Abstract
The objective of this work was to produce drug-loaded nanometre- and micrometre-scale particles using a single-step process that provides control over particle size and size distribution. Co-axial electrohydrodynamic processing was used, at ambient temperature and pressure, with poly(lactic-co-glycolic acid) as the polymeric coating material and oestradiol as the encapsulated drug. The particle diameter was varied from less than 120 nm to a few micrometres, by simple methodical adjustments in the processing parameters (polymer concentration and applied voltage). In vitro studies were performed to determine the drug release profile from the particles during unassisted and ultrasound-stimulated degradation in simulated body fluid. An encapsulation efficiency of approximately 70% was achieved and release of the drug was sustained for a period of over 20 days. Exposing the particles to ultrasound (22.5 kHz) increased the rate of release by approximately 8 per cent. This processing method offers several advantages over conventional emulsification techniques for the preparation of drug-loaded particles. Most significantly, process efficiency and the drug's functionality are preserved, as complex multistep processing involving harsh solvents, other additives and elevated temperatures or pressures are avoided. Production rates of 1012 particles min−1 can be achieved with a single pair of co-axial needles and the process is amenable to being scaled up by using multiple sets.
Keywords: biodegradable polymeric particles, encapsulation, drug delivery, electrohydrodynamic processing
1. Introduction
Encapsulation of drugs in biodegradable polymeric carriers is one of the most significant areas of current pharmaceutical and medical science research (Dai et al. 2005; Freitas et al. 2005). Enabling drugs to be released in a controlled manner helps to maintain a constant therapeutic concentration in body fluids over many hours or days from the moment of administration (Sinha & Trehan 2003). Different fabrication methods such as solvent evaporation and single and double emulsification are commonly employed to fabricate drug-loaded polymeric particles (Zhu et al. 2000; Yang et al. 2001; Zhang et al. 2008). However, there are inevitably certain disadvantages associated with these methods. For example, conventional emulsification methods result in a broad particle size distribution (Kissel et al. 1995). Also, some non-degradable additives such as surfactants or polymers are commonly used as emulsifiers (Freitas et al. 2005) and the separation of particles from the solvent can be time-consuming and expensive but needs to be carried out to reduce the amount of residual solvent to a safe level (Luan et al. 2006). Most importantly, the biofunctionality of drugs, especially in the case of biomacromolecules, can be greatly reduced during processing owing to exposure to, for example, elevated temperatures and high shear stresses (Rasiel et al. 2002; Vandervoort & Ludwig 2002; Kohane et al. 2006).
Electrohydrodynamic processing (EHP) is an attractive method for generating particles suitable for use as drug delivery vehicles (Ding et al. 2005; Pareta & Edirisinghe 2006). In EHP, a liquid is fed to a nozzle at the end of which a droplet is formed. When the droplet is exposed to a strong electric field, a charge is induced on its surface. Provided the liquid has sufficient electrical conductivity, there will be a range of combinations of the liquid flow rate and the applied voltage for which the drop will assume a conical shape (Xie et al. 2006) at the apex of which a narrow jet is formed (cone-jet mode) that subsequently breaks up into fine droplets. The co-axial method used in this work, whereby two concentric liquid jets are formed simultaneously, provides a means of directly encapsulating a drug inside a polymeric carrier of pre-determined size in a single step, unlike many existing methods that require two or more steps to achieve the encapsulated product. Particles are produced at the ambient temperature and pressure, thus overcoming the problems associated with defunctionalization of drugs under extreme processing conditions. A further significant feature of this technique is its ability to generate particles with a narrow size distribution, and a mean diameter that can be varied between hundreds of micrometres and tens of nanometres by varying the processing parameters for a particular system, e.g. flow rate(s), needle diameter(s) and applied voltage (Yeo et al. 2005; Ahmad et al. 2008; Samarasinghe et al. 2008; Xie et al. 2008) as well as the chemical composition and concentration of the encapsulating polymer solution.
A number of polymers have been investigated for drug delivery systems, but poly(lactic-co-glycolic acid) (PLGA), a copolymer of polyglycolide and polylactide units, has been most extensively used because of its biocompatibility, biodegradability and versatile degradation kinetics (Burton et al. 2000; Körber & Bodmeier 2008). The biodegradation products of PLGA (lactic and glycolic acids) are normal body metabolites, which are non-toxic, non-immunogenic, non-carcinogenic and non-teratogenic (Ravivarapu et al. 2000). These products are removed from the body by entering the tricarboxylic acid cycle and/or by kidney excretion and eventually eliminated as carbon dioxide and water. Furthermore, the composition of the polymer can be varied to obtain a desired release profile based on the rate of hydrolytic degradation. For these reasons, lactic/glycolic acid polymers have become increasingly important in the development of drug delivery systems (Jain 2000).
Another important feature of such delivery systems is the size of the particles themselves. Controlling the dimensions of the particles, from the micrometre to the nanometre scale, provides a means of varying the surface area to volume ratio for the particle population. The size of the individual particles is also of great importance, since it will determine their ability to penetrate tissue structures in vivo, which may be both desirable and undesirable in different situations (Sinha et al. 2006). This remains a contentious area and consequently it is highly desirable for processing techniques such as the one used in this study to be able to facilitate the generation of a range of particle sizes (Gelperina et al. 2005).
Oestradiol is used as a model drug in the present work and is a sex hormone which is often mislabelled the ‘female’ hormone. Oestrogens are endogenous substances with a variety of effects on the human body, including growth and maintenance of the female reproductive system and female sex characteristics (Variankaval et al. 2002; Pazol et al. 2004). Oestradiol is the principal oestrogen and is substantially more potent than oestrone or oestriol (its major metabolites) and is prescribed for moderate-to-severe menopausal symptoms and for the prevention of post-menopausal osteoporosis sometimes in combination with progestins (Deady 2004). It is a hydrophobic compound that has low aqueous solubility (1 in 10 000) and undergoes high gut wall and first-pass metabolism, contributing to poor (approx. 10%) oral bioavailability. In such cases, polymer-encapsulated nanometre and micrometre particles are promising candidates as drug-delivery carriers as they allow the drug to be shielded from these two biological processes (Hariharan et al. 2006).
This study demonstrates the feasibility of fabricating drug-loaded particles of various sizes ranging from tens of micrometres to tens of nanometres, using co-axial EHP. The process is a single-step fabrication method and was optimized so that the particle size range was controlled by systematic variation of the operating factors, specifically polymer concentration and applied voltage. For this study, PLGA (monomer ratio 50∶50) was used, which is known to hydrolyse at a faster rate than those containing a higher proportion of polylactic acid (Jain 2000). The effect of polymer concentration on the morphology, structure and size distribution of the drug-loaded particles produced was investigated using three different polymer/solvent ratios (2, 5 and 10 wt%). The influence of polymer concentration on the release profiles was also studied in vitro using ultraviolet (UV) spectrophotometry for various conditions, including following exposure to ultrasound.
2. Material and methods
2.1. Materials
PLGA (copolymer 50 : 50, Resomer RG503H, molecular weight = 33 000 kDa, inherent viscosity 0.41 dl g−1) was purchased from Boehringer Ingelheim (Ingelheim, Germany). Dimethylacetamide (DMAC), methanol, ethanol, oestradiol and Evans blue dye were purchased from Sigma Aldrich (Poole, UK).
2.2. Particle fabrication
PLGA solutions of different concentrations (2, 5 and 10 wt%) were prepared by combining appropriate amounts of polymer and DMAC and mechanically stirring for 900 s to obtain clear solutions indicating the total dissolution of the PLGA. Oestradiol (10 wt%) was dissolved in methanol. To assist visualization in the preliminary experiments, Evans blue dye (0.01 g) was dissolved in distilled water (5 ml) and then added to the methanol solution and thoroughly mixed to prepare a drug–dye solution. This was used to determine firstly the feasibility of encapsulating the solution and secondly whether the cone-jet mode was achievable using both the polymer and drug solutions. Subsequently, the drug solution was used without dye.
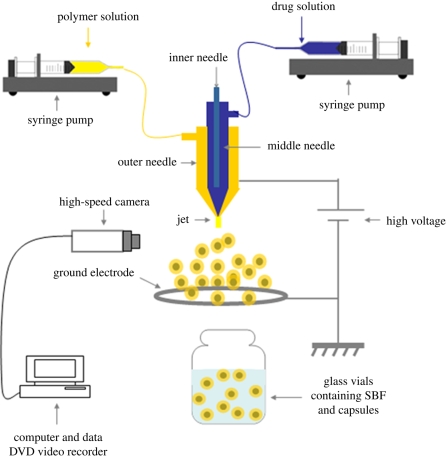
In this study, co-axial jets (of two immiscible liquids) were used to prepare polymeric drug-loaded particles with different size ranges. A three-needle stainless steel co-axial device (Ahmad et al. 2008) was coupled to a high-power voltage supply (Glassman Europe Ltd, Tadley, UK) (figure 1). In this case, the central needle and outer needles were used and the inner needle was disabled. The central needle carried the drug solution while the outer needle was perfused with a given PLGA solution to produce the polymeric coating. The two liquids were introduced simultaneously into the device using precision syringe pumps (PHD 4400, Harvard Apparatus, Edenbridge, UK) at inner and outer needle flow rates of 2 and 10 µl min−1, respectively. This combination of flow rates was determined empirically to provide a suitable compromise between jet stability and the required range of particle sizes. The external and internal diameters of the central needle were, respectively, 1.5 and 0.9 mm and those of the outer needle were 2.8 and 1.9 mm. The tip of the inner needle was held 0.5 mm inside and above the tip of the outer needle. The device was connected to the high-power voltage supply, and at an applied voltage of between 6 and 7 kV (figure 2) between the needles and a ground ring electrode, a stable cone jet was obtained, with fine encapsulated droplets emerging from the cone apex. The droplets were collected at a working distance of 20 mm below the device exit directly into glass vials containing 50 per cent simulated body fluid (SBF) and 50 per cent ethanol. Samples were collected for 600 s and were used for the in vitro release studies. Specimen samples were also collected on glass slides covered by a thin film of ethanol for microscopy. The jet and droplet formation processes were monitored using a Leica S6D JVC-colour video camera connected to a data DVD video recorder MP-600.
Figure 1.
Experimental set-up used for the production of drug-loaded particles.
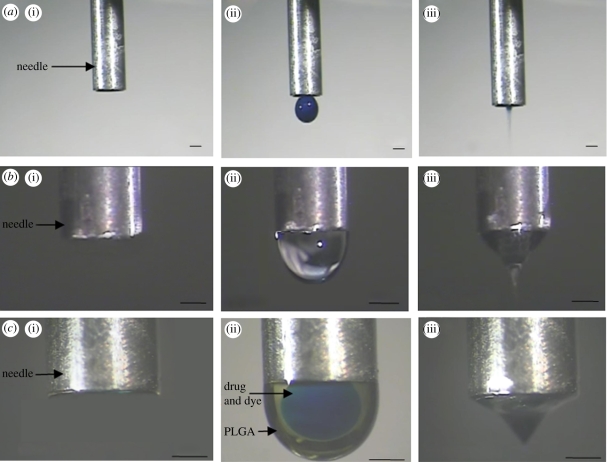
Figure 2.
Flow of liquid under an electric field using a single (central) needle showing (a)(i) no flow, (ii) dripping mode and (iii) cone-jet mode. Single needle (outer needle) showing (b)(i) no flow, (ii) dripping mode and (iii) cone-jet mode. Co-flowing solutions showing (c)(i) no flow, (ii) dripping mode demonstrating immiscibility and (iii) cone-jet for encapsulation. The applied voltage ranges were 4–5, 5–6 and 5–6 kV for a(iii), b(iii), c(iii), respectively. Scale bars, (a–c) 1 mm.
2.3. Characterization of particles
The morphology and structure of the prepared particles were determined using scanning electron microscopy (Jeol JSM-6301F field emission SEM) and optical microscopy (Nikon Eclipse ME600). The particles were collected on glass microscope slides and dried under ambient conditions (25°C) in a desiccator. Prior to the SEM studies, dried samples were sputter coated with gold for 60 s. The accelerating voltage ranged from 5 to 10 kV during scanning.
2.4. In vitro release study
For the in vitro release studies, samples were collected in glass vials and kept at 37°C with continuous stirring, and 50 : 50 SBF : ethanol (v/v) solution was chosen because of the low solubility of β-oestradiol in water or buffered solutions. At discrete time intervals, samples were centrifuged for 45 min at 4300 r.p.m. and the entire supernatant was extracted and its absorbance was measured using a UV spectrophotometer (UV-2401PC spectrophotometer, Shimadzu) at 280 nm following the protocol of Hariharan et al. (2006). Fresh SBF : ethanol solution was added to the vial immediately after the solution to be measured was removed to maintain a constant volume. Samples without β-oestradiol were also monitored so that any contributions to the measured absorbance from the polymer could be evaluated. The oestradiol calibration curve was obtained by dissolving the drug in an SBF : ethanol (50∶50 v/v) mixture after checking that the presence of ethanol (up to 50% by volume) modified neither the specific extinction nor the wavelength at which the maximum in UV absorbance appears.
The amount of unencapsulated drug in the supernatant obtained immediately following particle generation was measured to determine the encapsulation efficiency. This was calculated as the mass ratio between the amount of oestradiol incorporated into the particles and that which was used in the particle preparation.
The prepared particles were exposed to ultrasound using an ultrasonic cell disruptor (XL2000 Misonix Inc., Farmingdale, NY, USA; probe diameter 3 mm, operating centre frequency 22.5 kHz). Particles were collected in the same manner as above and then gently poured into a waterproof elastic rubber (latex) tube which is relatively transparent to ultrasound at kilohertz frequencies (Fatemi & Greenleaf 1998). The tube and the ultrasound probe were immersed in a beaker of water with a distance of 40 mm between them. The probe was held at an angle of approximately 45° to the vertical so that the particles were not directly in line with the probe axis and thus exposed to relatively low-intensity ultrasound.1 Continuous-wave ultrasound was applied for either 15 or 30 s, after which the sample was returned to the vial and UV spectrophotometry measurements were carried out as before. Control measurements were carried out to ensure that there was no change in the UV spectrophotometry readings owing to transfer of the particles between the vial and the tube.
3. Results and discussion
3.1. Encapsulation process
The jetting modes generated for flowing liquids under the influence of an electric field are functions of the applied voltage, the ‘working distance’ between the needle outlet and the collecting plane, the flow rate of the liquid, needle diameter and the properties of the flowing liquids (Jaworek & Krupa 1999). As the applied electric field strength increases either by increasing the applied voltage or by reducing the working distance, the atomization mode can be transformed from the dripping to the cone-jet mode. The cone-jet mode is the preferred atomization mode because it can produce uniform, stable and continuous particle generation with a controllable jet size (Amsden & Gossen 1997). A stable cone jet can be obtained for liquids satisfying the condition σi > σo where σ is the liquid-dielectric atmosphere surface tension and subscripts i and o refer to the inner and outer solution, respectively (Ku & Kim 2002). Another factor influencing the stability of the cone jet is the ability of the electric field to be able to interact with at least one of the solutions, which is sometimes referred to as a driving medium, and must be selected carefully to satisfy the requirements for electrical conductivity and surface tension (Lopez-Herrera et al. 2003). As shown in figure 2, stable cone-jet generation was found to be easily achievable for both solutions used in this study separately (figure 2a(iii),b(iii)) and under simultaneous flow (figure 2c(iii)). The immiscibility of the co-flowing media is clearly shown in figure 2c(ii), and this is essential for the encapsulation process. The inner blue-coloured solution (drug with dye added to enable visualization) was clearly encapsulated by the outer straw-yellow-coloured polymeric solution. As the applied voltage was increased, the desired co-axial cone jet formed (figure 2c(iii)). By the time the particles formed from the break up of the jet had reached the SBF : ethanol surface, most of the solvent had evaporated, leaving particles consisting of a PLGA matrix in which the oestradiol was incorporated.
3.2. Particle characteristics
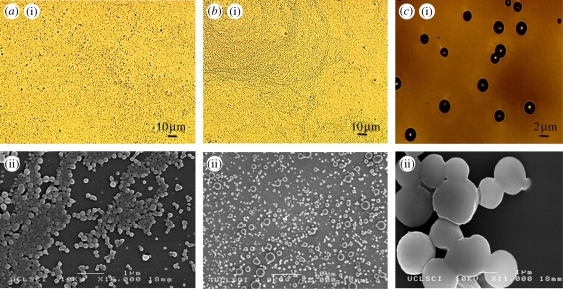
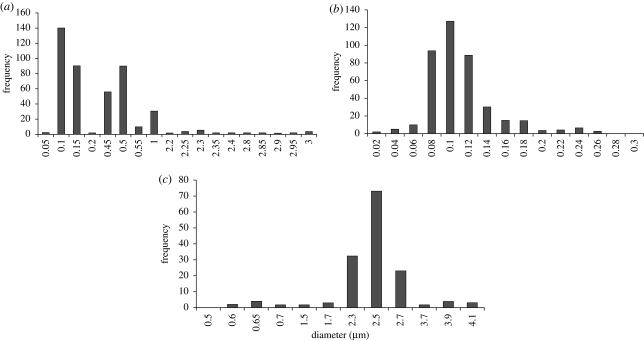
The optical images (figure 3a(i)–c(i)) demonstrate that very small particles were obtained when polymer concentrations of 2 and 5 per cent were used. Detailed analysis using SEM showed that all particles were spherical with a smooth outer surface (figure 3a(ii)–c(ii)). It was also observed that the mean particle diameter decreased as the polymer concentration was reduced. Particles with a diameter of 120 nm were produced using 2 and 5 wt% polymer solution. Particles prepared from the higher polymer concentration (10 wt%) agglomerated owing to the presence of residual solvent during drying. The higher polymer concentration leads to larger particles being produced and thus reduces the degree to which the solvent can diffuse out of the particles over the fixed working distance. The diameter of the particles was measured from the SEM images using the image-processing program UTHSCSA (Image Tool v. 2, University of Texas, USA). Particles prepared from 2 wt% PLGA had a bimodal size distribution with two peaks. Seventy per cent of the particles were less than 120 nm in diameter and 20 per cent of them were greater than 500 nm (figure 4a). In this case, the wider variation in particle size can be attributed to the generation of different types of droplets (‘primary’ and ‘satellite’) during the processing (Xu & Hanna 2006). More than 90 per cent of the particles prepared using 5 wt% PLGA were less than 120 nm in diameter (figure 4b). For the particles produced from the 10 wt% PLGA, 95 per cent had diameters greater than 2 µm (figure 4c). The polydispersivity index of the 5 wt% PLGA was the lowest (table 1). These results confirmed that the concentration of polymer in the liquid has a significant impact on the size of the particles produced and this is due primarily to the increase in viscosity with polymer concentration which results in larger droplets being formed (Jayasinghe & Edirisinghe 2002).
Figure 3.
(a(i)–c(i)) Optical microscopy and (a(ii)–c(ii)) SEM images of particles prepared with different polymer concentrations. (a): 2 wt% PLGA; (b): 5 wt% PLGA; (c): 10 wt% PLGA.
Figure 4.
Size distribution of particles prepared. (a) 2 wt% PLGA, (b) 5 wt% PLGA and (c) 10 wt% PLGA with corresponding applied voltage ranges of 4–5, 4–5 and 5–6 kV.
Table 1.
Size characteristics of the prepared particles.
| sample (wt%) | number of particles studied | mean size (nm) | standard deviation (nm) | polydispersivity (%) |
|---|---|---|---|---|
| PLGA 2 | 300 | 250 | 100 | 40 |
| PLGA 5 | 300 | 100 | 10 | 10 |
| PLGA 10 | 150 | 2500 | 400 | 15 |
3.3. In vitro release
Drug release from polymeric particles can take place by several mechanisms including diffusion, surface and bulk erosion, desorption and disintegration. In the case of PLGA particles, it occurs initially by diffusion from the polymer matrix, whereas during the later phases the release is mediated through both drug diffusion and degradation of the polymer matrix itself. PLGA degrades by means of hydrolysis (Schliecker et al. 2003). The inwards diffusion of the aqueous phase causes degradation of the polymer chains which in turn facilitates outwards diffusion of the entrapped drug (Zhang et al. 2008).
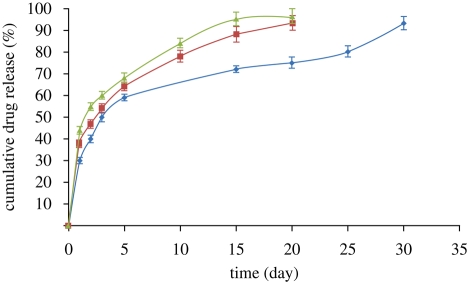
The in vitro release behaviour of the drug (oestradiol) from the PLGA particles was determined using UV spectrophotometry. Clear absorbance peaks were achieved at the expected wavelength for oestradiol (280 nm) indicating that the drug was not damaged during the encapsulation process. It was observed that oestradiol release was time dependent and all the drug-loaded nano- and microparticulate formulations showed a biphasic release pattern wherein there is an initial ‘burst’ release followed by a period of sustained release (figure 5). The release rate for the particles produced from the more concentrated solution (PLGA∶DMAC 10 wt%) was lower than that for those produced from the less concentrated solutions (PLGA : DMAC 2 and 5 wt%). This could be explained by the fact that the particles fabricated from the more dilute solutions were smaller in size and, with a decrease in particle size, the surface area to volume ratio increases leading to increased buffer penetration and faster drug diffusion. In the last stage, the release rate for all the samples increased significantly as degradative hydrolysis of the polymer particles took place and the central region, which would be expected to contain a higher concentration of drug, was exposed. The encapsulation efficiencies for the particles prepared from 2, 5 and 10 wt% polymer solution were 65, 68 and 75 per cent, respectively. This slight increase with polymer concentration may be due simply to the fact that an increase in the polymer concentration would result in higher resistance to outwards drug diffusion during processing.
Figure 5.
Oestradiol release profile from the PLGA particles produced in this work. Blue diamond, PLGA 10 wt%; red square, PLGA 5 wt%; green triangle, PLGA 2 wt%.
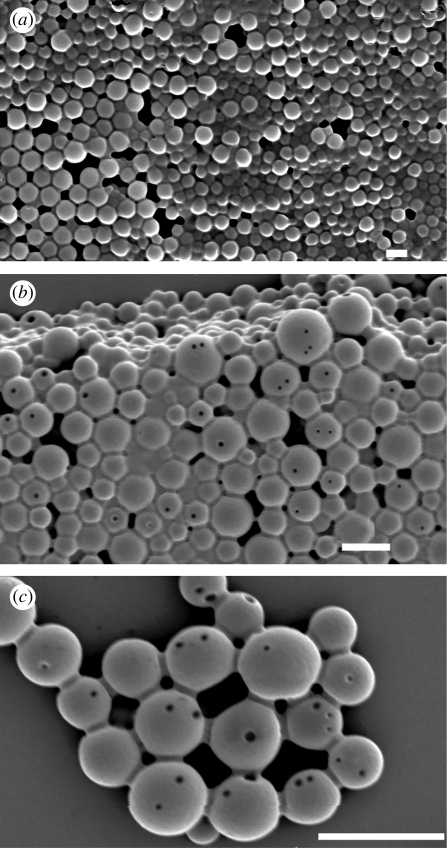
Another interesting feature of the surface of these particles during degradative hydrolysis was the variation in porosity as a function of time. The quantity and the size of pores have been observed to increase for PLGA-based implantable devices over prolonged periods in vivo (Schliecker et al. 2003). The surface morphology of the 10 wt% PLGA particles was studied over 30 days. The surface of the particles became increasingly porous (figure 6), characteristic of PLGA degradation, and this would explain the drug release patterns as above.
Figure 6.
Surface morphology of a particle prepared from 10 wt% PLGA as a function of time. (a) t = 0, (b) t = 15 days, (c) t = 30 days. Scale bars, (a–c) 5 µm.
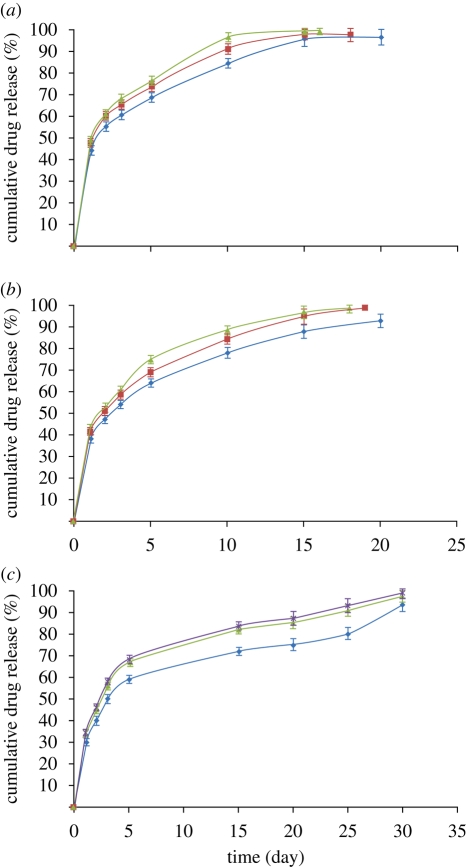
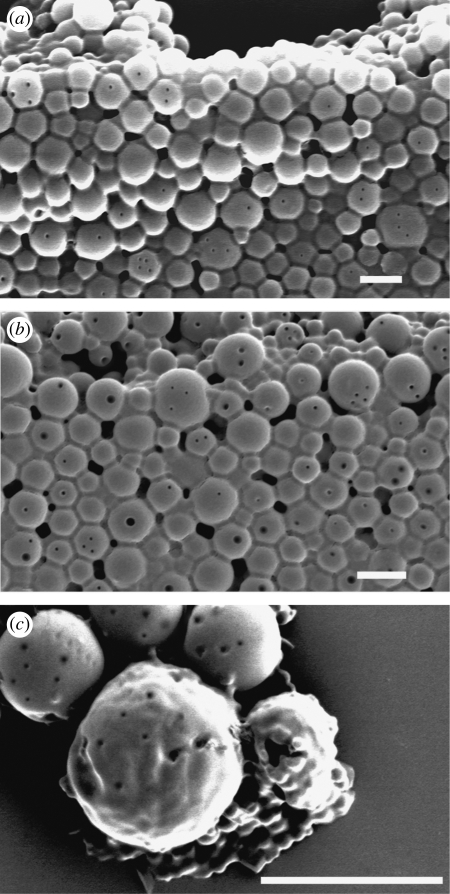
The release profile was clearly affected by exposing the particles to ultrasound. Ultrasound has been used in various drug delivery applications to enhance the release of pharmaceuticals in target tissues (Mitragotri 2005). The main mechanism for increased release is thought to be related to acoustic cavitation, which is the formation and/or activity of gas- or vapour-filled bubbles in the medium exposed to ultrasound (Larina et al. 2005). Such bubbles can collapse violently and disrupt the structure of the particles, because of the very high shear stresses in the region of the collapse, the shock wave produced by the collapse and/or the emission of a high-speed liquid jet from the bubble towards the particle surface (Nyborg 2001). There may also be chemical effects owing to free radicals produced by the high temperatures reached inside the bubble during collapse (Mornstein 1997). The collapsed bubbles often fragment into smaller bubbles that serve as cavitation nuclei, grow in size and eventually collapse again, thus sustaining the erosion process (Madersbacher & Marberger 2003). In this study, an increase in the rate of drug release was observed for all the samples exposed to ultrasound (figure 7 and table 2) with a greater increase obtained for longer exposures as would be expected. It was found that ultrasound had a slightly greater effect on the larger particles. This may be due to the higher probability of either gas entrapment in or on the particle's surface, or simply of its encountering a bubble owing to its larger size. The surfaces of the particles exposed to ultrasound were also seen to be rougher and contain more pores than the samples that were not exposed to ultrasound (figure 8). This may have been due to both mechanical damage (e.g. formation of liquid jets from collapsing bubbles) and also faster polymer degradation as a result of increased agitation of the surrounding liquid owing to the motion of the bubbles.
Figure 7.
Oestradiol release profile for PLGA particles following exposure to ultrasound (15 and 30 s). (a) PLGA : DMAC 2 : 98 (blue diamond, PLGA 2 wt%; red square, PLGA 2 wt% + 15 s US; green triangle, PLGA 2 wt% + 30 s US), (b) PLGA : DMAC 5 : 95 (blue diamond, PLGA 5 wt%; red square, PLGA 5 wt% + 15 s US; green triangle, PLGA 5 wt% + 30 s US) and (c) PLGA : DMAC 10 : 90 (blue diamond, PLGA 10 wt%; red square, PLGA 10 wt% + 15 s US; green triangle, PLGA 10 wt% + 30 s US). US, ultrasound.
Table 2.
Release characteristics of particles exposed to ultrasound.
| sample (wt%) | release difference after 15 s US (%) | release difference after 30 s US (%) |
|---|---|---|
| PLGA 2 | +3.5 | +5.7 |
| PLGA 5 | +4.4 | +6.8 |
| PLGA 10 | +6.3 | +7.8 |
Figure 8.
Surface morphology of a particle exposed to ultrasound as a function of time. (a) t = 0, (b) t = 15 days, (c) t = 30 days. Scale bars, (a–c) 5 µm.
4. Conclusions
This investigation demonstrates that co-axial EHP can successfully produce polymeric particles containing a drug via a single step process under ambient conditions. Particles with a mean size ranging from 100 nm to 2.5 µm and corresponding polydispersivity of 10–40% were produced with encapsulation efficiency ranging from 65% to 75%. The drug release from the particles was biphasic, starting with an initial short burst phase and followed by a longer period characterized by a lower release rate. The release rate from particles produced from PLGA : DMAC 10 wt% solution was markedly slower than from particles produced from PLGA : DMAC 2 and 5 wt% solutions. Exposing the particles to 22.5 kHz ultrasound for 30 s enhanced the rate of release by 7.8 per cent and was also seen to increase the porosity of the particles' surface.
Acknowledgements
This investigation was supported by a 2008 UCL Research Challenges award.
Footnotes
With the available apparatus, it was not possible to determine the intensity of the ultrasound to which the particles were exposed with a sufficient degree of accuracy but a more detailed study of the effect of ultrasound exposure parameters is currently in progress. The aim of this study was to see only whether any effect upon the rate of drug release could be observed upon ultrasound exposure.
References
- Ahmad Z., Zhang H. B., Farook U., Edirisinghe M., Stride E., Colombo P. 2008. Generation of multilayered structures for biomedical applications using a novel tri-needle coaxial device and electrohydrodynamic flow. J. R. Soc. Interface 27, 1255–1261. ( 10.1098/rsif.2008.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsden B. G., Gossen M. F. A. 1997. An examination of factors affecting the size, distribution and release characteristics of polymer microbeads made using electrostatics. J. Control. Release 43, 183–196. ( 10.1016/S0168-3659(96)01483-6) [DOI] [Google Scholar]
- Burton K. W., Shameem M., Thanoo B. C., Deluca P. P. 2000. Extended release peptide delivery systems through the use of PLGA microsphere combinations. J. Biomater. Sci 11, 715–729. ( 10.1163/156856200743977) [DOI] [PubMed] [Google Scholar]
- Dai C., Wang B., Zhao H. 2005. Microencapsulation peptide and protein drugs delivery system. Coll. Surf. B Biointerfaces 41, 117–120. ( 10.1016/j.colsurfb.2004.10.032) [DOI] [PubMed] [Google Scholar]
- Deady J. 2004. Clinical monograph: hormone replacement therapy. J. Manag. Care Pharm. 10, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Lee T., Wang C. 2005. Fabrication of monodisperse Taxol loaded particles using electro hydro dynamic atomization. J. Control. Release 102, 395–413. ( 10.1016/j.jconrel.2004.10.011) [DOI] [PubMed] [Google Scholar]
- Fatemi M., Greenleaf J. F. 1998. Ultrasound-stimulated vibro-acoustic spectrography. Science 280, 82–85. ( 10.1126/science.280.5360.82) [DOI] [PubMed] [Google Scholar]
- Freitas S., Merkle H. P., Gander B. 2005. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. J. Control. Release 102, 313–332. ( 10.1016/j.jconrel.2004.10.015) [DOI] [PubMed] [Google Scholar]
- Gelperina S., Kisich K., Iseman M. D., Heifets L. 2005. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 172, 1487–1490. ( 10.1164/rccm.200504-613PP) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan S., Bhardwaj V., Bala I., Sitterberg J., Bakowasky U., Kumar M. N. V. R. 2006. Design of estradiol loaded PLGA nanoparticulate formulations: a potential oral delivery system for hormone therapy. Pharm. Res. 23, 184–195. ( 10.1007/s11095-005-8418-y) [DOI] [PubMed] [Google Scholar]
- Jain A. 2000. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 21, 2475–2490. ( 10.1016/S0142-9612(00)00115-0) [DOI] [PubMed] [Google Scholar]
- Jaworek A., Krupa A. 1999. Classification of the modes of EHD spraying. J. Aerosol Sci. 30, 873–893. ( 10.1016/S0021-8502(98)00787-3) [DOI] [Google Scholar]
- Jayasinghe S. N., Edirisinghe M. J. 2002. Effect of viscosity on the size of relics produced by electrostatic atomization. J. Aerosol Sci. 33, 1379–1388. ( 10.1016/S0021-8502(02)00088-5) [DOI] [Google Scholar]
- Kissel T., Li Y. X., Volland C., Görich S., Koneberg R. 1995. Parenteral protein delivery systems using biodegradable polyesters of ABA block structure, containing hydrophobic poly(lactide-co-glycolide) A blocks and hydrophilic poly(ethylene oxide) B blocks. J. Control. Release 39, 315–326. ( 10.1016/0168-3659(95)00163-8) [DOI] [Google Scholar]
- Kohane D. S., Tse J. Y., Yeo Y., Padera R., Shubina M., Langer R. 2006. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. J. Biomed. Mater. Res. A 77A, 351–361. ( 10.1002/jbm.a.30654) [DOI] [PubMed] [Google Scholar]
- Körber M., Bodmeier R. 2008. Development of an in situ forming PLGA drug delivery system. I. Characterization of a non-aqueous protein precipitation. Eur. J. Pharm. Sci. 35, 283–292. ( 10.1016/j.ejps.2008.07.007) [DOI] [PubMed] [Google Scholar]
- Ku B. K., Kim S. S. 2002. Electrospray characteristics of highly viscous liquids. Aerosol Sci. Technol. 33, 1361–1378. ( 10.1016/S0021-8502(02)00075-7) [DOI] [Google Scholar]
- Larina I. V., Evers B. M., Ashitkov T. V., Bartels C., Larin K. V., Esenaliev R. O. 2005. Enhancement of drug delivery in tumors by using interaction of nanoparticles with ultrasound radiation. Technol. Cancer Res. Treat. 4, 217–226. [DOI] [PubMed] [Google Scholar]
- Lopez-Herrera J. M., Barrero A., Lopez A., Loscertales I. G., Marqueze M. 2003. Coaxial jets generated from electrified Taylor cones. Scaling laws. J. Aerosol Sci. 34, 535–552. ( 10.1016/S0021-8502(03)00021-1) [DOI] [Google Scholar]
- Luan X., Skupin M., Siepmann J., Bodmeier R. 2006. Key parameters affecting the initial release (burst) and encapsulation efficiency of peptide-containing poly(lactide-co-glycolide) microparticles. Int. J. Pharm. 324, 168–175. ( 10.1016/j.ijpharm.2006.06.004) [DOI] [PubMed] [Google Scholar]
- Madersbacher S., Marberger M. 2003. High-energy shockwaves and extracorporeal high-intensity focused ultrasound. J. Endourol. 17, 667–672. ( 10.1089/089277903322518680) [DOI] [PubMed] [Google Scholar]
- Mitragotri S. 2005. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 4, 255–260. ( 10.1038/nrd1662) [DOI] [PubMed] [Google Scholar]
- Mornstein V. 1997. Cavitation-induced risks associated with contrast agents used in ultrasonography. Eur. J. Ultrasound 5, 101–111. ( 10.1016/S0929-8266(97)00214-0) [DOI] [Google Scholar]
- Nyborg W. 2001. Biological effects of ultrasound: development of safety guidelines. Part II. General review. Ultrasound Med. Biol. 27, 301–333. ( 10.1016/S0301-5629(00)00333-1) [DOI] [PubMed] [Google Scholar]
- Pareta R., Edirisinghe M. J. 2006. A novel method for the preparation of biodegradable microspheres for protein drug delivery. J. R. Soc. Interface 3, 573–582. ( 10.1098/rsif.2006.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazol K., Kaplan J. R., Abbott D., Appt S. E., Wilson M. E. 2004. Practical measurement of total and bioavailable estradiol in female macaques. Clin. Chim. Acta 340, 117–126. ( 10.1016/j.cccn.2003.10.010) [DOI] [PubMed] [Google Scholar]
- Rasiel A., Sheskin T., Bergelson L., Domb A. J. 2002. Phospholipid coated poly(lactide acid) microspheres for the delivery of LHRH analogues. Polym. Adv. Technol. 13, 127–136. ( 10.1002/pat.164) [DOI] [Google Scholar]
- Ravivarapu H. B., Lee H., DeLuca P. P. 2000. Enhancing initial release of peptide from poly(d, l-lactide-co-glycolide) (PLGA) microspheres by addition of a porosigen and increasing drug load. Pharm. Dev. Technol. 5, 287–296. ( 10.1081/PDT-100100543) [DOI] [PubMed] [Google Scholar]
- Samarasinghe S. R., Balasubramanian K., Edirisinghe M. J. 2008. Encapsulation of silver particles using co-axial jetting. J. Mater. Sci. Mater. Elect. 19, 33–38. ( 10.1007/s10854-007-9278-5) [DOI] [Google Scholar]
- Schliecker G., Schmidt C., Fuchs S., Wombacher R., Kissel T. 2003. Hydrolytic degradation of poly(lactide-co-glycolide) films: effect of oligomers on degradation rate and crystallinity. Int. J. Pharm 266, 39–49. ( 10.1016/S0378-5173(03)00379-X) [DOI] [PubMed] [Google Scholar]
- Sinha V. R., Trehan A. 2003. Biodegradable microspheres for protein delivery. J. Control. Release 90, 261–280. ( 10.1016/S0168-3659(03)00194-9) [DOI] [PubMed] [Google Scholar]
- Sinha R., Kim G. J., Nie S., Shin D. M. 2006. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 5, 1909–1917. ( 10.1158/1535-7163.MCT-06-0141) [DOI] [PubMed] [Google Scholar]
- Vandervoort J., Ludwig A. 2002. Biocompatible stabilizers in the preparation of PLGA nanoparticles: a factorial design study. Int. J. Pharm. 238, 77–92. ( 10.1016/S0378-5173(02)00058-3) [DOI] [PubMed] [Google Scholar]
- Variankaval N. E., Jacob K. I., Dinh S. M. 2002. Polymorphism of 17-β estradiol in a transdermal drug delivery system. J. Mater. Sci. Mater. Med. 13, 271–280. ( 10.1023/A:1014058817352) [DOI] [PubMed] [Google Scholar]
- Xie J., Lim L. K., Phua Y. Y., Hua J. S., Wang C. H. 2006. Electrohydrodynamic atomization for biodegradable polymeric particle production. J. Coll. Interface Sci. 302, 103–112. ( 10.1016/j.jcis.2006.06.037) [DOI] [PubMed] [Google Scholar]
- Xie J., Lee W. N. L., Wang C. 2008. Encapsulation of protein drugs in biodegradable microparticles by co-axial electrospray. J. Coll. Interface Sci. 317, 469–476. ( 10.1016/j.jcis.2007.09.082) [DOI] [PubMed] [Google Scholar]
- Xu Y., Hanna M. A. 2006. Electrospray encapsulation of water-soluble protein with polylactide. Effects of formulations on morphology, encapsulation efficiency and release profile of particles. Int. J. Pharm. 320, 30–36. ( 10.1016/j.ijpharm.2006.03.046) [DOI] [PubMed] [Google Scholar]
- Yang Y. Y., Chung T. S., Ng N. P. 2001. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials 22, 231–241. ( 10.1016/S0142-9612(00)00178-2) [DOI] [PubMed] [Google Scholar]
- Yeo Y. L., Gagnon Z., Chang H. C. 2005. AC electrospray biomaterials synthesis. Biomaterials 26, 6122–6128. ( 10.1016/j.biomaterials.2005.03.033) [DOI] [PubMed] [Google Scholar]
- Zhang H., Lu Y., Zhang G., Gao S., Sun D., Zhong Y. 2008. Bupivacaine-loaded biodegradable poly(lactic-co-glycolic) acid microspheres. I. Optimization of the drug incorporation into the polymer matrix and modelling of drug release. Int. J. Pharm. 351, 244–249. ( 10.1016/j.ijpharm.2007.10.004) [DOI] [PubMed] [Google Scholar]
- Zhu G. Z., Mallery S. R., Schwendeman S. P. 2000. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide). Nat. Biotechnol. 18, 152–157. ( 10.1038/71916) [DOI] [PubMed] [Google Scholar]