. 85, 121–133 (doi:10.1023/A:1003049105464) [Google Scholar]
- Costello M. J., Altieri M. A.1995Abundance, growth rate and parasitism of Brevicoryne brassicae and Myzus persicae (Homoptera, Aphididae) on broccoli grown in living mulches. Agric. Ecosyst. Environ. 52, 187–196 (doi:10.1016/0167-8809(94)00535-M) [Google Scholar]
- Crawley M. J.2007The R book Chichester, UK: Wiley [Google Scholar]
- Culliney T. W., Pimentel D.1986Ecological effects of organic agricultural practices on insect populations. Agric. Ecosyst. Environ. 15, 253–266 (doi:10.1016/0167-8809(86)90124-6) [Google Scholar]
- Dangour A. D., Dodhia S. K., Hayter A., Allen E., Lock K., Uauy R.2009Nutritional quality of organic foods: a systematic review. Am. J. Clin. Nutr. (doi:10.3945/ajcn.2009.28041) [DOI] [PubMed] [Google Scholar]
- Eigenbrode S. D., Pimentel D.1988Effects of manure and chemical fertilizers on insect pest populations on collards. Agric. Ecosyst. Environ. 20, 109–125 (doi:10.1016/0167-8809(88)90151-X) [Google Scholar]
- Fischer J.1992The influence of different nitrogen and potassium fertilization on the chemical flavour composition of Kohlrabi (Brassica oleracea var gongylodes L). J. Sci. Food Agric. 60, 465–470 (doi:10.1002/jsfa.2740600410) [Google Scholar]
- Francis F., Lognay G., Wathelet J. P., Haubruge E.2001Effects of allelochemicals from first (Brassicaceae) and second (Myzus persicae and Brevicoryne brassicae) trophic levels on Adalia bipunctata. J. Chem. Ecol. 27, 243–256 (doi:10.1023/A:1005672220342) [DOI] [PubMed] [Google Scholar]
- Heaney R. K., Spinks E. A., Hanley B., Fenwick G. R.1986Technical bulletin: analysis of glucosinolates in rapeseed Crowe, Norwich, UK: AFRC, Food Research Institute. [Google Scholar]
- Hopkins R. J., van Dam N. M., van Loon J. J. A.2009Role of glucosinolates in insect–plant relationships and multitrophic interactions. Annu. Rev. Entomol. 54, 57–83 (doi:10.1146/annurev.ento.54.110807.090623) [DOI] [PubMed] [Google Scholar]
- Hsu Y. T., Shen T. C., Hwang S. Y.2009Soil fertility management and pest responses: a comparison of organic and synthetic fertilization. J. Econ. Entomol. 102, 160–169 (doi:10.1603/029.102.0123) [DOI] [PubMed] [Google Scholar]
- Jansson R. K., Leibee G. L., Sanchez C. A., Lecrone S. H.1991Effects of nitrogen and foliar biomass on population parameters of cabbage insects. Entomol. Exp. Appl. 61, 7–16 (doi:10.1007/BF00367163) [Google Scholar]
- Kazana E., Pope T. W., Tibbles L., Bridges M., Pickett J. A., Bones A. M., Powell G., Rossiter J. T.2007The cabbage aphid: a walking mustard oil bomb. Proc. R. Soc. B 274, 2271–2277 (doi:10.1098/rspb.2007.0237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Lee B. W., Schroeder F. C., Jander G.2008Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J. 54, 1015–1026 (doi:10.1111/j.1365-313X.2008.03476.x) [DOI] [PubMed] [Google Scholar]
- Kramer S. B., Reganold J. P., Glover J. D., Bohannan B. J. M., Mooney H. A.2006Reduced nitrate leaching and enhanced denitrifier activity and efficiency in organically fertilized soils. Proc. Natl Acad. Sci. USA 103, 4522–4527 (doi:10.1073/pnas.0600359103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau D. K., Drinkwater L. E., Shennan C.1996Effects of soil management on crop nitrogen and insect damage in organic vs conventional tomato fields. Agric. Ecosyst. Environ. 57, 179–187 (doi:10.1016/0167-8809(96)01027-4) [Google Scholar]
- Marazzi C., Stadler E.2005Influence of sulphur plant nutrition on oviposition and larval performance of the cabbage root fly. Agric. Forest Entomol. 7, 277–282 (doi:10.1111/j.1461-9555.2005.00272.x) [Google Scholar]
- Mattson W. J.1980Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Evol. Syst. 11, 119–161 [Google Scholar]
- Phelan P. L., Mason J. F., Stinner B. R.1995Soil-fertility management and host preference by European corn borer, Ostrinia nubilalis (Hubner), on Zea mays L: a comparison of organic and conventional chemical farming. Agric. Ecosyst. Environ. 56, 1–8 (doi:10.1016/0167-8809(95)00640-0) [Google Scholar]
- Phelan P. L., Norris K. H., Mason J. F.1996Soil-management history and host preference by Ostrinia nubilalis: evidence for plant mineral balance mediating insect–plant interactions. Environ. Entomol. 25, 1329–1336 [Google Scholar]
- Poelman E. H., Galiart R., Raaijmakers C. E., Van Loon J. J. A., van Dam N. M.2008aPerformance of specialist and generalist herbivores feeding on cabbage cultivars is not explained by glucosinolate profiles. Entomol. Exp. Appl. 127, 218–228 (doi:10.1111/j.1570-7458.2008.00700.x) [Google Scholar]
- Poelman E. H., Broekgaarden C., Van Loon J. J. A., Dicke M.2008bEarly season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol. Ecol. 17, 3352–3365 (doi:10.1111/j.1365-294X.2008.03838.x) [DOI] [PubMed] [Google Scholar]
- Ponti L., Altieri M. A., Gutierrez A. P.2007Effects of crop diversification levels and fertilization regimes on abundance of Brevicoryne brassicae (L.) and its parasitization by Diaeretiella rapae (M'Intosh) in broccoli. Agric. Forest Entomol. 9, 209–214 (doi:10.1111/j.1461-9563.2007.00330.x) [Google Scholar]
- Ratzka A., Vogel H., Kliebenstein D. J., Mitchell-Olds T., Kroymann J.2002Disarming the mustard oil bomb. Proc. Natl Acad. Sci. USA 99, 11 223–11 228 (doi:10.1073/pnas.172112899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed D. W., Pivnick K. A., Underhill E. W.1989Identification of chemical oviposition stimulants for the diamondback moth, Plutella xylostella, present in three species of Brassicaceae. Entomol. Exp. Appl. 53, 277–286 (doi:10.1007/BF00162859) [Google Scholar]
- Renwick J. A. A.1989Chemical ecology of oviposition in phytophagous insects. Experientia 45, 223–228 (doi:10.1007/BF01951807) [Google Scholar]
- Renwick J. A. A., Haribal M., Gouinguene S., Stadler E.2006Isothiocyanates stimulating oviposition by the diamondback moth, Plutella xylostella. J. Chem. Ecol. 32, 755–766 (doi:10.1007/s10886-006-9036-9) [DOI] [PubMed] [Google Scholar]
- Rodrigues A. S., Rosa E. A. S.1999Effect of post-harvest treatments on the level of glucosinolates in broccoli. J. Sci. Food Agric. 79, 1028–1032 (doi:10.1002/(SICI)1097-0010(19990515)79:7<1028::AID-JSFA322>3.0.CO;2-I) [Google Scholar]
- R Team 2006R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Schonhof I., Blankenburg D., Muller S., Krumbein A.2007Sulphur and nitrogen supply influence growth, product appearance, and glucosinolate concentration of broccoli. J. Plant Nutr. Soil Sci. 170, 65–72 (doi:10.1002/jpln.200620639) [Google Scholar]
- Siemens D. H., Mitchell-Olds T.1998Evolution of pest-induced defences in Brassica plants: tests of theory. Ecology 79, 632–646 [Google Scholar]
- Sokal R. R., Rohlf F. J.1995Biometry: the principles and practice of statistics in biological research New York, NY: W. H. Freeman and Company [Google Scholar]
- Wittstock U., Agerbirk N., Stauber E. J., Olsen C. E., Hippler M., Mitchell-Olds T., Gershenson J., Vogel H.2004Successful herbivore attack due to metabolic diversion of a plant chemical defence. Proc. Natl Acad. Sci. USA 101, 4859–4864 (doi:10.1073/pnas.0308007101) [DOI] [PMC free article] [PubMed] [Google Scholar]