Abstract
Cryptic coloration is assumed to be beneficial to predators because of an increased encounter rate with unwary prey. This hypothesis is, however, very rarely, if ever, studied in the field. The aim of this study was to quantify the encounter rate and capture success of an ambush predator, in the field, as a function of its level of colour-matching with the background. We used the crab spider Misumena vatia, which varies its body colour and can thereby match the colour of the flower it hunts upon. We carried out a manipulative field experiment using a complete factorial design resulting in six different colour combinations of crab spiders and flowers differing in their degree of colour-matching. A rich and diverse set of naturally occurring insects visited the flowers while we continuously video-recorded the spider's foraging activity. This enabled us to test the crypsis, the spider avoidance and the flower visitor attraction hypotheses, all three supported by previous studies. Flower visitors of different groups either avoided crab spiders independent of colour-matching, such as solitary bees and syrphid flies, or ignored them, such as bumble-bees and honeybees. Moreover, colour-matched spiders did not have a higher encounter rate and capture success compared to the visually apparent ones. Thus, our results support the spider avoidance hypothesis, reject the two other hypotheses and uncovered a fourth behaviour: indifference to predators. Because flower visitors reacted differently, a community approach is mandatory in order to understand the function of background colour-matching in generalist predators. We discuss our results in relation to the size and sociality of the prey and in relation to the functional significance of colour change in this predator.
Keywords: capture success, Misumena vatia, generalist predator, colour-matching, spider avoidance, flower visitor attraction
1. Introduction
Cryptic animals are thought to avoid detection by their potential prey or their predators (Oxford & Gillespie 1998; Heiling et al. 2005). Thus, colour-matching predators are assumed to have an advantage over unmatched conspecifics, for example, in terms of an increased prey encounter rate or a higher prey capture rate. Astonishingly, this fundamental assumption has seldom been tested for prey (e.g. Majerus et al. 2000) and has never, to our knowledge, been assessed for cryptic predators. This is the overall aim of our work.
Adult females of several crab spider species in the Thomisidae are able to change their colour between white and yellow (in rare cases also pink/purple). This ability has been studied for over one century and was claimed to have evolved as a strategy to minimize the colour contrast on inflorescences where they wait for flower visitors (Angus 1882; Rabaud 1919; Gabritschevsky 1927; Weigel 1941; Morse 1979, 1981, 2007; Schmalhofer 2001; Théry & Casas 2002; Heiling & Herberstein 2004; Théry 2007). The duration of colour change to adapt body colour reported in these studies ranges from 2 to 20 days with a mean of 4–7 days; it is therefore a morphological colour change (Oxford & Gillespie 1998; Insausti & Casas 2008, in press). In combination with this ability, these crab spiders are also reported to settle preferentially on inflorescences that match their body colour. Thus, white crab spiders mostly hunt on white inflorescences (e.g. 75% in Weigel 1941; 69% in Heiling et al. 2005), while yellow crab spiders almost exclusively forage on yellow inflorescences (e.g. 94% in Heiling et al. 2005).
However, the outcome of the latest works on this system has been increasingly discomforting for the tenants of the crypsis hypothesis. Chittka (2001) found that only white spiders closely match the background colour of white inflorescences, while the other colour combinations of spiders and inflorescences are not matching (Chittka 2001; see also Heiling et al. 2005). Thus, it became questionable whether one should still consider these crab spiders as cryptic. These misgivings are in line with the studies of Heiling et al. (2003, 2005), who found that, under certain circumstances, the Australian crab spider Thomisus spectabilis may even be attractive to some flower visitors. The most recent systematic field survey conclusively showed that Misumena vatia is not cryptic in the visual system of bees, one of the most important prey, and that the striking cases of perfect colour-matching occur with low probability, not different from that obtained through a random assortment of spider and flower colours (Defrize et al. submitted). It is therefore mandatory to assess prey behaviour and the added value of the lack of chromatic contrast by manipulation rather than by simply observing the degree of matching, and by quantifying the rate of visits by flower visitors and the capture success of the spiders. This is the specific aim of our work.
We formulated three hypotheses: the crypsis hypothesis, the spider avoidance hypothesis and the flower visitor attraction hypothesis. According to the crypsis hypothesis, flower visitors cannot perceive colour-matching crab spiders on inflorescences or perceive them with more difficulty and errors. The spider avoidance hypothesis stipulates that inflorescences, harbouring a spider are generally avoided, regardless of colour-matching. According to the flower visitor attraction hypothesis, inflorescences harbouring a crab spider should be visited more often compared to spider-free inflorescences. All three hypotheses are contingent on the degree of colour-matching between crab spiders and inflorescences, and all three have received support in previous studies. However, these studies either focused on crab spiders hunting on flowers of the same colour, and therefore neglected the ability of these spiders to adapt their body colour, or the colour adaptation was taken into account, but the studies focused on large social bees only (Fritz & Morse 1985; Dukas 2001; Schmalhofer 2001; Heiling et al. 2003, 2005; Heiling & Herberstein 2004). Nearly all neglected the more species-rich, non-social flower visitors, such as solitary bees and syrphid flies (but see Schmalhofer 2001). The latter groups are also common visitors to flowers, and so are potential prey items for crab spiders, and several studies have suggested that flower visitor identity might play an important role in the responses shown towards spiders (Reader et al. 2006; Brechbühl et al. in press). Thus, including the entire flower visitor community is essential in studies regarding crab spider–flower visitor interactions.
In order to test the three hypotheses, we placed outdoors white and yellow crab spiders (M. vatia) on three different coloured flower species—white, yellow and violet—resulting in six different colour combinations of spiders and inflorescences. Responses from different local flower visitor species towards the settled crab spiders were then recorded using continuous video surveillance and compared to spider-free inflorescences. Furthermore, as more insect visits towards an inflorescence do not necessarily result in a higher capture success by crab spiders, we measured capture rates and biomass of captured prey as both may translate into fitness benefits for the spider.
2. Material and methods
(a). Study area and species
The experiment was set up in the garden of the Zoological Institute in Bern (Switzerland) from May to August 2007. Female spiders were caught in wildflower fields around Bern by sweep-netting and kept in Drosophila tubes (5 cm diameter) that were partially filled with soil (1–2 cm). The caught spiders were brought to the rearing room; a tool shed just beside the experimental area (unregulated climate). Once a week, the spiders were fed (Acheta domestica: 4–6 mm) and some water was sprinkled into the tubes.
Three native plant species were chosen: Chrysanthemum frutescens (white inflorescences; Asteraceae), Anthemis tinctoria (yellow inflorescences; Asteraceae) and Knautia arvensis (violet inflorescences; Dipsacaceae), all of which are the regular hunting sites for M. vatia spiders (R. Brechbühl 2005–2009, personal observation). Seedlings were planted in plastic pots (16 l) in spring 2007. Twenty pots for each plant species were used, resulting in 60 pots with experimental plants. The experiment consisted of 20 patches distributed uniformly over the garden with a minimum distance of 3 m between them. Each patch included one pot of each of the three plant species, placed in a triangle as close together as possible.
Common flower visitor species were caught by sweep-netting in the experimental field, frozen at −20°C and dried to determine their dry mass (mg) using a Mettler MT5 balance. We used the average dry mass of each flower visitor species or genus in order to estimate the captured biomass from capture rates.
(b). Experimental design
We recorded the behaviour of flower visitors for nine different spider–inflorescence combinations in a complete factorial design (three spider treatments×three flower species): either white or yellow crab spiders were individually placed on one inflorescence of each of the three flower species. The term ‘inflorescence’ is used here to describe a flowering display unit (i.e. the typical ‘flower head’ of the Asteraceae). In addition, we also had treatments of each flower species without spiders. The experiment was repeated ten times for C. frutescens and A. tinctoria, but only eight times for K. arvensis owing to the lower flower numbers of this plant species. In order to prevent flower visitation activities before the experiment, we covered flower buds with gauze bags until the recordings started. We included this manipulation because preliminary studies and other published work on Apis mellifera foraging behaviour (e.g. Williams 1998) indicated that the probability of acceptance of a flower by a bee was likely to be influenced strongly by previous visits from other flower visitors.
Ten digital surveillance cameras were used to continuously monitor experimental inflorescences for a period of three consecutive sunny days in summer 2007. We used cameras that transmitted pictures to a wireless server via an Internet access point. The technical details of the surveillance system are fully described elsewhere (Brechbühl et al. in press). We conducted the experiment during 10 different three-day periods, which were used as temporal blocks in the analysis (see below). At the beginning of each temporal block, individual pots were randomly assigned to spider treatments. Three replicates of each spider treatment were observed simultaneously, plus an additional randomly chosen treatment. In the morning of the first day at 11.00, crab spiders were placed on inflorescences. We tried to select equally sized inflorescences within flower species, but flower size (diameter) is used later as covariate in the analyses to account for the differences in size that still remained. The flowers used in the experiments were bound to bamboo sticks to minimize flower movements owing to wind, as the cameras were equipped with a movement sensor. After placing the crab spiders, the experimental inflorescences were checked every two hours (at 13.00, 15.00 and 17.00). If the crab spider had left the inflorescence, it was put back, or replaced by another M. vatia of the same colour if it could not be re-located (spiders on control flowers were removed). Spiders sometimes also hide beneath the petals of inflorescences, thereby complicating the issue of conspicuousness, but this was only rarely the case in our experiment and we ignored this in the analysis. All crab spiders were used only once in the experiment. On days 2 and 3, the inflorescences were checked the same way as on day 1 (every two hours from 11.00 until 17.00). The experiment ended at 17.00 on day 3. Cameras recorded pictures continuously during the three day intervals. However, for data analysis, we used only data recorded from 09.00 to 19.00, when most of the flower visitors were active. Furthermore, we noted whether spiders caught prey and, if so, the identity of the prey.
We calculated the number and duration of visits to inflorescences per hour for each flower visitor taxon. Periods when spiders had left experimental inflorescences and periods during which cameras did not send pictures to the Internet because of connectivity problems were excluded.
Flower visitors were determined to species or genus level from the video recordings. In addition, we measured the height of each experimental inflorescence above the soil surface (cm) and its diameter (mm), and each patch received an x- and y-coordinate in order to account for the spatial heterogeneity in insect visits.
(c). The hypotheses
Given our setup, we can predict the outcome of the experiments according to the three hypotheses. In the crypsis hypothesis, spiders with the same colour as the flower and spider-free flowers should gain more insect visits than unmatched pairs. Accordingly, white crab spiders on white inflorescences and yellow spiders on yellow inflorescences should have a higher foraging success than the converse colour combinations. The two colours of crab spiders on violet inflorescences should gain the same amount of prey, as they are both conspicuous. In the spider avoidance hypothesis, we would expect considerably more insect visits on spider-free inflorescences. However, the foraging success should not depend on the colour combinations of crab spiders and inflorescences. Finally, in the flower visitor attraction hypothesis, contrasting spiders should have an appealing effect on flower visitors. Thus, we would expect more visits on inflorescences harbouring spiders than on control inflorescences. Here, again, we would not expect differences between different colour combinations of spiders and inflorescences.
(d). Statistical analysis
We tested for the preferences of different flower visitor groups with linear mixed effects models (function lme in the statistical software R v.2.7.2; R Development Core Team 2007) fitted by maximum likelihood with flower species and spider treatment (yes or no, independent of the spider's colour) and their interaction as fixed factors. In a second analysis, in which we used only data from inflorescences harbouring crab spiders, we tested (i) whether flower visitors showed higher or lower visitation rates to inflorescences on which the crab spider's colour matched the background colour of the flowers and (ii) whether crab spiders profited from colour-matching in terms of the number and dry mass of flower visitors caught. Here, spider colour, flower colour and their interactions were used as independent variables. The number of flower visitor visits per hour, the duration of visits per hour and the dry mass caught per hour (all log-transformed to conform to the assumptions of normality) were used as dependent variables. Inflorescence height, diameter of the inflorescences and the spatial patch position (x–y variables) were covariates in all analyses. The temporal block was included as a random factor. It should be noted that in some cases, we analysed the data for one flower species only, if no or very few visiting events occurred on the other flower species (e.g. for bumble-bees that exclusively visited K. arvensis; see appendices SA and SB, electronic supplementary material). We started with a full model, containing all variables, and used a backward procedure to obtain minimum adequate models by removing variables that did not improve the fit of the model (tested by the Bayesian information criterion (BIC), Schwarz 1978). All calculations were done in R v.2.7.2.
3. Results
During our experiment, a total of 8358 insect visits were observed on the inflorescences (table 1). Solitary bees were the most frequent visitors and made up almost half of all visitation events (3984 visits). Within the group of solitary bees, Hylaeus sp. (1927 visits) and Lasioglossum sp. (1118 visits) were the most common visitors, but two other genera also occurred in considerable numbers (Halictus sp. and Colletes sp.). The second most common visitors were bumble-bees, with 1906 visits, dominated by Bombus terrestris. Honeybees, with 706 visits, and syrphid flies, with 433 visits, also foraged regularly on the experimental inflorescences (table 1). All the other insect visitors either occurred in small numbers or were not typical pollinators (e.g. ants). We therefore concentrated our analyses on four flower visitor groups: bumble-bees, honeybees, solitary bees and syrphid flies.
Table 1.
Visitors to the experimental inflorescences: the total number of visits to experimental inflorescences (with and without crab spiders); the total number of visits to inflorescences harbouring a hunting crab spider; the number of insects caught by the spiders; the chance of being caught during a random visit towards an inflorescence harbouring a crab spider; and the average individual dry mass (in mg ± SE) of the different observed flower visitor taxa (in brackets, the number of weighed individuals per taxon).
| visits total | visits with spiders | insects caught | % caught | dry mass (mg) | |
|---|---|---|---|---|---|
| bumble-bees | 1906 | 821 | 2 | 0.24 | 98.2 ± 3.4 (11) |
| Bombus campestris | 284 | 59 | — | — | 105.8 ± 0.7 (2) |
| Bombus lapidarius | 26 | 15 | — | — | 81.5 ± 0.3 (2) |
| Bombus pascuorum | 374 | 208 | 1 | 0.48 | 93.6 ± 7.4 (3) |
| Bombus terrestris | 1089 | 470 | — | — | 108.8 ± 2.8 (3) |
| other Bombus | 133 | 69 | 1 | 1.45 | 98.9 ± 0.0 (1) |
| honeybees | 706 | 242 | 14 | 5.79 | 29.5 ± 1.1 (6) |
| solitary bees | 3984 | 777 | 51 | 6.56 | 7.1 ± 1.1 (18) |
| Colletes sp. | 357 | 67 | 3 | 4.48 | 15.6 ± 0.2 (3) |
| Halictus sp. | 557 | 162 | 2 | 1.23 | 6.2 ± 0.7 (3) |
| Hylaeus sp. | 1927 | 403 | 31 | 7.69 | 5.3 ± 0.3 (6) |
| Lasioglossum sp. | 1118 | 145 | 15 | 10.34 | 5.2 ± 0.5 (6) |
| other solitary bees | 25 | 0 | — | — | 5.9 ± 0.0 (1) |
| syrphid flies | 433 | 57 | 1 | 1.75 | 10.3 ± 2.5 (13) |
| Eristalis tenax | 164 | 21 | — | — | 17.2 ± 2.6 (5) |
| Sphaerophoria sp. | 106 | 8 | — | — | 2.6 ± 0.4 (3) |
| Syritta sp. | 89 | 7 | — | — | 1.3 ± 0.1 (3) |
| other syrphid flies | 74 | 21 | 1 | 4.76 | 11.8 ± 0.3 (2) |
| others | 1329 | 301 | 10 | 3.32 | 14.7 ± 2.2 (16) |
| ants | 392 | 93 | 1 | 1.08 | 1.7 ± 0.2 (6) |
| Coleoptera | 390 | 60 | 2 | 3.33 | 26.3 ± 11.8 (3) |
| other Diptera | 81 | 18 | 5 | 27.78 | 7.7 ± 1.4 (6) |
| wasps | 349 | 99 | — | — | 22.3 ± 0.0 (1) |
| undetermined | 117 | 31 | 2 | 6.45 | — |
| total | 8358 | 2198 | 78 | 3.55 | 24.5 ± 4.0 (64) |
The time spent by the flower visitors on the inflorescences was correlated with the number of visits (linear regression: n = 90 inflorescences, r2 = 0.47, p < 0.001). Thus, attractive inflorescences not only gained more insect visits, but were also visited for longer. As the results for the average number of insect visits and the average duration revealed similar results, we only present the results for the average number of visits in the paper (duration is treated in the electronic supplementary material). Bumble-bees and honeybees showed a clear preference for K. arvensis compared to the other two flower species used in the experiment. In fact, K. arvensis was the most often visited flower species by all groups (3704 visits) and a visit lasted on average 24.0 ± 2.8 s. Solitary bees and syrphid flies preferred A. tinctoria, the inflorescences of which were visited second most (3353 visits; 14.7 ± 1.8 s/visit). With 1301 visits, C. frutescens gained the fewest visits, but the longest (26.2 ± 5.8 s/visit).
We did not observe a uniform spatial distribution of the flower visitors, meaning that certain patches were preferred by different flower visitor taxa. This heterogeneity was seen when analysing the data for all flower visitors together (appendix SA, electronic supplementary material), but disappeared when the flower visitor groups were split into subgroups and genera. Furthermore, bumble-bees generally preferred taller inflorescences and solitary bees showed a slight preference for inflorescences with a larger diameter in their duration of visits (appendices SA and SB, electronic supplementary material).
(a). Responses to crab spiders
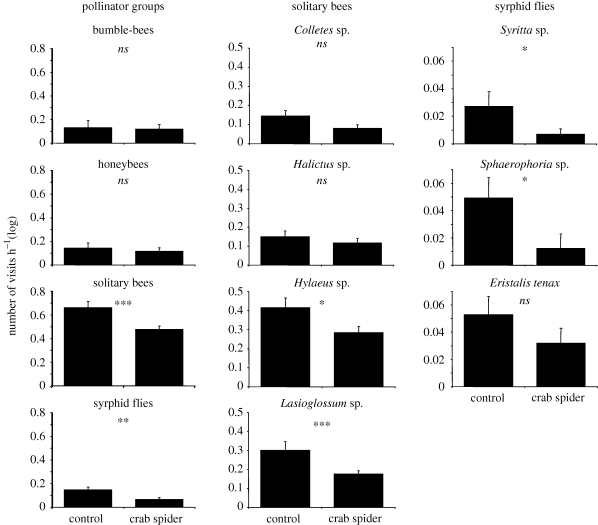
Solitary bees and syrphid flies strongly avoided inflorescences harbouring crab spiders, independent of colour combinations, and spent significantly less time on these inflorescences (figure 1; appendix SA, electronic supplementary material). Within the solitary bees and syrphid flies, we found differences between the observed genera. While the solitary bees Hylaeus sp. and Lasioglossum sp. avoided spider-harbouring inflorescences, the other two observed genera (Colletes and Halictus) did not significantly reduce their visits to inflorescences with crab spiders (figure 1). Although the number of visits of Colletes bees did not significantly decrease towards spider-harbouring inflorescences, they spent less time on them (appendix SA, electronic supplementary material). In the group of syrphid flies, only two of the three observed taxa (the Syritta and Sphaerophoria genera) avoided crab spiders. They visited inflorescences harbouring crab spiders less frequently and for shorter durations than spider-free inflorescences. No reaction towards crab spiders was observed for Eristalis tenax (figure 1).
Figure 1.
Total number of visits per hour (mean + SE; log-transformed) according to spider treatment (control, crab spider) of the different flower visitor groups, solitary bee taxa and syrphid fly taxa: *p < 0.05; **p < 0.01; ***p < 0.001; for exact values see appendix SA, electronic supplementary material.
By contrast, none of the observed bumble-bee species or honeybees showed a reaction towards M. vatia spiders. In both groups, the average number and duration of visits did not significantly differ between inflorescences with and without crab spiders (figure 1; appendix SA, electronic supplementary material). Furthermore, it is noteworthy that in our study, all flower-visiting groups either avoided or ignored crab spiders, but were never attracted to them (appendix SA, electronic supplementary material).
(b). Responses to different colour combinations of crab spiders and inflorescences
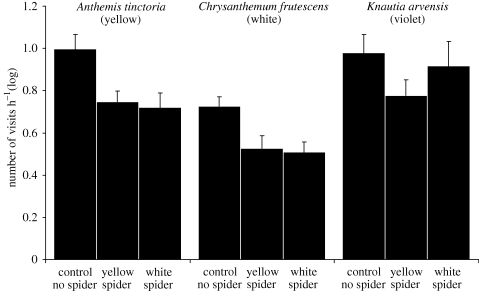
When analysing the data of inflorescences harbouring crab spiders only, and focusing on the different colour combinations of spiders and inflorescences, we found no evidence that crab spiders profit from adapting their colour to that of inflorescences (figure 2; appendix SB, electronic supplementary material). Neither white crab spiders on white inflorescences nor yellow spiders on yellow inflorescences gained significantly more insect visits compared to the respective unmatched colour combination. On the violet K. arvensis inflorescences, no significant differences between white and yellow crab spiders were observed. The average duration of the visits yielded similar results as the average number of visits (appendix SB, electronic supplementary material).
Figure 2.
Total number of insect visits (mean + SE; log-transformed) towards control inflorescences and different colour combinations of crab spiders and inflorescences.
(c). Prey capture success
Crab spiders were able to catch and feed on 78 insects. As 2198 visits occurred on spider-harbouring inflorescences (when a spider was present), the chance of a visiting insect being caught was on average 3.55 per cent (table 1). Although we observed only 18 visits of non-syrphid flies to inflorescences harbouring a crab spider, these had the highest probability of being caught (27.78%). Their small sizes prevented us from identifying them to the family or the genus level. Honeybees (5.79%) and solitary bees (7.40%) were also relatively common prey items. During the experiment, only two bumble-bees (0.24%) and one syrphid fly (1.75%) were caught (table 1).
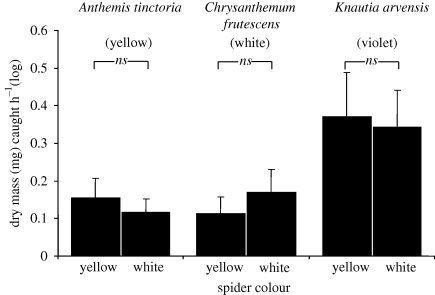
Colour-matched crab spiders did not catch significantly higher prey biomass (lme: t < 0.71, p > 0.48). Furthermore, crab spiders caught more insects (dry mass) on inflorescences with a larger diameter (lme: t = 3.88, p < 0.001). All the other variables did not remain in the minimum adequate models. Flower species did not appear as a significant factor, but there were clear differences in prey biomass caught on different flower species (figure 3), explained mostly by the differences in the diameter of the inflorescences of the three species (A. tinctoria: 30.2 ± 1.0 mm; C. frutescens: 35.2 ± 0.8 mm; K. arvensis: 38.8 ± 1.9 mm).
Figure 3.
Dry mass (mg) caught per hour (mean + SE; log-transformed) by white and yellow crab spiders on differently coloured inflorescences.
4. Discussion
Of the three hypotheses tested, we found support for only one: the spider avoidance hypothesis. If flower visitors reacted at all, they generally avoided crab spiders hunting on inflorescences, independently of the colour combination of spiders and inflorescences. While solitary bees and syrphid flies support this spider avoidance hypothesis, bumble-bees and honeybees displayed a fourth behaviour: they were indifferent to crab spiders, despite a high likelihood that they were able to detect them. Avoidance could be either innate or learnt. Recent work has shown that bumble-bees and honeybees can learn to avoid crab spiders, but only after they have had exposure to predation attempts, and the same might apply to flies (Ings & Chittka 2008; Abbott & Dukas 2009). Unfortunately, we have no information about the origin or the age of the bees that visited our garden. However, we have no reason to believe that the different flower visitor groups differed in their level of experience with crab spiders, as they experienced the same environment. Even if honeybees and bumble-bees, and maybe other flower visitors as well, are capable of learning to avoid predators, it remains to be shown how often this situation arises in nature. In our study, we artificially increased crab spider densities throughout the flowering season at the experimental site and still we did not find indications for the avoidance of spider-harbouring flowers by bumble-bees and honeybees. There may also be less evolutionary pressure in social insects to develop an avoidance reaction, because the death of a worker only marginally reduces its fitness (Hamilton's rule; Clark & Dukas 1994). A third reason may lie in the different body sizes of the flower visitor groups (Dukas & Morse 2003, 2005). The bumble-bees might be better protected from predation by their size alone, which is three times greater than that of the other flower visitors. As a consequence, bumble-bees were very frequent visitors but had the lowest probability of being captured.
In addition to the differences in flower visiting behaviour between flower visitor groups, we also found differences within these groups: some members of the solitary bee and syrphid fly groups strongly avoided crab spiders (e.g. Lasioglossum sp. and Syritta sp.), but others did not show significant avoidance reactions (e.g. Halictus sp. and E. tenax). This general result is in line with an increasing number of other studies mentioning that crab spider and flower visitor identities (also flower species) have to be taken into account in order to fully understand predator–prey–plant interactions (e.g. Reader et al. 2006; Brechbühl et al. in press). As the flower visitors were continuously video-recorded, we gained some indications of what might be responsible for the behavioural differences within the flower visitor groups. The syrphid flies of the genus Syritta and Sphaerophoria, for example, displayed characteristic hovering and systematic examination of an inflorescence before landing. They usually avoided a flower when a crab spider was present. In contrast, this hovering behaviour was not observed in E. tenax, which did not show avoidance reaction towards spiders. Inside the group of solitary bees, the behavioural avoidance reaction towards crab spiders could be observed before landing (spiders might be a visual cue) and also after landing (solitary bees spent less time on spider-harbouring inflorescences). The latter behaviour has been shown in a study with vertebrate ambush predators (lizards), where flower visitors fled as soon as they were attacked and thus spent less time on plants beside which lizards were hunting (Muñoz & Arroyo 2004). Therefore, a prey community approach is mandatory to understand crypsis in a generalist predator, as each prey has evolved specific visual abilities and behavioural responses to the same stimulus.
We found no support for the other two hypotheses (crypsis hypothesis and spider attraction hypothesis). Focusing first on the crypsis hypothesis, M. vatia spiders clearly did not profit from having the same colour as the inflorescence on which they were settled. Colour-matched spiders did not have more encounters with flower visitors and, more importantly, they did not have a higher foraging success in terms of the biomass captured. Bearing in mind the predominance of the crypsis hypothesis in the literature for over a century, this is a surprising result, but one which is in line with the findings of Chittka (2001), who often found poorly matching spiders, and Defrize et al. (submitted), who observed a very low degree of perfect matching in the field. As in our study system, most large flower visitors (bumble-bees and honeybees) were observed on the violet K. arvensis inflorescences, crab spiders should place themselves on these inflorescences in order to be most successful (most dry mass caught per hour), despite not matching there. Prey capture success seems therefore not to depend on the degree of colour-matching, but much more on the insects visiting the inflorescences—a fact that Morse & Fritz (1982) have reported a long time ago.
As we found no evidence that colour change would increase predation success, why do these spiders change their body colour? The colour change might be a protection mechanism against radiation (Venner & Casas 2005; Insausti & Casas 2008, in press; Théry & Casas 2009). The photo-protection role of these colour pigments (ommochromes) has been shown in insect eyes (Langer 1975; Stavenga 1989). As these crab spiders settle themselves for long periods on top of the inflorescences, a protection against intense sunlight might be necessary, most of all because they have a transparent cuticle. Thus, incidental colour adaptation (crypsis) might only be a by-product, with the driving force being the protection of the crab spiders against radiation. However, as flower colour choice in the field is not random (Weigel 1941; Heiling et al. 2005), protection against radiation alone cannot explain the colour adaptation of the spiders. Another argument that has been suggested is predator avoidance. If crypsis is involved in predator avoidance, one would expect higher predation rates on non-matching colour combinations. However, in three years of video observations (Brechbühl et al. in press; R. Brechbühl 2006–2008, personal observation), we only recorded one predation event (by a bird—a black redstart Phoenicurus ochruros), despite the presence of a multitude of potential predators at the experimental site—among others, spider wasps (Pompilidae), common and paper wasps (Vespidae), a variety of birds and assassin bugs. Although our study is restricted to one site only, we doubt that crypsis plays a major role in avoiding predation.
The third hypothesis tested in this work—the flower visitor attraction hypothesis (Heiling et al. 2003, 2005)—found no support in our study system. However, in contrast to the crab spider species (T. spectabilis) used by Heiling et al. (2003, 2005), M. vatia does not reflect ultraviolet light (Chittka 2001; Théry & Casas 2002).
In conclusion, we found no support for the two hypotheses of crypsis and flower visitor attraction. The spider avoidance hypothesis gained support for several flower visitor species; in particular solitary bees and different fly species, and a fourth mechanism, indifference to spiders, was found for the large social bees. Thus, deriving conclusions from the study of only a subset of flower visitor species is fraught with difficulties, and a community approach towards crypsis in a generalist predator seems mandatory.
Acknowledgements
We thank A. Strauss and A. Siegenthaler for assistance in technical matters. Research benefited from discussions with J. Defrize, R. Foelix and two reviewers who provided valuable comments on the earlier versions of this manuscript. F. Amiet is acknowledged for help in the determination of the hymenopterans. This study was funded by the National Center of Competence in Research (NCCR) Plant Survival, a research programme of the Swiss National Science Foundation.
References
- Abbott K. R., Dukas R.2009. Honeybees consider flower danger in their waggle dance. Anim. Behav. 78, 633–635 (doi:10.1016/j.anbehav.2009.05.029) [Google Scholar]
- Angus J.1882Protective change of color in a spider. Am. Nat. 16, 1010 [Google Scholar]
- Brechbühl R., Kropf C., Bacher S.In press Impact of flower dwelling crab spiders on plant pollinator mutualisms. Basic Appl. Ecol. (doi:10.1016/j.baae.2009.07.001) [Google Scholar]
- Chittka L.2001Camouflage of predatory crab spiders on flowers and the colour perception of bees (Aranida: Thomisidae/Hymenoptera: Apidae). Entomol. Gen. 25, 181–187 [Google Scholar]
- Clark C. W., Dukas R.1994Balancing foraging and antipredator demands—an advantage of sociality. Am. Nat. 144, 542–548 (doi:10.1086/285693) [Google Scholar]
- Defrize J., Théry M., Casas J.Submitted Background colour matching by a crab spider in the field: a community sensory ecology perspective. [DOI] [PubMed] [Google Scholar]
- Dukas R.2001Effects of perceived danger on flower choice by bees. Ecol. Lett. 4, 327–333 (doi:10.1046/j.1461-0248.2001.00228.x) [Google Scholar]
- Dukas R., Morse D. H.2003Crab spiders affect flower visitation by bees. Oikos 101, 157–163 (doi:10.1034/j.1600-0706.2003.12143.x) [Google Scholar]
- Dukas R., Morse D. H.2005Crab spiders show mixed effects on flower visiting bees and no effect on plant fitness. Ecoscience 12, 244–247 (doi:10.2980/i1195-6860-12-2-244.1) [Google Scholar]
- Fritz R. S., Morse D. H.1985Reproductive success, growth rate and foraging decisions of the crab spider Misumena vatia. Oecologia 65, 194–200 (doi:10.1007/BF00379217) [DOI] [PubMed] [Google Scholar]
- Gabritschevsky E.1927Experiments on color changes and regeneration in the crab-spider Misumena vatia. J. Exp. Zool. 47, 251–267 (doi:10.1002/jez.1400470207) [Google Scholar]
- Heiling A. M., Herberstein M. E.2004Floral quality signals lure pollinators and their predators. Ann. Zool. Fenn. 41, 421–428 [Google Scholar]
- Heiling A. M., Herberstein M. E., Chittka L.2003Crab-spiders manipulate flower signals. Nature 421, 334 (doi:10.1038/421334a) [DOI] [PubMed] [Google Scholar]
- Heiling A. M., Chittka L., Cheng K., Herberstein M. E.2005Colouration in crab spiders: substrate choice and prey attraction. J. Exp. Biol. 208, 1785–1792 (doi:10.1242/jeb.01585) [DOI] [PubMed] [Google Scholar]
- Ings T. C., Chittka L.2008Speed–accuracy tradeoffs and false alarms in bee responses to cryptic predators. Curr. Biol. 18, 1520–1524 (doi:10.1016/j.cub.2008.07.074) [DOI] [PubMed] [Google Scholar]
- Insausti T. C., Casas J.2008The functional morphology of color changing in a spider: development of ommochrome pigment granules. J. Exp. Biol. 211, 780–789 (doi:10.1242/jeb.014043) [DOI] [PubMed] [Google Scholar]
- Insausti T. C., Casas J.In press Turnover of pigment granules: cyclic catabolism and anabolism of ommochromes within epidermal cells. Tissue Cell (doi:10.1016/j.tice.2009.05.002) [DOI] [PubMed] [Google Scholar]
- Langer H.1975Properties and functions of screening pigments in insects eyes. In Photoreceptor optics (eds Snyder A. W., Menzel R.), pp. 429–455 Berlin, Germany; New York, NY: Springer-Verlag [Google Scholar]
- Majerus M. E. N., Brunton C. F. A., Stalker J.2000A bird's eye view of the peppered moth. J. Evol. Biol. 13, 155–159 (doi:10.1046/j.1420-9101.2000.00170.x) [Google Scholar]
- Morse D. H.1979Prey capture by the crab spider Misumena calycina (Araneae: Thomisidae). Oecologia 39, 309–319 (doi:10.1007/BF00345442) [DOI] [PubMed] [Google Scholar]
- Morse D. H.1981Prey capture by the crab spider Misumena vatia (Clerck) (Thomisidae) on three common native flowers. Am. Midl. Nat. 105, 358–367 (doi:10.2307/2424754) [Google Scholar]
- Morse D. H.2007. In Predator upon a flower: life history and fitness in a crab spider. Cambridge, MA: Harvard University Press [Google Scholar]
- Morse D. H., Fritz R. S.1982Experimental and observational studies of patch choice at different scales by the crab spider Misumena vatia. Ecology 63, 172–182 (doi:10.2307/1937042) [Google Scholar]
- Muñoz A. A., Arroyo M. T. K.2004Negative impacts of a vertebrate predator on insect pollinator visitation and seed output in Chuquiraga oppositifolia, a high Andean shrub. Oecologia 138, 66–73 (doi:10.1007/s00442-003-1405-2) [DOI] [PubMed] [Google Scholar]
- Oxford G. S., Gillespie R. G.1998Evolution and ecology of spider coloration. Ann. Rev. Entomol. 43, 619–643 (doi:oi:10.1146/annurev.ento.43.1.619) [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2007. In R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Rabaud E.1919Deuxième note sur l'adaptation chromatique des Thomisides. Bull. Soc. Zool. Fr. 44, 327–329 [Google Scholar]
- Reader T., Higginson A. D., Barnard C. J., Gilbert F. S.The Behavioural Ecology Field Course 2006The effects of predation risk from crab spiders on bee foraging behaviour. Behav. Ecol. 17, 933–939 (doi:10.1093/beheco/arl027) [Google Scholar]
- Schmalhofer V. R.2001Tritrophic interactions in a pollination system: impacts of species composition and size of flower patches on the hunting success of a flower-dwelling spider. Oecologia 129, 292–303 (doi:10.1007/s004420100726) [DOI] [PubMed] [Google Scholar]
- Schwarz G.1978Estimating dimension of a model. Ann. Stat. 6, 461–464 (doi:10.1214/aos/1176344136) [Google Scholar]
- Stavenga D. G.1989Pigments in compound eyes. In Facets of vision (eds Stavenga D. G., Hardie R. C.), pp. 152–172 Berlin, Germany: Springer-Verlag [Google Scholar]
- Théry M.2007Colours of background reflected light and of the prey's eye affect adaptive coloration in female crab spiders. Anim. Behav. 73, 797–804 (doi:10.1016/j.anbehav.2006.06.015) [Google Scholar]
- Théry M., Casas J.2002Predator and prey views of spider camouflage. Nature 415, 133 (doi:10.1038/415133a) [DOI] [PubMed] [Google Scholar]
- Théry M., Casas J.2009The multiple disguises of spiders: web colour and decorations, body colour and movement. Phil. Trans. R. Soc. B 364, 471–480 (doi:10.1098/rstb.2008.0212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venner S., Casas J.2005Spider webs designed for rare but life-saving catches. Proc. R. Soc. B 272, 1587–1592 (doi:10.1098/rspb.2005.3114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel G.1941Färbung und Farbwechsel der Krabbenspinne Misumena vatia (L.). J. Comp. Physiol. 29, 195–248 (doi:10.1007/BF00304448) [Google Scholar]
- Williams C. S.1998The identity of the previous visitor influences flower rejection by nectar-collecting bees. Anim. Behav. 56, 673–681 (doi:10.1006/anbe.1998.0794) [DOI] [PubMed] [Google Scholar]