Abstract
The mechanisms by which group-living animals collectively exploit resources, and the role of individuals in group decisions, are central issues for understanding animal distribution patterns. We investigated the extent to which boldness and shyness affect the distribution of social herbivores across vegetation patches, using sheep as a model species. Using an experimental and a theoretical approach, we show that collective choices emerge through the nonlinear dynamics of interactions between individuals, at both short and long distances. Within a range of parameter values derived from the observation of homogeneous groups of each behavioural type, we propose a simple mechanism whereby the same interaction rules can result in different patterns of distribution across patches for bold and shy individuals. We present a mathematical model based on behavioural rules derived from experiments, in which crowding and conspecific attraction affect the probability of entering or leaving patches. Variation in the strength of social attraction is sufficient to account for differences in spatial distribution across patches. The model predicts that resource fragmentation more strongly affects the distribution patterns of shy groups, and suggests that the presence of both bold and shy individuals within groups would result in more flexible behaviour at the population level.
Keywords: boldness, collective behaviours, social foraging, cohesion, sheep
1. Introduction
In group-living species, integrated decisions made by individuals result in collective behaviours which may, in turn, influence interactions between individuals and shape the resulting social system. Since the early 1970s, collective decision-making has been studied in several taxa (Camazine et al. 2001; Conradt & Roper 2005; Sumpter 2006), and in a variety of contexts including collective movements in primates (Petit et al. 2009), ungulates (Gueron & Levin 1993; Ramseyer et al. 2009), fishes (Parrish et al. 2002; Sumpter et al. 2008), birds (Ballerini et al. 2008; Daruka 2009), insects (Dussutour et al. 2004; Buhl et al. 2006) and unicellular organisms (Palsson & Othmer 2000); the selection of feeding sites or shelters in ants (Depickère et al. 2004; Jeanson et al. 2004), honeybees (Camazine & Sneyd 1991; Britton et al. 2002), cockroaches (Amé et al. 2006; Jeanson & Deneubourg 2007) and bats (Kerth et al. 2006); nest building in termites (Deneubourg 1977) and ants (Franks et al. 1992; Rasse & Deneubourg 2001), and activity synchronization in ants (Goss & Deneubourg 1988; Boi et al. 1999) and sheep (Gautrais et al. 2007). With a few noticeable exceptions, studies carried out with social or gregarious insects and in fishes have provided most of the experimental support for principles underlying collective decisions, while theoretical models have advanced ahead of empirical evidence in other vertebrates (Couzin & Krause 2003). Empirical studies typically consist of the quantification of individual interactions and the average performance of a collection of individuals, established under experimental conditions. However, variation among individuals within social groups is being increasingly recognized as an important determinant driving group behaviours and shaping collective decisions. Attention has notably been paid to the existence of particular individuals behaving as leaders, or as informed individuals, and to their having a greater effect on collective decision-making than other group members (Couzin et al. 2005; King et al. 2008; Stueckle & Zinner 2008; Conradt et al. 2009). Differences in the needs or preferences of one or a few individuals for a resource have also been shown to affect the outcome of collective decisions, without involving hierarchical interactions (Scott et al. 1995; Biro et al. 2006; Fischhoff et al. 2007). But other differences are likely to modulate collective decision-making: over the past few years, the concepts of personality, temperament and coping style have all received increasing attention. In many vertebrates, including birds, fishes and rodents, individuals differ in aggressiveness, sociability, level of activity, reaction to novelty and fearfulness (Koolhaas et al. 1999; Gosling 2001; Sih et al. 2004; Réale et al. 2007). Such personality traits have been used to characterize behavioural types and gave rise to the concept of ‘bold’ and ‘shy’ individuals. The ‘shy–bold continuum’ is now recognized as a fundamental axis of behavioural variation in animals (Wilson et al. 1994), and is associated with the response of an individual to risk-taking and novelty (Réale et al. 2000; Brick & Jakobsson 2002). While research has focused on context dependence (e.g. Van Oers et al. 2005) or the adaptative values of personality traits (e.g. Dingemanse & Réale 2005) and, to a lesser extent, on their evolutionary history (Dall et al. 2004; Bell 2007; Cote et al. 2008), comparatively little is known about the extent to which personality influences collective decision-making and their dynamics.
In the context of social foraging, the coexistence of individuals within groups differing in their behavioural strategies has mainly been addressed in terms of game theory and has received both theoretical and empirical attention (Giraldeau & Caraco 2000). The producer–scrounger model postulates that groups can include a mixture of strategies with some individuals (the scroungers) specializing in exploiting food resources discovered by others (the producers) (Vickery et al. 1991; Giraldeau & Beauchamp 1999). In addition, it has been proposed that individuals can adjust their foraging strategies depending on environmental constraints such as food patch size or social context (Barta & Giraldeau 1998; King et al. 2009). Although foraging economy provides valuable insights into social dynamics, few studies have addressed the proximal mechanisms underlying the influence of personality on groups foraging in fragmented environments.
The aim of the present study was thus to investigate the links between animal personality and the individual and collective decision-making processes in groups of large vertebrates foraging across patchy resources. We address two questions about collective decisions: (i) how the decision of an individual to enter or to leave a patch depends on the local density of conspecifics, and (ii) how personality affects decisions at both individual and collective level. We propose a mathematical model of individual decision-making, based on data from an experiment which used sheep (Ovis aries) as a model species (Michelena et al. 2009), to make predictions about the extent to which boldness and shyness affect foraging dynamics in patchy resources. In the experiment, we standardized conditions in terms of sex, age and familiarity between individuals, as well as animal density and pasture conditions. The model, using parameter values derived from the experimental data, provides a formal link between individual and collective behaviour.
2. Material and methods
(a). Experiments
The subjects of the experiment were 40 one-year-old, female Scottish blackface sheep, previously identified as either shy (n = 20) or bold (n = 20) personality types from their performance in an indoor exploration test where the individuals that explored the least were considered to be shy and those that explored the most were considered to be bold (Michelena et al. 2009; Sibbald et al. 2009). In the experiment, the distribution of groups of two, four, six or eight individuals, made up of either exclusively shy or exclusively bold sheep, were observed in a series of 30 min grazing tests. The tests were carried out in identical 45 × 5 m outdoor arenas, each consisting of a background of short grass (4.6 ± 0.6 cm) with patches (5 × 5 m) of taller grass (11.9 ± 1.6 cm) at each end. Behaviour was recorded on a videotape, and the numbers of sheep on each patch and on the background were counted every second. The times at which individual sheep entered or left the patches were also recorded, and the duration of bouts spent on patches and background was measured. Complete details of the arenas and experimental procedures can be found in Michelena et al. (2009). Each group size was tested for each personality type in each of the five replications of the experiment. Each individual animal was tested once in each replication and in each group size at least once, with group composition balanced to include as many different combinations of individuals as possible.
(b). Data analysis
Mean bout durations on a patch or on the background were calculated for single individuals and for groups of two, four, six and eight individuals. For each group size, the lifetime of a group was defined as the elapsed time between the point at which a new individual entered the patch or the background and the point at which any individual left the patch or the background. Lifetimes were calculated using the survival package of R software. Experimental data were fitted using nonlinear least-squares regressions performed with SPSS (v. 11.0, SPSS, Chicago, IL). The probability of leaving a patch or the background was calculated for each group size, assuming that the probability was constant per unit time (i.e. a Markovian process). In order to estimate the probability of entering a patch, we calculated the proportion of cases where an individual joined the largest group, as a function of the number of sheep located on each patch. Data for one group of eight bold sheep were excluded from the analyses because a sheep caught its horn in the fence during the observations.
3. Results
(a). Characterization of individual decision rules
(i). Probability of leaving a patch or the background
The probability of an individual leaving either a patch or the background decreased with the number of sheep located on the patch, whether the sheep were bold or shy (figure 1a). The theoretical probability of leaving corresponds to the ratio of the sensitivity to crowding and social attraction exerted by conspecifics within the individual's immediate vicinity. The experimental data were fitted with the function
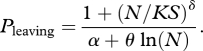 |
3.1 |
Figure 1.
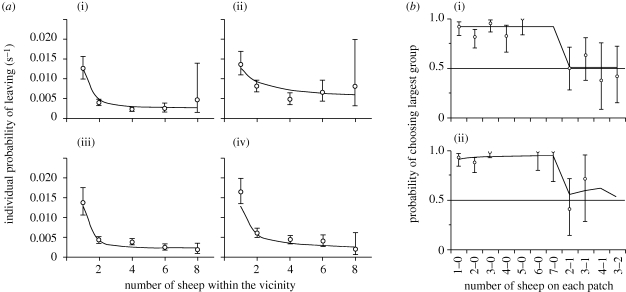
Individual behaviours: (a) represents the experimental (open circles: mean ± CI 95%) and theoretical (black lines; equation (3.1)) probabilities of leaving a patch or the background, for a bold or a shy sheep, as a function of the number of other sheep within the vicinity. (i) Bold sheep on the patch, (ii) bold sheep on the background, (iii) shy sheep on the patch, and (iv) shy sheep on the background; and (b) shows the experimental (open circles: mean ± CI 95%) and theoretical (black lines; equation (3.2)) probabilities of a (i) bold or a (ii) shy sheep leaving the background to join the largest group on a patch, as a function of the number of conspecifics on each of the patches.
This function formulates the individual attraction effect and calculates the probability of leaving a patch (or background) according to its area and the number of animals present. In equation (3.1), N is the number of sheep located on the patch (or background), S (m2) is the area of the patch (or background), K is the maximal crowding density (individual m−2), δ is a coefficient of sensitivity to crowding, α is the mean spontaneous time before an isolated individual leaves the patch (or background), and θ represents a coefficient of social attraction. In order to select the most parsimonious model, we constrained the parameter set and fitted the experimental curves with equation (3.1) by minimizing the residual sum of squares to estimate parameter values. Assuming that, in our experiment, the main difference between bold and shy sheep was their sensitivity to social attraction, the α, K and δ were all kept constant. This implies that bold and shy sheep were equally sensitive to crowding effects, whether they were on patches or on the background. The best fit to the experimental data (r2 = 0.80) was obtained with α = 78, K = 0.53 and δ = 2.5, and with θ = 188 for bold and θ = 245.7 for shy sheep on the patches and with θ = 42 for bold and θ = 145 for shy sheep on the background (figure 1a). Interestingly, these results suggest that social attraction is higher for shy sheep than for bold sheep, on both the patches and the background. The lower values for θ on the background compared to the patches might be explained by a different trade-off being made when animals are grazing short versus tall and more appetizing grass.
(ii). Probability of entering a patch
The probability of a sheep entering a particular patch i (Pi), was related to the number of conspecifics on each of the patches (figure 1b). The experimental data were fitted with the function
| 3.2 |
Equation (3.2) is a choice function, which quantifies the way a sheep makes the decision to enter a patch, as a function of the numbers Ni and Nj of conspecifics on each of the patches. The parameter ε determines the sensitivity to social attraction at long distances and the nonlinearity of the choice. ki and kj represent the intrinsic degree of attraction to each patch. Assuming the two patches are equally attractive when empty, the inherent probability of entering either of the patches will be 0.5. When only one of the two patches contains conspecifics, the probability of entering that patch is close to 1 (figure 1b). When there are equal numbers of conspecifics on both patches, the probability of entering either patch is 0.5. However, when the number of conspecifics on one patch is slightly higher, the sheep will have a higher probability of entering that patch. The best fit to the experimental data was obtained with ki = kj = 0.1, and with ε = 0.001 for bold sheep (nonlinear regression: F8,1 = 600.3, p < 0.05, r2 = 0.85) and ε = 0.4 for shy sheep (nonlinear regression: F6,1 = 727.2, p < 0.05, r2 = 0.83).
(b). Implementation of individual decision rules
In the individual-based model (implemented in Java), three compartments are considered: the two patches (i and j) and the background. For each sheep, probabilities are assigned depending on personality type (shy or bold) and parameters derived from empirical data. At the beginning of a simulation run, sheep are initialized randomly in any one of the compartments. The probability of leaving a compartment then depends on the number of conspecifics in the same compartment (equation (3.1)). When leaving a patch, sheep can only enter the background, whereas individuals on the background can enter either patch. The probability of entering patch i or j is given by the choice function (equation (3.2)), which depends on the relative number of sheep on the two patches. At each time step, these probabilities are updated and the individual decision to change compartments depends on a comparison between calculated probabilities and a random number between 0 and 1. In the simulation, the area of each compartment and the time scale (1 s per cycle) were preserved. A total of 1000 simulations were run for each combination of personality type and group size.
(c). Simulation and predictions of the model
(i). Simulation and experimental results for group sizes up to eight sheep
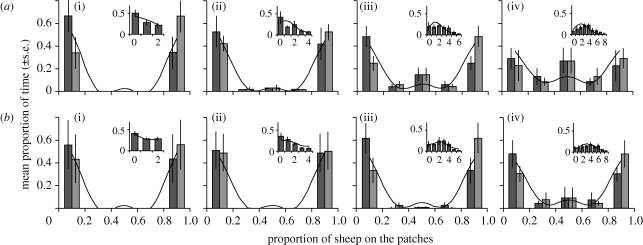
The simulated and experimental distributions of sheep across patches were qualitatively similar. In both cases, the smallest groups (i.e. N = 2 or 4) always grazed together on a patch, but there was an increasing likelihood of splitting into subgroups as group size increased, and bold sheep split into subgroups at smaller group sizes than shy sheep. Similarly, group splitting in the experiment mostly led to an equal number of sheep on each patch (figure 2), as predicted by the model.
Figure 2.
Collective behaviours: experimental (grey bars: mean ± s.e.) and simulated (black lines) frequency distributions of sheep across the patches for groups of two, four, six and eight animals and for bold and shy groups. Dark and light grey bars represent the right and left patches. The insets indicate the experimental (grey bars: mean ± s.e.) and simulated (black lines) frequency distribution of the different numbers of sheep on the background in each case. (a) Bold sheep: (i) group size 2, (ii) 4, (iii) 6, and (iv) 8. (b) Shy sheep: (i) group size 2, (ii) 4, (iii) 6, and (iv) 8.
(ii). Prediction of the model for larger group sizes
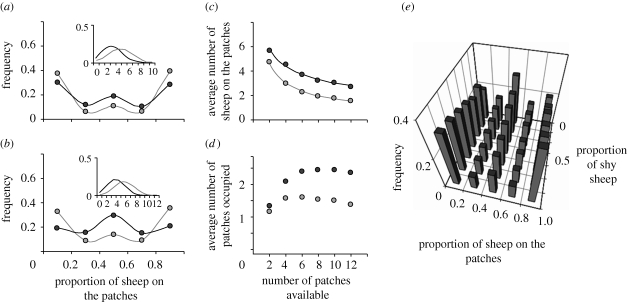
When group size increases to N = 10 and 12, the model predicts a greater difference between the distribution of bold and shy groups, with bold sheep distributed across both patches most of the time and shy sheep more likely to remain in single, cohesive groups (figure 3a,b).
Figure 3.
Model properties: (a,b) show predictions of the model for groups of 10 and 12 sheep, respectively, confronted with two patches. Dark and light grey circles represent the simulated frequency distribution for the proportions of sheep on patches for bold and shy groups, respectively. The insets indicate the frequency distribution of different numbers of sheep on the background in each case (black and grey lines for bold and shy groups, respectively); (c,d) show model predictions for the distribution of sheep across patches for simulated groups of eight bold (dark grey circles) or shy (light grey circles) individuals confronted with an increasing number of patches, when the total patch area is kept constant; (c) shows that the average number of sheep located on the patches at any time decreases as the square root of the number of patches, with black and grey lines showing fitted values for bold (y = 7.8x−0.4, r2 = 0.99) and shy (y = 7.1x−0.6, r2 = 0.99) simulated groups; (d) shows the average number of patches that contain sheep at any time as patch number increases; and (e) shows predicted frequency distributions of the proportion of sheep on the patches, for simulated mixed groups of 12 individuals, as a function of the proportion of shy sheep in the group.
(iii). Prediction of the model for larger numbers of patches
For groups of eight individuals, when the number of patches increases but the total patch area is constant, the model predicts that the total number of sheep on patches will decrease as the square root of the number of patches. Although crowding is kept constant across the resource, simulated fragmentation leads to a decreasing number of sheep grazing on patches, but this decay may evolve more slowly than the rate of fragmentation. This probably reflects the increased crowding effects that would result from smaller patches, combined with reduced social attraction effects owing to a greater dispersion of sheep across the patches. However, bold sheep, which are less sensitive to social attraction, are predicted to exploit the patches better, with a larger number of individuals distributed across more patches (figure 3c,d).
(iv). Prediction of the model for varying proportions of bold and shy individuals
For groups of 12 sheep, the model predicts an increasing likelihood of splitting into subgroups as the proportion of bold sheep increases, suggesting that collective decision-making within a mixed group reflects the different propensities of the individuals within the group, leading to intermediate foraging patterns (figure 3e).
4. Discussion
Our model, based on experimental results, demonstrates how social attraction and sensitivity to crowding can act as key factors in decisions to enter or leave patches. Animal distribution patterns emerge from the combination of individual resource preferences, social attraction and the effects of crowding, which will limit the density of animals at feeding sites. Unlike the model of collective decision-making processes seen in cockroaches facing similar environmental heterogeneity (Amé et al. 2006; Jeanson & Deneubourg 2007), our model does not only assume local, short-range interactions, but also considers long-range interactions between individuals. These long-range interactions increase the speed at which collective choices can be made. The effects of social attraction depend on a threshold response to the presence of conspecifics on patches. The model predicts that a slight difference in the sensitivity to conspecifics between bold and shy individuals (bold individuals having a lower value for the parameter ε than shy ones) has a strong impact on collective decision-making as group size increases. This is consistent with the key role for nonlinearity processes found in recent theoretical models of collective movements (Couzin et al. 2005; Conradt et al. 2009).
Our model is based on parsimonious and similar rules of interaction for bold and shy individuals which is in agreement with the assumptions of the shy–bold continuum. It suggests that no fundamentally different sets of behavioural rules need to be invoked for explaining the different distribution patterns of bold and shy groups of sheep. The magnitude of the behavioural differences between individuals, eventually amplified by social dynamics, rather than the existence of qualitatively different rules, may also account for the emergence of individuals behaving as leaders or informed individuals (Couzin et al. 2005; Stueckle & Zinner 2008; Conradt et al. 2009; Harcourt et al. 2009a). For instance, behavioural type is highly predictive of leadership in pairs of foraging barnacle geese Branta leucopsis where, as expected, it is the bold individuals that mainly take the role of leader (Kurvers et al. 2009).
The organization of social groups has been shown to benefit from the existence of individual variation. For instance, variability among individuals in their sensibility to environmental stimuli contributes significantly to the regulation of division of labour and improves the productivity and fitness of eusocial insects (Beshers & Fewell 2001; Mattila & Seeley 2007; Oldroyd & Fewell 2007). Similarly, the coexistence of different behavioural types within groups can positively influence mating success or foraging efficiency (Ward et al. 2004; Sih & Watters 2005; Dussutour et al. 2008; Nicolis et al. 2008; Pike et al. 2008; Harcourt et al. 2009a). In shoals of guppies, it has been proposed that boldness and shyness may represent a producer–scrounger system (Dyer et al. 2009). Similarly, our system with sheep could, to some extent, be likened to a producer–scrounger situation and it provides some evidence that the different tactics may arise mainly from differences in conspecific attraction, saving the cost of distinct phenotypes with qualitatively different behavioural rules.
Our model suggests that the coexistence of bold and shy individuals within a group influences collective decision-making. The theoretical explanation of the spatial distribution of sheep between patches in mixed groups relies on the assumption that individuals do not change their behaviour when interacting with others of different personality. However, empirical evidence suggests that individuals may adjust their strategies depending on group composition (Magnhagen & Staffan 2005) and that assortative preference for personality exists in mixed groups (Croft et al. 2009; Harcourt et al. 2009b). Further experiments are thus needed to compare the predictions of our model to the distribution patterns of experimental mixed groups in order to determine the extent to which the behavioural composition of the group can affect the behaviour of shy and bold individuals. Our model also provides a useful tool to make predictions about the behaviour of larger group sizes confronted with more fragmented resources than experimentally tested. Future experiments will be an opportunity to validate these predictions.
Our study suggests that variations in the proportion of behavioural types with groups may account for between-group variability observed at the population level (Aureli et al. 2008), leading to increased collective behavioural plasticity. In fostering social coordination, personality differences might thus help to optimize the exploitation of environmental resources. Our results provide a general framework for addressing generic issues related to collective movement patterns emerging from the interplay between social and ecological motivations, such as group fission–fusion dynamics in response to habitat fragmentation.
Acknowledgements
Animal care and experimental manipulations were applied in conformity with the rules of the Guidelines for the use of Animals in Research, the legal requirements of UK and the corresponding institutional guidelines.
We thank Richard Bon, Fanny Busson, Hans Erhard, Jean-François Gérard, Russell Hooper and Jim McLeod for help and comments during field experiments and data collection. We also acknowledge Hans Erhard's contribution in designing and carrying out the indoor exploration test. We are grateful to two anonymous referees for their constructive and helpful comments. This study was supported by the Fondation les Treilles, The LAVOISIER programme of the Ministère des Affaires Étrangères in France and the Center National de la Recherche Scientifique (Programme PICS 4423).
References
- Amé J. M., Halloy J., Rivault C., Detrain C., Deneubourg J. L.2006Collegial decision making based on social amplification leads to optimal group formation. Proc. Natl Acad. Sci. USA 103, 5835–5840 (doi:10.1073/pnas.0507877103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aureli F., et al. 2008Fission–fusion dynamics new research frameworks. Curr. Anthropol. 49, 627–654 (doi:10.1086/586708) [Google Scholar]
- Ballerini M., et al. 2008Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc. Natl Acad. Sci. USA 105, 1232–1237 (doi:10.1073/pnas.0711437105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta Z., Giraldeau L. A.1998The effect of dominance hierarchy on the use of alternative foraging tactics: a phenotype-limited producing-scrounging game. Behav. Ecol. Sociobiol. 42, 217–223 (doi:10.1007/s002650050433) [Google Scholar]
- Bell A. M.2007Evolutionary biology: animal personalities. Nature 447, 539–540 (doi:10.1038/447539a) [DOI] [PubMed] [Google Scholar]
- Beshers S. N., Fewell J. H.2001Models of division of labor in social insects. Ann. Rev. Entomol. 46, 413–440 (doi:10.1146/annurev.ento.46.1.413) [DOI] [PubMed] [Google Scholar]
- Biro D., Sumpter D. J. T., Meade J., Guilford T.2006From compromise to leadership in pigeon homing. Curr. Biol. 16, 2123–2128 (doi:10.1016/j.cub.2006.08.087) [DOI] [PubMed] [Google Scholar]
- Boi S., Couzin I. D., Del Buono N., Franks N. R., Britton N. F.1999Coupled oscillators and activity waves in ant colonies. Proc. R. Soc. Lond. B 266, 371–378 (doi:10.1098/rspb.1999.0647) [Google Scholar]
- Brick O., Jakobsson S.2002Individual variation in risk taking: the effect of a predatory threat on fighting behavior in Nannacara anomala. Behav. Ecol. 13, 439–442 (doi:10.1093/beheco/13.4.439) [Google Scholar]
- Britton N. F., Franks N. R., Pratt S. C., Seeley T. D.2002Deciding on a new home: how do honeybees agree? Proc. R. Soc. Lond. B 269, 1383–1388 (doi:10.1098/rspb.2002.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl J., Sumpter D. J. T., Couzin I. D., Hale J. J., Despland E., Miller E. R., Simpson S. J.2006From disorder to order in marching locusts. Science 312, 1402–1406 (doi:10.1126/science.1125142) [DOI] [PubMed] [Google Scholar]
- Camazine S., Sneyd J.1991A model of collective nectar source selection by honeybees: self-organization through simple rules. J. Theor. Biol. 149, 547–571 (doi:10.1016/S0022-5193(05)80098-0) [Google Scholar]
- Camazine S., Deneubourg J. L., Franks N. R., Sneyd J., Theraulaz G., Bonabeau E.2001Self-organization in biological systems Princeton, NJ: Princeton University Press [Google Scholar]
- Conradt L., Roper T. J.2005Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456 (doi:10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- Conradt L., Krause J., Couzin I. D., Roper T. J.2009‘Leading according to need’ in self-organizing groups. Am. Nat. 173, 304–312 (doi:10.1086/596532) [DOI] [PubMed] [Google Scholar]
- Cote J., Dreiss A., Clobert J.2008Social personality trait and fitness. Proc. R. Soc. B 275, 2851–2858 (doi:10.1098/rspb.2008.0783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin I. D., Krause J.2003Self-organization and collective behavior in vertebrates. Adv. Study Behav. 32, 1–75 (doi:10.1016/S0065-3454(03)01001-5) [Google Scholar]
- Couzin I. D., Krause J., Franks N. R., Levin S. A.2005Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516 (doi:10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- Croft D., Krause J., Darden S., Ramnarine I., Faria J., James R.2009Behavioural trait assortment in a social network: patterns and implications. Behav. Ecol. Sociobiol. 63, 1495–1503 (doi:10.1007/s00265-009-0802-x) [Google Scholar]
- Dall S. R. X., Houston A. I., McNamara J. M.2004The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 (doi:10.1111/j.1461-0248.2004.00618.x) [Google Scholar]
- Daruka I.2009A phenomenological model for the collective landing of bird flocks. Proc. R. Soc. B 276, 911–917 (doi:10.1098/rspb.2008.1444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneubourg J. L.1977Application de l'ordre par fluctuations à la description de certaines étapes de la construction du nid chez les termites. Insect. Soc. 24, 117–130 (doi:10.1007/BF02227166) [Google Scholar]
- Depickere S., Fresneau D., Deneubourg J. L.2004A basis for spatial and social patterns in ant species: dynamics and mechanisms of aggregation. J. Insect. Behav. 17, 81–97 (doi:10.1023/B:JOIR.0000025134.06111.be) [Google Scholar]
- Dingemanse N. J., Réale D.2005Natural selection and animal personality. Behaviour 142, 1159–1184 (doi:10.1163/156853905774539445) [Google Scholar]
- Dussutour A., Fourcassié V., Helbing D., Deneubourg J. L.2004Optimal traffic organization in ants under crowded conditions. Nature 428, 70–73 (doi:10.1038/nature02345) [DOI] [PubMed] [Google Scholar]
- Dussutour A., Nicolis S. C., Despland E., Simpson S. J.2008Individual differences influence collective behaviour in social caterpillars. Anim. Behav. 76, 5–16 (doi:10.1016/j.anbehav.2007.12.009) [Google Scholar]
- Dyer J. R. G., Croft D. P., Morrell L. J., Krause J.2009Shoal composition determines foraging success in the guppy. Behav. Ecol. 20, 165–171 (doi:10.1093/beheco/arn129) [Google Scholar]
- Fischhoff I. R., Sundaresan S. R., Cordingley J., Larkin H. M., Sellier M. J., Rubenstein D. I.2007Social relationships and reproductive state influence leadership roles in movements of plains zebra, Equus burchellii. Anim. Behav. 73, 825–831 (doi:10.1016/j.anbehav.2006.10.012) [Google Scholar]
- Franks N. R., Wilby A., Silverman B. W., Tofts C.1992Self-organizing nest construction in ants: sophisticated building by blind bulldozing. Anim. Behav. 44, 357–375 (doi:10.1016/0003-3472(92)90041-7) [Google Scholar]
- Gautrais J., Michelena P., Sibbald A., Bon R., Deneubourg L.2007Allelomimetic synchronization in Merino sheep. Anim. Behav. 74, 1443–1454 (doi:10.1016/j.anbehav.2007.02.020) [Google Scholar]
- Giraldeau L. A., Beauchamp G.1999Food exploitation: searching for the optimal joining policy. Trends Ecol. Evol. 14, 102–106 (doi:10.1016/S0169-5347(98)01542-0) [DOI] [PubMed] [Google Scholar]
- Giraldeau L. A., Caraco T.2000Social foraging theory Princeton, NJ: Princeton University Press [Google Scholar]
- Gosling S. D.2001From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86 (doi:10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- Goss S., Deneubourg J. L.1988Autocatalysis as a source of synchronised rhythmical activity in social insects. Insect. Soc. 35, 310–315 (doi:10.1007/BF02224063) [Google Scholar]
- Gueron S., Levin S.1993Self-organization of front patterns in large wildebeest herds. J. Theor. Biol. 165, 541–552 (doi:10.1006/jtbi.1993.1206) [Google Scholar]
- Harcourt J. L., Ang T. Z., Sweetman G., Johnstone R. A., Manica A.2009aSocial feedback and the emergence of leaders and followers. Curr. Biol. 19, 248–252 (doi:10.1016/j.cub.2008.12.051) [DOI] [PubMed] [Google Scholar]
- Harcourt J. L., Sweetman G., Johnstone R. A., Manica A.2009bPersonality counts: the effect of boldness on shoal choice in three-spined sticklebacks. Anim. Behav. 77, 1501–1505 (doi:10.1016/j.anbehav.2009.03.004) [Google Scholar]
- Jeanson R., Deneubourg J. L.2007Conspecific attraction and shelter selection in gregarious insects. Am. Nat. 170, 47–58 (doi:10.1086/518570) [DOI] [PubMed] [Google Scholar]
- Jeanson R., Deneubourg J.-L., Grimal R., Theraulaz G.2004Modulation of individual behavior and collective decision-making during aggregation site selection by the ant Messor barbarus. Behav. Ecol. Sociobiol. 55, 388–394 (doi:10.1007/s00265-003-0716-y) [Google Scholar]
- Kerth G., Ebert C., Schmidtke C.2006Group decision making in fission–fusion societies: evidence from two-field experiments in Bechstein's bats. Proc. R. Soc. B 273, 2785–2790 (doi:10.1098/rspb.2006.3647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. J., Douglas C. M. S., Huchard E., Isaac N. J. B., Cowlishaw G.2008Dominance and affiliation mediate despotism in a social primate. Curr. Biol. 18, 1833–1838 (doi:10.1016/j.cub.2008.10.048) [DOI] [PubMed] [Google Scholar]
- King A. J., Isaac N. J. B., Cowlishaw G.2009Ecological, social, and reproductive factors shape producer–scrounger dynamics in baboons. Behav. Ecol. 20, 1039–1049 (doi:10.1093/beheco/arp095) [Google Scholar]
- Koolhaas J. M., Korte S. M., de Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., de Jong I. C., Ruis M. A. W., Blokhuis H. J.1999Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- Kurvers R., Eijkelenkamp B., Van Oers K., Van Lith B., Van Wieren S. E., Ydenberg R. C., Prins H. H. T.2009Personality differences explain leadership in barnacle geese. Anim. Behav. 78, 447–453 (doi:10.1016/j.anbehav.2009.06.002) [Google Scholar]
- Magnhagen C., Staffan F.2005Is boldness affected by group composition in young-of-the-year perch (Perca fluviatilis)? Behav. Ecol. Sociobiol. 57, 295–303 (doi:10.1007/s00265-004-0834-1) [Google Scholar]
- Mattila H. R., Seeley T. D.2007Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317, 362–364 (doi:10.1126/science.1143046) [DOI] [PubMed] [Google Scholar]
- Michelena P., Sibbald A. M., Erhard H. W., McLeod J. E.2009Effects of group size and personality on social foraging: the distribution of sheep across patches. Behav. Ecol. 20, 145–152 (doi:10.1093/beheco/arn126) [Google Scholar]
- Nicolis S. C., Despland E., Dussutour A.2008Collective decision-making and behavioral polymorphism in group living organisms. J. Theor. Biol. 254, 580–586 (doi:10.1016/j.jtbi.2008.06.028) [DOI] [PubMed] [Google Scholar]
- Oldroyd B. P., Fewell J. H.2007Genetic diversity promotes homeostasis in insect colonies. Trends Ecol. Evol. 22, 408–413 (doi:10.1016/j.tree.2007.06.001) [DOI] [PubMed] [Google Scholar]
- Palsson E., Othmer H. G.2000A model for individual and collective cell movement in Dictyostelium discoideum. Proc. Natl Acad. Sci. USA 97, 10 448–10 453 (doi:10.1073/pnas.97.19.10448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish J. K., Viscido S. V., Grünbaum D.2002Self-organized fish schools: an examination of emergent properties. Biol. Bull. 202, 296–305 (doi:10.2307/1543482) [DOI] [PubMed] [Google Scholar]
- Petit O., Gautrais J., Leca J. B., Theraulaz G., Deneubourg J. L.2009Collective decision-making in white-faced capuchin monkeys. Proc. R. Soc. B 276, 3495–3503 (doi:10.1098/rspb.2009.0983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike T. W., Samanta M., Lindstrom J., Royle N. J.2008Behavioural phenotype affects social interactions in an animal network. Proc. Natl Acad. Sci. USA 275, 2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseyer A., Boissy A., Dumont B., Thierry B.2009Decision making in group departures of sheep is a continuous process. Anim. Behav. 78, 71–78 (doi:10.1016/j.anbehav.2009.03.017) [Google Scholar]
- Rasse P., Deneubourg J. L.2001Dynamics of nest excavation and nest size regulation of Lasius niger (Hymenoptera: Formicidae). J. Insect. Behav. 14, 433–449 (doi:10.1023/A:1011163804217) [Google Scholar]
- Réale D., Gallant B. Y., Leblanc M., Festa-Bianchet M.2000Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim. Behav. 60, 589–597 (doi:10.1006/anbe.2000.1530) [DOI] [PubMed] [Google Scholar]
- Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J.2007Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- Scott C. B., Provenza F. D., Banner R. E.1995Dietary habits and social interactions affect choice of feeding location by sheep. Appl. Anim. Behav. Sci. 45, 225–237 (doi:10.1016/0168-1591(95)00605-R) [Google Scholar]
- Sibbald A. M., Erhard H. W., McLeod J. E., Hooper R. J.2009Individual personality and the spatial distribution of groups of grazing animals: an example with sheep. Behav. Proc. 82, 319–326 (doi:10.1016/j.beproc.2009.07.011) [DOI] [PubMed] [Google Scholar]
- Sih A., Watters J. V.2005The mix matters: behavioural types and group dynamics in water striders. Behaviour 142, 1417–1431 (doi:10.1163/156853905774539454) [Google Scholar]
- Sih A., Bell A., Johnson J. C.2004Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- Stueckle S., Zinner D.2008To follow or not to follow: decision making and leadership during the morning departure in chacma baboons. Anim. Behav. 75, 1995–2004 (doi:10.1016/j.anbehav.2007.12.012) [Google Scholar]
- Sumpter D. J. T.2006The principles of collective animal behaviour. Phil. Trans. R. Soc. B 361, 5–22 (doi:10.1098/rstb.2005.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter D. J. T., Krause J., James R., Couzin I. D., Ward A. J. W.2008Consensus decision making by fish. Curr. Biol. 18, 1773–1777 (doi:10.1016/j.cub.2008.09.064) [DOI] [PubMed] [Google Scholar]
- Van Oers K., de Jong G., Van Noordwijk A. J., Kempenaers B., Drent P. J.2005Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142, 1185–1206 (doi:10.1163/156853905774539364) [Google Scholar]
- Vickery W. L., Giraldeau L. A., Templeton J. J., Kramer D. L., Chapman C. A.1991Producers, scroungers, and group foraging. Am. Nat. 137, 847–863 (doi:10.1086/285197) [Google Scholar]
- Ward A. J. W., Thomas P., Hart P. J. B., Krause J.2004Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus). Behav. Ecol. Sociobiol. 55, 561–568 (doi:10.1007/s00265-003-0751-8) [Google Scholar]
- Wilson D. S., Clark A. B., Coleman K., Dearstyne T.1994Shyness and boldness in humans and other animals. Trends Ecol. Evol. 9, 442–446 (doi:10.1016/0169-5347(94)90134-1) [DOI] [PubMed] [Google Scholar]