Abstract
In 1959, P. Karlson and M. Lüscher introduced the term ‘pheromone’, broadly used nowadays for various chemicals involved in intraspecific communication. To demonstrate the term, they depicted the situation in termite societies, where king and queen inhibit the reproduction of nest-mates by an unknown chemical substance. Paradoxically, half a century later, neither the source nor the chemical identity of this ‘royal’ pheromone is known. In this study, we report for the first time the secretion of polar compounds of proteinaceous origin by functional reproductives in three termite species, Prorhinotermes simplex, Reticulitermes santonensis and Kalotermes flavicollis. Aqueous washes of functional reproductives contained sex-specific proteinaceous compounds, virtually absent in non-reproducing stages. Moreover, the presence of these compounds was clearly correlated with the age of reproductives and their reproductive status. We discuss the putative function of these substances in termite caste recognition and regulation.
Keywords: Isoptera, neotenic secondary reproductives, primer pheromones, fertility signal, caste regulation, MALDI-TOF
1. Introduction
The reproductive division of labour in termite societies has reached a high level of complexity, represented by several caste phenotypes with greatly skewed reproductive potentials. On one hand, winged imagoes, the ancestral isopteran phenotype and the only adults, undergo a risky dispersal to become the primary reproductives in new colonies. At the other extreme, permanently sterile soldiers rely solely on inclusive benefits. In addition, immature stages, such as larvae, true or false workers (pseudergates) or nymphs, can eventually enter upon a way of secondary reproduction as neotenics within their natal nest.
Neoteny is a widespread phenomenon in lower termites, evidenced in more than 60 per cent of genera (Myles 1999). Its significance for isopteran social evolution has been broadly discussed as a prime mover for the occurrence of helpers, i.e. pseudergates (Bartz 1979; Myles 1988; Thorne 1997) and soldiers (Myles 1986; Thorne et al. 2003). Although these ambitious hypotheses were subjected to serious criticisms (Roisin 1999), the philopatric reproduction by neoteny undoubtedly represents a crucial aspect of the reproductive biology in numerous species. Neotenics develop in two modal social contexts: (i) as replacement reproductives in response to orphaning and (ii) as supplementary reproductives even in the presence of functional reproductives. A plethora of species-specific situations has been described with respect to the readiness to produce neotenics, their number, fecundity and ontogenetic origin (reviewed in Myles 1999). Despite the theoretical accessibility of this alternative reproduction to most individuals, only a few of them become neotenics. A question therefore arises about the mechanism controlling the decision between neotenic reproduction and other options within the network of developmental pathways. The social aspects of this mechanism are known from the pioneering studies on Kalotermes flavicollis (Kalotermitidae), Zootermopsis (Termopsidae) and Reticulitermes (Rhinotermitidae) (Pickens 1932; Light 1944; Grassé & Noirot 1946, 1960; Lüscher 1949, 1955), summarized by Lüscher (1961) in the famous model of social control: king(s) and queen(s) inhibit in concert the formation of neotenics via a putative inhibitory substance, distributed among nest-mates by mutual contact. Absence of this inhibition results in the formation of neotenics, eliminated to species-specific optimum by siblicidal fights and cannibalism (Lüscher 1952; Ruppli 1969; Nagin 1972; Lenz 1985).
This concept was used by Karlson & Lüscher (1959) to define the term ‘pheromone’, universally used nowadays for chemicals involved in communication within one species. Paradoxically, neither the source nor the chemistry of this putative pheromone has been revealed in the last 50 years. During an extensive quest, several alternatives were hypothesized, such as the distribution of the substance by proctodeal feeding from reproductives (Lüscher 1955), later disclaimed several times (Nagin 1972; Stuart 1979; Greenberg & Stuart 1980, 1982), its secretion via the cuticle on the body surface (Lüscher 1974; Bordereau 1985; Šobotník et al. 2003) or the attribution of pheromonal action to juvenile hormone (Lüscher 1972; Myles & Chang 1984). None of these hypotheses has received decisive support, and the major enigma of termite biology still remains unresolved.
From the modern point of view, the non-reproducing individuals in insect societies are not necessarily helpless victims of manipulation, but rather decision-making subjects choosing the best option in terms of inclusive fitness. The queen signals can therefore be viewed as honest signals, announcing the presence of fertile individuals (Keller & Nonacs 1993). In eusocial hymenopterans, such signalling of fertility has been repeatedly attributed to cuticular hydrocarbons (CHCs) (reviewed, e.g. in Monnin 2006). In termites, CHCs represent a useful and frequent tool in the discrimination of species and populations (reviewed, e.g. in Clément & Bagnères 1998; Howard & Blomquist 2005). Only a few papers, however, have focused on inter-caste differences (Howard et al. 1982; Haverty et al. 1988, 1996; Watson et al. 1989; Brown et al. 1996; Bagnères et al. 1998; Sevala et al. 2000) and none has addressed in detail the reproductive status of individuals. Very recently, two independent studies observed CHC patterns specific to functional neotenics and concluded that in termites CHCs could also be involved in fertility signalling or caste regulation (Liebig et al. 2009; Weil et al. 2009).
Nevertheless, one should not overemphasize the powerful CHC paradigm and forget the banal fact that other compounds are also secreted by insects. Namely, the function of polar substances of peptidic (proteinaceous) origin in insect communication is unexplored, although their presence on the cuticular surface has been documented in various insects (Zupko et al. 1993; Korchi et al. 1998; Cornette et al. 2002; Turillazzi et al. 2006a). They may act as antibacterial agents (Turillazzi et al. 2006a), but experiments have also indicated their role in signalling (Cornette et al. 2002, 2003; Turillazzi et al. 2006b). Recently, distinct patterns of peptides were found in paper wasp females with different social status, suggesting that these compounds might be, beside CHCs, involved in caste and status signalling (Dapporto et al. 2008). We investigated the chemical patterns of polar compounds in body washes of neotenic reproductives and non-reproducing castes and stages in Prorhinotermes simplex (Rhinotermitidae). For comparison, two other termite species were included in this study: K. flavicollis and Reticulitermes santonensis.
2. Material and methods
(a). Termites: origin and laboratory breeding
Colonies of K. flavicollis Fabr. were collected in Salau, Catalonia, Spain, in 1993. Colonies of P. simplex (Hagen 1858) were collected in Soroa, Piñar del Rio, Cuba, in 1964 and 1989 and in Florida, USA, in 2003. Colonies of R. santonensis De Feytaud (this species is now considered as an introduced population of Reticulitermes flavipes; Austin et al. 2005) were collected in Ile d'Oléron, Charente-Maritime, France, in 2007. Colonies were kept in the laboratory in permanent darkness at the following temperatures and relative humidity (r.h.): 26°C and 90 per cent r.h. for P. simplex, 24°C and 85 per cent r.h. for R. santonensis and 25°C and 75 per cent r.h. for K. flavicollis.
(b). Termites: selection for body washes
The case of P. simplex was studied in detail; preliminary work (choice of the solvent, choice of MALDI-TOF matrix and methods, selection of methods for cuticular brushes) was performed on three mature colonies, two from Cuba (1964) and one from Florida (2003). We removed neotenics, nymphs, pseudergates and soldiers and returned them back after analysis. Each colony was inspected only once.
All results presented in this article were obtained from one mature colony (Cuba 1989), from which we removed during an inspection 18 male and 18 female neotenics, three soldiers, three pseudergates and two nymphs. We classified the neotenics as follows (figure 1): young—yellowish, non-physogastric, collected on nest periphery, and mature—from brownish to dark brown with various levels of physogastry in females, removed from the chambers with eggs and larvae (figure 1).
Figure 1.
Neotenic females of P. simplex. (a) Young, non-reproducing neotenic female, usually found on nest periphery or surface; (b–d) neotenic females of various ages found in chambers with eggs and early larvae. Scale bar, 5 mm.
Three mature male and female neotenics were used for brushes of the cuticular surface, two of the three females were subsequently dissected (see below). The remaining individuals were used for the main part of the experiment described below, i.e. the body washes, analysis of body weight and coloration, MALDI-TOF analysis and data analysis.
Two neotenic females were removed together with four pseudergates and four soldiers from two mature colonies of K. flavicollis. In R. santonensis, three functional physogastric neotenic females were removed from one mature colony together with three workers and three soldiers.
(c). Chemicals
Sinapinic acid (SA), Protein Calibration Standard I (both Bruker Daltonik GmbH Bremen, Germany), water (ultrapure water system, Millipore, Billerica, MA, USA), gradient grade methanol (Sigma-Aldrich, Buchs, Switzerland), acetonitrile (ACN, Lab-Scan, Dublin, Ireland), acetic acid p.a. (Lach-Ner, Neratovice, Czech Republic) and sodium dodecyl sulfate (SDS, Sigma-Aldrich).
(d). Body washes
During preliminary stages, a set of six solvents was tested (ultrapure water; H2O + 1 per cent CH3COOH; H2O + 0.1 per cent SDS; CH3OH; H2O + CH3OH 1 : 1; H2O + ACN 1 : 1). Ultrapure water finally proved to be the best solvent for the given purpose. Individual termites were washed alive for 5 min in 15 µl of water in tipped glass vials and shaken twice on vortex for 10 s. After their removal, the extracts were evaporated to dryness in a refrigerated centrifugal vacuum concentrator (Labconco, Kansas City, USA) at 4°C and then dissolved in 5 µl water.
(e). Weight and body coloration in neotenic reproductives
The washed P. simplex neotenics (15 of each sex) were weighed and photographed with a digital camera Olympus C-5060 (with constant exposition settings) mounted on a binocular loupe prior to their return to colonies. The photographs were converted to grey scale images, and the mean grey scale value from 10 pixels, randomly selected on the third abdominal tergite, was calculated.
(f). Source of polar compounds
To identify the source of the compounds from body washes, three living mature P. simplex neotenics of both sexes were gently rubbed 300 times on the tergal and sternal part of the abdomen with a small piece of cleaned cotton wool mounted on forceps and moistened with ultrapure water. Dry pieces of cotton wool were used to absorb the droplets appearing on the abdominal tip. The cotton wool pieces were inserted into tipped vials, washed with 15 µl of ultrapure water and further treated as the body washes. Two mature neotenic females were carefully dissected to localize the source of female-specific compounds in the following structures: rectum, ovaries, accessory glands and haemolymph. The organs cut into pieces and a drop of haemolymph were introduced into tipped vials, washed with 15 µl of ultrapure water and further treated as the body washes.
(g). MALDI-TOF analysis
Extracts were analysed using MALDI-TOF performed on Reflex IV (Bruker Daltonik GmbH) operated in a linear mode with the acceleration voltage of 20 kV and 200 ns extraction pulse. Desorption and ionization were achieved using a nitrogen UV laser (337.1 nm, 4 ns pulse of 300 µJ, maximum frequency 20 Hz) with laser power adjusted to 30–35%. Matrix ions were suppressed below m/z 3000. Data were collected from m/z 3800 to 25 000 and analysed with FlexAnalysis 3.0 (Bruker Daltonik GmbH). The mass spectra were externally calibrated using Protein Calibration Standard I (Bruker Daltonik). All spectra were averaged from 300 laser shots (10 × 30 shots) taken from at least four distinct places on a spot. For data analysis, background subtraction and smoothing were performed. SA was used as a matrix. A saturated solution of SA in acetone (1 µl) was applied to the target plate and the solvent was allowed to evaporate. Sample solution (1 µl) was applied on top of the first matrix crystal layer, followed by the deposition of the second layer of the matrix from 1 µl of saturated solution of SA in ACN: 0.1 per cent TFA in water, 1 : 1.
(h). Data analysis
In P. simplex, the peaks with prominent intensities were manually selected from the data for 38 individuals: young and mature neotenics (15 of both sexes), three soldiers, three pseudergates and two nymphs. The peak intensities were converted to relative percentages and evaluated with multivariate exploratory techniques (PCA) performed with Statistica 8.1. Body mass and coloration were included as independent variables.
3. Results
(a). Polar compounds in body washes of P. simplex
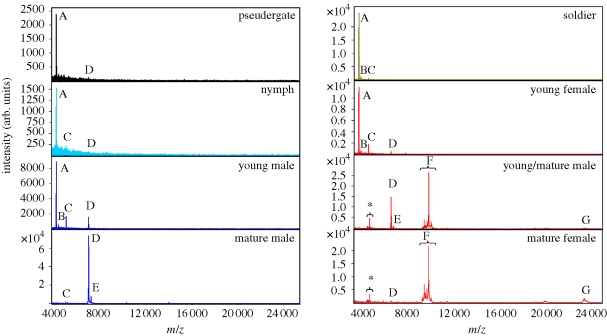
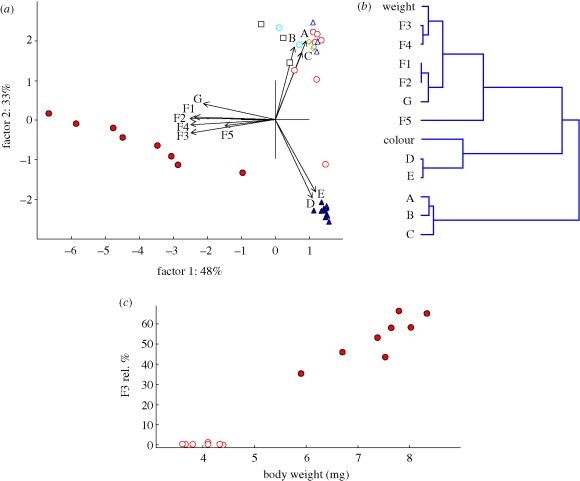
MALDI-TOF analysis revealed the presence of polar compounds of high molecular weight in all samples (figure 2). Prominent peaks were further studied with respect to caste- and sex specificity: A (m/z = 4168), B (m/z = 4376), C (m/z = 5035), D (m/z = 6994), E (m/z = 7200), F1–F5 = cluster of correlated peaks from m/z 9752 to 10 540 and G (m/z = 23 830). The diversity in relative intensities of these 11 peaks among individuals is depicted in figure 3a.
Figure 2.
Characteristic mass spectra of aqueous body washes of non-reproducing stages and neotenic reproductives from mature colonies of P. simplex. Lettering indicates following masses (m/z): A = 4168, B = 4376, C = 5035, D = 6994, E = 7200, F = cluster of five peaks from 9752 to 10 540, G = 23 830. Peaks marked with asterisks represent doubly charged ions of F. Note the peaks D + E characteristic for mature male and female neotenics and F + G specific to mature female neotenics.
Figure 3.
Intercaste differences in chemical profiles of polar compounds in body washes of reproductives and non-reproducing stages of P. simplex. (a) Factor scores were computed by PCA analysis based on relative percentages of 11 major peak intensities (A–G) from MALDI-TOF. Blue open circles, nymph; green open diamonds, soldier; open squares, pseudergate; open blue triangles, young male; filled blue triangles, mature male; open red circles, young female; filled red circles, mature female. (b) Cluster tree visualizing the correlations among relative proportions of 11 major peak intensities (A–G) and body weight and coloration in male and female neotenic reproductives (UPGMA method based on inverted correlation matrix 1 − R). (c) Correlation between body weight and the relative proportion of the major female-specific peak F3. Open circles, young female; filled circles, mature female.
The spectra in non-reproducing castes and stages, i.e. pseudergates, nymphs, soldiers and majority of young neotenics, were similar: one major (A) and three minor peaks (B–D) consistently occurred in all individuals. On the other hand, mature neotenics displayed a very different and sex-specific composition of polar compounds. Mature males formed a homogeneous group well separated by the presence of largely dominant peaks D + E. In mature females, a group of exclusively female peaks (F1–5 + G) occurred as a dominant component of the profile beside the peaks D + E. Mature females were therefore distributed along the changing ratio of peaks (D + E)/(F1–5 + G), from individuals with prominent peaks D + E to individuals with dominant peaks F1–5 + G (figures 2 and 3a).
In male neotenics, the polar compounds of high molecular weights allowed us to clearly distinguish between two homogeneous groups (young and mature) corresponding well with their body coloration and spatial distribution in the colony (figure 3b). The chemical profiles of female neotenics were more heterogenous, we therefore tested whether the observed gradual change is related to their age and fertility. Indeed, the relative proportion of female-specific peaks (F1–5 + G) proved to be clearly positively correlated with the body weight, reflecting the level of physogastry (figure 3b,c).
(b). Source of neotenic-specific polar compounds in P. simplex
The compounds corresponding to peaks D + E proved to be located on the cuticular surface of the abdomen in both males and females and absent in the liquid droplets collected on the tip of the abdomen. On the contrary, the peaks detected exclusively in body washes of mature females (F1–5, G) were found in high concentrations in the transparent fluid appearing on the tip of their abdomens, while only in traces or not at all in the cuticular brushes, gonads, accessory glands, rectum and haemolymph. The transparent liquid was regularly secreted by the neotenics a few seconds after manipulation with forceps; it is therefore highly probable that it was also secreted during the body washes, the female-specific compounds being subsequently detected in the samples.
(c). Polar compounds in body washes of K. flavicollis and R. santonensis
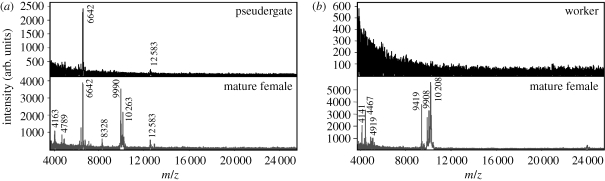
The comparative samples of K. flavicollis and R. santonensis also proved that the body washes of functional female neotenics differ in the polar compounds of high molecular weight from non-reproducing individuals (figure 4). Among other female-specific compounds, the cluster of peaks with m/z about 10 000 was also detected just as in females of P. simplex.
Figure 4.
Characteristic mass spectra of aqueous body washes of non-reproducing stages (pseudergates or workers) and female neotenic reproductives in (a) Kalotermes flavicollis and (b) R. santonensis.
4. Discussion
In the body washes of P. simplex, we have demonstrated the presence of polar compounds of high molecular weight specific to mature neotenic reproductives, and at the same time sex specific. The molecular weights and the detection methods used suggest that these compounds are of proteinaceous origin. Two of these compounds (D + E) were common to both sexes and were located on the cuticular surface, whereas mature females additionally secreted several female-specific compounds (F1–5 + G) in the transparent liquid from the anus. The proportion of these compounds is correlated with the reproductive status: D + E is elevated in dark mature males found within the chambers with eggs and larvae, while the ratio of female-specific compounds is tightly correlated with the body mass of females removed from the breeding chambers. These compounds are lacking in immature yellowish neotenics found on nest peripheries or surfaces. During the preliminary stages of the research, we observed this pattern of distribution of polar compounds in three other unrelated colonies of P. simplex. In addition, we confirmed the presence of reproductive-specific compounds in functional neotenic females in two other termite species: R. santonensis and K. flavicollis.
Despite the well-understood social context of neotenic formation in termites, little is known about the proximate mechanisms involved in (i) their recognition by nest-mates linked with the onset of appropriate behaviour, such as feeding and egg care (releaser function) and (ii) the inhibition of the development of (further) neotenics (primer function). These two functions may be ensured by a single signalling pathway or by multiple independent signals, and should also include the information about the sex of the neotenic. There is little doubt that this signalling is mediated chemically, though we still lack an unambiguous answer as to what these putative substances are, where they are synthesized and how they are spread across the colony.
The signalling of fertility is better understood in eusocial Hymenoptera. Its research is hugely dominated by the paradigm of CHCs as major recognition and fertility cues, with evidence for the specificity of CHC patterns linked with social and fertility status (Monnin 2006), documented proof of antennal perception of CHCs (D'Ettorre et al. 2004; Saïd et al. 2005) and a proposed mechanism of linkage between the dynamics in the titre of the juvenile hormone and changes in CHC composition (Trabalon et al. 1990; Sevala et al. 2000; Sledge et al. 2004; Lengyel et al. 2007). Therefore, it was rather surprising that studies postulating an analogous function of CHCs in termites appeared as late as 2009 (Liebig et al. 2009; Weil et al. 2009). The studies demonstrated that the neotenic queens in the kalotermitid Cryptotermes secundus and both sexes of fertile reproductives in the termopsid Zootermopsis nevadensis bear CHC profiles easily distinguishable from those of sterile castes. In Cryptotermes, the authors have drawn ambitious conclusions that, just as in some social Hymenoptera, the fertility cue in termites could consist in an increased abundance of methyl-branched and long-chained alkanes (Weil et al. 2009). In Zootermopsis, four reproductive-specific polyunsaturated alkenes were identified (Liebig et al. 2009).
Unfortunately, despite these promising results, both studies lack evidence for the sex specificity of the signal, which seems necessary for both releaser and primer function. Our previous analyses of CHCs in Prorhinotermes brought similar conclusions: we were able to distinguish between sterile castes and functional reproductives, but not between sexes of reproductives (Hanus et al. 2008). Moreover, a broader comparison of several colonies in time did not allow us to extract any consistent universal reproductive-specific factor, the chemical profiles being unstable in time as well as the pattern of intercaste differences (R. Hanus 2008, unpublished data).
On the other hand, our present results clearly demonstrate the fertility-related secretion of proteinaceous substances in P. simplex. The observed patterns comply with all prerequisites for a cue involved in fertility recognition and signalling: the selected compounds are tightly correlated with anatomic and behavioural symptoms of fertility, they are sex-specific, and universally present in time across several studied colonies. Also, the finding of similar patterns in females of two other termite species is in agreement with the presumed stability of fertility signalling across termite taxa. If we tentatively assign to these proteinaceous substances a role in fertility signalling, other observed characteristics also correspond to the putative pheromone as it was inferred from bioassays (e.g. Lüscher 1961). First, the detected amounts of reproductive-specific compounds are relatively high; they exceed by far (by more than one order) the total amount of polar compounds in non-reproducing individuals. This would agree, together with the low volatility of concerned compounds, with the presumed mode of distribution of the substances by grooming/anal trophallaxis from reproductives to numerous nest-mates (and subsequently from nest-mate to nest-mate). Second, the candidate compounds are secreted (i) in droplets from the anus in the case of female-specific compounds and (ii) on the cuticular surface probably from glandular cells of the epidermis (both males and females). Both sources were hypothesized (though not confirmed) in the past to be the possible sources of inhibitory substances (Lüscher 1974, 1955; Bordereau 1985). The presence of proteinaceous substances on the cuticle of neotenics is more expected than surprising: in our previous study, we reported a massive transformation of normal epidermal cells into glandular ones with partially proteinaceous secretion in neotenics of P. simplex (Šobotník et al. 2003).
Nevertheless, we can only speculate about the functional mechanism of the hypothetical proteinaceous recognition/inhibitory factors; they may act via ingestion or through the sensoric pathway, and they may be the stimuli themselves or rather pheromone modulators, carriers or releasers. Peptides and proteins are known to act as pheromones in water environments or as contact pheromones in terrestrial animals; reported organisms include bacteria, fungi, worms, copepods, molluscs and vertebrates, such as amphibians and rodents (Altstein 2004; Wyatt 2006; Touhara 2008). Reports of proteinaceous pheromones in insects are rare; they have been described, e.g. in Drosophila (Kubli 1992). But some evidence suggests that not enough is known about insect communication via peptides and proteins rather than it being absent, peptides and proteins having been detected on bodies of various species, such as locusts, honeybees, paper wasps and cockroaches (Zupko et al. 1993; Korchi et al. 1998; Cornette et al. 2002; Turillazzi et al. 2006a).
A few records of surface peptides/proteins in insects are particularly important in the context of this study. First, termite relatives, Leucophaea maderae cockroaches, are known to secrete epicuticular proteins that are supposed to act (i) as pheromone binding and activating agents (Korchi et al. 1998; Cornette et al. 2001, 2003) or (ii) even directly as contact stimuli (Cornette et al. 2002). The former hypothesis is explored in the recent study by Korb et al. (2009): by the suppression of a gene for β-glycosidase, queen-specific protein known also from the cockroach surface, the queen dominance in the group of C. secundus appeared to be suppressed, at least at the behavioural level. Second, in Polistes wasps, distinct patterns of surface peptides were found in females with different reproductive and social status (Dapporto et al. 2008). Moreover, previous studies revealed that epicuticular and venom peptides are perceived by Polistes dominulus and used in communication, which suggests that peptides could be, beside the CHCs, involved in fertility signalling (Turillazzi et al. 2006a,b). This case represents a perfect analogy with the situation observed in P. simplex. Given the molecular weights of neotenic-specific compounds (ranging from 7000 to 24 000), either or both mechanisms proposed above for cockroaches and paper wasps could hypothetically be involved in the signalling in P. simplex.
At the current state of our knowledge, we can only speculate about the function of the observed fertility-related chemical specificity in proteinaceous compounds; a direct proof for the reproductive inhibition is particularly difficult to obtain as it requires a long-term bioassay with numerous social and environmental factors to be controlled. Nevertheless, our results at least help to drive the imagination beyond the well-known field of cuticular lipids and routine analytical approaches to unexplored areas of insect chemical ecology and to propose alternative scenarios in the so far unsuccessful quest for the termite primer pheromone.
Acknowledgements
This research was supported by the Grant Agency of the Academy of Sciences of the Czech Republic (project no. A600550614) and by the Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Prague (project no. Z4 055 0506). We would like to thank Dr David Sillam-Dussès (IOCB, Prague) for critical reading of this manuscript and Nathan Fields for proofreading of the manuscript. We thank two anonymous reviewers for valuable comments on the manuscript.
References
- Altstein M.2004Peptide pheromones: an overview. Peptides 25, 1373–1376 (doi:10.1016/j.peptides.2004.07.002) [DOI] [PubMed] [Google Scholar]
- Austin J. W., Szalanski A. L., Scheffrahn R. H., Messenger M. T., Dronnet S., Bagnères G.2005Genetic evidence for the synonymy of two Reticulitermes species: Reticulitermes flavipes and Reticulitermes santonensis. Ann. Entomol. Soc. Am. 98, 395–401 (doi:10.1603/0013-8746(2005)098[0395:GEFTSO]2.0.CO;2) [Google Scholar]
- Bagnères A.-G., Rivière G., Clément L.1998Artificial neural network modeling of caste odor discrimination based on cuticular hydrocarbons in termites. Chemoecology 8, 201–209 (doi:10.1007/s000490050026) [Google Scholar]
- Bartz S. H.1979Evolution of eusociality in termites. Proc. Natl Acad. Sci. USA 76, 5764–5768 (doi:10.1073/pnas.76.11.5764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordereau C.1985The role of pheromones in termite caste differentiation. In Current themes in tropical science, vol. 3. Caste differentiation in social insects (eds Watson J. A. L., Okot-Kotber B. M., Noirot C.), pp. 221–226 Oxford, UK: Pergamon Press [Google Scholar]
- Brown W. V., Watson J. A. L., Lacey M. J., Morton S. R., Miller L. R.1996Composition of cuticular hydrocarbons in the Australian harvester termite Drepanotermes perniger (Isoptera: Termitidae): variation among individuals, castes, colonies and locations. Sociobiology 27, 181–197 [Google Scholar]
- Clément J.-L. A., Bagnères G.1998Nestmate recognition in termites. In Pheromone communication in social insects: ants, wasps, bees and termites (eds Van der Meer R. K., Breed M. D., Espelie K. E., Winston M. L.), pp. 126–155 Boulder, CO: Westview Press [Google Scholar]
- Cornette R., Farine J.-P., Quennedey B., Brossut R.2001Molecular characterization of a new adult male putative calycin specific to tergal aphrodisiac secretion in the cockroach Leucophaea maderae. FEBS Lett. 507, 313–317 (doi:10.1016/S0014-5793(01)02997-0) [DOI] [PubMed] [Google Scholar]
- Cornette R., Farine J.-P., Quennedey B., Riviere S., Brossut R.2002Molecular characterization of Lma-p54, a new epicuticular surface protein in the cockroach Leucophaea maderae (Dictyoptera, Oxyhaloinae). Insect Biochem. Mol. Biol. 32, 1635–1642 (doi:10.1016/S0965-1748(02)00103-0) [DOI] [PubMed] [Google Scholar]
- Cornette R., Farine J.-P., Abed-Viellard D., Quennedey B., Brossut R.2003Molecular characterization of a male-specific glycosyl hydrolase, Lma-p72, secreted on to the abdominal surface of the Madeira cockroach Leucophaea maderae (Blaberidae, Oxyhaloinae). Biochem. J. 372, 535–541 (doi:10.1042/BJ20030025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapporto L., Lambardi D., Turillazzi S.2008Not only cuticular lipids: first evidence of differences between foundresses and their daughters in polar substances in the paper wasp Polistes dominulus. J. Insect Physiol. 54, 89–95 (doi:10.1016/j.jinsphys.2007.08.005) [DOI] [PubMed] [Google Scholar]
- D'Ettorre P., Heinze J., Schulz C., Francke W., Ayasse M.2004Does she smell like a queen? Chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inverse. J. Exp. Biol. 207, 1085–1091 (doi:10.1242/jeb.00865) [DOI] [PubMed] [Google Scholar]
- Grassé P. P., Noirot C.1946La production des sexués néoteniques chez le termite à cou jaune (Calotermes flavicollis F.): inhibition germinale et inhibition somatique. C. R. Acad. Sci. 223, 869–871 [Google Scholar]
- Grassé P. P., Noirot C.1960L'isolement chez le termite à cou jaune (Calotermes flavicollis Fab.) et ses conséquences. Insectes Soc. 7, 323–331 (doi:10.1007/BF02225768) [Google Scholar]
- Greenberg S. L. W., Stuart A. M.1980Control of neotenic development in a primitive termite (Isoptera: Hodotermitidae). J. NY Entomol. Soc. 88, 49–50 [Google Scholar]
- Greenberg S. L. W., Stuart A. M.1982Precocious reproductive development (neoteny) by larvae of a primitive termite Zootermopsis angusticollis (Hagen). Insectes Soc. 29, 535–547 (doi:10.1007/BF02224223) [Google Scholar]
- Hanus R., Piskorski R., Šobotník J., Urbanová K., Valterová I.2008Epicuticular lipids as caste-recognition cues in the termite Prorhinotermes simplex (Isoptera: Rhinotermitidae). ICE2008 (XXIII Int. Congress of Entomology 2008), Durban, South Africa, 6–12 July 2008. Abstract on CD, no. 1910 [Google Scholar]
- Haverty M. I., Page M., Nelson L. J., Blomquist G. J.1988Cuticular hydrocarbons of dampwood termites, Zootermopsis: intra- and intercolony variation and potential as taxonomic characters. J. Chem. Ecol. 14, 1035–1058 (doi:10.1007/BF01018791) [DOI] [PubMed] [Google Scholar]
- Haverty M. I., Grace J. K., Nelson L. J., Yamamoto R. T.1996Intercaste, intercolony, and temporal variation in cuticular hydrocarbons of Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). J. Chem. Ecol. 22, 1813–1834 (doi:10.1007/BF02028506) [DOI] [PubMed] [Google Scholar]
- Howard R. W., Blomquist G. J.2005Ecological, behavioral and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393 (doi:10.1146/annurev.ento.50.071803.130359) [DOI] [PubMed] [Google Scholar]
- Howard R. W., McDaniel C. A., Nelson D. R., Blomquist G. J., Gelbaum L. T., Zalkow L. H.1982Cuticular hydrocarbons of Reticulitermes virginicus (Banks) and their role as potential species- and caste-recognition cues. J. Chem. Ecol. 8, 1227–1239 (doi:10.1007/BF00990755) [DOI] [PubMed] [Google Scholar]
- Karlson P., Lüscher M.1959‘Pheromones’: a new term for a class of biologically active substances. Nature 183, 55–56 (doi:10.1038/183055a0) [DOI] [PubMed] [Google Scholar]
- Keller L., Nonacs P.1993The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 45, 787–794 (doi:10.1006/anbe.1993.1092) [Google Scholar]
- Korb J., Weil T., Hoffmann T., Foster K. R., Rehli M.2009A gene necessary for reproductive suppression in termites. Science 324, 758 (doi:10.1126/science.1170660) [DOI] [PubMed] [Google Scholar]
- Korchi A., Farine J.-P., Brossut R.1998Characterization of two male-specific polypeptides in the tergal glands secretions of the cockroach Leucophaea maderae (Dictyoptera, Blaberidae). Insect Biochem. Mol. Biol. 28, 113–120 (doi:10.1016/S0965-1748(97)00104-5) [DOI] [PubMed] [Google Scholar]
- Kubli E.1992The sex-peptide. Bioessays 14, 779–784 (doi:10.1002/bies.950141111) [DOI] [PubMed] [Google Scholar]
- Lengyel F., Westerlund S. A., Kaib M.2007Juvenile hormone III influences task-specific cuticular hydrocarbon profile changes in the ant Myrmicaria eumenoides. J. Chem. Ecol. 33, 167–181 (doi:10.1007/s10886-006-9185-x) [DOI] [PubMed] [Google Scholar]
- Lenz M.1985Is inter- and intraspecific variability of lower termite neotenic numbers due to adaptive thresholds for neotenic elimination?—considerations from studies on Porotermes adamsoni (Froggatt) (Isoptera: Termopsidae). In Current themes in tropical science, vol. 3. Caste differentiation in social insects (eds Watson J. A. L., Okot-Kotber B. M., Noirot C.), pp. 125–145 Oxford, UK: Pergamon Press [Google Scholar]
- Liebig J., Eliyahu D., Brent C. S.2009Cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav. Ecol. Sociobiol. 63, 1799–1807 (doi:10.1007/s00265-009-0807-5) [Google Scholar]
- Light S. F.1944Experimental studies on ectohormonal control of the development of supplementary reproductives in the termite genus Zootermopsis (formerly Termopsis). Univ. Calif. Publ. Zool. 53, 1–40 [Google Scholar]
- Lüscher M.1949Continuous observation of termites in laboratory cultures. Acta Trop. 6, 161–165 [PubMed] [Google Scholar]
- Lüscher M.1952Die Production und Elimination von Ersatzgeschlechtstieren bei der Termite Kalotermes flavicollis Fabr. Z. Vgl. Physiol. 34, 123–141 [Google Scholar]
- Lüscher M.1955Zur Frage der Übertragung socialer Wirkstoffe bei Termiten. Naturwissenschaften 42, 186 (doi:10.1007/BF00595320) [Google Scholar]
- Lüscher M.1956Hemmende und fördernde Faktoren bei der Entstehung der Ersatzgeschlechtstiere bei der Termite Kalotermes flavicollis Fabr. Rev. Suisse Zool. 63, 261–267 [Google Scholar]
- Lüscher M.1961Social control of polymorfism in termites. In Insect polymorfism (ed. Kennedy J. S.), pp. 57–67 London, UK: Royal Entomological Society of London [Google Scholar]
- Lüscher M.1972Environmental control of juvenile hormone (JH) secretion and caste differentiation in termites. Gen. Comp. Endocrinol. 3, 509–514 (doi:10.1016/0016-6480(72)90181-5) [Google Scholar]
- Lüscher M.1974Kasten und Kastendifferenzierung bei niederen Termiten. In Sozialpolymorphismus bei Insekten (ed. Schmidt G. H.), pp. 694–739 Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft [Google Scholar]
- Monnin T.2006Chemical recognition of reproductive status in social insects. Ann. Zool. Fennici 43, 515–530 [Google Scholar]
- Myles T. G.1986Reproductive soldiers in the Termopsidae (Isoptera). Pan-Pac. Entomol. 62, 293–299 [Google Scholar]
- Myles T. G.1988Resource inheritance in social evolution from termites to man. In The ecology of social behavior (ed. Slobodchikoff C. N.), pp. 379–423 New York, NY: Academic Press Inc [Google Scholar]
- Myles T. G.1999Review of secondary reproduction in termites (Insecta: Isoptera) with comments on its role in termite ecology and social evolution. Sociobiology 33, 1–91 [Google Scholar]
- Myles T. G., Chang F.1984The caste system and caste mechanisms of Neotermes connexus (Isoptera: Kalotermitidae). Sociobiology 9, 1–321 [Google Scholar]
- Nagin R.1972Caste determination in Neotermes jouteli (Banks). Insectes Soc. 19, 39–61 (doi:10.1007/BF02223214) [Google Scholar]
- Pickens A. L.1932Observation on the genus Reticulitermes Holmgren. Pan-Pac. Entomol. 8, 178–180 [Google Scholar]
- Roisin Y.1999Philopatric reproduction, a prime mover in the evolution of termite sociality? Insectes Soc. 46, 297–305 (doi:10.1007/s000400050149) [Google Scholar]
- Ruppli E.1969Die Elimination uberzahliger Ersatzgeschlechtstiere bei der Termite Kalotermes flavicollis (Fabr.). Insectes Soc. 16, 235–248 (doi:10.1007/BF02223411) [Google Scholar]
- Saïd I., Gaertner C., Renou M., Rivault C.2005Perception of cuticular hydrocarbons by the olfactory organs in Periplaneta americana (L.) (Insecta: Dictyoptera). J. Insect Physiol. 51, 1384–1389 (doi:10.1016/j.jinsphys.2005.09.001) [DOI] [PubMed] [Google Scholar]
- Sevala V. L., Bagnères A.-G., Kuenzli M., Blomquist G. J., Schal C.2000Cuticular hydrocarbons of the dampwood termite, Zootermopsis nevadensis: caste differences and role of lipophorin in transport of hydrocarbons and hydrocarbon metabolites. J. Chem. Ecol. 26, 765–789 (doi:10.1023/A:1005440624678) [Google Scholar]
- Sledge M. F., Trinca I., Massolo A., Boscaro F., Turillazzi S.2004Variation in cuticular hydrocarbon signatures, hormonal correlates and establishment of reproductive dominance in a polistine wasp. J. Insect Physiol. 50, 73–83 (doi:10.1016/j.jinsphys.2003.10.001) [DOI] [PubMed] [Google Scholar]
- Šobotník J., Weyda F., Hanus R.2003Ultrastructure of epidermal glands in neotenic reproductives of the termite Prorhinotermes simplex (Isoptera: Rhinotermitidae). Arthropod Struct. Dev. 32, 201–208 (doi:10.1016/S1467-8039(03)00051-3) [DOI] [PubMed] [Google Scholar]
- Stuart A. M.1979The determination and regulation of the neotenic reproductive caste in the lower termites (Isoptera): with special reference to the genus Zootermopsis (Hagen). Sociobiology 4, 223–237 [Google Scholar]
- Thorne B. L.1997Evolution of eusociality in termites. Annu. Rev. Ecol. Syst. 28, 27–54 (doi:10.1146/annurev.ecolsys.28.1.27) [Google Scholar]
- Thorne B. L., Breisch N. L., Muscedere M. L.2003Evolution of eusociality and the soldier caste in termites: influence of intraspecific competition and accelerated inheritance. Proc. Natl Acad. Sci. USA 100, 12 808–12 813 (doi:10.1073/pnas.2133530100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touhara K.2008Sexual communication via peptide and protein pheromones. Curr. Opin. Pharmacol. 8, 759–764 (doi:10.1016/j.coph.2008.09.001) [DOI] [PubMed] [Google Scholar]
- Trabalon M., Campan M., Porcheron P., Clément J. L., Baehr J. C., Moriniere M., Joulie C.1990Relationships among hormonal changes, cuticular hydrocarbons, and attractiveness during the first gonadotropic cycle of the female Calliphora vomitoria (Diptera). Gen. Comp. Endocrinol. 80, 216–222 (doi:10.1016/0016-6480(90)90166-J) [DOI] [PubMed] [Google Scholar]
- Turillazzi S., et al. 2006aDominulin A and B: two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/ TOF, and ESI-Ion Trap. J. Am. Soc. Mass. Spectrom. 17, 376–383 (doi:10.1016/j.jasms.2005.11.017) [DOI] [PubMed] [Google Scholar]
- Turillazzi S., Dapporto L., Pansolli C., Boulay R., Dani F. R., Moneti G., Pieraccini G.2006bHabitually used hibernation sites of paper wasps are marked with venom and cuticular peptides. Curr. Biol. 16, R530–R531 (doi:10.1016/j.cub.2006.06.050) [DOI] [PubMed] [Google Scholar]
- Watson J. A. L., Brown W. V., Miller L. R., Carter F. L., Lacey M. J.1989Taxonomy of Heterotermes (Isoptera: Rhinotermitidae) in south-eastern Australia: cuticular hydrocarbons of workers, and soldier and alate morphology. Syst. Entomol. 14, 299–325 (doi:10.1111/j.1365-3113.1989.tb00287.x) [Google Scholar]
- Weil T., Hoffmann K., Kroiss J., Strohm E., Korb J.2009Scent of a queen-cuticular hydrocarbons specific for female reproductives in lower termites. Naturwissenschaften 96, 315–319 (doi:10.1007/s00114-008-0475-8) [DOI] [PubMed] [Google Scholar]
- Wyatt T. D.2006Pheromones: convergence and contrasts in insects and vertebrates. In Chemical signals in vertebrates, vol. 10 (eds Mason R. T., LeMaster M. P., Müller-Schwartze D.), pp. 7–19 New York, NY: Springer; (doi:10.1007/0-387-25160-X_2) [Google Scholar]
- Zupko K., Sklan D., Lensky Y.1993Proteins of the honeybee (Apis mellifera L.) body surface and exocrine gland secretions. J. Insect Physiol. 39, 41–46 (doi:10.1016/0022-1910(93)90016-K) [Google Scholar]