Abstract
Habitat clearance remains the major cause of biodiversity loss, with consequences for ecosystem services and for people. In response to this, many global conservation schemes direct funds to regions with high rates of recent habitat destruction, though some also emphasize the conservation of remaining large tracts of intact habitat. If the pattern of habitat clearance is highly contagious, the latter approach will help prevent destructive processes gaining a foothold in areas of contiguous intact habitat. Here, we test the strength of spatial contagion in the pattern of habitat clearance. Using a global dataset of land-cover change at 50 × 50 km resolution, we discover that intact habitat areas in grid cells are refractory to clearance only when all neighbouring cells are also intact. The likelihood of loss increases dramatically as soon as habitat is cleared in just one neighbouring cell, and remains high thereafter. This effect is consistent for forests and grassland, across biogeographic realms and over centuries, constituting a coherent global pattern. Our results show that landscapes become vulnerable to wholesale clearance as soon as threatening processes begin to penetrate, so actions to prevent any incursions into large, intact blocks of natural habitat are key to their long-term persistence.
Keywords: habitat loss, global change biology, conservation, wilderness
1. Introduction
During the past 10 000 years, vegetation across about half of Earth's ice-free land surface has been cleared or become otherwise dominated by human activity (Vitousek et al. 1997; Lambin et al. 2003). It has been predicted that a further 109 ha of natural habitat will be converted to cultivation or grazing by 2050, in what may be the final expansion phase of global agriculture (Tilman et al. 2001). Habitat loss resulting from this clearance is at the heart of the current biodiversity crisis, causing extinctions of populations and species. Habitat destruction and degradation are implicated in the decline of over 85 per cent of the world's threatened mammals, birds and amphibians (Baillie et al. 2004).
Conservation organizations and environmental planners have responded to this rampant habitat loss by prioritizing those regions under imminent threat, such as places with high levels of habitat clearance in the recent past (Margules & Pressey 2000). Several influential conservation priority-setting schemes explicitly use high levels of past threat as a criterion for allocating conservation effort (e.g. biodiversity hotspots: Myers et al. 2000; Mittermeier et al. 2004; crisis ecoregions: Hoekstra et al. 2005). In addition to these reactive approaches to conservation, the importance of conserving large tracts of intact habitat has been widely recognized (Bryant et al. 1997; Mittermeier et al. 1998; Rothenberg & Ulvaeus 2001; Sanderson et al. 2002). While these ‘wilderness’ areas often do not support high levels of biodiversity (Mittermeier et al. 2003), they are certainly important for global ecological resilience. Large areas of intact habitat are important for maintaining key ecosystem services such as climate and water regulation (Daily 1997; Lee & Jetz 2008), and for sustaining ecological and evolutionary processes central to the long-term persistence of biodiversity (Pressey et al. 2007; Klein et al. 2009).
In response to these two perspectives, calls have been made for a two-pronged conservation strategy that simultaneously emphasizes the most and least vulnerable landscapes (Mittermeier et al. 1998; Brooks et al. 2006). However, large-scale patterns of habitat clearance are poorly understood, so the extent to which past habitat loss predicts future vulnerability remains an open question. If clearance is highly contagious, a given amount of habitat conversion will have a much greater impact in an intact landscape than in a heavily cleared one, because it opens the way for rapid incursion of additional threatening processes.
Extensive clearance of vegetation by burning occurred as early as the Neolithic period (5000–3000 BC), but the rate of habitat clearance has accelerated dramatically in the past few centuries (Williams 1989). From 1700 onward, the global human population grew from approximately 600 million to six billion at the end of the twentieth century (Demeny 1990; United Nations 1999), and habitat clearance during this period, to fuel growing economies and a spreading human population, dwarfed that occurring previously (Meyer & Turner 1992). Here, we test the strength of spatial contagion in the pattern of global habitat clearance since 1700. Using a half-degree map of vegetation change, we measure how the likelihood of habitat loss in a grid cell varies with the number of its neighbouring cells that have been cleared.
2. Methods
We obtained maps of global land-cover from the History Database of the Global Environment (HYDE 2.0; Klein Goldewijk 2001). The database maps the distribution of cultivated land, pasture and 14 types of natural vegetation, from 1700 to 1950 in 50-yr intervals, and in 1990 at half degree resolution. The mapping was constructed by superimposing historic data about the locations of agricultural landscapes onto estimates of the natural distribution of vegetation communities prior to human transformation. Subsequent expansion of these cleared landscapes was modelled using historical human population density data, derived from Tober et al. (1995). The resulting estimates of current and pre-agricultural land-cover agree well with those generated by other models and measurements (Matthews 1983; Ramankutty & Foley 1999; Klein Goldewijk 2001; Gaston et al. 2003). Full details of the land-cover mapping can be found in Klein Goldewijk (2001). We used HYDE 2.0 as opposed to the much finer-scale HYDE 3.0 or 3.1 since we were specifically interested in relatively large-scale patterns of habitat change.
We tested whether the likelihood of habitat within an intact grid cell being cleared depended on the clearance of surrounding cells. The models used to generate the HYDE land-cover database did not use this type of spatial dependency to determine whether natural vegetation had been cleared (Klein Goldewijk 2001). Since our hypothesis was based on potentially scale-dependent processes, we standardized the grain size of our analyses by re-projecting the HYDE 2.0 dataset into a Behrmann equal area projection, and generated a grid with cells measuring 48.24 × 48.24 km, approximating to a half degree resolution. Grid cells with sea or ocean covering more than 50 per cent of their area were excluded from all analyses. We investigated patterns of clearance for two natural vegetation types: forest and grassland. The HYDE 2.0 habitat categories were re-classified into ‘forest’ (boreal forest, cool conifer forest, temperate mixed forest, temperate deciduous forest, warm mixed forest, tropical woodland, tropical forest), ‘grassland’ (grassland, steppe, scrubland, savannah) and ‘cleared’ (cultivated land, pasture). Dominant coverage of the land area by forest or grassland was used to assign habitat type to our equal area grid cells for each time step between 1700 and 1990. Open natural habitat types considered neither forest nor grassland (ice, tundra, wooded tundra and hot desert) were treated as equivalent to cultivated land and pasture (i.e. as cleared cells), since such habitats represent a similarly abrupt transition to a more open habitat.
In each time interval of t0 to t1, n forested cells were cleared. We counted Ox the observed number of these n cells with x (0, 1, … , 8) non-forest neighbours at time t0. We then calculated Ex, the expected distribution of the number of n cells with x non-forest neighbours were deforestation to occur at random, by randomly selecting n forested cells from the t0 grid and counting how many of these cells had x (0, 1, … , 8) non-forest neighbours. We repeated this process 1000 times and averaged the counts. We compared O with E using a χ2-test, pooling counts to ensure O and E always exceeded five. Our observed–expected approach incorporates the fact that cells with different numbers of neighbours occur at differing frequencies, since we divide by expected values when calculating χ2 statistics. The analysis for forest and grassland was identical except that we only considered a grassland cell to be on an ‘edge’ if it was bordered by neither grassland nor forest. In the grassland analysis we therefore defined O and E as the number of neighbouring cells classified as either forest or grassland. For both analyses we investigated the pattern of habitat clearance both globally and by biogeographic realm (from Olson et al. 2001), and over combinations of time steps between 1700, 1750, 1800, 1850, 1900, 1950 and 1990.
3. Results
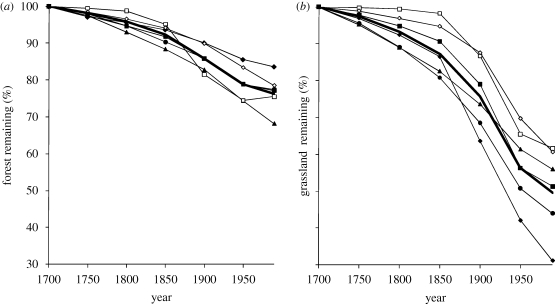
A quarter of the world's forest and half its grassland has been converted to agriculture since 1700 (figure 1). Variation in the rate of clearance among realms is marked, with highest forest loss in the Indo-Malay and Nearctic realms (32% and 25%, respectively) and grassland loss in Australasia and the Palaearctic (69% and 56%). Proportional forest and grassland loss are negatively related, though not significantly so (rs = −0.543, n = 6, p = 0.266). For example, Australasia has proportionally lost the most grassland, but the least forest (figure 1).
Figure 1.
Percentage loss of (a) forest and (b) grassland, both globally and by realm, over the period 1700–1990. Black diamond, Australasia; black square, Afrotropic; black triangle, Indo-Malay; white square, Nearctic; white diamond, Neotropic; black circle, Palaearctic; bold line with circles, global.
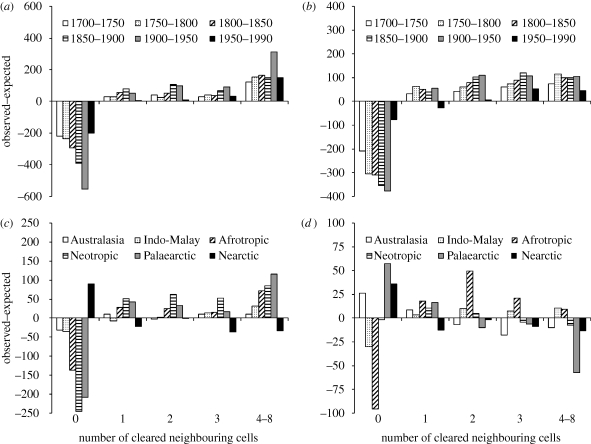
Clearance of natural vegetation in just one of an intact cell's neighbours dramatically increased the likelihood of the intact cell itself being transformed (figure 2a,b). Cells with no cleared neighbours were far less likely to be converted than expected under the random model. If even one adjoining cell was converted, the likelihood of the intact cell also being converted rose quickly; only cells with all their neighbouring cells intact appeared to have lower-than-expected rates of loss. This effect was consistent for forests and grasslands over almost all temporal periods both globally and across biogeographic realms, constituting a coherent global pattern (figure 2; electronic supplementary material). The pattern for forest clearance was reversed in the Nearctic (figure 2c), and was less clear for grassland among realms over the period 1700–1990 (figure 2d). However, over shorter time scales, contagion in grassland clearance was readily apparent.
Figure 2.
Contagion in global habitat clearance. Plots show the difference between the observed numbers of converted grid cells with x cleared neighbours and those that would be expected by chance for global (a) forest and (b) grassland conversion between 1700 and 1990 at 50-yr intervals. Where O < E, fewer deforested cells than expected have x cleared neighbours, indicating these intact cells' robustness to clearance. Conversion switched to a rate much greater than expected by chance as soon as one neighbouring cell had been cleared (all p < 0.001). Data on conversion in the period 1700–1990 by biogeographic realm show a consistent pattern for forest (c) but are less clear for grassland (d).
In the few exceptional instances in which the likelihood of clearance in cells with no converted neighbours switched from less than to greater than expected by chance, all but one occurred in time periods ending in 1990 (electronic supplementary material). In these cases, some buffering against clearance was associated with having one to seven cleared neighbours, perhaps reflecting decreased accessibility of the remaining habitat fragments or the very few intact areas remaining in those time periods limiting protection overall.
4. Discussion
Our results demonstrate that the global pattern of habitat clearance since humans began to dominate the planet is characterized by extreme contagion. A habitat block is much more likely to be cleared as soon as a single adjacent block is cleared, consistent with the observed acceleration in habitat loss over the past 300 years. Such contagion in habitat clearance will lead to non-random distribution of threat across space and will contribute to the observed spatial structure in extinction probabilities (Orme et al. 2005). However, empirical studies of patterns of extinctions across species' geographic ranges have produced conflicting results (Channell & Lomolino 2000; Donald & Greenwood 2001; Wilson et al. 2004), and the mechanisms associated with alternative patterns of geographic range decline remain largely unknown.
Contagion in global habitat clearance at the coarse scale we report here cannot continue indefinitely, although it has shown little sign of abating over the time period we examined (1700–1990; figure 2a,b). Eventually, conversion of surviving habitat fragments will be uneconomic (e.g. inaccessibility, poor soil quality), and they will be increasingly resistant to clearance. Indeed, during the most recent time period (1950–1990), relaxation of contagion occurred in the regions with most heavily cleared forest (Indo-Malay) and grassland (Australasia). A reversed pattern was also seen over the 1700–1990 time period in the Nearctic, the realm with by far the most rapid rate of deforestation (1850–1900) and the only one in which net reforestation has occurred (1950–1990). This might be explained by rapid deforestation following European colonization.
In common with our large-scale finding, studies of local and regional patterns of habitat clearance have usually found non-random patterns of habitat conversion (for a review see Lepers et al. 2005). For example, recent forest clearance in the Caquetá region of the Colombian Amazon has occurred in waves emanating from human settlements (Etter et al. 2006), and a comprehensive synthesis of information on land cover change between 1981 and 2000 showed that rapid habitat conversion is concentrated around the edges of large forest blocks and along major transport networks (Lepers et al. 2005). Almost all selective logging and total deforestation in the Brazilian Amazon between 1999 and 2002 was concentrated within 25 km of main roads, and once forest had been selectively logged, the probability of it being deforested rose fourfold (Asner et al. 2006). Predicting future patterns of change at any scale involves understanding the mechanisms that control the balance between positive feedbacks in which land clearance leads to further accelerating change in a region, and negative feedbacks in which management measures dampen or reverse a trajectory of land cover change (Lambin et al. 2003).
Much conservation activity is reactive, focusing on threatened species and landscapes that have undergone recent degradation (Myers et al. 2000). This is of course logical in the face of ongoing threats, but our results show that large continuous blocks of intact habitat have been disproportionately resistant to clearance. Thus, depending on additional factors such as irreplaceability and cost, it might be efficient to invest in efforts to prevent incursions into large areas of intact natural habitat. Preserving the largest intact areas could significantly reduce future rates of habitat loss because initial clearance of small areas within them leads to rapidly spreading habitat loss. Two major global prioritization schemes, Frontier Forests (Bryant et al. 1997) and Last of the Wild (Sanderson et al. 2002), are explicitly proactive, focusing on regions that have remained more or less intact.
Habitat conservation is critical to species conservation: many small-bodied species can be preserved simply by maintaining key habitats (Cardillo et al. 2005), but additionally many of the species that are expected to be most vulnerable to future threats occupy relatively intact habitats; their current less-threatened status is in part a result of their relative isolation from anthropogenic impacts (Cardillo et al. 2006). Species–area approaches to conservation planning emphasize that the rate of extinctions from a habitat accelerates as it declines in area, placing a premium on minimizing further loss in already small habitat fragments (Desmet & Cowling 2004). However, our results suggest that such approaches could be misleading in areas where substantial intact landscapes remain, because the rate of habitat clearance accelerates dramatically once threatening processes penetrate intact areas. Put simply, if habitat clearance does not start, it cannot continue.
We echo previous calls (Mittermeier et al. 1998; Brooks et al. 2006) for a two-pronged conservation strategy that reactively captures samples of biodiversity remaining in highly threatened and fragmented landscapes, but also proactively minimizes the penetration of threatening processes into our remaining wildernesses. We have shown that such areas become vulnerable to wholesale clearance soon after threatening processes begin to penetrate. Our results underline the importance of extra vigilance and effective management and enforcement to maintain the integrity of the remaining large intact blocks of natural habitat.
Acknowledgements
We thank R. G. Davies, K. K. Goldewijk, R. Grenyer, A. Phillimore, H. P. Possingham, O. Venter and J. E. M. Watson for discussion and assistance, and we are grateful to two anonymous referees for helpful comments on a previous version of the manuscript. This work was funded by grant number F/07058/AK from the Leverhulme Trust. R.A.F. is additionally supported by the Applied Environmental Decision Analysis research hub, funded through the Commonwealth Environment Research Facilities programme, Australia, and a grant from the University of Queensland.
References
- Asner G. P., Broadbent E. N., Oliveira P. J. C., Keller M., Knapp D. E., Silva J. N. M.2006Condition and fate of logged forests in the Brazilian Amazon. Proc. Natl Acad. Sci. USA 103, 12 947–12 950 (doi:10.1073/pnas.0604093103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie J. E. M., Hilton-Taylor C., Stuart S. N.20042004 IUCN red list of threatened species Gland, Switzerland: IUCN [Google Scholar]
- Brooks T. M., Mittermeier R. A., da Fonseca G. A. B., Gerlach J., Hoffmann M., Lamoreux J. F., Mittermeier C. G., Pilgrim J. D., Rodrigues A. S. L.2006Global biodiversity conservation priorities. Science 313, 58–61 (doi:10.1126/science.1127609) [DOI] [PubMed] [Google Scholar]
- Bryant D., Nielsen D., Tangley L.1997Last frontier forests Washington, DC: World Resources Institute [Google Scholar]
- Cardillo M., Mace G. M., Jones K. E., Bielby J., Bininda-Emonds O. R. P., Sechrest W., Orme C. D. L., Purvis A.2005Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 (doi:10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- Cardillo M., Mace G. M., Gittleman J. L., Purvis A.2006Latent extinction risk and the future battlegrounds of mammal conservation. Proc. Natl Acad. Sci. USA 103, 4157–4161 (doi:10.1073/pnas.0510541103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channell R., Lomolino M. V.2000Dynamic biogeography and conservation of endangered species. Nature 403, 84–86 (doi:10.1038/47487) [DOI] [PubMed] [Google Scholar]
- Daily G.1997Nature's services: societal dependence on natural ecosystems Washington, DC: Island Press [Google Scholar]
- Demeny P.1990Population. In The Earth as transformed by human action (eds Turner B. L., II, Clark W. C., Kates R. W., Richards J. F., Matthews J. T., Meyer W. B.), pp. 41–54 New York, NY: Cambridge University Press [Google Scholar]
- Desmet P., Cowling R.2004Using the species–area relationship to set baseline targets for conservation. Ecol. Soc. 9, 11 [Google Scholar]
- Donald P. F., Greenwood J. J. D.2001Spatial patterns of range contraction in British breeding birds. Ibis 143, 593–601 (doi:10.1111/j.1474-919X.2001.tb04887.x) [Google Scholar]
- Etter A., McAlpine C., Phinn S., Pullar D., Possingham H.2006Characterizing a tropical deforestation wave: a dynamic spatial analysis of a deforestation hotspot in the Colombian Amazon. Global Change Biol. 12, 1409–1420 (doi:10.1111/j.1365-2486.2006.01168.x) [Google Scholar]
- Gaston K. J., Blackburn T. M., Klein Goldewijk K.2003Habitat conversion and global avian biodiversity loss. Proc. R. Soc. Lond. B 270, 1293–1300 (doi:10.1098/rspb.2002.2303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra J. M., Boucher T. M., Ricketts T. H., Roberts C.2005Confronting a biome crisis: global disparities of habitat loss and protection. Ecol. Lett. 8, 23–29 (doi:10.1111/j.1461-0248.2004.00686.x) [Google Scholar]
- Klein C., Wilson K., Watts M., Stein J., Berry S., Carwardine J., Smith M. S., Mackey B., Possingham H.2009Incorporating ecological and evolutionary processes into continental-scale conservation planning. Ecol. Appl. 19, 206–217 (doi:10.1890/07-1684.1) [DOI] [PubMed] [Google Scholar]
- Klein Goldewijk K.2001Estimating global land-use change over the past 300 years: the HYDE database. Global Biogeochem. Cycles 15, 417–433 [Google Scholar]
- Lambin E. F., Geist H. J., Lepers E.2003Dynamics of land-use and land-cover change in tropical regions. Annu. Rev. Environ. Resour. 28, 205–241 (doi:10.1146/annurev.energy.28.050302.105459) [Google Scholar]
- Lee T. M., Jetz W.2008Future battlegrounds for conservation under global change. Proc. R. Soc. B 275, 1261–1270 (doi:10.1098/rspb.2007.1732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepers E., Lambin E. F., Janetos A. C., DeFries R., Achard F., Ramankutty N., Scholes R. J.2005A synthesis of information on rapid land-cover change for the period 1981–2000. BioScience 55, 115–124 (doi:10.1641/0006-3568(2005)055[0115:ASOIOR]2.0.CO;2) [Google Scholar]
- Margules C. R., Pressey R. L.2000Systematic conservation planning. Nature 405, 243–253 (doi:10.1038/35012251) [DOI] [PubMed] [Google Scholar]
- Matthews E.1983Global vegetation and land use: new high-resolution data bases for climate studies. J. Clim. Appl. Meteorol. 22, 474–487 (doi:10.1175/1520-0450(1983)022<0474:GVALUN>2.0.CO;2) [Google Scholar]
- Meyer W. B., Turner B. L., II1992Human population growth and global land-use/cover change. Annu. Rev. Ecol. Syst. 23, 39–61 (doi:10.1146/annurev.es.23.110192.000351) [Google Scholar]
- Mittermeier R. A., Myers N., Thomsen J. B., da Fonseca G. A. B., Olivieri S.1998Biodiversity hotspots and major tropical wilderness areas: approaches to setting conservation priorities. Conserv. Biol. 12, 516–520 (doi:10.1046/j.1523-1739.1998.012003516.x) [Google Scholar]
- Mittermeier R. A., Mittermeier C. G., Brooks T. M., Pilgrim J. D., Konstant W. R., da Fonseca G. A. B., Kormos C.2003Wilderness and biodiversity conservation. Proc. Natl Acad. Sci. USA 100, 10 309–10 313 (doi:10.1073/pnas.1732458100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittermeier R. A., Gil P. R., Hoffmann M., Pilgrim J., Brooks T., Mittermeier C. G., Lamoreux J., da Fonseca G. A. B.2004Hotspots revisited: Earth's biologically richest and most endangered terrestrial ecoregions Mexico City, Mexico: CEMEX [Google Scholar]
- Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A. B., Kent J.2000Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (doi:10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- Olson D. M., et al. 2001Terrestrial ecoregions of the world: a new map of life on Earth. BioScience 51, 933–938 (doi:10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2) [Google Scholar]
- Orme C. D. L., et al. 2005Global hotspots of species richness are not congruent with endemism or threat. Nature 436, 1016–1019 (doi:10.1038/nature03850) [DOI] [PubMed] [Google Scholar]
- Pressey R. L., Cabeza M., Watts M. E., Cowling R. M., Wilson K. A.2007Conservation planning in a changing world. Trends Ecol. Evol. 22, 583–592 (doi:10.1016/j.tree.2007.10.001) [DOI] [PubMed] [Google Scholar]
- Ramankutty N., Foley J. A.1999Estimating historical changes in global land cover: croplands from 1700 to 1992. Global Biogeochem. Cycles 13, 997–1027 (doi:10.1029/1999GB900046) [Google Scholar]
- Rothenberg D., Ulvaeus M.2001The world and the wild Tucson, AZ: University of Arizona Press [Google Scholar]
- Sanderson E. W., Jaiteh M., Levy M. A., Redford K. H., Wannebo A. V., Woolmer G.2002The human footprint and the last of the wild. BioScience 52, 891–904 (doi:10.1641/0006-3568(2002)052[0891:THFATL]2.0.CO;2) [Google Scholar]
- Tilman D., et al. 2001Forecasting agriculturally driven global environmental change. Science 292, 281–284 (doi:10.1126/science.1057544) [DOI] [PubMed] [Google Scholar]
- Tober W., Deichmann U., Gottsegen J., Maloy K.1995The global demography project Technical Report TR-95-6 Santa Barbara, CA: National Centre for Geographic Information and Analysis (NCGIA) [Google Scholar]
- United Nations. The world at six billion. New York, NY: United Nations.: 1999. [Google Scholar]
- Vitousek P. M., Mooney H. A., Lubchenco J., Melillo J. M.1997Human domination of Earth's ecosystems. Science 277, 494–499 (doi:10.1126/science.277.5325.494) [Google Scholar]
- Williams M.1989Deforestation: past and present. Prog. Hum. Geog. 13, 176–208 (doi:10.1177/030913258901300202) [Google Scholar]
- Wilson R. J., Thomas C. D., Fox R., Roy D. B., Kunin W. E.2004Spatial patterns in species distributions reveal biodiversity change. Nature 432, 393–396 (doi:10.1038/nature03031) [DOI] [PubMed] [Google Scholar]