Abstract
The alternation of short/coastal and long/pelagic foraging trips has been proposed as a strategy for seabirds to reconcile self-feeding and parental care. Both types of foraging trips may result in different foraging efforts and diet qualities, and consequently are likely to modify the oxidative status of seabirds.
We examined the relationship between the oxidative status of Adélie penguins and (i) the duration of their foraging trips and (ii) their plasma δ13C values reflecting their spatial distribution.
The oxidative status did not correlate with the foraging trip duration but with the δ13C values: high values being associated with high levels of oxidative damage.
This relationship is likely to be related to the prey properties of penguins as both parameters are largely determined by the diet. Two non-exclusive hypotheses can be proposed to explain this relationship: (i) penguins foraging in coastal areas feed on a diet enriched in 13C and depleted in antioxidant compounds; (ii) birds with low antioxidant capacity are constrained to forage in coastal areas.
Our study is the first to show that the adoption of different foraging strategies is associated with different levels of oxidative stress. However, further studies are needed to investigate the underlying mechanisms of this intriguing relationship.
Keywords: foraging, stable isotope, oxidative stress, seabirds
1. Introduction
One strategy adopted by seabirds to reconcile self-feeding and parental care consists in performing foraging trips of different durations. Short foraging trips result in increased provisioning rate to offspring while long foraging trips result in decreased provisioning rate but allow parents to restore their body reserves (Chaurand & Weimerskirch 1994; Weimerskirch 1995). The most plausible reason for why foraging trips are short or long is that seabirds adopt different spatial foraging distributions at sea: short trips are more coastal while long trips are more oceanic (Weimerskirch et al. 1997, 1998; Catard et al. 2000; Hamer et al. 2001).
This alternation in spatial foraging distribution may be associated with a change in the diet of seabirds (Weimerskirch et al. 1998; Cherel et al. 2005a). For instance, white-chinned petrels Procellaria aequinoctialis feed mainly on fish during short/coastal trips while they feed on krill and fish during long/pelagic trips (Catard et al. 2000). The biochemical composition of these prey items may differ and thereby influence the biochemistry and the physiology of the consumer. For example, two of the most common prey in the Austral Ocean, Antarctic krill Euphausia superba and fish, exhibit close energy and protein contents but differ both quantitatively and qualitatively in their fat and antioxidant contents. Krill contains less fat than fish (Yanagimoto et al. 1979; Friedrich & Hagen 1994) but more polyunsaturated fatty acids (Tierney et al. 2008) and antioxidants (Tou et al. 2007). As the antioxidant capacity of birds reflects the antioxidant content of their diet (as shown by the positive relationship between dietary antioxidants and blood antioxidant capacity in birds; Cohen et al. 2009), it is likely that seabirds feeding predominantly on krill exhibit higher antioxidant capacity than those feeding on fish. The selection of krill during long/pelagic trips, as observed in white-chinned petrels (Catard et al. 2000), may consequently be associated with a low oxidative stress, i.e. a high antioxidant capacity relative to the production of reactive oxygen species (ROS) (Finkel & Holbrook 2000). In contrast, because polyunsaturated fatty acids are more susceptible to peroxidation than monounsaturated fatty acids (Hulbert 2008), a diet with a high content of polyunsaturated fatty acids such as krill may be associated with higher oxidative stress (Jenkinson et al. 1999). Therefore, by selecting prey with different compositions during short/coastal and long/pelagic trips, seabirds may modulate their oxidative status, depending on the antioxidant content of their prey relative to the pro-oxidant effect of this prey (for instance, through its content in polyunsaturated fatty acids).
Moreover, the cost associated with the different foraging trips may also impact the oxidative status of seabirds. Indeed, short trips, energetically more costly than long trips (Weimerskirch et al. 2003), are likely to be related to higher oxygen consumption and consequently to greater ROS production (Loft et al. 1994).
In the present study, we examined whether the oxidative status of Adélie penguins Pygoscelis adeliae was related to (i) the duration of their foraging trips and (ii) their plasma ratio 13C/12C (further referred to as δ13C) giving an index of the spatial distribution of birds (Kelly 2000; Inger & Bearhop 2008).
2. Material and methods
We first examined the relationship between oxidative status and foraging strategies in penguins subject to the same environmental conditions but performing foraging trips of different durations. We took into consideration potential confounding factors susceptible to modulate foraging efforts such as the brood size, the sex of the parent and the sex of the chicks (Beaulieu et al. 2009). Then, we examined the relationship between oxidative status and foraging strategies for 2 years by considering when the same individuals changed the duration of their foraging trips as well as their spatial distribution because of different environmental conditions (Beaulieu et al. in press).
(a). Study species
The Adélie penguin is a long-lived species (maximum lifespan: 20 years; Ainley 2002) where breeding cycle comprises four phases: (i) the courtship from mid-October to early November; (ii) the incubation of one or two eggs for 30–36 days; (iii) the guard stage (from mid-December to mid-January) when both parents alternate foraging at sea and chick attendance at nest; and (iv) the crèche stage (from mid-January to mid-February) when both parents can forage at the same time leaving the chick(s) alone in the colony.
(b). Fieldwork
The study took place in Dumont d'Urville (66°40′S; 140°01′E), Adélie Land, Antarctica, in summers of 2006–2007 and 2007–2008. In 2006–2007, penguins performed short foraging trips and fed in more coastal areas than in 2007–2008 (Beaulieu et al. in press).
Eleven stable pairs were followed during the two consecutive summers. These pairs were visually identified during the courtship period and their nests were observed every 2 h to obtain the duration of their foraging trips throughout the breeding cycle (Beaulieu et al. in press). Penguins were weighed during the chick-rearing period, 40–45 days after egg-laying, when parents alternate periods at sea (duration: 1–2 days) and periods on the nest. At the same time, approximately 1.5 ml of blood was collected in heparinized syringes in less than 5 min after capture. Blood samples were then centrifuged and plasma samples were frozen at −20°C for further analyses.
In 2007–2008, among the 11 pairs, we selected those that had only one chick during the chick-rearing period. We completed this group with new pairs also with only one chick to avoid the potential bias owing to different brood size on foraging effort (Beaulieu et al. 2009). A sample of 18 pairs with one chick was then constituted and underwent the same procedure as that described above.
Adults were sexed by cloacal inspection and by observation of copulations. As foraging effort may be modulated by the sex of the chick, we also determined the sex of the chicks of the pairs monitored in 2007–2008, by molecular sexing from feathers collected at the end of the season (Beaulieu et al. 2009).
(c). Laboratory analyses
As previously described in birds (e.g. Costantini 2008; Costantini et al. 2007), oxidative stress was measured in plasma samples by using the d-reactive oxygen metabolites (d-ROM) and the oxy-adsorbent tests (Diacron International).
The d-ROM test measures plasmatic hydroperoxydes, a reactive oxygen metabolite (ROM) resulting from the attack of ROS on organic substrates (carbohydrates, lipids, amino acids, proteins, nucleotides). The plasma (4 µl) was first diluted in 200 µl of an acidic buffer solution (pH = 4.8) and 2 µl of chromogen (N,N-diethyl-p-phenylenediamine) and then incubated at 37°C for 75 min. These acidic conditions favour the release of iron ions from plasma proteins, which catalyse the breakdown of hydroperoxyde into alkoxyl and peroxyl radicals. These final products in turn react with the chromogen and produce a complex where colour intensity, read with a microplate reader (490 nm, Statfax3200, Awareness Technology Inc.), is proportional to its concentration. The concentration of hydroperoxyde was then calculated by comparison with a standard solution whose oxidative activity on the chromogen is equivalent to the activity of H2O2 (0.08 mg dl−1). Measurements were therefore expressed as mg dl−1 H2O2 equivalents. Intra- and inter-assay coefficients of variations were 8 per cent and 6 per cent, respectively.
The oxy-adsorbent test measures the total plasma antioxidant capacity. This test evaluates the plasma ability to oppose the massive oxidative action of a hypochlorous acid (HClO) solution. The plasma (2 µl) was first diluted 1 : 100 with distilled water; 5 µl of this solution was then incubated with 200 µl of a titred HClO solution at 37°C for 10 min. Then, 5 µl of chromogen (N,N-diethyl-p-phenylenediamine) was added to measure the excess of HClO in plasma. The resulting coloured complex, read with a spectrophotometer (490 nm, Statfax3200, Awareness Technology Inc.), is inversely related to the antioxidant power. The plasmatic antioxidant capacity was then calculated by comparison with a standard solution. Measurements were expressed as mmol−1 HOCL neutralized. Intra- and inter-assay coefficients of variations were 7 per cent and 4 per cent, respectively.
As phytoplankton, at the base of marine foodwebs, is richer in 13C values in coastal than in pelagic areas (France 1995), animals foraging and feeding in coastal areas exhibit higher δ13C values than pelagic foragers (Cherel & Hobson 2007). Moreover, isotopic values mirror the diet throughout the period of tissue synthesis (Kelly 2000; Inger & Bearhop 2008). Therefore, our 13C measurements on plasma, whose turnover is about 3 days (Hobson & Clark 1993), reflected the diet of penguins during the foraging trip preceding blood sample. Isotopic analyses were carried out at the Centre de Recherche sur les Ecosystèmes Littoraux Anthropisés (CRELA, France). As recommended by Cherel et al. (2005b), plasma samples (200 µl) were delipidated, as lipids, depleted in 13C, decrease plasmatic δ13C values (Cherel et al. 2005a). Then, they were lyophilized (48 h) and powdered (Hobson et al. 1997). Results are expressed in the standard δ notation (‰) relative to PDB (PeeDee Belemnite). Intra- and inter-assay coefficients of variation were 0.88 per cent and 0.42 per cent, respectively. This technique has already allowed us to highlight different spatial distributions of Adélie penguins according to sea-ice conditions: in 2007–2008, when fast ice remains for longer in Adélie Land than in 2006–2007, penguins foraged in more oceanic areas (Beaulieu et al. in press).
(d). Statistical analyses
First, we assessed in 2007–2008 whether body mass, δ13C values, oxidative status and the duration of the foraging trip preceding blood sampling differed between males and females. As the sex of the chick affects foraging trip duration (Beaulieu et al. 2009), we also considered this parameter and we used general linear models (GLMs) with the sex of the adult, the sex of the young and their interaction as fixed factors. For the duration of foraging trips, data were log-transformed to obtain normality of residuals. Second, we conducted Pearson or Spearman correlations (according to normality of data) to investigate the relationships between parameters in males and females. When the GLMs indicated no differences between sexes, we conducted the same correlations including males and females together. Inter-annual comparison for oxidative status was carried out by using general linear mixed models (GLMMs) to avoid the problem of pseudoreplication as our statistical analyses involved the same penguins. Individuals were considered as a random factor while the year, the sex and their interaction were used as fixed factors. Normality of residuals was assessed with a Shapiro–Wilk test.
All analyses were conducted using SPSS 16.02 (SPSS Inc.). Results are expressed as means ± s.e. and significance level was set at α = 0.05.
3. Results
(a). Intra-annual analyses (2007–2008)
Males were heavier than females when blood was sampled (5.16 ± 0.11 and 4.46 ± 0.11 kg, respectively) and they had performed shorter foraging trips than females (1.10 ± 0.15 and 1.74 ± 0.15 days, respectively) before blood sampling (table 1). Neither the adult sex nor the chick sex affected δ13C values or the oxidative status of parents (table 1): δ13C values (−25.74 ± 0.12 and −25.63 ± 0.12‰, respectively), ROM levels (6.64 ± 0.52 and 6.90 ± 0.51 mg H2O2 dl−1, respectively) and the antioxidant capacity (185.37 ± 18.56 and 171.13 ± 18.28 mmol−1 HOCL neutralized, respectively) were similar in males and females.
Table 1.
Results of general linear models (GLMs) assessing the influence of the sex of the adult and the chick on adult body mass, δ13C ratio, oxidative status and the duration of the foraging trip preceding blood sampling.
| adult sex |
chick sex |
interaction |
||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| body mass | 13.342 | 0.001 | 1.527 | 0.226 | 1.301 | 0.263 |
| δ13C | 0.416 | 0.524 | 0.251 | 0.620 | 0.010 | 0.920 |
| ROM | 0.122 | 0.730 | 1.483 | 0.232 | 0.170 | 0.683 |
| OXY | 0.299 | 0.589 | 0.873 | 0.357 | 0.180 | 0.674 |
| foraging trip duration | 11.446 | 0.002 | 2.217 | 0.147 | 0.287 | 0.596 |
As δ13C and oxidative values were independent of adult sex, data of male and female were first pooled. ROM levels were positively correlated with δ13C values and negatively correlated with the antioxidant capacity of birds (figure 1). These trends were found in males and females although they were not significant for females (figure 2). In contrast, body mass and foraging trip duration before blood sampling were not correlated with δ13C values or the oxidative status (all p > 0.05).
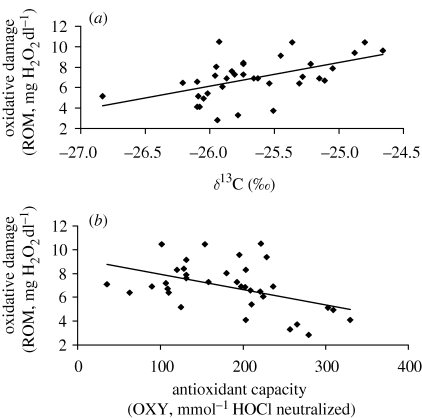
Figure 1.
Scatter plots showing the relationships between (a) δ13C values and oxidative damage: r = 0.531; p = 0.001. (b) Oxidative damage and antioxidant capacity: r = −0.463; p = 0.005.
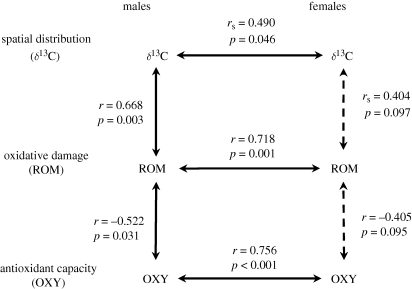
Figure 2.
Relationships between the spatial distribution and the oxidative status in male and female Adélie penguins and between males and females within pairs. Solid arrows: significant relationships; dashed arrows: non-significant relationships.
Within pairs, the males' and females' body mass were not related (Pearson correlation: r = 0.034, p = 0.896). In contrast, there was a significant positive relationship between males' and females' δ13C values and between males' and females' oxidative status (figure 2).
(b). Inter-annual analyses (2006–2007 versus 2007–2008)
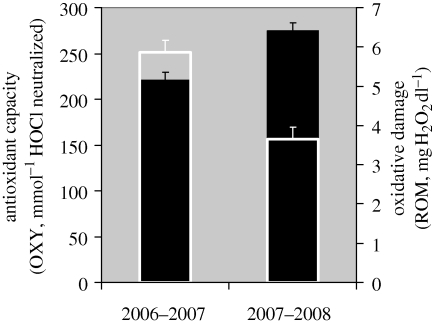
The sex of penguins had no effect on ROM levels (GLMM: F1,18 = 1.42, p = 0.25) and on their antioxidant capacity (GLMM: F1,18 = 1.06, p = 0.32). However, there was a strong inter-annual effect (figure 3): in 2006–2007, when penguins foraged in more coastal areas, ROM levels were higher (GLMM: F1,18 = 27.00, p < 0.001) and their antioxidant capacity was lower (GLMM: F1,18 = 27.03, p < 0.001) than in 2007–2008 when sea-ice conditions forced the penguins to forage in more oceanic areas (Beaulieu et al. in press).
Figure 3.
Oxidative damage (white histograms) and antioxidant capacity (black histograms) in the same group of 11 stable pairs of Adélie penguins in 2006–2007 and 2007–2008.
4. Discussion
Foraging trip duration has been found to be related to energy expenditure with short foraging trips being more costly than long foraging trips (Weimerskirch et al. 2003). As high energy expenditure is likely to increase oxidative stress through increased oxygen consumption (Loft et al. 1994), we expected foraging trip duration and oxidative status also to be related. However, in our study, foraging trip duration was not related to the oxidative status of penguins.
Instead of a relationship between the oxidative status and the foraging trip duration, we found a relationship between the oxidative status and δ13C values of penguins: penguins with higher plasmatic δ13C values also experience greater oxidative damage. To our knowledge, no other study has previously described this relationship at the scale of the organism. As δ13C values and the antioxidant capacity of birds are largely determined by food intake (Inger & Bearhop 2008; Cohen et al. 2009), differences in diet quality can be implicated.
Firstly, a direct impact of food 13C content on the likelihood to suffer from more oxidative stress can be suggested. It has been proposed that a diet enriched in 13C might decrease damage caused by ROS on proteins, nucleic acids or lipids, as biomolecules that incorporate heavier isotopes such as 13C may be more stable and successfully resist to oxidative stress (Shchepinov 2007). In disagreement with this hypothesis, Adélie penguins, feeding on a diet richer in 13C than terrestrial birds (Inger & Bearhop 2008), exhibit higher ROM levels than most terrestrial bird species (reviewed in Costantini et al. 2007). Moreover, in our study, penguins with higher δ13C values also had higher oxidative damage. This suggests that the potential beneficial direct effect of 13C on oxidative status is negligible compared with other parameters such as the antioxidant properties of the diet (Cohen et al. 2009).
In Adélie Land, Adélie penguins rely mainly on Antarctic krill and Antarctic silverfish (Pleuragramma antarcticum, Ridoux & Offredo 1989). Antioxidant levels are higher in krill than in fish (Tou et al. 2007) and may explain the inter-annual differences in the antioxidant status of penguins observed in our study. Indeed, the contribution of fish, poorer in antioxidants, was slightly more important in the penguins' diet in 2006–2007 (Beaulieu et al. in press) when their antioxidant capacity was lower. In addition, Antarctic silverfish inhabit more coastal areas (with higher δ13C values) than Antarctic krill (Cherel 2008). This suggests that the relationship between the δ13C values and the oxidative status of penguins is likely to be due to the different antioxidant levels of their prey living predominantly either in coastal (fish) or in oceanic (krill) areas.
Krill also contains higher levels of polyunsaturated fatty acids than fish (Tierney et al. 2008), a class of lipids known to have a pro-oxidant impact at the cell (Mazière et al. 1999) and organism levels (Jenkinson et al. 1999). In humans, a diet enriched with 15 per cent of polyunsaturated fatty acids adversely affects lipid peroxidation levels. However, the coupling of antioxidant treatments to diets rich in polyunsaturated fatty acids has been suggested to re-equilibrate the oxidative balance (Jenkinson et al. 1999). This is likely to be the case in penguins, as krill, rich in polyunsaturated fatty acids, is also characterized by high antioxidant contents.
Another hypothesis explaining the relationship between oxidative status and δ13C values of penguins (and therefore their foraging distribution) could be that birds with high antioxidant capacity and thus low oxidative damage were able to forage in oceanic waters while those with low antioxidant capacity and high oxidative damage were constrained to forage in coastal waters. In birds, age affects the antioxidant capacity (Bize et al. 2008; Costantini 2008), the foraging effort and the spatial foraging range of seabirds (Catry et al. 2006) that, in turn, may also affect oxidative status. As there is an assortative mating by age in Adélie penguins (Reid 1988), this may explain the positive correlation between males’ and females’ oxidative statuses and between males’ and females’ δ13C values. This suggests that male and female Adélie penguins of similar age and oxidative status share the same foraging spatial distribution.
In conclusion, our study revealed that, in contrast to foraging trip duration, the oxidative status of Adélie penguins was related to δ13C ratios and therefore presumably to the spatial distribution of their prey. To go further into the understanding of the respective influences of diet and age on the oxidative status of penguins, an experimental approach appears necessary. In this context, it would be worthwhile to measure the oxidative status of penguins in captivity fed with different controlled diets (e.g. krill versus fish), in parallel with a longitudinal study examining the changes in oxidative status of known-age penguins over their lifetime.
Acknowledgements
This study was approved and supported by the French Polar Institute Paul-Emile Victor (IPEV) and the Terres Australes et Antarctiques Françaises (TAAF). We are grateful to T. Raclot, D. Lazin and A.M. Thierry for their great help in the field and to L. Joassard for isotopic measurements.
References
- Ainley D. G.2002The Adélie penguin. Bellwether of climate change New York, NY: Columbia University Press [Google Scholar]
- Beaulieu M., Thierry A. M., Raclot T., Maho Y., Ropert-Coudert Y., Gachot-Neveu H., Ancel A.2009Sex-specific parental strategies according to the sex of offspring in the Adélie penguin. Behav. Ecol. 20, 878–883 (doi:10.1093/beheco/arp076) [Google Scholar]
- Beaulieu M., Dervaux A., Thierry A.-M., Lazin D., Le Maho Y., Ropert-Coudert Y., Spee M., Raclot T., Ancel A.In press When sea-ice clock is ahead of Adélie penguins' clock. Funct. Ecol. (doi:10.1111/j.1365-2435.2009.01638.x) [Google Scholar]
- Bize P., Devevey G., Monaghan P., Doligez B., Christe P.2008Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology 89, 2584–2593 (doi:10.1890/07-1135.1) [DOI] [PubMed] [Google Scholar]
- Catard A., Weimerskirch H., Cherel Y.2000Exploitation of distant Antarctic waters and close shelf-break waters by white-chinned petrels rearing chicks. Mar. Ecol. Prog. Ser. 194, 249–261 (doi:10.3354/meps194249) [Google Scholar]
- Catry P., Phillips R. A., Phalan B., Croxall J. P.2006Senescence effects in an extremely long-lived bird: the grey-headed albatross Thalassarche chrysostoma. Proc. R. Soc. B 273, 1625–1630 (doi:10.1098/rspb.2006.3482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurand T., Weimerskirch H.1994The regular alternation of short and long foraging trips in the blue petrel Halobaena caerulea: a previously undescribed strategy of food provisioning in a pelagic seabird. J. Anim. Ecol. 63, 275–282 (doi:10.2307/5546) [Google Scholar]
- Cherel Y.2008Isotopic niches of emperor and Adélie penguins in Adélie Land, Antarctica. Mar. Biol. 154, 813–821 (doi:10.1007/s00227-008-0974-3) [Google Scholar]
- Cherel Y., Hobson K. A.2007Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar. Ecol. Prog. Ser. 329, 281–287 (doi:10.3354/meps329281) [Google Scholar]
- Cherel Y., Hobson K. A., Weimerskirch H.2005aUsing stable isotopes to study resource acquisition and allocation in procellariiform seabirds. Oecologia 145, 533–540 (doi:10.1007/s00442-005-0156-7) [DOI] [PubMed] [Google Scholar]
- Cherel Y., Hobson K. A., Bailleul F. R., Groscolas R.2005bNutrition, physiology, and stable isotopes: new information from fasting and molting penguins. Ecology 86, 2881–2888 (doi:10.1890/05-0562) [Google Scholar]
- Cohen A. A., McGraw K. J., Robinson W. D.2009Serum antioxidant levels in wild birds vary in relation to diet, season, life history strategy, and species. Oecologia 161, 673–683 (doi:10.1007/s00442-009-1423-9) [DOI] [PubMed] [Google Scholar]
- Costantini D.2008Oxidative stress in ecology and evolution: lessons from avian studies. Ecol. Lett. 11, 1238–1251 (doi:10.1111/j.1461-0248.2008.01246.x) [DOI] [PubMed] [Google Scholar]
- Costantini D., Cardinale M., Carere C.2007Oxidative damage and anti-oxidant capacity in two migratory bird species at a stop-over site. Comp. Biochem. Phys. C 144, 363–371 [DOI] [PubMed] [Google Scholar]
- Costantini D., Dell'Ariccia G., Lipp H. P.2008Long flights and age affect oxidative status of homing pigeons (Columba livia). J. Exp. Biol. 211, 377–381 (doi:10.1242/jeb.012856) [DOI] [PubMed] [Google Scholar]
- Finkel T., Holbrook N. J.2000Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 (doi:10.1038/35041687) [DOI] [PubMed] [Google Scholar]
- France R. L.1995Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar. Ecol. Prog. Ser. 124, 307–312 (doi:10.3354/meps124307) [Google Scholar]
- Friedrich C., Hagen W.1994Lipid contents of five species of notothenioid fish from high-Antarctic waters and ecological implications. Polar Biol. 14, 359–369 (doi:10.1007/BF00240256) [Google Scholar]
- Hamer K. C., Phillips R. A., Hill J., Wanless S., Wood A.2001Contrasting foraging strategies of gannets Morus bassanus at two North Atlantic colonies: foraging trip duration and foraging area fidelity. Mar. Ecol. Prog. Ser. 224, 283–290 (doi:10.3354/meps224283) [Google Scholar]
- Hobson K. A., Clark R. G.1993Turnover of 13C in cellular and plasma fractions of blood: implications for nondestructive sampling in avian dietary studies. Auk 110, 638–641 [Google Scholar]
- Hobson K. A., Gibbs H. L., Gloutney M. L.1997Preservation of blood and tissue samples for stable-carbon and stable nitrogen analysis. Can. J. Zool. 75, 1720–1723 (doi:10.1139/z97-799) [Google Scholar]
- Hulbert A. J.2008Explaining longevity of different animals: is membrane fatty acid composition the missing link? Age 30, 89–97 (doi:10.1007/s11357-008-9055-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inger R., Bearhop S.2008Applications of stable isotope analyses to avian ecology. Ibis 150, 447–461 (doi:10.1111/j.1474-919X.2008.00839.x) [Google Scholar]
- Jenkinson A., Franklin M. F., Wahle K., Duthie G. G.1999Dietary intakes of polyunsaturated fatty acids and indices of oxidative stress in human volunteers. Eur. J. Clin. Nutr. 53, 523–528 [DOI] [PubMed] [Google Scholar]
- Kelly J. F.2000Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 78, 1–27 (doi:10.1139/cjz-78-1-1) [Google Scholar]
- Loft S., Astrup A., Bueman B., Poulsen H. E.1994Oxidative DNA damage correlates with oxygen consumption in Humans. FASEB J. 8, 534–537 [DOI] [PubMed] [Google Scholar]
- Mazière C., Conte M. A., Degonville J., Ali D., Mazière J. C.1999Cellular enrichment with polyunsaturated fatty acids induces an oxidative stress and activates the transcription factors AP1 and NF kappa B. Biochem. Biophys. Res. Comm. 265, 116–122 (doi:10.1006/bbrc.1999.1644) [DOI] [PubMed] [Google Scholar]
- Reid W. V.1988Age correlations within pairs of breeding birds. Auk 105, 278–285 [Google Scholar]
- Ridoux V., Offredo C.1989The diets of five summer breeding seabirds in Adélie Land, Antarctica. Polar Biol. 9, 137–145 (doi:10.1007/BF00297168) [Google Scholar]
- Shchepinov M.2007Reactive oxygen species, isotope effect, essential nutrients, and enhanced longevity. Rejuv. Res. 10, 47–59 (doi:10.1089/rej.2006.0506) [DOI] [PubMed] [Google Scholar]
- Tierney M., Southwe C., Emmerson L. M., Hindell M. A.2008Evaluating and using stable-isotope analysis to infer diet composition and foraging ecology of Adélie penguins Pygoscelis adeliae. Mar. Ecol. Prog. Ser. 355, 297–307 (doi:10.3354/meps07235) [Google Scholar]
- Tou J. C., Jaczynski J., Chen Y. C.2007Krill for human consumption: Nutritional value and potential health benefits. Nutr. Rev. 65, 63–77 (doi:10.1111/j.1753-4887.2007.tb00283.x) [DOI] [PubMed] [Google Scholar]
- Weimerskirch H.1995Regulation of foraging trips and incubation routine in male and female wandering albatrosses. Oecologia 102, 37–43 [DOI] [PubMed] [Google Scholar]
- Weimerskirch H., Cherel Y.1998Feeding ecology of short-tailed shearwaters: breeding in Tasmania and foraging in the Antarctic? Mar. Ecol. Prog. Ser. 167, 261–274 (doi:10.3354/meps167261) [Google Scholar]
- Weimerskirch H., Cherel Y., Cuenot-Chaillet F., Ridoux V.1997Alternative foraging strategies and resource allocation by male and female wandering albatrosses. Ecology 78, 2051–2063 [Google Scholar]
- Weimerskirch H., Ancel A., Caloin M., Zahariev A., Spagiari J., Kersten M., Chastel O.2003Foraging efficiency and adjustment of energy expenditure in a pelagic seabird provisioning its chick. J. Anim. Ecol. 72, 500–508 (doi:10.1046/j.1365-2656.2002.00720.x) [Google Scholar]
- Yanagimoto M., Kato N., Yokoyama Y., Kobayashi T., Kimura S.1979Chemical compositions of Antarctic Krill (Euphausia superba) for the evaluation of processing. B. Jpn. Soc. Sci. Fish. Oceanogr. 45, 369–374 [Google Scholar]