Abstract
Variation in reactions to aposematic prey is common among conspecific individuals of bird predators. It may result from different individual experience but it also exists among naive birds. This variation may possibly be explained by the effect of personality—a complex of correlated, heritable behavioural traits consistent across contexts. In the great tit (Parus major), two extreme personality types have been defined. ‘Fast’ explorers are bold, aggressive and routine-forming; ‘slow’ explorers are shy, non-aggressive and innovative. Influence of personality type on unlearned reaction to aposematic prey, rate of avoidance learning and memory were tested in naive, hand-reared great tits from two opposite lines selected for exploration (slow against fast). The birds were subjected to a sequence of trials in which they were offered aposematic adult firebugs (Pyrrhocoris apterus). Slow birds showed a greater degree of unlearned wariness and learned to avoid the firebugs faster than fast birds. Although birds of both personality types remembered their experience, slow birds were more cautious in the memory test. We conclude that not only different species but also populations of predators that differ in proportions of personality types may have different impacts on survival of aposematic insects under natural conditions.
Keywords: aposematic insects, avoidance learning, naive predators, Parus, personality, Pyrrhocoris
1. Introduction
Various bird species may react differently towards a warning signal of the same species of aposematic prey (e.g. Exnerová et al. 2003; Endler & Mappes 2004). Even closely related species may have different impacts on populations of aposematic prey owing to specific foraging strategies (Brower 1988) or may differ in the origin of avoidance (Exnerová et al. 2007).
Among predators, there is a considerable intraspecific variation in reactions to aposematic prey. In experiments testing the reactions of passerine birds (e.g. great tits, robins, blackbirds) to aposematic true bugs (Lygaeus equestris, Pyrrhocoris apterus), some birds refused to attack the prey on sight, while others seized and dropped it, and some consumed it (Sillén-Tullberg et al. 1982; Exnerová et al. 2003, 2006). In wild-caught birds, the variation may have resulted from different individual experience. However, similar differences exist also among naive birds (Sillén-Tullberg 1985; Exnerová et al. 2007; Svádová et al. 2009) and among wild birds presented with novel food (Marples et al. 1998, 2005).
Attitude to a novel prey, especially to an aposematic one, may be potentially linked with individual personality. However, the link between individual behavioural traits of predators (such as their different personalities) and predator reactions to aposematic prey has never been tested. Personality (alternatively termed ‘coping style’ or ‘behavioural syndrome’) is a complex of correlated behavioural traits that are consistent across time and ecological situations (Benus et al. 1990; Sih et al. 2004).
Personality characteristics have been extensively studied in great tits. Individual great tits differ in the way they explore a novel environment and, at the extremes of the variation, may be assessed as ‘slow’ or ‘fast’ explorers (Verbeek et al. 1994; Drent et al. 2003). This difference is correlated with differences in behaviour towards novel objects (Verbeek et al. 1994), risk-taking (van Oers et al. 2004a), aggressiveness (Verbeek et al. 1996), foraging behaviour (Verbeek et al. 1994), use of social information (Marchetti & Drent 2000; van Oers et al. 2005), response to stress (Carere et al. 2003a; Carere & van Oers 2004; Fucikova et al. 2009) and other behavioural and physiological traits (Carere & van Oers 2004). Personality traits are determined partly genetically (Drent et al. 2003; van Oers et al. 2004b).
Fast explorers are bold, risk-taking, aggressive, routine forming, explore new environment quickly but superficially and tend to copy other individuals’ foraging behaviour. Slow explorers are shy, less risk-taking, non-aggressive, innovative, explore new environment slowly but thoroughly and are active and independent foragers. Neophobic individuals are expected to belong to the slow personality type because they are more cautious, less risk-taking and shy of novel objects. Consequently, it could be expected that personality may cause differences in individual reactions to the aposematic prey—unlearned wariness, rate of avoidance learning and memory for the aposematic signal.
To test this hypothesis, we subjected naive great tits to a sequence of trials, in which they were offered aposematic adult firebugs (P. apterus). We compared the degree of unlearned wariness, the rate of avoidance learning and the memory of experience with aposematic prey in birds originating from two lines, which were selected for differences in early exploration (fast against slow) as indicators of personality (Drent et al. 2003).
2. Material and methods
The experiments were carried out in the Netherlands Institute of Ecology, Heteren, in July 2007.
(a). Birds
We tested altogether 42 naive, hand-reared great tits (Parus major) descended from the F4 generation of ‘fast’ (22 birds) and ‘slow’ (20 birds) bi-directional artificial selection lines (see Drent et al. 2003 for details). The birds came from 18 broods (mostly two or three birds from the same brood). All birds were tested by standard tests for early exploratory behaviour (reaction to novel objects—a penlight battery and a pink panther toy—and behaviour in a novel environment; see Drent et al. 2003) when they were 25–35 days old. Birds were housed individually in wooden cages (90 × 40 × 50 cm) with wire-mesh front wall and three perches. They were kept under natural light conditions and temperature, and provided with ad libitum drinking and bathing water and commercial food mixture (containing mainly proteins, trace elements, vitamins and minerals) supplemented with mealworms. The tests with the aposematic prey were carried out when the birds were 64–90 days old. The birds had no experience with aposematic insects or other warningly coloured food items prior to the experiments. The experimental groups were balanced in terms of sex (slow personality group: 12 males and 8 females; fast personality group: 11 males and 11 females; Fisher's exact test: n = 42, p = 0.551) and age of the birds (slow personality group: mean ± s.d. = 78.11 ± 7.93 days; fast personality group: mean ± s.d. = 76.95 ± 7.92 days; ANOVA: F1,38 = 0.21, p = 0.649). After the experiment, all birds were returned to the pool of birds used for breeding the selection lines.
(b). Prey
Adult brachypterous firebugs (P. apterus; Heteroptera: Pyrrhocoridae), conspicuously coloured red and black, were used as aposematic prey since they are unpalatable for small passerines (Exnerová et al. 2003). Their defensive secretion is composed of over 35 chemicals, mainly aldehydes (Farine et al. 1992), produced in metathoracic glands. Firebugs are widespread in the Palaearctic region in a variety of habitats, feeding on seeds of Tilia spp., various mallows (Malvaceae) and Robinia pseudacacia (Fabaceae). The firebugs were collected at several localities in Prague, Czech Republic, from populations feeding on Tilia cordata. They were reared on its seeds and water ad libitum, under a long-day photoperiod (18L : 6D), at a temperature of 26 ± 1°C.
(c). Experimental design
Experiments were carried out in wooden cages (70 × 70 × 70 cm) with wire mesh walls, a perch, a circular feeding tray with beige (pine wood) cups and front wall made of one-way glass (see Exnerová et al. 2003 for details). Cage illumination (Biolux Combi 18W, Osram) was used to simulate the full daylight spectrum. Birds were trained to search for mealworms (Tenebrio molitor larvae) in one of the cups of the feeding tray and then deprived of food for 2 h before the experiment. Each bird was tested alone in the whole sequence of tests and used only once.
The experiment was carried out in two phases: an avoidance-learning session and memory test. (i) The avoidance-learning session consisted of a sequence of 5 min trials. Each bird was successively offered one mealworm (odd trials) and one firebug (even trials) in turn, starting with the mealworm. Mealworms, which were familiar to the birds, were used to check their foraging motivation. The trials were repeated until the bird refused to attack the firebug in three successive trials; this was considered the learning criterion. If the bird refused to attack the first firebug, the sequence continued until a maximum of 10 firebugs were left untouched; in such a case, the learning session was repeated the following day. Birds that refused to attack the firebugs during both sessions were used only in the analysis of unlearned wariness but excluded from the learning and memory experiment. (ii) The memory test was carried out the day after the learning session. Each bird was offered a mealworm and a firebug in two successive 5 min trials.
During every trial we recorded (i) whether the bird attacked (seized or pecked) the prey, (ii) attack latency and (iii) whether the prey was killed. Attack latencies were measured as the time from the beginning of the trial to the first handling (pecking or seizing) of the prey.
(d). Data analysis
Our first aim was to find out whether two personality groups differed specifically in their responses to aposematic prey, because their responses could merely reflect their difference in foraging motivation and general attack tendency. ANOVA was used to compare their latencies to attack the first mealworm offered in the avoidance-learning session. The same comparison was done for latencies to attack the mealworm offered in the memory test. Attack latencies were log-transformed to fit the normal distribution.
To assess the unlearned wariness for firebugs, we (i) compared the counts of fast and slow birds that attacked or did not attack the firebugs during first ten trials of the avoidance-learning session, using the Fisher exact test; and (ii) used the Mann–Whitney U test for comparison of latencies to attack the first firebug in the avoidance-learning session.
To compare the rate of avoidance learning, we used two different dependent variables: (i) number of firebug trials required by the bird to reach the learning criterion (counted from the first trial in which the bird attacked the firebug until the third successive firebug trial without attacking); and (ii) number of trials in which the bird attacked the firebug. The data were analysed by GLM ANOVA with the Poisson distribution and log-link function. To evaluate the relative mortality of attacked firebugs when confronted with birds of different personality types, we compared the proportions of attacked firebugs that were killed by individual slow and fast birds using ANOVA. The proportions were arcsin-transformed to fit the normal distribution.
To assess the birds’ memory, we used the Wilcoxon signed-ranks test for within-group comparisons of latencies to attack the first firebug between avoidance-learning session and memory test. Then we applied the Mann–Whitney U test for comparison of latencies to attack the firebug between the two personality groups. We used the Fisher exact test to compare proportions of slow and fast birds that attacked and killed firebugs in the memory test.
All the p-values are two-tailed. Bonferroni correction was applied to comparisons of avoidance-learning rate (α = 0.017) and to comparisons of numbers of birds that attacked and killed firebugs in the memory test (α = 0.025). All calculations were made in S-PLUS 4.0 (MathSoft 1997).
3. Results
(a). Reactions to familiar prey
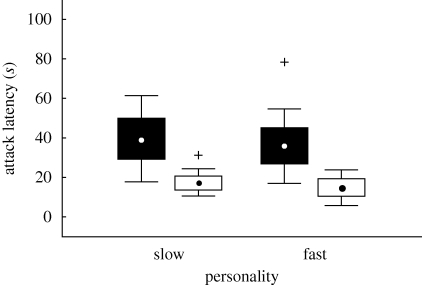
All the birds, irrespective of their personality, killed and ate all the mealworms they were offered during the avoidance-learning session, as well as during the memory test. Latencies to attack the mealworm in the first trial of avoidance-learning did not differ between the two personality groups (ANOVA: F1,40 = 0.58, p = 0.46; figure 1), and the same was true for latencies to attack the mealworm in the memory test (ANOVA: F1,38 = 2.02, p = 0.17).
Figure 1.
Attack latencies of naive great tits of the two personality types measured from the beginning of the first mealworm trial to first handling the mealworm in the avoidance-learning session (black bars) and in the memory test (white bars). Point, mean; box, mean ± s.e.; whiskers, confidence intervals (0.95); crosses, outliers.
(b). Unlearned wariness
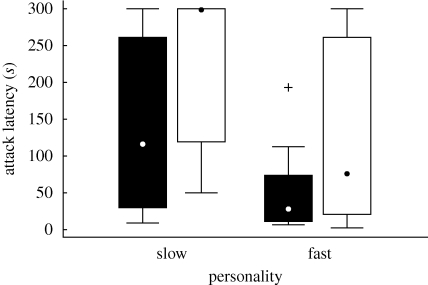
Birds of the two personality groups differed in their willingness to attack a novel aposematic prey. During the first 10 trials of the avoidance-learning session, all 22 fast birds attacked at least one of the offered firebugs, while 5 out of 20 slow birds refused to attack the firebugs at all (Fisher's exact test: n = 42, p = 0.018). Three of these five slow birds started to attack the firebugs during the second-day session. Latencies to attack the first differed as well (Mann–Whitney U test: U = 117, n1 = 18, n2 = 22, p = 0.028); the slow birds hesitated longer (figure 2).
Figure 2.
Attack latencies of naive great tits of the two personality types measured from the beginning of the first firebug trial to first handling the firebug in the avoidance-learning session (black bars) and in the memory test (white bars). Point, median; box, lower and upper quartile (inter-quartile range); whiskers, non-outlier range (values within 1 times the inter-quartile range outside the closest quartile); crosses, outliers.
(c). Avoidance learning
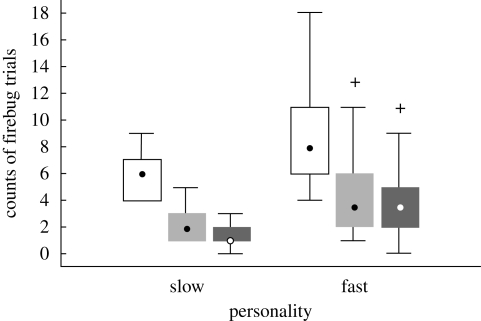
All the birds that attacked the firebugs achieved the learning criterion within the sequence of the maximum 18 trials. The number of trials required by the bird to reach the learning criterion differed between the two personality groups (GLM ANOVA: F1,38 = 14.76, p < 0.001); slow birds took fewer trials to learn the avoidance than fast birds (figure 3).
Figure 3.
Counts of firebug trials required by the naive great tits of the two personality types to reach the avoidance learning criterion (white bars), and counts of trials in which the birds attacked (grey bars) and killed (black bars) the firebugs during the avoidance-learning session. Point, median; box, lower and upper quartile (inter-quartile range); whiskers, non-outlier range (values within 1 times the inter-quartile range outside the closest quartile); crosses, outliers.
Personality type affected also the number of firebugs attacked by individual birds during the avoidance learning (GLM ANOVA: F1,38 = 13.59, p < 0.001); slow birds attacked fewer firebugs than fast birds before they reached the learning criterion (figure 3).
Personality type of the birds did not influence the relative mortality of the firebugs attacked (ANOVA: F1,38 = 1.02, p = 0.33). The average probability of a firebug being killed by a bird was around 0.8, irrespective of the personality of the bird. However, the overall firebug mortality caused by fast birds was considerably higher than that caused by slow birds (figure 3) because of the greater number of firebugs attacked by them.
(d). Memory
Both slow and fast birds hesitated longer before attacking the firebug in the memory test than they did in the first firebug trial of the avoidance-learning session (Wilcoxon signed-ranks test: slow birds: Z = 2.61, n = 18, p = 0.009; fast birds: Z = 2.52, n = 22, p = 0.012; figure 2). However, latencies to attack the firebug in the memory test were longer in the slow birds than in the fast birds (Mann–Whitney U test: U = 120.5, n1 = 18, n2 = 22, p = 0.035; figure 2).
The number of birds that attacked the firebug in the memory test did not differ between the slow and fast personality groups (Fisher's exact test: n = 40, p = 0.203); approximately half the birds in each group attacked the firebugs. The number of birds that killed the firebugs in the memory test differed between the two personality groups (Fisher's exact test: n = 40, p = 0.016); half of the fast birds killed the firebugs, whereas only two slow birds did.
4. Discussion
Birds of the two personality groups did not differ in their tendency to attack and consume familiar palatable prey. Consequently, we may consider the differences in their reactions to firebugs to be specific for newly encountered aposematic prey and not only reflecting different foraging motivation or general attack tendency.
Slow and fast birds differed in their willingness to attack a novel aposematic prey; slow birds hesitated longer than fast birds. Once the birds attacked the prey in the avoidance-learning session, birds of both personality types did it forcefully, and the bug was most likely to be killed. However, slow birds learned to avoid the firebugs considerably faster, attacking and therefore killing fewer firebugs than the fast birds.
Both slow and fast birds hesitated longer before attacking the first firebug in the memory test performed on the second experimental day than they had done in the avoidance-learning session. Consequently, both groups remembered their experience. Nevertheless, the attack latencies of slow birds were longer than those of fast birds. In contrast to the fast birds, slow birds handled the firebugs in the memory test carefully, and usually did not kill them.
(a). Unlearned wariness
The slow birds hesitated longer to approach and attack novel aposematic prey than the fast birds. This is consistent with Verbeek et al. (1994), who found that slow explorers had longer latencies than the fast explorers in approaching novel objects placed in their home cages. We assume that approaching, attacking and consuming an unknown prey may be considered a risk-taking behaviour. Fast explorers are known to risk more than slow explorers also in other situations (van Oers et al. 2004a, 2005).
Our results are in accordance with experimental evidence that response to novelty is correlated across different situations involving novel environment, various kinds of novel objects, and novel food (Jones et al. 1991; Verbeek et al. 1994; Webster & Lefebvre 2001). However, results of other studies suggest that neophobia could be specific in a particular context (Coleman & Wilson 1998; van Oers et al. 2005; Boogert et al. 2006).
Unlearned wariness plays a significant role in the relation of predators to novel, especially aposematic prey (Ruxton et al. 2004; Marples et al. 2005). Birds of different species differ in their level of neophobia (Greenberg 1990; Mettke-Hofmann et al. 2005), and these differences could be partly responsible for interspecific differences in behaviour towards the aposematic prey (Exnerová et al. 2007). Our results show that variation in neophobic reaction to aposematic prey at an individual level may be associated with personality of the predator. We hypothesize that different proportions of personality types in different predator species tested may explain part of interspecific differences in unlearned reactions to the aposematic prey.
Unlearned wariness of aposematic prey may also involve dietary conservatism (Marples et al. 2005), which differs from neophobia by considerably longer duration (Marples et al. 1998; Marples & Kelly 1999) and more difficult deactivation by experience (Marples et al. 2007). Because the level of dietary conservatism varies greatly among conspecific individuals (Marples et al. 1998), it is possible that it may be linked with personality—a hypothesis to be tested.
(b). Avoidance learning and memory
Slow explorers appeared to be better than fast explorers in avoidance learning with aposematic prey as an aversive stimulus. We offer two hypotheses to explain this difference. (i) The difference could be specific to a particular avoidance-learning task, and training with positive stimuli or other learning tasks could lead to different results. (ii) Slow birds are generally more flexible. They are thorough explorers, more sensitive to environmental changes and better (or at least faster) in gathering information about the environment (Verbeek et al. 1994). This may also play a role in using information on the palatability of the prey. Slow birds change their foraging routines more easily than fast birds (Marchetti & Drent 2000), and they may quickly return to their initial wariness when facing aversive stimuli.
Compared with the avoidance learning, a different situation was found with other cognitive tasks in great tits, particularly with observational learning (Marchetti & Drent 2000) and with using spatial and visual cues in foraging (Carere et al. 2003b). When using social information about location of food supplies, fast birds copied quickly the behaviour of tutors, while slow birds did not (Marchetti & Drent 2000). Carere et al. (2003b) found no difference between slow and fast birds in their ability to retain spatial and visual cues indicating food location in their memory, both groups making similar number of errors in the test. Our results partly correspond with theirs. Both slow and fast birds remembered their experience with aposematic prey, which can be seen in prolonged attack latencies of both groups in the memory test. However, slow birds performed better in the memory test, having longer attack latencies and handling the prey more carefully than fast birds. It is therefore possible that the relation between cognition and personality is context-dependent—slow birds perform better in some tasks and fast birds in others.
Relations between personality and cognitive abilities have been studied in only a limited number of animal species. Nevertheless, relevant information can be found in studies of the relationship between cognition and neophobia, without specific reference to personality. In contrast to our results, some published studies do not show any correlation between boldness or neophobia and performance in learning tasks (Boogert et al. 2006 in starlings Sturnus vulgaris; Brydges et al. 2008 in sticklebacks Gasterosteus aculeatus). In other studies, the performance in learning tasks correlated positively with boldness or negatively with neophobia (Dugatkin & Alfieri 2003 in guppies Poecilia reticulata; Sneddon 2003 in rainbow trout Oncorhynchus mykiss; Arnold et al. 2007 in blue tits Cyanistes caeruleus). Similarly, a negative correlation between the level of neophobia and performance in learning tasks was found in a comparison across several bird species (Webster & Lefebvre 2001). There are at least two possible explanations of the differences: (i) the relationship between personality and learning may be different in different species, as suggested by Brydges et al. (2008), or (ii) the relationship between learning performance and personality depends on the type of the learning task and stimuli used. In all the above-mentioned studies, the learning task involved positive stimuli (usually food as a reward), and in the majority of them (except Boogert et al. 2006), the animals were learning in the context of an environment novel to them. This type of learning task favours bold, less neophobic individuals (Webster & Lefebvre 2001; Greenberg 2003). On the contrary, the avoidance-learning task could favour shy, more neophobic individuals, which are more sensitive to negative stimuli.
(c). The implications of personality for the predator–aposematic prey interactions
The following discussion is based on three premises. (i) Great tit personality types occur throughout the species range (e.g. Korsten et al. in press). (ii) Personality traits occur in other bird species as well (cf. Garamszegi et al. 2008). (iii) The interactions between birds and aposematic Heteroptera are diverse because of the variation in antipredatory defences of true bugs (Aldrich 1988) and corresponding reactions of birds (Exnerová et al. 2008).
When an aposematically coloured insect meets a naive bird predator, it matters which of the personality types the predator belongs to.
A fast explorer hesitates less to attack unknown prey, needs more attempts to learn to avoid it, if it proves to be noxious, and may cause higher prey mortality. A slow explorer shows a greater degree of unlearned wariness, learns fast to avoid noxious prey and becomes more cautious in handling the prey after the negative experience. Since the prey has greater chance to escape or employ its defensive mechanisms, it may often survive the attack (Sillén-Tullberg 1985; Exnerová et al. 2007) and fewer individuals will be killed. Consequently, slow exploring birds differ from fast explorers in their lower effectiveness in negative selection aimed at aposematic insect prey.
Different behaviour of slow and fast explorers towards aposematic prey would have probably a negligible effect on survival of large populations of P. apterus. The situation may be different in small populations that may be found particularly along the border of its range—prevailing personality of the predators could add to other factors influencing their survival. However, other predator species would also be involved, and interspecific variation in their behaviour towards aposematic prey exceeds the individual variation that exists among great tits (Exnerová et al. 2003, 2008). Local presence of species belonging to a red-and-black hemipteran mimetic complex (see Exnerová et al. 2008) would probably facilitate persistence of small populations of P. apterus.
The usual red colour of P. apterus may be affected by recessive mutations turning it to orange, yellow or white (Socha & Němec 1992). There is a considerable intraspecific variation in behaviour of birds towards these mutants (Exnerová et al. 2006), part of which could be explained by personality of birds. If we extend this interpretation also to the origins of novel aposematic signals in other insects, we may expect different roles of predators of the two personality types in survival of new aposematic morphs. An assumption of different representations of great tit personality types in geographically distant populations may also help to elucidate differences in the results of experiments concerning attitudes of naive tits towards aposematic prey (e.g. Lindström et al. 1999; Svádová et al. 2009).
Acknowledgements
This study was supported by Czech Science Foundation (project 206/07/0507) and Ministry of Education (project 0021620828). Permission for maintaining the selection lines for great tit hand rearing and the behavioural tests was granted by the Dutch Committee KNAW Dier Experimenten Commissie (DEC-KNAW), license no. CTO.07-06. We thank the Netherlands Institute for Ecology (NIOO-KNAW) for giving the opportunity to use their birds and equipment and for support to carry out the tests. Kees van Oers and Piet de Goede have assisted by testing personality of the birds. Ab and Chiel Wijlhuizen constructed the test cages. We are also grateful to Marylou Aaldering, Janneke Venhorst and Floor Petit for hand rearing and taking care of the experimental birds. Olga Kukal (Queen's University, Canada) and Michael R. Wilson (National Museum of Wales, Cardiff) kindly revised our English. Two anonymous referees and the editors of the journal greatly assisted in improving the quality of our paper.
References
- Aldrich J. R.1988Chemical ecology of the Heteroptera. Ann. Rev. Entomol. 33, 211–238 (doi:10.1146/annurev.en.33.010188.001235) [Google Scholar]
- Arnold K. E., Ramsay S. L., Donaldson C., Adam A.2007Parental prey selection affects risk-taking behaviour and spatial learning in avian offspring. Proc. R. Soc. B 274, 2563–2569 (doi:10.1098/rspb.2007.0687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benus R. F., den Daas S., Koolhaas J. M., van Oortmerssen G. A.1990Routine formation and flexibility in social and non-social behaviour of aggressive and non-aggressive male mice. Behaviour 112, 176–193 (doi:10.1163/156853990X00185) [Google Scholar]
- Boogert N. J., Reader S. M., Laland K. N.2006The relation between social rank, neophobia and individual learning in starlings. Anim. Behav. 72, 1229–1239 (doi:10.1016/j.anbehav.2006.02.021) [Google Scholar]
- Brower L. P.1988Avian predation on the monarch butterfly and its implications for mimicry theory. In Mimicry and the evolutionary process (ed. Brower L. P.), pp. 4–6 Chicago, IL: University of Chicago Press [Google Scholar]
- Brydges N. M., Colegrave N., Heathcote R. J. P., Braithwaite V. A.2008Habitat stability and predation pressure affect temperament behaviours in populations of three-spined sticklebacks. J. Anim. Ecol. 77, 229–235 (doi:10.1111/j.1365-656.2008.01343.x) [DOI] [PubMed] [Google Scholar]
- Carere C., van Oers K.2004Shy and bold great tits (Parus major): body temperature and breath rate in response to handling stress. Physiol. Behav. 82, 905–912 (doi:10.1016/j.physbeh.2004.07.009) [DOI] [PubMed] [Google Scholar]
- Carere C., Groothuis T. G. G., Möstl E., Daan S., Koolhaas J. M.2003aFecal corticosteroids in a territorial bird selected for different personalities: daily rhythm and the response to social stress. Horm. Behav. 43, 540–548 (doi:10.1016/S0018-506X(03)00065-5) [DOI] [PubMed] [Google Scholar]
- Carere C., Havekes R., Oorebeek M., Koolhaas J. M., Groothuis T. G. G.2003b. Food finding abilities in two lines of great tits (Parus major) selected for different ‘personalities’. Personalities as epigenetic suites of traits. A study on a passerine Bird (C. Carere). PhD dissertation, University of Groningen, pp. 42–56
- Coleman K., Wilson D. S.1998Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim. Behav. 56, 927–936 (doi:10.1006/anbe.1998.0852) [DOI] [PubMed] [Google Scholar]
- Drent P. J., van Oers K., van Noordwijk A. J.2003Realized heritability of personalities in the great tit (Parus major). Proc. R. Soc. Lond. B 270, 45–51 (doi:10.1098/rspb.2002.2168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugatkin L. A., Alfieri M. S.2003Boldness, behavioural inhibition and learning. Ethol. Ecol. Evol. 15, 43–49 [Google Scholar]
- Endler J. A., Mappes J.2004Predator mixes and the conspicuousness of the aposematic signal. Am. Nat. 163, 232–247 [DOI] [PubMed] [Google Scholar]
- Exnerová A., Landová E., Štys P., Fuchs R., Prokopová M., Cehláriková P.2003Reactions of passerine birds to aposematic and non-aposematic firebugs (Pyrrhocoris apterus; Heteroptera). Biol. J. Linn. Soc. 78, 517–525 (doi:10.1046/j.0024-4066.2002.00161.x) [Google Scholar]
- Exnerová A., Svádová K., Štys P., Barcalová S., Landová E., Prokopová M., Fuchs R., Socha R.2006Importance of colour in the reaction of passerine predators to aposematic prey: an experiment with mutants of Pyrrhocoris apterus (Heteroptera). Biol. J. Linn. Soc. 88, 143–153 (doi:10.1111/j.1095-8312.2006.00611.x) [Google Scholar]
- Exnerová A., Štys P., Fučíková E., Veselá S., Svádová K., Prokopová M., Jarošík V., Fuchs R., Landová E.2007Avoidance of aposematic prey in European tits (Paridae): learned or innate? Behav. Ecol. 18, 148–156 (doi:10.1093/beheco/arl061) [Google Scholar]
- Exnerová A., Svádová K., Fousová P., Fučíková E., Ježová D., Niederlová A., Kopečková M., Štys P.2008European birds and aposematic Heteroptera: review of comparative experiments. Bull. Insectol. 61, 163–165 [Google Scholar]
- Farine J. P., Bonnard O., Brossut R., Le Quere J. L.1992Chemistry of defensive secretion in nymphs and adults of firebug, Pyrrhocoris apterus L. (Heteroptera, Pyrrhocoridae). J. Chem. Ecol. 8, 1673–1682 [DOI] [PubMed] [Google Scholar]
- Fucikova E., Drent P. J., Smits N., van Oers K.2009Handling stress as a measurement of personality in great tit nestlings (Parus major). Ethology 115, 366–374 (doi:10.1111/j.1439-0310.2009.01618.x) [Google Scholar]
- Garamszegi L. Z., Eens M., Török J.2008Birds reveal their personality when singing. PLoS ONE 3, e2647 (doi:10.1371/journal.pone.0002647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R.1990Ecological plasticity, neophobia, and resource use in birds. Stud. Avian Biol. 13, 431–437 [Google Scholar]
- Greenberg R.2003The role of neophobia and neophilia in the development of innovative behaviour of birds. In Animal innovation (eds Reader S. M., Laland K. N.), pp. 175–196 Oxford, UK: Oxford University Press [Google Scholar]
- Jones R. B., Mills A. D., Faure J. M.1991Genetic and experiential manipulation of fear-related behavior in Japanese quail chicks (Coturnix coturnix japonica). J. Comp. Psychol. 105, 15–24 (doi:10.1037/0735-7036.105.1.15) [DOI] [PubMed] [Google Scholar]
- Korsten P., et al. In press Association between DRD4 gene polymorphism and personality variation in great tits: a test across four wild populations. Mol. Ecol. [DOI] [PubMed] [Google Scholar]
- Lindström L., Alatalo R. V., Mappes J.1999Reactions of hand-reared and wild-caught predators toward warningly colored, gregarious, and conspicuous prey. Behav. Ecol. 10, 317–322 (doi:10.1093/beheco/10.3.317) [Google Scholar]
- Marchetti C., Drent P. J.2000Individual differences in the use of social information in foraging by captive great tits. Anim. Behav. 60, 131–140 (doi:10.1006/anbe.2000.1443) [DOI] [PubMed] [Google Scholar]
- Marples N. M., Kelly D. J.1999Neophobia and dietary conservatism: two distinct processes? Evol. Ecol. 13, 641–653 (doi:10.1023/A:1011077731153) [Google Scholar]
- Marples N. M., Roper T. J., Harper D. G. C.1998Responses of wild birds to novel prey: evidence of dietary conservatism. Oikos 83, 161–165 (doi:10.2307/3546557) [Google Scholar]
- Marples N. M., Kelly D. J., Thomas R. J.2005The evolution of warning coloration is not paradoxical. Evolution 59, 933–940 [PubMed] [Google Scholar]
- Marples N. M., Quinlan M., Thomas R. J., Kelly D. J.2007Deactivation of dietary wariness through experience of novel food. Behav. Ecol. 18, 803–810 (doi:10.1093/beheco/arm053) [Google Scholar]
- MathSoft 1997S-plus 4. Guide to statistics: data analysis Seattle, WA: MathSoft Inc [Google Scholar]
- Mettke-Hofmann C., Ebert C., Schmidt T., Steiger S., Stieb S.2005Personality traits in resident and migratory warbler species. Behaviour 42, 1363–1381 [Google Scholar]
- Ruxton G. D., Sherratt T. N., Speed M. P.2004Avoiding attack New York, NY: Oxford University Press [Google Scholar]
- Sih A., Bell A. M., Johnson J. C., Ziemba R. E.2004Behavioural syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 [DOI] [PubMed] [Google Scholar]
- Sillén-Tullberg B.1985Higher survival of an aposematic than of a cryptic form of a distasteful bug. Oecologia 67, 411–415 (doi:10.1007/BF00384948) [DOI] [PubMed] [Google Scholar]
- Sillén-Tullberg B., Wiklund C., Järvi T.1982Aposematic coloration in adults and larvae of Lygaeus equestris and its bearing on Müllerian mimicry: an experimental study on predation on living bugs by the great tit Parus major. Oikos 39, 131–136 (doi:10.2307/3544476) [Google Scholar]
- Sneddon L. U.2003The bold and the shy: individual differences in rainbow trout. J. Fish Biol. 62, 971–975 (doi:10.1046/j.1095-8649.2003.00084.x) [Google Scholar]
- Socha R., Němec V.1992Pteridine analysis in five body-colour mutations of Pyrrhocoris apterus (Heteroptera, Pyrrhocoridae). Acta Entomol. Bohemoslov. 89, 195–203 [Google Scholar]
- Svádová K., Exnerová A., Štys P., Landová E., Valenta J., Fučíková A., Socha R.2009Role of different colours of aposematic insects in learning, memory and generalization of naive bird predators. Anim. Behav. 77, 327–336 (doi:10.1016/j.anbehav.2008.09.034) [Google Scholar]
- van Oers K., Drent P. J., de Goede P., van Noordwijk A. J.2004aRealized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proc. R. Soc. Lond. B 271, 65–73 (doi:10.1098/rspb.2003.2518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers K., de Jong G., Drent P. J., van Noordwijk A. J.2004bGenetic correlations of avian personality traits: correlated response to artificial selection. Behav. Genet. 34, 611–619 (doi:10.1007/s10519-004-5588-z) [DOI] [PubMed] [Google Scholar]
- van Oers K., Klunder M., Drent P. J.2005Context dependence of avian personalities: risk-taking behavior in a social and a non-social situation. Behav. Ecol. 16, 716–723 (doi:10.1093/beheco/ari045) [Google Scholar]
- Verbeek M. E. M., Drent P. J., Wiepkema P. R.1994Consistent individual differences in early exploratory behaviour of male great tits. Anim. Behav. 48, 1113–1121 (doi:10.1006/anbe.1994.1344) [Google Scholar]
- Verbeek M. E. M., Boon A., Drent P. J.1996Exploration, aggressive behaviour and dominance in pair-wise confrontations of juvenile male great tits. Behaviour 133, 945–963 (doi:10.1163/156853996X00314) [Google Scholar]
- Webster S. J., Lefebvre L.2001Problem solving and neophobia in a columbiform-passeriform assemblage in Barbados. Anim. Behav. 62, 23–32 (doi:10.1006/anbe.2000.1725) [Google Scholar]