Abstract
Sexual selection can facilitate divergent evolution of traits related to mating and consequently promote speciation. Theoretically, independent operation of sexual selection in different populations can lead to divergence of sexual traits among populations and result in allopatric speciation. Here, we show that divergent evolution in sexual morphology affecting mating compatibility (body size and genital morphologies) and speciation have occurred in a lineage of millipedes, the Parafontaria tonominea species complex. In this millipede group, male and female body and genital sizes exhibit marked, correlated divergence among populations, and the diverged morphologies result in mechanical reproductive isolation between sympatric species. The morphological divergence occurred among populations independently and without any correlation with climatic variables, although matching between sexes has been maintained, suggesting that morphological divergence was not a by-product of climatic adaptation. The diverged populations underwent restricted dispersal and secondary contact without hybridization. The extent of morphological difference between sympatric species is variable, as is diversity among allopatric populations; consequently, the species complex appears to contain many species. This millipede case suggests that sexual selection does contribute to species richness via morphological diversification when a lineage of organisms consists of highly divided populations owing to limited dispersal.
Keywords: body size, genitalia, millipede, reproductive isolation, sexual selection
1. Introduction
Since Darwin's (1871) initial insight into sexual selection, the idea that sexual selection promotes speciation has been repeatedly considered (West-Eberhard 1983; Panhuis et al. 2001; Ritchie 2007). However, the role of sexual selection in speciation in nature is often obscured by prominent ecological diversification, with which some differential mate choice may be associated (Ritchie 2007). Thus, how often sexual selection acts to promote speciation without the collaboration of natural selection remains unclear (Hendry 2009). Evolutionary models of sexual selection predict that male and female sexual traits related to mating will show correlated evolution along a line of equilibrium (Lande 1982; Iwasa & Pomiankowski 1995; Gavrilets 2000). It follows that speciation owing to divergent evolution of sexual traits is most likely to occur among allopatric populations experiencing varying intensities and directions of sexual selection. However, the relationship between allopatric divergence in sexual traits by sexual selection and speciation has not been clearly revealed.
Sexual selection can act on various traits involved in pre-zygotic stages of sexual reproduction (Andersson 1994). Empirical studies have focused on the divergent evolution of male courtship signals and female preference, which constitute major pre-zygotic isolation mechanisms. However, divergent evolution in morphology, especially the genitalia of internally fertilized animals, also can be caused by sexual selection (Eberhard 1985), and the resultant morphological difference can lead to mechanical reproductive isolation (Coyne & Orr 2004). Comparative studies suggest that sexual selection promotes divergence of genital morphologies (Arnqvist 1998; Takami & Sota 2007), but the relationship between divergence in genital morphology and speciation has not been well understood. The effectiveness of species-specific genital morphology in reproductive isolation has been questioned repeatedly (Eberhard 1985; Shapiro & Porter 1989). Nevertheless, cases of parapatric or sympatric species pairs exist, in which pre-mating isolation is incomplete; yet, mechanical isolation occurs owing to incompatible genital morphologies, resulting in a high cost of interspecific mating (Sota & Kubota 1998; Tanabe & Sota 2008). These cases suggest that divergent genital evolution can occur rapidly enough to precede the evolution of pre-mating isolation and to be the primary process leading to speciation.
Here we elucidate the link between divergent evolution of mechanical agents of reproductive isolation and speciation in a millipede lineage consisting of highly diverged populations in sympatry as well as allopatry. The tonominea species complex of the genus Parafontaria is endemic to Japan (figure 1c) and exhibits marked variations in body size, genital size and genital morphology among populations, which hinder delimiting species boundaries based on morphology (Tanabe et al. 2001). In this species complex, two different forms occur together in some localities, and differences between two sympatric forms are evidenced by morphological discontinuity with an absence of intermediate forms indicating hybridization (Tanabe et al. 2001). Furthermore, experimental evidence has indicated mechanical isolation between sympatric forms owing to body size and genital mismatch (Tanabe & Sota 2008). The mating behaviour in this millipede group consists of alignment of the genitalia between sexes, preliminary intromission of the gonopods without sperm transfer and true intromission with sperm transfer (Tanabe & Sota 2008). Mismatches in body and genital size/shape hinder these copulatory behaviours because body length differences prevent alignment of genital openings, and genital size/shape differences prevent proper intromission of male gonopods into female genital cavities (Tanabe & Sota 2008). Corresponding structures in male and female devices may function in interactions between sexes and may be subject to coevolution by sexual selection. Notably, Parafontaria millipedes are detritivores that consume leaf litter and are confined to forest floors in the temperate zone. No ecological divergence is recognized among the members of Parafontaria. Thus, the tonomiea species complex provides an intriguing case in which to examine the relationship between sexual selection and speciation, possibly excluding the predominant effect of ecologically divergent selection.
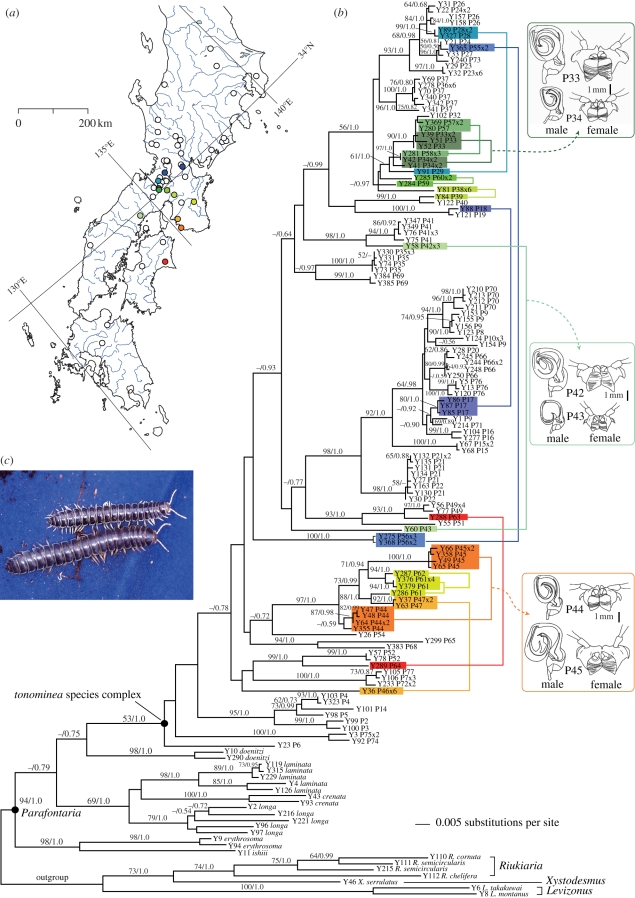
Figure 1.
(a) Sampling sites of the tonominea species complex in Japan. Open circles are single-species sites. Coloured circles are two-species sites. (b) Phylogenetic tree of Parafontaria millipedes resulting from a maximum-likelihood analysis of mitochondrial COI–COII sequences. Node supports are indicated by bootstrap percentages (when >50%) and Bayesian posterior probabilities. Sympatric species pairs are connected by coloured lines corresponding to the colours in (a). Differences in genital morphologies between sympatric species are illustrated for three example sites. (c) Males of sympatric species of the tonominea species complex from Rurikei, Kyoto.
We examined whether the divergence pattern in the tonominea species complex adheres to the model of allopatric speciation by sexual selection. We focused on body and genital sizes of both sexes as key mating traits, and conducted analyses of genetic and morphological differentiation of the millipede populations using mitochondrial and nuclear gene sequences. We show that population divergence in this millipede lineage has occurred as a result of contiguous dispersion and isolation, as would be expected from the low mobility of millipedes; morphological divergence has not been affected by environmental clines and has occurred without interdependency among neighbour populations, as would be expected from allopatric divergence by sexual selection; and by assessing phylogenetically independent contrasts, correlated evolution in mating traits between sexes has occurred to maintain matching between sexes, whereas the evolution of genital traits was partly independent from that in body size, providing broader phenotypes to produce mechanically isolated species.
2. Material and methods
(a). Sampling
The P. tonominea species complex (including P. falcifera and P. tokaiensis) was sampled from 54 locations, 14 of which harboured two sympatric species, in Honshu, Shikoku and Kyushu, Japan (figure 1a; table S1a in the electronic supplementary material). Other Parafontaria and outgroup taxa from four xystodesmid genera (Riukiaria, Xystodesmus and Levizonus) were collected in Hokkaido through Ryukyu, Japan (table S1b in the electronic supplementary material), for phylogenetic analysis.
(b). Molecular methods
Total genomic DNA was extracted from legs preserved in 99 per cent or 80 per cent ethanol. To amplify the mitochondrial COI–COII gene region, we used primers COS2183N (5′-CAR CAY YTA TTY TGR TTY TTY GG-3′) and COA3662 (5′-CCA CAA ATT TCT GAA CAT TGA CC-3′), and additional primers in between COA2745Y (5′-T YAA MCC YAA RAA ATG YTG AG-3′); COSY1 (5′-GCT ATT ACT TTT TGR TTT CC); COAY1 (5′-CGA TTY AAY ATY ATA TTT CT-3′); and COAY2 (5′-ACA GCA TCY AAY TTW ACY CC-3′). The data matrix consisted of 1275 bp sequences that are unambiguously aligned. About 600 bp of the nuclear elongation factor 1-alpha gene (EF-1a) was amplified using primers DiploEF1aF (5′-GCC TGG GTT TTG GAT AAA CTT AAG GC-3′) and DiploEF1aR3 (5′-CCT CCA ATC TTG TAA ACG TC-3′). Sequences have been deposited in GenBank (accession numbers: FJ775184–FJ775328; FJ775329–FJ775526). The aligned data matrix had 614 sites with a 519 bp exon and 95 bp (including gaps) intron sequence. For COI–COII and EF-1α data, maximum likelihood (ML) trees were obtained by a genetic algorithm implemented in Garli v. 0.96 (http://www.bio.utexas.edu/faculty/antisense/garli/Garli.html). The GTR + I + G substitution model was used, and the credibility of nodes was assessed by 1000 bootstrap replicates. Bayesian analyses with MrBayes v. 3.1.1 (Huelsenbeck & Ronquist 2001) were also conducted to obtain posterior probabilities that different nodes appeared in the ML trees. Also, the average sequence difference between populations was obtained from Arlequin v. 3.11 (Excoffier et al. 2005) for use in Mantel tests.
(c). Estimation of divergence time
Divergence times of populations within the tonominea species complex were estimated using mitochondrial gene sequences from eight species outside the tonominea species complex and 66 tonominea species complex sequences. An ML tree was constructed using Garli, and the divergence times were estimated using a relaxed clock model using Multidivtime (Thorne & Kishino 2002). Since no fossil or geohistoric evidence was available for calibrating the millipede divergence time, we referred to the evolutionary rate of the COI gene of Plateumaris beetles in Japan (Sota & Hayashi 2007), because the sequence region in the Plateumaris data was identical to the early part of the millipede sequence data and both organisms occur in the same geographical region. Accounting for a time-dependent rate of sequence divergence (Ho et al. 2005), the rate of sequence divergence per million years (Myr) at t Myr BP was expressed as 0.032 × exp(−1.984t) + 0.018. Divergence times corresponding to the sequence divergences (uncorrected pairwise distances) predicted under this evolutionary rate were used to set priors and constraints for nodes and the evolutionary rate. The root node of Parafontaria had a mean sequence divergence corresponding to 3.6 Myr, which was used for rttm, the mean of the prior distribution for the age of the ingroup root (with s.d., rttmsd of 1.8 Myr). Both the mean and s.d. (rtrate, rtratesd) of the prior distribution of the evolutionary rate at the root node were set at 0.016. The sequence divergence at the root node of the tonominea species complex corresponded to 2.7–2.1 Myr and this range was used to constrain the root node. Regarding 11 nodes for the coalescence of sympatric species, we set conservative lower and upper ages as the mean age ± 0.5 × mean age, where the mean age was estimated from the mean sequence divergence between sympatric species using the above equation.
(d). Morphological analysis
Male and female body sizes were expressed as the tenth metatergal width. Dried genital organs (male gonopods and female genital segment) were weighed. Genital weight is closely correlated with other elaborate measures of genital dimensions such as the length of the male genital appendage and centroid size of female genitalia (Tanabe et al. 2001).
(e). Statistical methods
The relationship between geographical distance and genetic distance between populations was tested with a Mantel test implemented in the R-package (Casgrain & Legendre 2004). An extension of the Mantel test, the partial matrix correspondence test (Manly 1986) implemented in RT-MANT v. 2.1 (Manly 1997) was used to determine the contributions of geographical distance, genetic distance and morphological difference to variation in genital morphology. A Mantel test for correlations among mitochondrial genetic distance, geographical distance and morphological distance between populations was performed with the R-package.
To assess environmental factors affecting body and genital size variation, stepwise multiple regression was performed using three geographic (latitude, longitude and altitude) and two climatic (annual mean temperature and annual rainfall) variables. These variables potentially show significant correlations with arthropod body size (e.g. Masaki 1967; Mousseau 1997; Sota et al. 2000; Bidau & Martí 2008).
To study the evolutionary rates and correlated evolution of sexual body size and genital sizes, phylogenetically independent contrasts (Felsenstein 1985) were obtained based on the ultrametric mitochondrial tree using the PDAP module (Midford et al. 2003) in Mesquite v. 2.6 (Maddison & Maddison 2004). Thirty-six populations with all four morphological measurements were included in this analysis. All measurements were log10-transformed. To adjust the dimension of measurements in the comparison of evolutionary rates, genital weight (but not metatergal width) was divided by 3 after log10 transformation. To standardize independent contrasts adequately, the branch lengths were log-transformed (Garland et al. 1992). To assess heterogeneity in the evolutionary rates among characters, a Friedman test was used, and absolute values of standardized contrasts at different nodes were compared with a Wilcoxon sign test (Takami & Sota 2007).
3. Results
(a). Genetic divergence among populations and divergence time
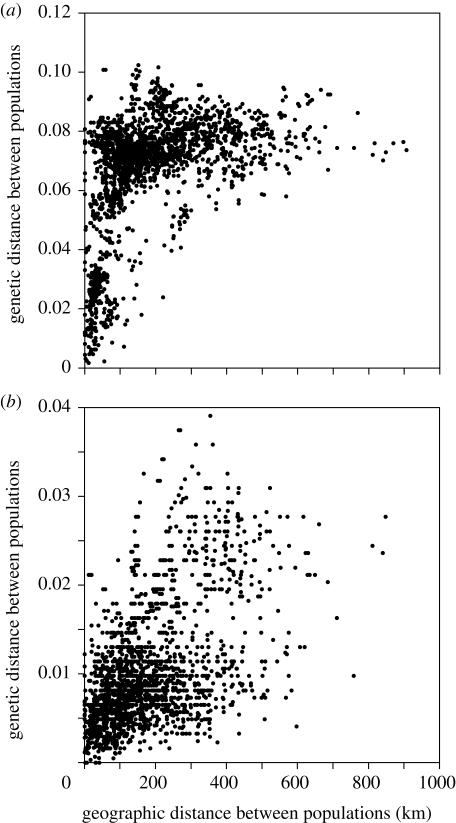
Mitochondrial and nuclear gene genealogies provided evidence for genetic differentiation among the sympatric and allopatric populations of the tonominea species complex. In the COI–COII gene sequence (figure 1b), the tonominea species complex consists of diverged haplotypes (maximum pairwise sequence divergence, 10.27%), and sympatric species pairs showed 0.45–7.69% sequence divergence. The gene tree indicates that sympatric species were not sisters in most cases. The nuclear EF-1α gene (exon+intron) sequences showed lower divergence (maximum pairwise sequence divergence 2.36%; figure S1a in the electronic supplementary material), but sympatric species had sequences with 0.24–0.77% difference. Within the tonominea species complex, the genetic distance between populations in mitochondrial and nuclear genes increased significantly with geographical distance (figure 2; Mantel test; COI–COII, r = 0.446, p = 0.0001; EF-1α, r = 0.522, p = 0.0001), indicating restricted gene flow and isolation by distance among geographical populations.
Figure 2.
Relationship between geographical and genetic distance in the tonominea species complex. (a) COI–COII gene sequence. (b) EF-1α gene sequence. Genetic distance is the uncorrected proportion of sequence difference.
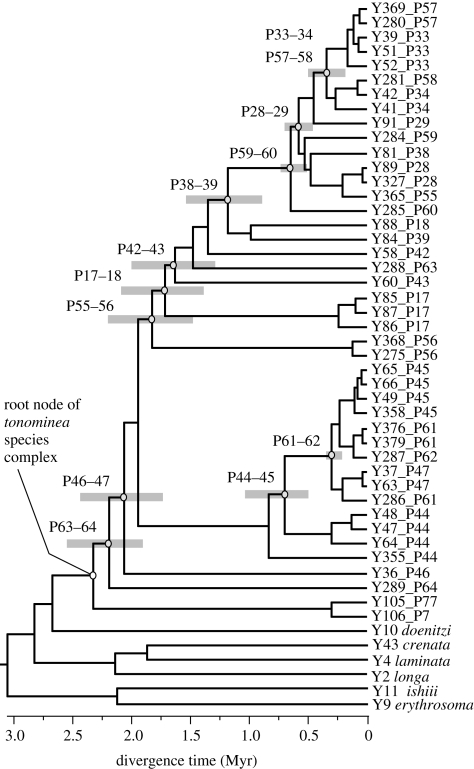
The common ancestor of the tonominea species complex was estimated to have occurred 2.4 Myr ago (95% credible interval, 2.8–2.1 Myr; figure 3). The coalescent times of sympatric populations were 0.15–2.0 Myr, indicating that morphological divergence occurred at various times during the past 2.4 Myr.
Figure 3.
Divergence times between populations of the tonominea species complex. For the nodes of the most recent common ancestors of two sympatric species, the 95% credible range of the estimated ages is indicated by grey bars.
(b). Factors of morphological variation
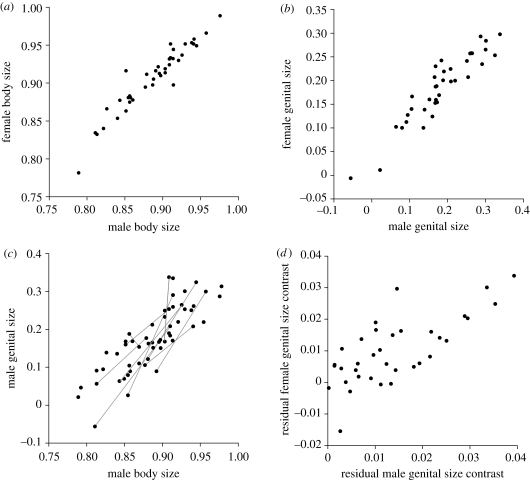
Both body size and genital size showed close matching between sexes (figure 4a, r = 0.954; figure 4b, r = 0.930; p < 0.0001 for both). Genital size was correlated with body size (figure 4c; r = 0.835, p < 0.0001 for males; females: r = 0.890, p < 0.0001); note that genital size varied more than body size and that differences in body and genital sizes between sympatric species were highly variable. The matching of genital sizes between sexes remained even after removing the effect of body size (r = 0.826, p < 0.0001). The body- or genital-size distribution did not differ between species in sympatry and those in allopatry (figure S2a,b in the electronic supplementary material), with equivalent levels of variation among populations. The size difference between sympatric species was not biased toward large values against the difference between random population pairs (figure S2c,d in the electronic supplementary material) and was not correlated with genetic distance (figure S2e,f in the electronic supplementary material).
Figure 4.
(a) Relationship between population mean body sizes (tenth metatergal width) of males and females of the tonominea species complex. (b) Relationship between population mean male and female genital sizes (dry weight). (c) Relationship between population mean male body size and genital size. The sympatric species are connected by grey lines. (d) Relationship between residuals of phylogenetically independent contrasts of male and female genital sizes, which are regressed on the phylogenetically independent contrasts of body sizes.
In the multiple regression analysis for environmental correlates of body- and genital-size variation, this variation was not explained by any of the five geographical or climatic parameters, although the effects of annual rainfall on male body size, male genital size and female genital size were marginally significant (table 1). In addition, the body-size difference between populations was not correlated with geographical or genetic distance between populations (table 2). Similarly, the genital-size difference between populations could not be explained by geographical or genetic distance, whereas it was clearly correlated with the genital size of the opposite sex and weakly with body size (table 2).
Table 1.
Multiple regression of body and genital sizes on three geographical and two climatic variables. Because no significant model was found with the stepwise regression, the effects (t with p-value in parentheses) in models with all variables are given.
| variable | male body size (n = 58) | male genital size (n = 55) | female body size (n = 47) | female genital size (n = 43) |
|---|---|---|---|---|
| latitude | −0.16 (0.875) | −0.22 (0.824) | 0.34 (0.737) | 0.16 (0.874) |
| longitude | 0.65 (0.519) | 1.01 (0.317) | 0.09 (0.927) | 0.52 (0.608) |
| altitude | −0.48 (0.633) | −0.29 (0.772) | 0.16 (0.875) | 0.30 (0.766) |
| annual mean temperature | −0.14 (0.893) | −0.03 (0.978) | 0.40 (0.688) | 0.38 (0.704) |
| annual rainfall | 1.97 (0.054) | 1.82 (0.075) | 1.38 (0.175) | 1.95 (0.059) |
Table 2.
Matrix correspondence tests for variation in (a) body size and (b) genital size among populations. In (a), geographical and genetic distance (mitochondrial gene sequence) are regressed on Euclidean distances of body size between populations for each sex. In (b), Euclidean distances of body size of one's own sex and genital size of the opposite sex, geographical distance and genetic distance are regressed on the Euclidean distance of genital size between populations for each sex.
| dependent variable |
||||||
|---|---|---|---|---|---|---|
| variable | t | p | t | p | ||
| (a) body size | male | female | ||||
| geographical distance | 4.21 | 0.141 | 2.78 | 0.300 | ||
| genetic distance | −1.21 | 0.578 | 1.16 | 0.537 | ||
| (b) genital size | male | female | ||||
| body size of own sex | 3.57 | 0.068 | 16.66 | 0.000 | ||
| genital size of opposite sex | 27.03 | 0.000 | 28.75 | 0.000 | ||
| geographical distance | −4.00 | 0.100 | 0.96 | 0.681 | ||
| genetic distance | 0.47 | 0.801 | −0.49 | 0.787 | ||
Finally, the evolutionary rates and correlated evolution of body and genital sizes were analysed using phylogenetically independent contrasts. The absolute value of the standardized phylogenetically independent contrast (n = 35) was 1.5 times larger for genital size than body size, suggesting faster evolution of genital size (table 3). The contrasts of body and genital sizes showed correlated evolution between sexes (body size: r = 0.811, p < 0.0001; genital size: r = 0.886; p < 0.0001). Although the evolution of genital size was correlated with that of body size in each sex (table 3), the residuals of the genital-size contrast regressed on body size were correlated between the sexes (figure 4d; r = 0.729; p < 0.0001), indicating that the correlated evolution in genital size was partly independent of that in body-size evolution.
Table 3.
Evolutionary rates and correlations of body and genital sizes based on phylogenetically independent contrasts. Standardized contrasts with the same letter do not differ significantly by Wilcoxon sign test (after sequential Bonferroni correction).
| regression of genital size on body size |
||||
|---|---|---|---|---|
| character | absolute value of standardized contrast (mean ± s.e.) | slope | t | p |
| male body size | 0.0140 ± 0.00176bc | |||
| male genital size | 0.0233 ± 0.00321a | 1.403 ± 0.171 | 8.21 | <0.0001 |
| female body size | 0.0136 ± 0.00159c | |||
| female genital size | 0.0196 ± 0.00257ab | 1.217 ± 0.150 | 8.12 | <0.0001 |
4. Discussion
Populations of the tonominea species complex have diverged extensively in key mating traits, body size and genital size. Notably, no significant correlation was observed between the differences in these sizes and the geographical/genetic distances between populations (table 2), despite the fact that the genetic divergence between populations as revealed by COI–COII and EF-1α sequences showed clear isolation-by-distance patterns, implying gradual dispersal of the millipedes (figure 2). In addition, climatic variables did not explain the body-size variation (table 1), suggesting that climatic adaptation (Masaki 1967; Roff 1980) is not the cause of the body-size variation. The climatic variables used were rather coarse and might not reflect soil microhabitat conditions appropriately. For example, the marginally significant effect of annual rainfall on body size (table 1) may imply an effect of soil moisture. However, the sympatry of different-sized millipedes (actually within the same plots) suggests that local environmental variables are not major determinants of the body size variation. Nevertheless, our study has not resolved the causes of body-size variation. A proximate cause of body size variation may be variation in the larval developmental period, which may require several years in Parafontaria (Fujiyama 1996), but the life cycle of the tonominea species complex is not well known. Obviously, detailed life history and population studies of sympatric species pairs are needed.
Despite the above reservation, the lack of phylogenetic, geographical and climatic correlates with body size, as well as the sympatry of species pairs with diverged body sizes, suggest that body size has diverged rapidly, primarily in response to intrinsic factors within populations, even though the range of body sizes has been constrained by natural selection. Similarly, although genital sizes show positive correlations with body size, the genital-size variation has occurred partly independently of body-size variation (figure 4c,d). Our previous study revealed that size matching between the sexes in both body and genital size is required to accomplish copulation and that complex interactions take place between male and female genitalia during insemination (Tanabe & Sota 2008), which suggests that sexual selection could be the primary process leading to morphological divergence in the tonominea species complex. The role of reinforcement or reproductive character displacement in morphological divergence has not been demonstrated because the degree of morphological difference between sympatric species was as variable as that between random pairs of species, similar to the conclusions of a previous analysis using multidimensional shape parameters (Tanabe et al. 2001).
Our observation that matching between the sexes in body size and in genital size is required, and hence probably selected for achievement of copulation (Tanabe & Sota 2008), implies that correlated evolution between the sexes has occurred in response to intersexual selection. Common genetic regulation and common natural selection on sex-limited characters also can affect correlated evolution (Arnqvist & Rowe 2002). Underlying common genetic regulation is invoked not only for body size but also for genital size. However, the male and female genitalia of millipedes are not homologous organs (Hopkin & Read 1992); in Parafontaria, the male gonopods are transformed eighth legs, whereas the female genitalia comprise a complex of modified genital openings, adjacent legs and a sternite (Tanabe et al. 2001). Therefore, their simultaneous evolution is not necessarily governed by a single genetic factor. In addition, genital size has evolved independently of body size to an appreciable extent (figure 4d), suggesting that body size only roughly constrains genital size. Matching of body size between the sexes is required in a different phase of copulation than matching of genital size (i.e. body alignment and intromission), and hence body size and genital size may be subject to different kinds of selection. For example, at intromission, the interplay between genitalia is complex, possibly involving tactile communication and mechanical interaction between sexes (Eberhard 1985; Tanabe & Sota 2008), and can be subject to an arms race towards exaggeration. Thus, divergent evolution, probably driven by sexual selection, in millipedes can result in various combinations of body sizes and genital sizes between once-diverged populations, and thus provides great potential to produce mechanically isolated species in morphological space.
The estimated divergence time of mitochondrial haplotypes from sympatric species ranged from 0.15 to 2.0 Myr, suggesting large variation in the divergence time. On the mitochondrial gene tree (figure 3), the time to the bifurcation leading to sympatric species was relatively short, 0.03–0.39 Myr (mean, 0.15 ± 0.12 s.d. Myr). Because our population sampling is far from complete, many lineages remain unsampled and hence the actual divergence of populations could be very rapid. For species pairs with longer divergence times, pre-zygotic isolation other than mechanical isolation and post-zygotic isolation may have evolved, as total isolation of 0.90 is attained in 1.1 Myr on average between allopatric Drosophila species (Coyne & Orr 2004). In our previous study on reproductive isolation (Tanabe & Sota 2008), we used a sympatric species pair with relatively large morphological differences (figure 1b; P33–34) and this pair had the second smallest genetic divergence (0.21 Myr; figure 3). The species pair did not discriminate against heterospecific mates and hybridization was hindered by mechanical barriers during copulation. This case demonstrates that substantial morphological divergence preceded divergence in mate recognition traits (e.g. sexual pheromones). However, the inability to discriminate heterospecific mates can incur direct costs (such as injury) when copulatory organs are mismatched between sexes (e.g. Sota & Kubota 1998). Therefore, species pairs with longer divergence times may have acquired pre-mating isolation mechanisms through divergence of some signal traits.
Explosive radiation of species within a lineage is known in Hawaiian Drosophila and African lake cichlids (Ritchie 2007). However, these examples of radiation are associated with marked divergence in ecological characters, particularly in the use of microhabitats and food. Thus, this millipede case is remarkable, although the animals are inconspicuous, because their radiation is based on morphological divergence in agents of mechanical reproductive isolation without marked sign of ecological divergence. Similar cases of explosive but cryptic speciation are likely in various arthropod groups with internal fertilization and low dispersal power, and such groups may be beyond the resolution of morphology-based taxonomy and escape the notice of evolutionary biologists. Our study suggests that genital and other morphologies in arthropods that can cause mechanical reproductive isolation are sensitive to sexual selection, contribute to mechanical reproductive isolation (at least before the evolution of pre-mating isolation) and enable speciation.
Acknowledgements
We thank Y. Takami for comments and Y. Kawakami, Y. Takai, T. Wada, N. Tsurusaki, Y. Nishikawa, G. Takaku, Y. Takashima, K. Yahata, M. Hashimoto, N. Nagata and H. Nishi for sampling. This study was supported by Grants-in-Aid for scientific research (nos 15207004, 20370011, 21570097) from the Japan Society for the Promotion of Sciences, and the Global COE programme A06 ‘Formation of a Strategic Base for Biodiversity and Evolutionary Research; from Genomics to Ecosystems' from the Ministry of Education, Culture, Sports and Technology, Japan.
References
- Andersson M.1994Sexual selection Princeton, NJ: Princeton University Press [Google Scholar]
- Arnqvist G.1998Comparative evidence for the evolution of genitalia by sexual selection. Nature 393, 784–786 (doi:10.1038/31689) [Google Scholar]
- Arnqvist G., Rowe L.2002Correlated evolution of male and female morphogenesis in water striders. Evolution 56, 936–947 [DOI] [PubMed] [Google Scholar]
- Bidau C. J., Martí D. A.2008Geographic and climatic factors related to a body-size cline in Dichroplus pratensis Bruner, 1900 (Acrididae, Melanoplinae). J. Orthptera Res. 17, 149–156 (doi:10.1665/1082-6467-17.2.149) [Google Scholar]
- Casgrain P., Legendre P. The R-package for multivariate and spatial analysis v. 4.0 5d-user's manual. Department Des Sciences Biologiques, Universite de Montreal; 2004. Available at http://www.fas.umontreal.ca/BIOL/legendre/ [Google Scholar]
- Coyne J. A., Orr H. A.2004Speciation Sunderland, MA: Sinauer Associates [Google Scholar]
- Darwin C.1871The descent of man and selection in relation to sex London, UK: J. Murray [Google Scholar]
- Eberhard W. G.1985Sexual selection and animal genitalia Cambridge, MA: Harvard University Press [Google Scholar]
- Excoffier L., Laval L. G., Schneider S.2005Arlequin v. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1, 47–50 [PMC free article] [PubMed] [Google Scholar]
- Fujiyama S.1996Annual thermoperiod regulating an eight-year life-cycle of a periodical diplopod, Parafontaria laminata armigera Verhoeff (Diplopoda). Pedobiologia 40, 541–547 [Google Scholar]
- Felsenstein J.1985Phylogenies and the comparative method. Am. Nat. 125, 1–15 (doi:10.1086/284325) [Google Scholar]
- Gavrilets S.2000Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403, 886–889 (doi:10.1038/35002564) [DOI] [PubMed] [Google Scholar]
- Garland T., Harvey P. H., Ives A. R.1992Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32 (doi:10.2307/2992503) [Google Scholar]
- Hendry A. P.2009Speciation. Nature 458, 162–164 (doi:10.1038/458162a) [DOI] [PubMed] [Google Scholar]
- Ho S. Y. W., Phillips M. J., Cooper A., Drummond A. J.2005Time dependency of molecular rate estimates and systematic overestimation of recent divergence times. Mol. Biol. Evol. 22, 1561–1568 (doi:10.1093/molbev/msi145) [DOI] [PubMed] [Google Scholar]
- Hopkin S. P., Read H. J.1992The biology of millipedes New York, NY: Oxford University Press [Google Scholar]
- Huelsenbeck J. P., Ronquist F.2001MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (doi:10.1093/bioinformatics/17.8.754) [DOI] [PubMed] [Google Scholar]
- Iwasa Y., Pomiankowski A.1995Continual change in mate preference. Nature 377, 420–422 (doi:10.1038/377420a0) [DOI] [PubMed] [Google Scholar]
- Lande R.1982Rapid origin of sexual isolation and character divergence in a cline. Evolution 36, 213–223 (doi:10.2307/2408039) [DOI] [PubMed] [Google Scholar]
- Maddison W. P., Maddison D. R.2004Mesquite: a modular system for evolutionary analysis, v. 2.6. Available at http://mesquiteproject.org
- Manly B. F. J.1986Randomization and regression methods for testing for associations with geographical, environmental and biological distances between populations. Res. Popul. Ecol. 28, 201–208 (doi:10.1007/BF02515450) [Google Scholar]
- Manly B. F. J.1997RT: a program or randomization testing Cheyenne, WY: West, Inc [Google Scholar]
- Masaki S.1967Geographic variation and climatic adaptation in a field cricket (Orthoptera: Gryllidae). Evolution 21, 725–741 (doi:10.2307/2406770) [DOI] [PubMed] [Google Scholar]
- Midford P. E., Garland T., Maddison W. P. PDAP package. 2003. http://mesquiteproject.org/pdap_mesquite .
- Mousseau T. A.1997Ectotherms follow the converse to Bergmann's rule. Evolution 51, 630–632 (doi:10.2307/2411138) [DOI] [PubMed] [Google Scholar]
- Panhuis T. M., Butlin R., Zuk M., Tregenza T.2001Sexual selection and speciation. Trends Ecol. Evol. 16, 364–371 (doi:10.1016/S0169-5347(01)02160-7) [DOI] [PubMed] [Google Scholar]
- Ritchie M. G.2007Sexual selection and speciation. Annu. Rev. Ecol. Evol. Syst. 38, 79–102 (doi:10.1146/annurev.ecolsys.38.091206.095733) [Google Scholar]
- Roff D.1980Optimizing development time in a seasonal environment: the ‘ups and downs’ of clinal variation. Oecologia 45, 202–208 (doi:10.1007/BF00346461) [DOI] [PubMed] [Google Scholar]
- Shapiro A. M., Porter A. H.1989The lock-and-key hypothesis: evolutionary and biosystematic interpretation of insect genitalia. Annu. Rev. Entomol. 34, 231–245 (doi:10.1146/annurev.en.34.010189.001311) [Google Scholar]
- Sota T., Hayashi M.2007Comparative historical biogeography of Plateumaris leaf beetles (Coleoptera: Chrysomelidae) in Japan: interplay between fossil and molecular data. J. Biogeogr. 34, 977–993 (doi:10.1111/j.1365-2699.2006.01672.x) [Google Scholar]
- Sota T., Kubota K.1998Genital lock-and-key as a selective agent against hybridization. Evolution 52, 1507–1513 (doi:10.2307/2411321) [DOI] [PubMed] [Google Scholar]
- Sota T., Takami Y., Kubota K., Ishikawa R.2000Geographic variation in the body size of some Japanese Leptocarabus species (Coleoptera, Carabidae): The ‘toppled-domino pattern’ in species along a geographic cline. Entomol. Sci. 3, 309–320 [Google Scholar]
- Takami Y., Sota T.2007Rapid diversification of male genitalia and mating strategies in Ohomopterus ground beetles. J. Evol. Biol. 20, 1385–1395 (doi:10.1111/j.1420-9101.2007.01338.x) [DOI] [PubMed] [Google Scholar]
- Tanabe T., Sota T.2008Complex copulatory behavior and the proximate effect of genital and body size differences on mechanical reproductive isolation in the millipede genus. Parafontaria. Am. Nat. 171, 692–699 (doi:10.1086/587075) [DOI] [PubMed] [Google Scholar]
- Tanabe T., Katakura H., Mawatari S. F.2001Morphological difference and reproductive isolation: morphometrics in the millipede Parafontaria tonominea and its allied forms. Biol. J. Linn. Soc. 72, 249–264 [Google Scholar]
- Thorne J. L., Kishino H.2002Divergence time and evolutionary rate estimation with molecular data. Syst. Biol. 51, 689–702 (doi:10.1080/10635150290102456) [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J.1983Sexual selection, social competition, and speciation. Quart. Rev. Biol. 58, 155–183 (doi:10.1086/413215) [Google Scholar]