Abstract
Current knowledge suggests that patterns of energy storage and depletion in animals are governed by behavioural trade-offs between risks associated with feeding and future energy demands. However, the length of adverse periods varies over geographical or climatic gradients. To explore the potential for genotypic sources of variation in behavioural trade-offs, we compared the winter energy-depletion patterns among 13 wild populations of juvenile Atlantic salmon (Salmo salar L.) along a latitudinal gradient (58–70°N) and performed common-environment experiments of energy-state-dependent feeding. In the wild, winter lipid-depletion rates were lower for northern than for southern populations. The variation in spring lipid levels among the population was lower than autumn variation, with storage lipid levels clustered close to critical limits for survival. In semi-natural stream channels with natural food supply, hatchery-reared fish originating from northern populations showed a positive scaling of feeding activity with decreasing energy levels, whereas southern populations did not. In conclusion, juvenile Atlantic salmon from northern populations defend their energy levels more strongly than fish from southern populations. Adaptive variation in feeding activity appears important for this difference. Thus, the present study shows a link between geographical patterns in storage energy trajectories and adaptive differences in state-dependent feeding motivation.
Keywords: energy storage, overwintering strategies, trade-off, lipid, starvation, behaviour
1. Introduction
State-dependent modification of risk-taking behaviour is well documented among a range of animal taxa (Bull et al. 1996; Lima 1998; Noren & Mangel 2004). A classic example is the effect of the level of storage lipids on feeding under predation risk (McNamara et al. 2005; Brodin 2007). Animals typically draw on reserves in the form of stored lipids in adverse periods when energy intake does not match the demand or when feeding is risky. As acquiring and catabolizing lipid reserves imposes costs, energy reserves are usually maintained below maximum levels (Witter & Cuthill 1993; Brodin 2007; Macleod et al. 2008). Several vertebrate groups are known to tailor their levels of storage lipids according to short- and long-term energy demands and to regulate their energy intake based on a projected energy state at the expected end of the adverse period (Bull et al. 1996; Polo et al. 2007). Both theoretical and empirical works (Bull et al. 1996; Polo et al. 2007) suggest that this state-dependent regulation of feeding motivation is an adaptive trait where the animal optimizes future survival. However, for many species with a wide geographical distribution, different populations will experience very different environmental and seasonal conditions.
There is both theoretical and empirical support for phenotypic plasticity to shape temporal trajectories of storage energy metabolism, tuning the optimal trade-off strategy according to ambient environmental conditions using external cues (e.g. photoperiod; McNamara & Houston 1990; Macleod et al. 2005; Brodin 2007; Polo et al. 2007). Cyclic daily patterns of fattening and starvation are found in small birds during winter, and photoperiodic responses suggest fine-scale adjustments of energy reserves (Polo et al. 2007). However, there is also experimental evidence for between-population differences in winter energy storage patterns (Schultz & Conover 1997), indicating adaptive differences between populations. Northern populations of Atlantic silverside (Menidia menidia), a small estuarine fish, have trajectories for energy accumulation and depletion that are different from those of southern populations, when kept under common environmental conditions (Schultz & Conover 1997). This suggests that local genetic adaptations may be a mechanism by which populations inhabiting different environments adjust storage energy trade-offs, possibly involving adaptive divergence in behavioural traits (Foster 1999). Although the selective pressure underpinning energy-state-dependent risk-taking has received considerable attention (Lima 1998; Biro et al. 2005, 2007), there is, to our knowledge, no study showing adaptive divergences and geographical patterns in the behavioural traits influencing winter storage energy trajectories.
In the present study, we map the geographical variation in winter storage energy trajectories of juvenile Atlantic salmon (Salmo salar L.) from wild populations and use common-environment experiments to test whether adaptive geographical patterns in feeding motivation may explain the variation. Atlantic salmon is a teleost fish that spawns in rivers or freshwater streams, where juveniles typically live from one to five years before migrating to sea (Klemetsen et al. 2003). After one to three years at sea, they usually return to their natal river to spawn. The natural distribution spans a broad environmental gradient from the Mediterranean climate regions of Spain to the subarctic northern Norway and Russia (MacCrimmon & Gots 1979). Thus, both the length and the hostility of the winter season vary considerably among populations. Juvenile Atlantic salmon undertake periods of reduced food intake and declines of energy reserves during wintertime (Gardiner & Geddes 1980; Metcalfe & Thorpe 1992; Berg & Bremset 1998). During this period, they may become periodically anorexic even though food is plentiful (Metcalfe et al. 1986; Metcalfe & Thorpe 1992). It has been suggested that increased predation risk at low temperatures and, thus, reduced scope for activity may be important for the seasonal pattern of feeding and energy intake (Metcalfe & Thorpe 1992). In accordance, experiments by Bull et al. (1996) demonstrated that the regulation of appetite depended on projected energy state at the end of the winter. This suggests an adaptive regulation of energy intake to match long-term requirements. Given that the fish project energy intake in order to reach a specific target at the end of the adverse season, it can be predicted that local adaptations should lead to a more conservative energy-depletion strategy with increasing latitude.
Using a combination of field data and common-environment experiments, we show that northern populations of juvenile Atlantic salmon have slower field energy-depletion rates than southern and amplify feeding behaviour comparably more at low energy levels when held in common-environment experiments. To our knowledge, this is the first demonstration of a link between geographical patterns in storage energy trajectories and adaptive differences in state-dependent feeding motivation.
2. Material and methods
(a). Lipid-depletion rates in the wild
We tested for a latitudinal gradient in field winter lipid-depletion rates of juvenile Atlantic salmon by measuring mean lipid content of fish from 13 rivers located from 58–70°N (figure 1 and table 1) in autumn and in the subsequent spring (see table 1 for sampling dates and sample sizes). Autumn samples were collected as close as possible to the onset of winter and spring samples as close as possible to the end of winter (between ice-cover removal and spring flooding). Sampling was performed with a 12 V backpack electroshocker, covering a minimum of a 300 m river stretch and a range of habitat conditions within each river. To avoid fish that prepare for migration to the sea the following spring, and thus potentially have a different lipid storage pattern (Thorpe et al. 1998), only fish smaller than 110 mm were included in the analyses.
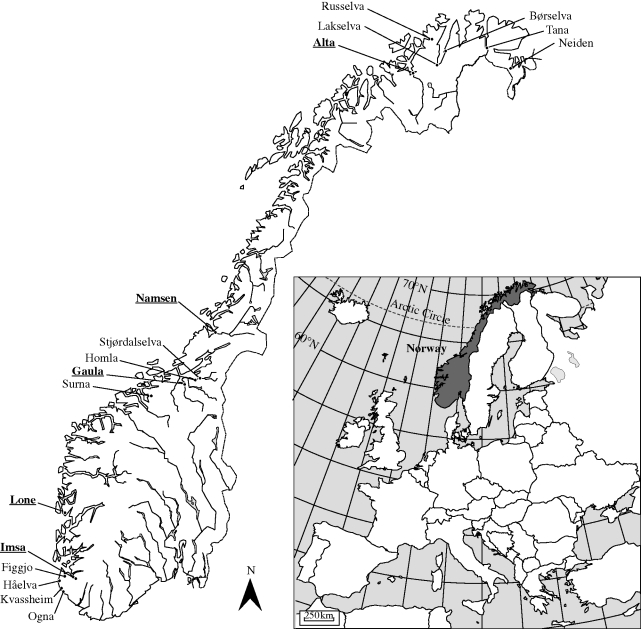
Figure 1.
Position of the rivers where fish were sampled for autumn and spring lipid levels, and the river of origin of populations included in the common-environment study (bold underline). The river Gaula population is included in both studies.
Table 1.
Latitude of river mouth (°N), sampling dates, number of fish (n) and mean fresh mass with standard deviation (mass ± s.d.) for rivers included in the latitudinal gradient of winter lipid-depletion rates. Also given are estimated intercept (β0), slope (β1) and r2 from least-square regressions between lipid mass and lipid-free dry mass (loge lipid mass = β0 + β1 loge lipid-free mass) used to size-standardize lipid content to a common mean lipid-free dry mass of 0.96 (4.04 g fresh mass). All p-values < 0.002.
| river | °N | sampling dates | n | mass ± s.d. | β0 | β1 | r2 |
|---|---|---|---|---|---|---|---|
| Børselva | 70.2 | 05/09/2007 | 55 | 3.58 (2.51) | −2.68 | 0.71 | 0.61 |
| 07/05/2008 | 56 | 4.60 (2.79) | −2.40 | 0.86 | 0.81 | ||
| Lakselva | 70.5 | 06/09/2007 | 41 | 4.55 (3.55) | −1.83 | 1.30 | 0.96 |
| 06/05/2008 | 37 | 4.67 (2.44) | −2.55 | 1.06 | 0.86 | ||
| Russelva | 70.4 | 05/09/2007 | 33 | 4.10 (2.62) | −1.70 | 0.82 | 0.86 |
| 09/05/2008 | 40 | 7.31 (2.92) | −2.53 | 1.26 | 0.69 | ||
| Neiden | 69.4 | 12/09/2007 | 33 | 4.35 (3.79) | −2.70 | 1.14 | 0.93 |
| 04/05/2008 | 45 | 4.89 (2.86) | −3.04 | 1.29 | 0.89 | ||
| Tana | 70.1 | 13/09/2007 | 48 | 3.13 (2.61) | −2.18 | 0.97 | 0.91 |
| 03/05/2008 | 47 | 3.18 (2.56) | −2.98 | 0.59 | 0.60 | ||
| Homla | 63.2 | 03/10/2007 | 50 | 2.94 (2.67) | −2.79 | 1.42 | 0.87 |
| 17/04/2008 | 50 | 3.35 (2.35) | −2.82 | 1.20 | 0.87 | ||
| Stjørdalselva | 63.3 | 29/09/2007 | 46 | 3.64 (2.67) | −2.19 | 1.04 | 0.84 |
| 21/03/2008 | 43 | 3.30 (2.74) | −2.77 | 0.89 | 0.88 | ||
| Gaula | 63.2 | 10/10/2007 | 56 | 3.05 (2.48) | −2.84 | 1.05 | 0.83 |
| 22/05/2008 | 53 | 3.53 (2.77) | −2.75 | 0.96 | 0.97 | ||
| Surna | 63.0 | 13/10/2007 | 82 | 5.00 (2.71) | −2.44 | 0.87 | 0.69 |
| 29/03/2008 | 73 | 3.60 (2.44) | −3.27 | 0.70 | 0.44 | ||
| Figgjo | 58.5 | 20/09/2007 | 30 | 4.70 (3.98) | −1.92 | 1.29 | 0.98 |
| 26/03/2008 | 30 | 3.70 (2.88) | −3.28 | 1.25 | 0.80 | ||
| Håelva | 58.4 | 19/09/2007 | 32 | 4.20 (2.88) | −2.68 | 1.53 | 0.96 |
| 26/03/2008 | 26 | 6.21 (2.97) | −2.92 | 1.09 | 0.59 | ||
| Kvassheim | 58.4 | 19/09/2007 | 29 | 3.76 (2.48) | −2.36 | 1.41 | 0.89 |
| 26/03/2008 | 28 | 4.35 (1.84) | −2.70 | 1.08 | 0.82 | ||
| Ogna | 58.3 | 19/09/2007 | 38 | 4.07 (3.64) | −2.63 | 1.17 | 0.88 |
| 26/03/2008 | 29 | 4.95 (3.03) | −3.38 | 0.81 | 0.34 |
Each fish was killed by a quick blow to the head or by an overdose of anaesthetic, and fresh mass was determined in the field (precision: ±0.005 g). In laboratory, the fish were dried (55°C for 5–30 days) until a stable dry mass was obtained, and lipid content was determined gravimetrically by extraction (Berg et al. 2009). This method determined reversible (non-polar lipids) energy stores (Dobush et al. 1985; Naesje et al. 2006).
Lipid storage capacity is allometrically related to body size (Post & Parkinson 2001). We used regressions between log-transformed lipid mass and lipid-free dry mass (proxy for structural mass) to standardize lipid content to mean body size among populations (see table 1 for details). Standardized values of lipid storage in autumn and spring samples from each population, expressed as mass-specific values (g lipid g lipid-free dry mass−1), were used to calculate mean-population-specific lipid-depletion rates (g g−1 d−1). Lipid-depletion rates are given as mass-specific autumn lipid values minus mass-specific spring lipid values, divided by the number of days between sampling.
The slopes of the lipid storage to body mass allometry were not homogeneous among populations (table 1), and the chosen common mass for size standardization may thus influence the analyses. We accordingly tested the robustness of the approach by repeating the analysis varying the standardization mass within the observed range (0.57–2.0 g lipid-free dry mass) across populations and sampling periods.
We tested for latitudinal effects on size-adjusted lipid energy and lipid-depletion rates by linear regression. If the fish adjusts its energy reserves according to a projected energy state at the end of the winter, populations with higher initial levels of storage energy should show higher depletion rates. We tested for this by entering autumn lipid levels before latitude in a linear model and tested the relationships with sequential sum of squares. Mean population mass (autumn lipid-free dry mass) was first entered as a covariate in the model, but removed after it was proven insignificant (F1,9 = 0.49, p = 0.501).
Observations of latitudinal patterns of lipid-depletion rates may be confounded by differences in energy-selective mortality (e.g. longer and more severe winters causing higher energy-selective mortality in the north). If so, spring distributions of lipid storage may be truncated at low levels and misinterpreted as slower depletion rates. We addressed this potential bias along two lines. First, we empirically tested if the truncation (measured as skewness; Sokal & Rohlf 1995) changes systematically with latitude during winter. Thereafter, we conducted a simulation study to test if the observed patterns in lipid loss rates theoretically could be caused by equal loss rates, but difference in lipid-selective mortality, across latitudes. Observed lipid-depletion rates from the field study were compared with rates from a simulated dataset, where lipid loss rates were held equal among populations, but where energy-selective mortality increased with increasing length of the winter season. The procedure was repeated 10 000 times using lipid-depletion rates sampled at random from a uniform distribution with maximum and minimum from the observed data (−0.0002 and 0.0006 g−1 d−1). For each simulation, 10 000 individuals were sampled with replacement from the population-specific distributions of lipid- and fat-free lipid mass. Distributions of spring storage lipids were then constructed by keeping depletion rates similar for all populations, but varying winter length according to time between samples in the field data, and retaining individuals that had not depleted storage lipids (corresponding to empirically demonstrate critical limit for survival; Finstad et al. 2004). A sample (n = spring samples of original data) was then drawn at random from this distribution and used together with observed autumn lipid- and fat-free dry-mass data to calculate inferred lipid-depletion rates resulting from energy-selective mortality alone.
(b). Feeding activity in stream channels
To test for adaptive patterns in energy depletion among populations, we compared the relationship between levels of storage energy and feeding activity in common-environment experiments. The fish used were first-generation hatchery-reared one-summer-old (0+) juvenile Atlantic salmon originating from five rivers spanning a latitudinal gradient from 59 to 70°N (table 2). The experiments were conducted at the NINA Research Station, Ims, in south-western Norway (59°N, 6°E) from January to March in 2003, 2004 and 2005. The set-up is described in Finstad & Forseth (2006), presenting results on the energy loss rates of the different populations resulting from ice-cover manipulations. Here, we focus on how lipid storage levels at the end of the experiment influenced observed feeding activity.
Table 2.
River of origin for the populations included in the common-environment experiment with latitude of river mouth, mean initial fresh mass (±s.d.) of the fish, number of replicates, duration (days) and year of experiments.
| population | latitude (°N) | fresh mass (g) | replicates | duration | year |
|---|---|---|---|---|---|
| Imsa | 59.0 | 23.5 (3.4) | 6 | 54 | 2004 |
| Lone | 60.0 | 6.7 (2.5) | 6 | 61 | 2005 |
| Gaula | 63.2 | 7.6 (2.7) | 6 | 61 | 2005 |
| Namsen | 64.5 | 13.2 (5.8) | 6 | 61 | 2005 |
| Alta | 70.0 | 17.2 (4.8) | 10 | 31 | 2003 |
The experimental set-up consisted of nine rectangular stream channels with gravel substrate (485 × 50 cm, water depth 30 cm, discharge 50 l min−1) blocked by a screen to prevent escapes. The channels were divided into two equal-sized compartments in the longitudinal direction using wooden planks. One compartment of the stream channel was covered with black light-impermeable plastic to simulate ice cover (Finstad & Forseth 2006), the other covered with clear plastic to prevent drift of terrestrial food items into the system while allowing natural daylight.
Mean temperature was 2.8°C during the experimental periods and day lengths increased from approximately 6 h at the start of the experiment to approximately 9 h at the end. Water was run in the channels for 10 weeks prior to the experiments to permit colonization of an invertebrate fauna. Drifting invertebrates were also naturally present in the supply water, which was drained from a nearby lake. At the beginning of experiments, 10 individually marked (Alcian blue in fins and adipose fin clipping) and weighed (precision: 0.01 g) fish were released into each replicate. In total, 18 of 340 fish died during the study. At the end of the study periods, the channels were drained and the fish were collected, killed and weighed, and presence of stomach content was registered. Dry-mass proportion (g dry mass g fresh mass−1 100) correlates well with storage lipid levels in hatchery-reared salmonids (r2 = 0.98; Finstad & Forseth 2006) and was used as a proxy for storage lipid levels.
The low ambient temperatures in the present experiments give very slow gastric evacuation rates, and presence of stomach content at termination of the experiment was therefore considered a good proxy for feeding activity (feeding frequency) the last few days before termination (Finstad 2005). We took a conservative approach, and rather than using individual data, we used the frequency of fish observed with stomach content in each stream channel as the proxy for feeding activity and mean dry-mass proportion (dryprop) in each channel as proxy for storage lipids. We tested for population differences in the feeding-frequency to dryprop relationship by entering mean mass, ice-cover treatment, dryprop, latitude of population and finally the dryprop to latitude interaction into a linear model with feeding frequency as dependent variable (table 3). The effect of the different explanatory variables was tested by sequential sum of squares where the variables were entered into the model in the order listed above.
Table 3.
ANOVA table for the effect of fresh mass, ice-cover treatment, latitude of population origin and dry-weight proportion (dryprop), as well as the latitude to dryprop interaction on frequency of feeding in stream channel experiments.
| d.f. | mean sq. | F values | p | |
|---|---|---|---|---|
| mass | 1 | 0.25 | 8.87 | 0.005 |
| ice cover | 1 | 0.01 | 0.26 | 0.609 |
| latitude | 1 | 0.05 | 1.71 | 0.202 |
| dryprop | 1 | 0.01 | 0.30 | 0.583 |
| latitude × dryprop | 1 | 0.14 | 5.35 | 0.028 |
| residuals | 28 | 0.03 |
3. Results
(a). Lipid-depletion rates in the wild
Mean levels of lipid storage energy depleted during the winter (figure 2a). In the autumn samples, there was large variation in mean lipid levels among populations (mean 0.10 ± 0.04 s.d.; range 0.06–0.21 g), but no significant relationship between population latitude and lipid storage level (F1,11 = 0.62, p = 0.447). Among populations, variation in mean lipid levels in spring samples was lower than in autumn samples (mean 0.06 ± 0.02 s.d.; range 0.04–0.09 g). In the spring samples, levels of stored lipid increased with latitude (F1,11 = 4.36, p = 0.068). Varying the range of mass used for size standardization did not change the relationship between spring lipid storage levels and latitude (all F1,11 > 3.87, all p < 0.076; figure 2a).
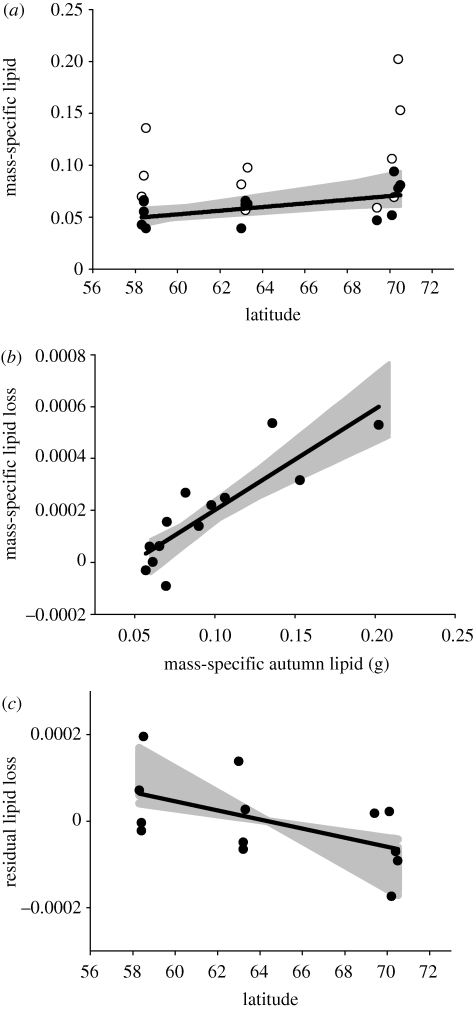
Figure 2.
(a) Mean mass-standardized-specific autumn (white circles) and spring (black circles) lipid levels for wild populations of juvenile Atlantic salmon along a latitudinal gradient in Norway (g lipid g lipid-free dry mass−1), p = 0.068. (b) Mean mass-standardized-specific lipid loss from autumn to spring (g lipid g lipid-free dry mass−1 d−1) plotted against mass-standardized-specific autumn lipid content, p = 0.001. (c) Residual lipid loss from the relationship in (b) plotted against latitude of the sampled population, p = 0.053. Solid lines are least-squares regression lines from the plotted values (standardized to grand mean across populations; 0.96 g), p = 0.053. p-values are significance levels for the least-square regression lines shown. Grey shaded areas are outcome space for regression lines when varying the common lipid-free dry mass used for size standardization across the observed among-population range (0.57–2.0 g).
Mean lipid-depletion rates (measured as average mass-specific daily loss rate) varied between −0.0002 and 0.0006 g d−1. There was no latitudinal trend in the rates of energy depletion per se (F1,11 < 0.15, p = 0.905). However, rates of energy depletion increased significantly with increasing levels of autumn storage energy (figure 2b). Lipid-depletion rates corrected for autumn lipid levels (residuals) scaled negatively with latitude (autumn lipid: F1,10 = 46.46, p < 0.001; latitude: F1,10 = 5.25, p = 0.045; figure 2c). Accordingly, populations with high initial levels of storage lipids had higher loss rates than populations with low levels, but northern populations were comparably more conservative with storage lipids at a given level of initial autumn energy. Varying the chosen mass for size standardization did not affect these results (figure 2b,c). Autumn lipid level significantly affected lipid-depletion rates throughout the mass range (all F1,10 > 52.54, all p < 0.001) and latitude significantly affected lipid-depletion rates (all F1,10 > 4.96, all p < 0.049) for all but the smallest observed masses (<0.69 g fat-free dry weight), where the relationship was marginally insignificant (all F1,10 > 4.64, all p < 0.056).
The observed latitudinal trend in lipid-depletion rates was not accounted for by energy-selective mortality alone. There was no latitudinal trend in the change in skewness of lipid storage distributions either before (latitude: F1,11 = 1.76, p = 0.21) or after correcting for autumn lipid values (autumn lipid: F1,10 = 1.16, p = 0.31; latitude: F1,10 = 1.02, p = 0.34). Moreover, results from the simulation model, assuming constant lipid-depletion rates, were inconsistent with results from field observations. Simulated slope coefficients for relationships between lipid loss and either autumn lipid content or latitude (after correcting for autumn lipid content) were different from observed relationships (figure 2b,c). Simulated slopes were more negative (autumn lipid content to loss rate) or more positive (latitude to loss rate, after correcting for autumn lipid content) than observed slopes in more than 99.9 per cent of the simulation runs. When re-running the analyses of relationships between latitude and loss rate in the simulated samples, there was also a low probability (<0.001) of observing a significant negative relationship between lipid loss and latitude (after correcting for autumn lipid content).
(b). Feeding activity in stream channels
All juvenile Atlantic salmon in the semi-natural stream channels experienced net mass losses during the experimental period, and mean-specific growth rates within each stream channel ranged from −0.005 to −0.001 g g−1 d−1. There was no detectable effect of mass at the start or the end of the experiment on energy status (all Pearson r < 0.23, d.f. = 32, all p > 0.178). Also, there was no detectable effect of simulated ice-cover treatment or interaction between ice cover and latitude of population origin on feeding frequency (ice cover: F1,30 = 0.01, p = 0.916; latitude: F1,30 = 7.48, p = 0.010; ice cover × latitude: F1,30 = 0.44, p = 0.509).
The proportion of fish with stomach content in each stream channel (proxy for feeding activity) varied between 0 and 0.8. Overall, feeding activity was affected by mean mass, but not by latitude or dry-mass proportion after correcting for mass differences (table 3). However, there was a significant interaction between latitudinal origin and energy status. The interaction resulted from a positive scaling of feeding activity with decreasing energy levels of northern populations and no relationship for the more southern populations (figure 3). One of the southern populations (river Imsa) had markedly higher energy status than the other populations at the end of the experiment. However, this did not affect the latitude and energy-level to feeding-frequency relationship. Reanalysing the data without this population yielded similar results (latitude × dryprop: F1,22 = 7.13, p = 0.013, compared with table 3).
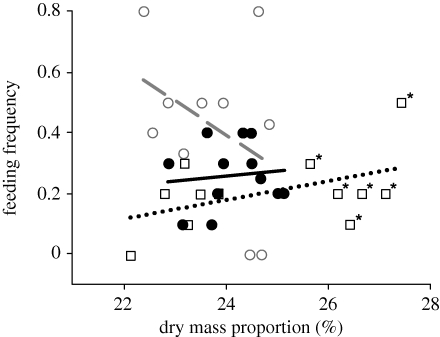
Figure 3.
Feeding frequency of hatchery-reared juvenile Atlantic salmon held in stream channels plotted against mean dry-mass proportion in the replicates. To illustrate the interaction effect between latitude and dry-mass proportion on feeding frequency, populations are grouped according to southern (Imsa and Lone), central (Gaula and Namsen) and northern (Alta) origin (squares, black circles and white circles, respectively). The Imsa population is indicated by asterisks. Predicted relationships between dry-mass proportion and feeding for the latitudinal groups are plotted using dotted black, solid black and stippled grey lines, for southern, central and northern populations, respectively. Dry-mass proportion × latitude interaction was still significant with latitudinal origin grouped as presented in the figure for illustrational purpose (dryprop: F1,30 = 0.39, p = 0.534; latitude: F1,30 = 8.70, p = 0.006; dryprop × latitude: F1,30 = 4.79, p = 0.036).
4. Discussion
Observed mean population lipid-depletion rates in wild populations of juvenile Atlantic salmon were faster for populations with higher autumn levels of storage energy. This resulted in low between-population variations in spring storage lipids, with lipid levels clustering close to critical levels needed for survival (Biro et al. 2004; Finstad et al. 2004). The estimated daily winter lipid-depletion rates were similar to previously reported rates (Berg & Bremset 1998; Berg et al. 2006). Northern populations appear to reduce lipid levels at a lower rate than southern, and there was a positive correlation between latitude and retained levels of storage energy. The observed latitudinal trend could not be explained by energy-selective mortality alone, indicating a more conservative storage energy strategy in northern populations experiencing longer winters.
When released in semi-natural stream channels with natural food supply, hatchery-reared fish with parents originating from northern populations had a stronger defence of their energy levels compared with southern populations. This was evident from generally higher feeding activity with decreasing energetic status in northern fish, a relationship lacking in southern fish.
In combination, the field and experimental data from the current study support previous experimental and theoretical work predicting that animals adjust their feeding in order to reach a specific target of energy reserves at the end of the adverse period (Bull et al. 1996). Furthermore, the observed difference in energy-state-dependent feeding motivation among populations supports a geographical adaptive diversity in behavioural traits (Foster 1999) along the investigated latitudinal gradient. Latitudinal patterns of autumn lipid accumulation have been documented for the estuarine fish Atlantic silverside (Schultz & Conover 1997) and for birds such as coal tits (Periparus ater L.; Polo et al. 2007; McNamara & Houston 2008; Wingfield 2008). Although previous studies have linked energy allocation to variation in seasonal length (Schultz & Conover 1997; Billerbeck et al. 2000), intraspecific geographical patterns in storage energy dynamics and local adaptations to seasonality have rarely been demonstrated (Schultz & Conover 1997; Polo et al. 2007).
An alternative explanation for the observed geographical pattern of storage lipid depletion is energy-dependent mortality (Biro et al. 2004; Finstad et al. 2004). Under such a scenario, fish may deplete stored energy independent of energy status or length of winter season. When reaching a critical limit for survival, the fish dies. The observed population mean lipid storage levels in spring will accordingly be based on the skewed distribution of surviving fish. Although energy-dependent mortality is likely to operate in many of the sampled populations (Finstad et al. 2004) and may influence our results, we were unable to replicate the observed latitudinal gradient in lipid-depletion rates under such a mechanism alone.
Juvenile Atlantic salmon appear to be unable to survive long winter periods solely on stored energy reserves, but rely on active feeding in order to meet their energy demands (Berg & Bremset 1998). In periods of inactivity, juvenile salmonids shelter between interstitial spaces in the substrate in order to avoid predators (Heggenes et al. 1993; Valdimarsson & Metcalfe 1998), whereas during foraging the fish are exposed to predation. Theoretically, the fish may therefore optimize its energy intake so that levels of storage energy at the end of winter are just sufficient to survive. The observed patterns of lipid-depletion rates in wild populations of juvenile Atlantic salmon support such a behavioural trade-off between predation risk and starvation, and indicate an optimization of energy intake according to expected energy levels at the end of winter (Bull et al. 1996). Juvenile Atlantic salmon may use simple rules (e.g. increase energy intake when approaching critical storage energy levels) or adjust their trajectories of storage energy based on general environmental cues to infer seasonal length or progression. Alternatively, fish from different populations may respond differently to the same environmental cues, use different cues or base their feeding motivation on intrinsic inherited winter length anticipations.
Our common-environment experiments support the view that northern fish anticipate a longer winter and adjust their feeding pattern accordingly. These results are also in accordance with previous theoretical and empirical work showing that animals should tailor their feeding activity in order to fit long-term energy needs (Bull et al. 1996). Like most animals at temperate latitudes, Atlantic salmon use photoperiod as a cue for seasonal changes (Villarreal et al. 1988; Paul et al. 2008). The current common-environment experiments were run at facilities located in southern Norway under ambient light conditions. However, the relatively shorter days on higher latitudes before spring equinox make it unlikely that northern fish interpret the ambient light conditions as earlier in the season than southern fish. The comparably higher feeding motivation at lower lipid levels of northern fish is thus unlikely to result from seasonal confusion.
In summary, juvenile Atlantic salmon show patterns of lipid storage energy depletion throughout winter, compatible with current theories of costs and benefits of lipid storage. The common-environment experiments demonstrate that the behavioural trade-offs underpinning this pattern have an adaptive basis where the fish makes adjustments of its intake on the basis of state-dependent intrinsic cues or inherited anticipation of the length of the winter season. Thus, our study demonstrates adaptive differences in feeding motivation between populations along a latitudinal gradient, indicating population differences in the genetic basis for the behavioural trade-off between feeding and sheltering.
Acknowledgements
We thank the staff at the NINA Research Station for technical assistance. The study was supported by the Norwegian Research Council (NFR 145208/210, 185109/S30 and 177954/S40), Statkraft Energy A/S and the Norwegian Directorate for Nature Management. The study was conducted according to national regulations for the treatment and welfare of experimental animals.
References
- Berg O. K., Bremset G.1998Seasonal changes in the body composition of young riverine Atlantic salmon and brown trout. J. Fish Biol. 52, 1272–1288 (doi:10.1111/j.1095-8649.1998.tb00971.x) [Google Scholar]
- Berg O. K., Arnekleiv J. V., Lohrmann A.2006The influence of hydroelectric power generation on the body composition of juvenile Atlantic salmon. Riv. Res. Applic. 22, 993–1008 (doi:10.1002/rra.949) [Google Scholar]
- Berg O. K., Finstad A. G., Solem Ø., Ugedal O., Forseth T., Niemela E., Arnekleiv J. V., Lohrmann A., Naesje T. F.2009Pre-winter lipid stores in young-of-year Atlantic salmon along a north–south gradient. J. Fish Biol. 74, 1383–1393 (doi:10.1111/j.1095-8649.2009.02193.x) [DOI] [PubMed] [Google Scholar]
- Billerbeck J. M., Schultz E. T., Conover D. O.2000Adaptive variation in energy acquisition and allocation among latitudinal populations of the Atlantic silverside. Oecologia 122, 210–219 (doi:10.1007/PL00008848) [DOI] [PubMed] [Google Scholar]
- Biro P. A., Morton A. E., Post J. R., Parkinson E. A.2004Over-winter lipid depletion and mortality of age-0 rainbow trout (Oncorhynchus mykiss). Can. J. Fish. Aquat. Sci. 61, 1513–1519 (doi:10.1139/f04-083) [Google Scholar]
- Biro P. A., Post J. R., Abrahams M. V.2005Ontogeny of energy allocation reveals selective pressure promoting risk-taking behaviour in young fish cohorts. Proc. R. Soc. B 272, 1443–1448 (doi:10.1098/rspb.2005.3096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro P. A., Abrahams M. V., Post J. R.2007Direct manipulation of behaviour reveals a mechanism for variation in growth and mortality among prey populations. Anim. Behav. 73, 891–896 (doi:10.1016/j.anbehav.2006.10.019) [Google Scholar]
- Brodin A.2007Theoretical models of adaptive energy management in small wintering birds. Phil. Trans. R. Soc. B 362, 1857–1871 (doi:10.1098/rstb.2006.1812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C. D., Metcalfe N. B., Mangel M.1996Seasonal matching of foraging to anticipated energy requirements in anorexic juvenile salmon. Proc. R. Soc. Lond. B 263, 13–18 (doi:10.1098/rspb.1996.0003) [Google Scholar]
- Dobush G. R., Ankney C. D., Krementz D. G.1985The effect of apparatus, extraction time, and solvent type on lipid extractions of snow geese. Can. J. Zool. 63, 1917–1920 (doi:10.1139/z85-285) [Google Scholar]
- Finstad A. G.2005Effect of sampling interval and temperature on the accuracy of food consumption estimates from stomach contents. J. Fish Biol. 66, 33–44 (doi:10.1111/j.0022-1112.2005.00577.x) [Google Scholar]
- Finstad A. G., Forseth T.2006Adaptation to ice-cover conditions in Atlantic salmon, Salmo salar L. Evol. Ecol. Res. 8, 1249–1262 [Google Scholar]
- Finstad A. G., Ugedal O., Forseth T., Naesje T. F.2004Energy-related juvenile winter mortality in a northern population of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 61, 2358–2368 (doi:10.1139/f04-213) [Google Scholar]
- Foster S. A.1999The geography of behaviour: an evolutionary perspective. Trends Ecol. Evol. 14, 190–195 (doi:10.1016/S0169-5347(98)01577-8) [DOI] [PubMed] [Google Scholar]
- Gardiner W. R., Geddes P.1980The influence of body-composition on the survival of juvenile salmon. Hydrobiologia 69, 67–72 (doi:10.1007/BF00016537) [Google Scholar]
- Heggenes J., Krog O. M. W., Lindas O. R., Dokk J. G., Bremnes T.1993Homeostatic behavioural responses in a changing environment: brown trout (Salmo trutta) become nocturnal during winter. J. Anim. Ecol. 62, 295–308 (doi:10.2307/5361) [Google Scholar]
- Klemetsen A., Amundsen P.-A., Dempson J. B., Jonsson B., Jonsson N., O'Connell M. F., Mortensen E.2003Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): review of aspects of their life histories. Ecol. Freshw. Fish 12, 1–59 (doi:10.1034/j.1600-0633.2003.00010.x) [Google Scholar]
- Lima S. L.1998Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. In Stress and Behavior, Advances in the Study of Behaviour, vol. 27, pp. 215–290 San Diego, CA: Academic Press Inc [Google Scholar]
- MacCrimmon H. R., Gots B. L.1979World distribution of Atlantic salmon, Salmo salar. J. Fish. Res. Board Can. 36, 422–457 [Google Scholar]
- Macleod R., Gosler A. G., Cresswell W.2005Diurnal mass gain strategies and perceived predation risk in the great tit (Parus major). J. Anim. Ecol. 74, 956–964 (doi:10.1111/j.1365-2656.2005.00993.x) [Google Scholar]
- Macleod R., Clark J., Cresswell W.2008The starvation–predation risk trade-off, body mass and population status in the Common Starling Sturnus vulgaris. Ibis 150, 199–208 (doi:10.1111/j.1474-919X.2008.00820.x) [Google Scholar]
- McNamara J. M., Houston A. I.1990The value of fat reserves and the tradeoff between starvation and predation. Acta Biotheoret. 38, 37–61 (doi:10.1007/BF00047272) [DOI] [PubMed] [Google Scholar]
- McNamara J. M., Houston A. I.2008Optimal annual routines: behaviour in the context of physiology and ecology. Phil. Trans. R. Soc. B 363, 301–319 (doi:10.1098/rstb.2007.2141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J. M., Barta Z., Houston A. I., Race P.2005A theoretical investigation of the effect of predators on foraging behaviour and energy reserves. Proc. R. Soc. B 272, 929–934 (doi:10.1098/rspb.2004.3037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe N. B., Thorpe J. E.1992Anorexia and defended energy-levels in over-wintering juvenile salmon. J. Anim. Ecol. 61, 175–181 (doi:10.2307/5520) [Google Scholar]
- Metcalfe N. B., Huntingford F. A., Thorpe J. E.1986Seasonal changes in feeding motivation of juvenile Atlantic salmon (Salmo salar). Can. J. Zool. 64, 2439–2446 (doi:10.1139/z86-364) [Google Scholar]
- Naesje T. F., Thorstad E. B., Forseth T., Aursand M., Saksgard R., Finstad A. G.2006Lipid class content as an indicator of critical periods for survival in juvenile Atlantic salmon (Salmo salar). Ecol. Freshw. Fish 15, 572–577 (doi:10.1111/j.1600-0633.2006.00173.x) [Google Scholar]
- Noren D. P., Mangel M.2004Energy reserve allocation in fasting northern elephant seal pups: inter-relationships between body condition and fasting duration. Func. Ecol. 18, 233–242 (doi:10.1111/j.0269-8463.2004.00840.x) [Google Scholar]
- Paul M. J., Zucker I., Schwartz W. J.2008Tracking the seasons: the internal calendars of vertebrates. Phil. Trans. R. Soc. B 363, 341–361 (doi:10.1098/rstb.2007.2143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo V., Carrascal L. M., Metcalfe N. B.2007The effects of latitude and day length on fattening strategies of wintering coal tits Periparus ater (L.): a field study and aviary experiment. J. Anim. Ecol. 76, 866–872 (doi:10.1111/j.1365-2656.2007.01270.x) [DOI] [PubMed] [Google Scholar]
- Post J. R., Parkinson E. A.2001Energy allocation strategy in young fish: allometry and survival. Ecology 82, 1040–1051 [Google Scholar]
- Schultz E. T., Conover D. O.1997Latitudinal differences in somatic energy storage: adaptive responses to seasonality in an estuarine fish (Atherinidae: Menidia menidia). Oecologia 109, 516–529 (doi:10.1007/s004420050112) [DOI] [PubMed] [Google Scholar]
- Sokal R. R., Rohlf F. J.1995Biometry: the principles and practice of statistics in biological research New York, NY: Freeman [Google Scholar]
- Thorpe J. E., Mangel M., Metcalfe N. B., Huntingford F. A.1998Modelling the proximate basis of salmonid life-history variation, with application to Atlantic salmon, Salmo salar L. Evol. Ecol. 12, 581–599 (doi:10.1023/A:1022351814644) [Google Scholar]
- Valdimarsson S. K., Metcalfe N. B.1998Shelter selection in juvenile Atlantic salmon or why do salmon seek shelter in winter? J. Fish Biol. 52, 42–49 (doi:10.1111/j.1095-8649.1998.tb01551.x) [Google Scholar]
- Villarreal C. A., Thorpe J. E., Miles M. S.1988Influence of photoperiod on growth changes in juvenile Atlantic salmon, Salmo salar L. J. Fish Biol. 33, 15–30 (doi:10.1111/j.1095-8649.1988.tb05445.x) [Google Scholar]
- Wingfield J. C.2008Organization of vertebrate annual cycles: implications for control mechanisms. Phil. Trans. R. Soc. B 363, 425–441 (doi:10.1098/rstb.2007.2149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter M. S., Cuthill I. C.1993The ecological costs of avian fat storage. Phil. Trans. R. Soc. Lond. B 340, 73–92 (doi:10.1098/rstb.1993.0050) [DOI] [PubMed] [Google Scholar]