Abstract
The adult hippocampus in birds and mammals undergoes neurogenesis and the resulting new neurons appear to integrate structurally and functionally into the existing neural architecture. However, the factors underlying the regulation of new neuron production is still under scrutiny. In recent years, the concept that spatial memory affects adult hippocampal neurogenesis has gained acceptance, although results attempting to causally link memory use to neurogenesis remain inconclusive, possibly owing to confounds of motor activity, task difficulty or training for the task. Here, we show that ecologically relevant, spatial memory-based experiences of food caching and retrieving directly affect hippocampal neurogenesis in mountain chickadees (Poecile gambeli). We found that restricting memory experiences in captivity caused significantly lower rates of neurogenesis, as determined by doublecortin expression, compared with captive individuals provided with such experiences. However, neurogenesis rates in both groups of captive birds were still greatly lower than those in free-ranging conspecifics. These findings show that ecologically relevant spatial memory experiences can directly modulate neurogenesis, separate from other confounds that may also independently affect neurogenesis.
Keywords: spatial memory, hippocampus, neurogenesis, food caching, chickadees, doublecortin
1. Introduction
Hippocampal neurons are continually produced in birds and mammals. These neurons appear to structurally integrate into the hippocampal neural network, possess characteristics of functional neurons and appear to be important for spatial discrimination (Markakis & Gage 1999; van Praag et al. 2002; Kempermann et al. 2004; Clelland et al. 2009). The use of spatial memory has been shown to affect hippocampal neurogenesis in mammals; however, the direction of the effect across studies is inconsistent (Greenough et al. 1999; Leuner et al. 2006). In some studies, neurogenesis rates were enhanced when rodents were trained on a spatially based water maze (Gould et al. 1999; Ambrogini et al. 2000; Döbrössy et al. 2003; Hariston et al. 2005). However, other studies found no effect or a negative effect of spatial learning on neurogenesis in the same water maze paradigm (van Praag et al. 1999a; Ambrogini et al. 2004; Snyder et al. 2005; Van der Borght et al. 2005). These conflicting reports may be partially attributed to other variables that can also affect neurogenesis such as training to accomplish the task itself (Cain 1997; Döbrössy et al. 2003) and motor stimulation (van Praag et al. 1999a,b). Thus, the importance of spatial memory for neurogenesis remains ambiguous because of difficulties demonstrating that neurogenesis is causally related to spatial learning rather than to other potentially confounding variables. Consequently, Ehninger & Kempermann (2006) suggested that the water maze cannot provide pure learning/memory experiences to investigate the effects of spatial memory on hippocampal neurogenesis. They and others (e.g. Gerlai & Clayton 1999) suggest that a natural, freely expressed behaviour may be needed to examine the effects of spatial learning on neurogenesis, so that variables that affect neurogenesis, but are associated with participating in the task itself, may be avoided (Abrous et al. 2005). Although all confounds may not be avoided with a freely expressed behaviour, using such may eliminate some confounds and expand the relevance of neurogenesis to naturally expressed and ecologically important behaviours, which may be important when studying the evolution of those behaviours and their neural correlates.
An animal's physical environment can also affect neurogenesis. Captivity has been shown to reduce neurogenesis relative to free-ranging conspecifics (Barnea & Nottebohm 1994), due possibly in part to reduced environmental complexity which restricts both memory-based experiences and motor stimulation (sensu van Praag et al. 2002). Thus, providing a more complex physical captive environment may allow for increased motor stimulation and demands on memory, both of which may independently or jointly increase neurogenesis (Kempermann et al. 1997). However, distinguishing between the effects of memory and motor stimulation has proven difficult, as a more complex environment necessitates increased motor stimulation and spatially based experiences. Therefore, motor stimulation must be adequately controlled to establish that diminished neurogenesis in captivity is attributable to a decrease in memory-based experiences.
Here, we tested whether a naturally occurring, spatial memory-based food-caching behaviour was causally related to neurogenesis, apart from other factors. We compared hippocampal neurogenesis among captive birds which were either allowed or denied food-caching spatial memory experiences and free-ranging conspecifics. To visualize neurogenesis, we used an endogenous marker, doublecortin, which is a protein expressed in newly produced and migrating neurons (Francis et al. 1999; Gleeson et al. 1999). Doublecortin appears to be a viable alternative to the traditional bromodeoxyuridine (BrdU) method for detecting and quantifying neurogenesis (Brown et al. 2003; Rao & Shetty 2004; Couillard-Despres et al. 2005; Balthazart et al. 2008), especially in wild-caught animals where injection of exogenous markers is difficult. In both birds and mammals, only new neurons express doublecortin (e.g. Francis et al. 1999; Gleeson et al. 1999; Balthazart et al. 2008), which eliminates the need for BrdU injections and double labelling to establish neuronal identity. Changes in neurogenesis rates measured with doublecortin in response to behavioural experiences known to affect adult neurogenesis mirror changes measured with BrdU labelling (Couillard-Despres et al. 2005). Unlike BrdU, doublecortin shows only transient expression, which lasts for about two weeks in rodents (Brown et al. 2003) and over 25 days in passerine birds (Balthazart et al. 2008). Therefore, neurogenesis rates estimated by using doublecortin only infer to these time periods in new neuron life.
2. Material and methods
(a). Subjects
Twenty-four male mountain chickadees (Poecile gambeli) of approximately three to four months of age were caught near Sagehen Creek in Tahoe National Forest, CA, in September 2007. Sex was determined genetically via PCR and confirmed by visual inspection of the gonads after sacrifice (for PCR methods, see Fox et al. 2009). We matched subjects in pairs based on body weight and randomly assigned each of the birds to one of two captive treatment groups. The two captive treatment groups comprised birds with either the opportunity to cache and retrieve food items, and thus engage in memory-based experiences (experienced group), or birds deprived of memory-based caching and retrieval experiences (deprived group). Except for the differences in memory-based food caching and retrieval, birds in both groups received exactly the same experiences. Experienced birds participated in spatial memory-based cache and retrieval tasks, as well as in an associative learning task, while deprived birds were permitted in the testing room for the same amount of time and were treated exactly the same, but without cache and retrieval or associative learning opportunities. By doing so, we could assure that the only difference between the two captive groups was the opportunity for spatial memory-based experiences (see LaDage et al. 2009).
The free-ranging group comprised additional males of the same age (three to four months) that were captured at the same time as the captive birds (September), colour banded and released. Twelve of these birds were recaptured in the same location in January 2008, at which time all birds were sacrificed for the brain analyses. Both captive and free-ranging birds were seven to eight months old at the time of sacrifice.
(b). Spatial memory-based tasks
The spatial memory-based tasks for the experienced captive birds were identical to those described in LaDage et al. (2009). Briefly, all captive birds were housed individually and maintained on a natural light schedule. All tasks were performed in a testing room adjacent to the home cages of the birds. The testing room contained two caching trees and caching blocks on the walls where the experienced group could cache and retrieve food (total of 72 caching sites). Individuals in the experienced group were allowed 30 min of caching and retrieval experience every other day, with either a caching–retrieving task or an associative learning task that exercised spatial memory (LaDage et al. 2009).
In the associative learning task, we stored one seed and allowed the bird to recover the seed using memory (Brodbeck 1994; Clayton & Krebs 1994; Clayton 1995). During the learning phase, a bird was allowed into the testing room in which all caching locations were open and only one of them had a clearly visible pine nut. When the bird discovered the nut, it was allowed to eat for 2 s, after which lights were turned off and the bird flew back into its home cage. After a 5 min retention interval during which the bird had no food, it was allowed back into the room but now all caching sites were covered by a knot at the end of the string so the bird had to use memory to locate previously found food.
In the cache and retrieval task (e.g. Shettleworth et al. 1990; Clayton & Krebs 1994; Pravosudov & Clayton 2002), a dish with pine nuts and sunflower seeds was provided in the testing room. The bird was allowed in the testing room, and we recorded the type and amount of food consumed, as well as where the bird stored food items within the caching array. All observations of testing occurred from behind a one-way mirror. After 10 min, the bird was returned to its home cage and all caches were removed from the caching array. After a 4 h retention interval, we replaced the bird's caches in the appropriate caching holes, covered the holes with the string and allowed the bird back in the testing room for 20 min but now the only food available was located in the bird's previous cache locations. We recorded the number of caches recovered and the number and order of caching holes investigated.
(c). Controlling for movement
Because hippocampal attributes can be affected by increased activity levels in more complex environments (van Praag et al. 1999a,b), we allowed individuals from the deprived group access to the testing room for the same amount of time as the experienced group (30 min every other day for three months), but without the opportunity for caching experience. We achieved this by not providing food in the testing room and restricting access to the caching sites. Therefore, the only difference between the experienced and deprived groups was in the opportunity to obtain memory-based cache retrieval experiences. None of the captive birds were given any opportunities to cache and retrieve in their home cages (LaDage et al. 2009).
To examine whether our captive treatments had differential effects on bird physical activity, we used an additional 22 birds to measure distance travelled within the testing room over a 10 min period. Half of the birds were allowed to cache while the remaining 11 birds were restricted from caching activity. Consequently, we could assess differences in motor stimulation between birds that were allowed to cache versus birds that were restricted from caching activity. Over 10 min, we recorded the total distance flown in the room and the number of caching/perch sites visited for each bird.
(d). Immunohistochemistry
In January 2008, at approximately seven to eight months of age, birds were perfused, their brains were removed and sectioned, and processed as previously described (LaDage et al. 2009). Briefly, birds were anesthetized with a lethal overdose of Nembutal (0.07 ml of 50 mg ml−1). The birds were transcardially perfused with 0.1 M phosphate buffered saline for 10 min followed by 15–20 min perfusion of 4 per cent paraformaldehyde in 0.1 M phosphate buffer. Brains were extracted and post-fixed in 4 per cent paraformaldehyde for 24 h before cryoprotection. Brains were cryoprotected in 15 per cent sucrose, then 30 per cent sucrose and flash-frozen on dry ice. Brains were stored at −80°C until sectioning. Brains were sectioned on a cryostat (Leica CM 3050S, Bannockburn, IL, USA) at −20°C in the coronal plane every 40 µm and every fourth section was subjected to immunohistochemistry. Sections were processed for doublecortin, an endogenous marker of immature neurons (Brown et al. 2003; Rao & Shetty 2004; Couillard-Despres et al. 2005; Hariston et al. 2005; Balthazart et al. 2008).
In passerine birds, doublecortin expression in new neurons is known to occur for at least 25–30 days after a neuron is born (Balthazart et al. 2008). Consequently, only the last month of our behavioural assays influenced the number of doublecortin-labelled cells we counted. It is also important to note that once new neurons start expressing NeuN, which is usually associated with fully mature neurons (Mullen et al. 1992), the expression of doublecortin ceases (Brown et al. 2003). Therefore, our analysis of neurogenesis only concerns the early immature stage of neuronal life. Although captivity could possibly depress caching and retrieval behaviour, thus depressing neurogenesis rates, we found that during the last month of testing when birds engaged in the caching and retrieving task, all 12 birds that participated in the memory-based activity cached seeds and retrieved those seeds using memory.
The doublecortin marker has a distinct advantage over traditional exogenous markers of neurogenesis in that it only labels new neurons (Brown et al. 2003; Rao & Shetty 2004; Balthazart et al. 2008). Since doublecortin is a relatively new marker used for visualizing neurogenesis, it warrants discussion as to its use for the examination of neurogenesis. First, the anti-doublecortin antibody has been validated as to its specificity in both birds and mammals. Western blot, negative control procedures and experiments with preabsorption of the antibody with synthetic peptides have all provided support that the antibody is specific to the doublecortin protein (e.g. Francis et al. 1999; Brown et al. 2003; Boseret et al. 2007). Further, Hannan et al. (1999) found that doublecortin in chickens (Gallus gallus) was homologous to mammalian doublecortin, and that expression of doublecortin corresponds to neuronal migration peaks. Thus, the use and specificity of the anti-doublecortin antibody appear to be applicable to both birds and mammals. Second, the doublecortin marker appears to label only newly produced neurons, as opposed to mature neurons and glia. Previous studies have found that doublecortin-labelled cells mostly exhibited a migratory morphology (Yang et al. 2004), expressed other markers indicative of immature neurons (Rao & Shetty 2004; Yang et al. 2004) and did not co-express with markers for astrocytes (Brown et al. 2003; Rao & Shetty 2004; Yang et al. 2004), microglia (Yang et al. 2004) or oligodendrocytes (Brown et al. 2003; Rao & Shetty 2004). Also, because doublecortin is an endogenous marker, it is not affected by variables associated with exogenous markers such as the amount or timing of injection (Greenough et al. 1999; Gould & Gross 2002) and avoids potential problems of toxicity at high doses (Kolb et al. 1999; Cameron & McKay 2001). This is advantageous in studies where pre-labelling with an exogenous marker is difficult or impossible, as with studies on wild-caught animals. Additionally, although we do know that stress can downregulate neurogenesis (Gould & Cameron 1996; Gould et al. 1998), we do not know if stress from multiple injections of an exogenous marker can also affect neurogenesis rates.
Although doublecortin has been found primarily in areas of the adult brain known to exhibit neurogenesis, doublecortin expression has also been found in other areas in which new neurons have not traditionally been observed (e.g. Kim et al. 2006). This has been suggested to be due to (i) low levels of neurogenesis occurring in areas of the brain not previously thought to undergo neurogenesis (e.g. Magavi et al. 2000; Bernier et al. 2002), or (ii) migration of new neurons to or through areas of the brain not known to exhibit neurogenesis (e.g. Arvidsson et al. 2002). Nacher et al. (2001) also suggested that differentiated, adult neurons may have the capacity to express doublecortin during changes in axon or dendrite growth or synaptogenesis, but they had no firm data supporting this conclusion. Brown et al. (2003) found that, within the dentate gyrus, cells which co-labelled for doublecortin, NeuN and BrdU, were primarily found during the time period where cells were transitioning from an immature phenotype to an adult phenotype. This suggests that co-expression of doublecortin and NeuN may be a product of ontogeny, during the time period when immature neurons are transitioning into adult neurons. Balthazart et al. (2008) also found that, in canaries, changes in immature and more mature cell morphology tracked the percentage of cells that co-labelled for doublecortin and BrdU. They found that cells with an immature morphology co-labelled more so at day 10 than at day 30, while cells with a more mature morphology co-labelled more at day 30 than at day 10. Therefore, evidence suggests that doublecortin is only expressed in new neurons in both mammals and birds.
To visualize doublecortin, sections were washed in Tris-buffered saline (TBS), incubated in 30 per cent hydrogen peroxide plus TBS (1 : 50) at room temperature for 30 min, washed in TBS, incubated in blocking buffer (normal horse serum (1 : 33.3), TX-100 (1 : 39.8) and TBS) at room temperature for 30 min, and then incubated in anti-doublecortin antibody plus blocking buffer (1 : 200; Santa Cruz Biotechnology, Santa Cruz, CA, SC-8066) overnight (approx. 18 h) at 4°C. The following day, sections were washed in TBS, incubated in biotinylated horse anti-goat antibody in blocking buffer (1 : 200; Vector Laboratories, Burlingame, CA, USA, BA-9500) at room temperature for 2 h, washed in TBS, incubated in ABC Elite kit (Vector Laboratories, PK-6100) at room temperature for 1 h, washed in TBS, reacted with DAB + nickel kit (Vector Laboratories, SK-4100) at room temperature for 2 min and again washed in TBS and mounted on slides. Slides were dried, lightly Nissl stained and coverslipped. We also performed a negative control to account for non-specific binding of the secondary antibody. To do so, we used the same protocol as above, but replaced the anti-doublecortin antibody with TBS during the overnight incubation. We found that the elimination of the primary antibody suppressed staining, indicating that our protocol specifically stained cells that expressed doublecortin, rather than staining non-specifically. The lateral hippocampal formation boundary was determined by the change in density of Nissl-stained cells at the boundary, as per Krebs et al. (1989) and as in our previous studies (Pravosudov & Clayton 2002; Pravosudov et al. 2002; Pravosudov & Omanska 2005a,b). The hippocampal formation is also bounded by the midline and ventrally by the septum (Krebs et al. 1989). Doublecortin-labelled cells within the hippocampus were easily identified, as they stained darkly compared with surrounding Nissl-stained cells. They were also morphologically similar to doublecortin-labelled cells described by Boseret et al. (2007)—round multipolar cells and fusiform cells, which are typical morphotypes of differentiating neurons and migrating neurons, respectively. From a previous study with these individuals, we had estimated hippocampal neuron numbers using the optical fractionator method (StereoInvestigator software, Microbrightfield, Inc., Colchester, VT, USA; microscope, Leica M4000B, Bannockburn, IL, USA) (values reported in and taken from LaDage et al. 2009).
The number of doublecortin-positive cells was high, preventing exhaustive counts throughout the entire hippocampal formation. Consequently, we used optical fractionator to estimate the number of doublecortin-positive cells as suggested by previous studies (Rao & Shetty 2004; Couillard-Despres et al. 2005). We have optimized our parameters for the optical fractionator to minimize the estimation errors (CE = 0.095 ± 0.002; mean ± s.e.; West et al. 1996). Doublecortin neuron counts were performed with an optimal grid size of 250 µm, counting frame of 70 × 70 µm and dissector height of 5 µm. The left and right hemispheres were both measured for neurons expressing doublecortin and then summed to produce the given values. There were no significant differences between left and right hemispheres of the hippocampus in terms of the number of neurons expressing doublecortin (paired t-test, t35 = 1.638, p = 0.110).
To ascertain if the effect of our treatment groups were specific to the hippocampus, we also estimated the number of doublecortin-positive cells and the density of doublecortin-positive cells in a control region, the hyperpallium apicale (HA; formerly the hyperstriatum accessorium), which is laterally adjacent to the hippocampus. We followed the protocol of Barnea & Nottebohm (1994), measuring the number of cells in the HA, up to a distance of 3 mm from the midline. Again, the left and right hemispheres of the HA were counted for doublecortin-positive cells and summed. There were no significant differences between the right and left hemispheres in number of doublecortin-positive cells (paired t-test, t35 = −1.138, p = 0.263).
(e). Statistical analysis
The number of neurons expressing doublecortin was log-transformed and conformed to the assumptions for parametric testing (Levene's test F2,33 = 1.777, p = 0.185). Differences among treatments in the log-transformed number of neurons expressing doublecortin were determined by general linear model (GLM), followed by planned comparisons when appropriate. The proportion of neurons expressing doublecortin was calculated by dividing the number of neurons expressing doublecortin by the total number of neurons in the hippocampus (data collected on total number of neurons from LaDage et al. 2009). Differences among treatments in the proportion of neurons expressing doublecortin were also determined by GLM, followed by planned comparisons when appropriate. In the HA control region, we also ascertained differences among treatments in the number of neurons expressing doublecortin and the density of doublecortin-positive cells. We considered all results to be statistically significant if p < 0.05 (α = 0.05).
3. Results
We found that all individuals allowed to cache and retrieve did so during their very first opportunity in the testing room. This indicates that individuals did not have to be trained to learn the task of caching and retrieving, a factor that has been implicated in modifying neurogenesis rates (Cain 1997; Döbrössy et al. 2003). Further, these birds continued to cache until perfusion, indicating that neurogenesis, in approximately 30 days in which we visualized doublecortin, was likely modified by our behavioural manipulation. In addition, distances travelled in the testing room and number of cache/perch sites visited (both indicators of motor stimulation) by birds who were restricted from caching were not significantly different from the distances travelled by birds allowed to cache (distance travelled: F1,21 = .0459, p = 0.506; cache/perch sites visited: F1,21 = 0.016, p = 0.899).
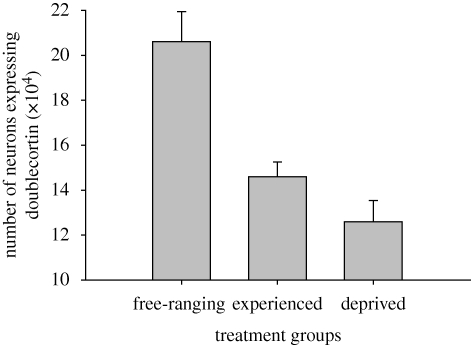
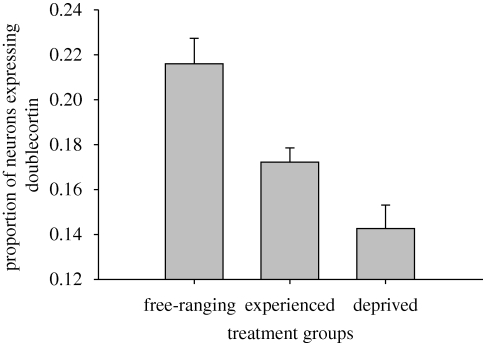
The number of neurons expressing doublecortin in the hippocampal formation and the proportion of hippocampal neurons expressing doublecortin significantly differed among free-ranging, experienced and deprived birds (number: F2,33 = 15.753, p < 0.001; proportion: F2,33 = 14.450, p < 0.001; table 1; examples of labelled cells in figure 1). Free-ranging birds had more neurons expressing doublecortin and a greater proportion of neurons expressing doublecortin than experienced birds (number: p < 0.001; proportion: p = 0.002) or deprived birds (number: p < 0.001; proportion: p < 0.001) (figures 2 and 3). The per cent of neurons expressing doublecortin ranged from 9 per cent in captive birds to 31 per cent in free-ranging birds. More importantly, we found that experienced birds had more neurons expressing doublecortin and a greater proportion of neurons expressing doublecortin than did experience deprived birds (number: p = 0.038; proportion: p = 0.019) (figures 2 and 3). We also found that the number and density of doublecortin-positive cells in the HA (the control region) were statistically indistinguishable, regardless of treatment group (number: F2,33 = 2.307, p = 0.115; density: F2,33 = 0.449, p = 0.642).
Table 1.
Mean ± s.e.m. stereological estimates of the number of doublecortin-labelled cells in the hippocampus, the total number of hippocampal neurons and the proportion of hippocampal neurons expressing doublecortin in three groups of birds.
| treatment | number of doublecortin-labelled cells (×105) | number of neurons (×105)a | proportion of neurons expressing doublecortin |
|---|---|---|---|
| deprived | 1.26 ± 0.09 | 8.92 ± 0.33 | 0.14 ± 0.011 |
| experienced | 1.46 ± 0.07 | 8.59 ± 0.48 | 0.17 ± 0.011 |
| free-ranging | 2.06 ± 0.13 | 9.51 ± 0.33 | 0.22 ± 0.006 |
aData taken from LaDage et al. (2009).
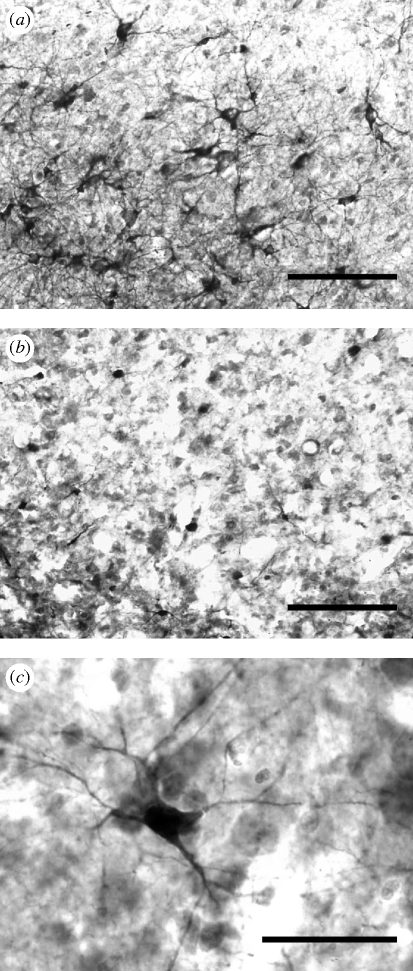
Figure 1.
Representative examples of hippocampal neurogenesis in (a) free-ranging birds and (b) captive birds deprived of memory-based experiences. (c) New neurons were visualized with a marker for the endogenous expression of doublecortin. Scale bars, (a,b) 100 µm, and (c) 25 µm.
Figure 2.
Total number of hippocampal neurons expressing doublecortin + s.e. Treatment groups were free-ranging birds (n = 12), captive birds with the opportunity for memory-based cache retrieval experiences (experienced, n = 12) and captive birds deprived of memory-based cache retrieval experiences (deprived, n = 12). Free-ranging birds had more neurons expressing doublecortin than either of the captive groups (both p < 0.001). Experienced birds had more neurons expressing doublecortin than deprived birds (p = 0.038).
Figure 3.
Proportion of new hippocampal neurons expressing doublecortin + s.e. Treatment groups were free-ranging birds (n = 12), captive birds with the opportunity for memory-based cache retrieval experiences (experienced, n = 12) and captive birds deprived of memory-based cache retrieval experiences (deprived, n = 12). Free-ranging birds had a greater proportion of neurons expressing doublecortin than either of the captive groups (both p < 0.001). Experienced birds had a greater proportion of neurons expressing doublecortin than deprived birds (p = 0.019).
4. Discussion
We found that, within captivity, restricting the opportunity to engage in spatial memory use via food caching and cache retrieval behaviour resulted in significantly fewer numbers of hippocampal neurons expressing doublecortin compared with captive conspecifics allowed to engage in spatial memory use. Further, we found that both the deprived and experienced captive groups had significantly fewer hippocampal neurons with doublecortin expression compared with free-ranging conspecifics. These differences do not appear to be global, as the number and density of doublecortin-positive cells in the adjacent control region (HA) did not differ among our treatment groups. The results of our comparison are similar to that of Barnea & Nottebohm (1994), in that free-ranging birds had greater rates of surviving neurons compared with captive counterparts. Further, the per cent of neurons expressing doublecortin track the number of surviving neurons in Barnea & Nottebohm (1994). Barnea & Nottebohm (1994) found that 0.9 per cent of neurons survived after six weeks after a single injection of BrdU. Assuming that one injection might label all cells that were produced within a 12 h period, at the most (this is a conservative estimate, as BrdU has been shown to label dividing cells generally within 1–2 h, e.g. Cameron & McKay 2001), and extrapolating a constant rate of neurogenesis, 36 per cent of cells would have been labelled if all cells produced during a 20-day time period had been labelled. Similarly, Cameron & McKay (2001) found that 9000 new cells were generated in the rat dentate gyrus within 1 day, potentially leading to 250 000 cells generated over a month in a structure that contains 1–2 million cells (12.5–25%). Thus, our findings that upward of 30 per cent of neurons were labelled are not unexpected when compared with previous literature (i.e. Barnea & Nottebohm 1994; Cameron & McKay 2001).
Moreover, our study has also identified the use of spatial memory as a contributing factor in determining neurogenesis rates, as exemplified by differences in the number of doublecortin-positive neurons between the two captive groups. Several mammalian studies have found that hippocampal neurogenesis rates can be modulated by spatial memory use. However, taken as a whole, the results have been equivocal, as some studies show a positive association between spatial memory use and neurogenesis rates (Gould et al. 1999; Ambrogini et al. 2000; Döbrössy et al. 2003; Hariston et al. 2005), some show a negative relationship (Ambrogini et al. 2004) and others have found no discernable relationship (van Praag et al. 1999a; Snyder et al. 2005; Van der Borght et al. 2005). Further, Epp et al. (2007) found that the age of new neurons determined whether spatial learning would increase their survival. They found that spatial learning between 6 and 10 days from neuronal birth increased neuron survival whereas spatial learning between days 1 and 5 or days 11 and 15 had no effect on neurogenesis rates. This suggests that there may also be a critical temporal stage in neuronal development where spatial learning can influence survival rate.
Some of the contradictory results reported in the mammal work may be related in part to the stress, difficulty and training required accomplishing the task itself. Several studies have found that the use of the Morris water maze, the traditional paradigm used in the aforementioned studies of spatial memory use and hippocampal neurogenesis, can affect neurogenesis rates independent of spatial memory use per se (e.g. Gerlai & Clayton 1999; Beiko et al. 2004; Ehninger & Kempermann 2006; Mohapel et al. 2006). Thus, depending on the protocol used, these variables may cause variation in the rate of hippocampal neurogenesis rates outside of the variable of interest (i.e. spatial memory use). By using a freely expressed, naturally occurring behaviour involved in spatial memory use, we can potentially reduce the confounding effects of training, stress and difficulty (Gould & Cameron 1996; Cain 1997; Gould et al. 1998; Döbrössy et al. 2003; Beiko et al. 2004; Mohapel et al. 2006; Gould 2007) on the rate of neurogenesis, which have previously been difficult to dissociate from the role of learning and memory.
In this study, birds traversed similar distances and visited similar numbers of cache/perch sites in the testing room, regardless of whether they were engaging in food-caching behaviour or not. This indicates that motor stimulation was similar between the two captive groups. Because we used a different set of birds to assess distance travelled and number of cache/perch sites visited, the possibility exists that the birds whose brains we analysed had different movement patterns in the testing room. However, because we measured movement in the same species of bird, which were maintained in captivity in exactly the same way and allowed the same experiences as subject birds, differences, if any, are probably minor.
We also found that captive birds, regardless of whether they were allowed or deprived of memory-based experiences, had rates of neurogenesis that were substantially lower than those of free-ranging birds. This result is similar to those found by Barnea & Nottebohm (1994) and suggests that there is some aspect of captivity and/or our experimental design that precluded neurogenesis rates in captivity to equal those seen in free-ranging birds. Captivity represents an extremely simple and constrained environment even in the best case, thereby restricting both physical movement and memory-based experiences and demands (van Praag et al. 2000). Animals in the wild undoubtedly encounter a far more complex physical environment that allows for greater physical stimulation, encourages increased activity levels and increased demands on memory, all of which may increase hippocampal neurogenesis rates (Kempermann et al. 1997).
This caveat limits our ability to draw firm conclusions as to the differences between captive and free-ranging birds. Although our experimental design allowed for memory-based experiences, these opportunities were probably much fewer than those experienced in the wild and were probably insufficient to induce neurogenesis rates similar to those found in free-ranging birds. In addition, captivity could downregulate proteins necessary for differentiation, migration or incorporation of new neurons, which would necessarily downregulate neurogenesis rates. Similarly, time in captivity could affect the downregulation of neurogenesis. Although we subjected birds to memory-based experiences for three months, we could only measure the youngest cohort (up to 30 days old at time of sacrifice, based on Balthazart et al. 2008) of the total number of new neurons generated during the three-month period. Depending on the time of sacrifice within that three-month period, we may have seen experienced, captive birds with neurogenesis rates closer to free-ranging birds when sacrificed earlier in their time in captivity compared with neurogenesis rates in those same birds after three months in captivity. The temporal effects of captivity on neurogenesis remain an interesting direction for future study.
An important aspect of our study concerns the temporal profile of the population of neurons expressing doublecortin. In mammals, time sequence experiments have shown that co-labelling of doublecortin and BrdU peaks within two weeks after BrdU administration. In rats, Brown et al. (2003) found that 90 per cent of cells co-expressed doublecortin and BrdU at 7 days, while Rao & Shetty (2004) found that 90 per cent co-expressed at 12 days. After this time, expression of doublecortin fell sharply between days 14 and 30 as the expression of NeuN rose sharply, and doublecortin was undetectable from day 60 until day 180 (Brown et al. 2003). However, in passerine birds, doublecortin expression appears to be maintained for a longer time period compared with rats and it may be related to longer neuron maturation period (Balthazart et al. 2008). Balthazart et al. (2008) found that 75 per cent of doublecortin-labelled cells in canaries co-labelled with BrdU 10 days after injection fell to 54 per cent around 30 days, leading them to conclude that new neurons express doublecortin for about 20 days in canaries. Unfortunately, this study only accounted for doublecortin cells that also co-labelled for BrdU, rather than reporting the total number of BrdU-positive cells labelled, and the labelling of BrdU-positive cells that co-expressed doublecortin. At 30 days after BrdU injections, there were probably many more new neurons with doublecortin expression that were born between BrdU injection and the time of sacrifice. As a result, the fact that only 54 per cent of doublecortin-positive cells also labelled for BrdU may reflect an increase in new neurons and hence a lower proportion of BrdU-labelled cells among doublecortin-positive cells. Hoshooley & Sherry (2007) found that, in chickadees, only one-third of BrdU-positive cells also expressed NeuN after six weeks. Expression of NeuN closely follows cessation of doublecortin expression (Brown et al. 2003) and thus it is likely that doublecortin expression in birds lasts for up to six weeks or more. Although these two studies may provide preliminary evidence suggesting that new neurons in birds take longer to differentiate, additional studies will be needed to verify this.
An additional temporal caveat to doublecortin is that it labels all cells produced within a particular time frame, which can either be a benefit or drawback, depending on the question asked. For instance, if one is primarily interested in the number of cells produced within the previous 15–30 days regardless of the stage of maturation or survival, doublecortin would be a viable option. Consequently, doublecortin cannot be used to provide a snapshot of a population of new cells. Because of this, an alternative marker such as BrdU would provide a better resolution of temporal issues surrounding neuronal proliferation and survival, when coupled with double-labelling for neuronal phenotype. In conclusion, our study showed that the population of new hippocampal neurons within their first few weeks of life and prior to their maturation was significantly affected by captivity and memory-based experiences in captivity. Such a result may be explained by differences in both new neuron production rates and new neuron survival rates prior to maturation. To our knowledge, this is the first study to show that an ecologically relevant behaviour that directly engages spatial memory affects hippocampal neurogenesis in captivity, independent of several confounding factors. Further work is needed to investigate whether similar conditions also affect new neuron survival rates after they are matured and incorporated into hippocampal neural circuits.
Acknowledgements
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Nevada, Reno (A06/07-25), and comply with the laws of the USA.
We thank Geniveve Hanson, Jodi Johnson and Ashley Rolfe for bird care and efficacious data collection. Birds were collected under the US Federal Fish and Wildlife (MB022532) and California State (802017-05) scientific collecting permits. This research was supported by grants from the National Institutes of Health (MH079892 and MH076797) and the National Science Foundation (IOB-0615021) to V.P. Comments by two anonymous reviewers greatly improved the manuscript.
References
- Abrous D. N., Koehl M., Le Moal M.2005Adult neurogenesis: from precursors to network and physiology. Physiol. Rev. 85, 523–569 (doi:10.1152/physrev.00055.2003) [DOI] [PubMed] [Google Scholar]
- Ambrogini P., Cuppini R., Cuppini C., Ciaroni S., Cecchini T., Ferri P., Sartini S., Del Grande P.2000Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci. Lett. 286, 21–24 (doi:10.1016/S0304-3940(00)01074-0) [DOI] [PubMed] [Google Scholar]
- Ambrogini P., Orsini L., Mancini C., Ferri P., Ciaroni S., Cuppini R.2004Learning may reduce neurogenesis in adult rat dentate gyrus. Neurosci. Lett. 359, 13–16 (doi:10.1016/j.neulet.2003.12.123) [DOI] [PubMed] [Google Scholar]
- Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O.2002Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970 (doi:10.1038/nm747) [DOI] [PubMed] [Google Scholar]
- Balthazart J., Boseret G., Konkle A. T. M., Hurley L. L., Ball G. F.2008Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur. J. Neurosci. 27, 801–817 (doi:10.1111/j.1460-9568.2008.06059.x) [DOI] [PubMed] [Google Scholar]
- Barnea A., Nottebohm F.1994Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl Acad. Sci. USA 91, 11 217–11 221 (doi:10.1073/pnas.91.23.11217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiko J., Lander R., Hampson E., Boon F., Cain D. P.2004Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behav. Brain Res. 151, 239–253 (doi:10.1016/j.bbr.2003.08.019) [DOI] [PubMed] [Google Scholar]
- Bernier P. J., Bedard A., Vinet J., Levesque M., Parent A.2002Newly generated neurons in the amygdale and adjoining cortex of adult primates. Proc. Natl Acad. Sci. USA 99, 11 464–11 469 (doi:10.1073/pnas.172403999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boseret G., Ball G. F., Balthazart J.2007The microtubule-associate protein doublecortin is broadly expressed in the telencephalon of adult canaries. J. Chem. Neuoranat. 33, 140–154 (doi:10.1016/j.jchemneu.2007.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck D. R.1994Memory for spatial and local cues: a comparison of a storing and a nonstoring species. Anim. Learn. Behav. 22, 119–133 [Google Scholar]
- Brown J. P., Couillard-Després S., Cooper-Kuhn C. M., Winkler J., Aigner L., Kuhn H. G.2003Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 467, 1–10 (doi:10.1002/cne.10874) [DOI] [PubMed] [Google Scholar]
- Cain D. P.1997Prior non-spatial pretraining eliminates sensorimotor disturbances and impairments in water maze learning caused by diazepam. Psychopharmacology 130, 313–319 (doi:10.1007/s002130050245) [DOI] [PubMed] [Google Scholar]
- Cameron H. A., McKay R. D. G.2001Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 435, 406–417 (doi:10.1002/cne.1040) [DOI] [PubMed] [Google Scholar]
- Clayton N. S.1995Development of memory and the hippocampus: comparison of food-storing and nonstoring birds on a one-trial associative memory task. J. Neurosci. 15, 2796–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton N. S., Krebs J. R.1994Memory for spatial and object-specific cues in food-storing and non-storing birds. J. Comp. Physiol. A 174, 371–379 (doi:10.1007/BF00240218) [Google Scholar]
- Clelland C. D., et al. 2009A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 (doi:10.1126/science.1173215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S., et al. 2005Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 21, 1–14 (doi:10.1111/j.1460-9568.2004.03813.x) [DOI] [PubMed] [Google Scholar]
- Döbrössy M. D., Drapeau E., Aurousseau C., Le Moal M., Piazza P. V., Abrous D. N.2003Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry 8, 974–982 (doi:10.1038/sj.mp.4001419) [DOI] [PubMed] [Google Scholar]
- Ehninger D., Kempermann G.2006Paradoxical effects of learning the Morris water maze on adult hippocampal neurogenesis in mice may be explained by a combination of stress and physical activity. Genes Brain Behav. 5, 29–39 (doi:10.1111/j.1601-183X.2005.00129.x) [DOI] [PubMed] [Google Scholar]
- Epp J. R., Spritzer M. D., Galea L. A. M.2007Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience 149, 273–285 (doi:10.1016/j.neuroscience.2007.07.046) [DOI] [PubMed] [Google Scholar]
- Fox R. A., LaDage L. L., Roth T. C., II, Pravosudov V. V.2009Behavioural profile predicts dominance status in mountain chickadees, Poecile gambeli. Anim. Behav. 77, 1441–1448 (doi:10.1016/j.anbehav.2009.02.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis F., et al. 1999Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23, 247–256 (doi:10.1016/S0896-6273(00)80777-1) [DOI] [PubMed] [Google Scholar]
- Gerlai R., Clayton N. S.1999Analysing hippocampal function in transgenic mice: an ethological perspective. Trends Neurosci. 22, 47–51 (doi:10.1016/S0166-2236(98)01346-0) [DOI] [PubMed] [Google Scholar]
- Gleeson J. G., Lin P. T., Flanagan L. A., Walsh C. A.1999Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23, 257–271 (doi:10.1016/S0896-6273(00)80778-3) [DOI] [PubMed] [Google Scholar]
- Gould E.2007Structural plasticity. In The hippocampus book (eds Andersen P., Morris J., O'Keefe J.), pp. 321–342 New York, NY: Oxford University Press [Google Scholar]
- Gould E., Cameron H. S.1996Regulation of neuronal birth, migration and death in the rat dentate gyrus. Dev. Neurosci. 18, 22–35 (doi:10.1159/000111392) [DOI] [PubMed] [Google Scholar]
- Gould E., Gross C. G.2002Neurogenesis in adult mammals: some progress and problems. J. Neurosci. 22, 619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., Tanapat P., McEwen B. S., Flugge G., Fuchs E.1998Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl Acad. Sci. USA 95, 3168–3171 (doi:10.1073/pnas.95.6.3168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., Beylin A., Tanapat P., Reeves A., Shors T. J.1999Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2, 260–265 (doi:10.1038/6365) [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Cohen N. J., Juraska J. M.1999New neurons in old brains: learning to survive? Nat. Neurosci. 2, 203–205 (doi:10.1038/6300) [DOI] [PubMed] [Google Scholar]
- Hannan A. J., Henke R. C., Seeto G. S., Capes-Davis A., Dunn J., Jeffrey P. L.1999Expression of doublecortin correlates with neuronal migration and pattern formation in diverse regions of the developing chick brain. J. Neurosci. Res. 55, 650–657 (doi:10.1002/(SICI)1097-4547(19990301)55:5<650::AID-JNR12>3.0.CO;2-S) [DOI] [PubMed] [Google Scholar]
- Hariston I. S., Little M. T. M., Scanlon M. D., Barakat M. T., Palmer T. D., Sapolsky R. M., Heller H. C.2005Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J. Neurophysiol. 94, 4224–4233 (doi:10.1152/jn.00218.2005) [DOI] [PubMed] [Google Scholar]
- Hoshooley J. S., Sherry D. F.2007Greater hippocampal neuronal recruitment in food-storing than in non-food-storing birds. Dev. Neurobiol. 67, 406–414 (doi:10.1002/dneu.20316) [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H. G., Gage F. H.1997More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 (doi:10.1038/386493a0) [DOI] [PubMed] [Google Scholar]
- Kempermann G., Wiskott L., Gage F. H.2004Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 14, 186–191 (doi:10.1016/j.conb.2004.03.001) [DOI] [PubMed] [Google Scholar]
- Kim Y.-H., Peregrine J., Arnold A. P.2006The distribution of expression of doublecortin (DCX) mRNA and protein in the zebra finch brain. Brain Res. 1106, 189–196 (doi:10.1016/j.brainres.2006.05.080) [DOI] [PubMed] [Google Scholar]
- Kolb B., Pedersen B., Ballermann M., Gibb R., Whishaw I. Q.1999Embryonic and postnatal injections of bromodeoxyuridine produces age-dependent morphological and behavioural abnormalities. J. Neurosci. 19, 2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs J. R., Sherry D. F., Healy S. D., Perry V. H., Vaccarino A. L.1989Hippocampal specialization of food-storing birds. Proc. Natl Acad. Sci. USA 86, 1388–1392 (doi:10.1073/pnas.86.4.1388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDage L. D., Roth T. C., II, Fox R. A., Pravosudov V. V.2009Effects of captivity and memory-based experiences on the hippocampus in mountain chickadees. Behav. Neurosci. 123, 284–291 (doi:10.1037/a0014817) [DOI] [PubMed] [Google Scholar]
- Leuner B., Gould E., Shors T. J.2006Is there a link between adult neurogenesis and learning? Hippocampus 16, 216–224 (doi:10.1002/hipo.20153) [DOI] [PubMed] [Google Scholar]
- Magavi S. S., Leavitt B. R., Macklis J. D.2000Induction of neurogenesis in the neocortex of adult mice. Nature 405, 951–955 (doi:10.1038/35016083) [DOI] [PubMed] [Google Scholar]
- Markakis E. A., Gage F. H.1999Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J. Comp. Neurol. 406, 449–460 (doi:10.1002/(SICI)1096-9861(19990419)406:4<449::AID-CNE3>3.0.CO;2-I) [PubMed] [Google Scholar]
- Mohapel P., Mundt-Petersen K., Brundin P., Frielingsdorf H.2006Working memory training decreases hippocampal neurogenesis. Neuroscience 142, 609–613 (doi:10.1016/j.neuroscience.2006.07.033) [DOI] [PubMed] [Google Scholar]
- Mullen R. J., Buck C. R., Smith A. M.1992NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201–211 [DOI] [PubMed] [Google Scholar]
- Nacher J., Crespo C., McEwen B.2001Doublecortin expression in the adult rat telencephalon. Eur. J. Neurosci. 14, 629–644 (doi:10.1046/j.0953-816x.2001.01683.x) [DOI] [PubMed] [Google Scholar]
- Pravosudov V. V., Clayton N. S.2002A test of the adaptive specialization hypothesis: population differences in caching, memory and the hippocampus in black-capped chickadees (Poecile atricapilla). Behav. Neurosci. 116, 515–522 (doi:10.1037/0735-7044.116.4.515) [PubMed] [Google Scholar]
- Pravosudov V. V., Omanska A.2005aProlonged moderate elevation of corticosterone does not affect hippocampal anatomy or cell proliferation rates in mountain chickadees (Poecile gambeli). J. Neurobiol. 62, 82–91 (doi:10.1002/neu.20069) [DOI] [PubMed] [Google Scholar]
- Pravosudov V. V., Omanska A.2005bDominance-related changes in spatial memory are associated with changes in hippocampal cell proliferation rates in mountain chickadees. J. Neurobiol. 62, 31–41 (doi:10.1002/neu.20065) [DOI] [PubMed] [Google Scholar]
- Pravosudov V. V., Lavenex P., Clayton N. S.2002Changes in spatial memory mediated by experimental variation in food supply do not affect hippocampal anatomy in mountain chickadees (Poecile gambeli). J. Neurobiol. 51, 142–148 (doi:10.1002/neu.10045) [DOI] [PubMed] [Google Scholar]
- Rao M. S., Shetty A. K.2004Efficacy of doublecortin as a marker to analyze the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci. 19, 234–246 (doi:10.1111/j.0953-816X.2003.03123.x) [DOI] [PubMed] [Google Scholar]
- Shettleworth S. J., Krebs J. R., Healy S. D., Thomas C. M.1990Spatial memory of food-storing tits (Parus ater and P. atricapillus): comparison of storing and nonstoring tasks. J. Comp. Psychol. 104, 71–81 (doi:10.1037/0735-7036.104.1.71) [Google Scholar]
- Snyder J. S., Hong N. S., McDonald R. J., Wojtowicz J. M.2005A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–852 (doi:10.1016/j.neuroscience.2004.10.009) [DOI] [PubMed] [Google Scholar]
- Van der Borght K., Wallinga A. E., Luiten P. G. M., Eggen B. J. L., Van der Zee E. A.2005Morris water maze learning in two rat strains increases the expression of the polysialylated form of the neural cell adhesion molecule in the dentate gyrus but has no effect on hippocampal neurogenesis. Behav. Neurosci. 119, 926–932 (doi:10.1037/0735-7044.119.4.926) [DOI] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F. H.1999aRunning increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270 (doi:10.1038/6368) [DOI] [PubMed] [Google Scholar]
- van Praag H., Christie B. R., Sejnowski T. J., Gage F. H.1999bRunning enhances neurogenesis, learning and long-term potentiation in mice. Proc. Natl Acad. Sci. USA 96, 13 427–13 431 (doi:10.1073/pnas.96.23.13427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F. H.2000Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198 (doi:10.1038/35044558) [DOI] [PubMed] [Google Scholar]
- van Praag H., Schinder A. F., Christie B. R., Toni N., Palmer T. D., Gage F. H.2002Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034 (doi:10.1038/4151030a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M. J., Ostergaard K., Andreassen O. A., Finsen B.1996Estimation of the number of somatostatin neurons in the striatum: an in situ hybridization study using the optical fractionator method. J. Comp. Neuorol. 370, 11–22 (doi:10.1002/(SICI)1096-9861(19960617)370:1<11::AID-CNE2>3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- Yang H. K. C., Sundholm-Peters N. L., Goings G. E., Walker A. S., Hyland K., Szele F. G.2004Distribution of doublecortin expressing cells near the lateral ventricles in the adult mouse brain. J. Neuorsci. Res. 76, 282–295 (doi:10.1002/jnr.20071) [DOI] [PubMed] [Google Scholar]