Abstract
The zona pellucida (ZP) is the spherical layer that surrounds the mammalian oocyte. The physical hardness of this layer plays a crucial role in fertilization and is largely unknown because of the lack of appropriate measuring and modelling methods. The aim of this study is to measure the biomechanical properties of the ZP of human/mouse ovum and to test the hypothesis that Young's modulus of the ZP varies with fertilization. Young's moduli of ZP are determined before and after fertilization by using the micropipette aspiration technique, coupled with theoretical models of the oocyte as an elastic incompressible half-space (half-space model), an elastic compressible bilayer (layered model) or an elastic compressible shell (shell model). Comparison of the models shows that incorporation of the layered geometry of the ovum and the compressibility of the ZP in the layered and shell models may provide a means of more accurately characterizing ZP elasticity. Evaluation of results shows that although the results of the models are different, all confirm that the hardening of ZP will increase following fertilization. As can be seen, different choices of models and experimental parameters can affect the interpretation of experimental data and lead to differing mechanical properties.
Keywords: ovum, zona pellucida, micropipette aspiration, Young's modulus
1. Introduction
The mechanical properties of living cells play an important role in helping to understand cell physiology and pathology. As such, a quantitative study in single-cell mechanics needs to be conducted. In this context some mechanical models have been developed to quantitatively evaluate the mechanical properties and responses of living cells when subjected to stimulation and/or perturbation (Lim et al. 2006). Recently, considerable biomedical attention has concentrated on determination of the mechanical properties of oocytes as a single cell. Numerous studies concerning the mechanical properties of oocytes have been reported. These studies show that the variation of the oocyte stiffness is strongly influenced by mechanical characteristics of the biomembranes that isolate these structures from their immediate surroundings (Mitchison & Swann 1955; Hiramoto 1967, 1976; Yoneda & Dan 1972; Nakamura & Hiramoto 1978; Ohtsubo & Hiramoto 1985; Yoshida et al. 2000). According to previous reports on various animal species, fertilization of an oocyte leads to a change in the mechanical behaviour of the cell's extracellular biomembrane (Sun et al. 2003; Murayama et al. 2004, 2006, 2008). Zona pellucida (ZP) is the extracellular layer of glycoprotein enveloping an oocyte. This layer is modified after fertilization in a process described as zona reaction (Braden et al. 1954; Wassarman et al. 2001; Zhao & Dean 2002). Immediately after a sperm triggers an oocyte, a cascade of calcium (Ca2+) floods the cell and the contents of cortical granules (CGs), a kind of special organelle in the egg, are released into the perivitelline space (PVS; cortical reaction), causing the ZP to become refractory to sperm binding and penetration (zona reaction; Okada et al. 1993; Tahara et al. 1996). The CG exudates cause zona sperm receptor modification and zona hardening thus blocking polyspermic penetration (Ducibella et al. 1993; Abbott & Ducibella 2001; Sun 2003). In zona reaction, increased resistance to dissolution by various biochemical agents has been evaluated in many species (Hatanaka et al. 1992; Iwamoto et al. 1999). This phenomenon has been termed zona hardening (Schmell et al. 1983). However, although the term zona hardening implies a mechanical change, to date the physical hardness of ZP has been examined with few techniques and is largely unknown.
In general, to measure the mechanical properties of a living cell, if the cell can be deformed in some way with a known force then its deformation can be measured. Until now several techniques have been developed to directly quantify the mechanical properties of single cells (Van Vliet et al. 2003; Lim et al. 2006). Besides various measurement systems, we have used the micropipette aspiration technique to quantify the ovum's mechanical properties. The micropipette aspiration technique in particular provides a versatile method for determining cellular properties such as Young's modulus (hardness) and viscosity (Evans & Yeung 1973, 1989; Hochmuth 2000), but has not previously been applied to the study of egg cells. With this technique, real-time measurements of pressure and deformation can be made, and in conjunction with theoretical models, can be used to determine the intrinsic mechanical and volumetric properties of a single cell. Here we have applied the micropipette aspiration technique to quantify the elasticity of the ZP layer.
In recent years, human in vitro fertilization (IVF) has become an important issue and better understanding of human oocyte behaviour in different physico-chemical environments is of major interest. As the penetration of the ZP shell by spermatozoa plays a crucial role in mammalian fertilization, hence any inability of spermatozoa to penetrate the ZP inevitably leads to infertility. IVF is the assisted therapeutic technique offered to patients suffering from reproduction difficulties. Therefore, it is inferred that to achieve improved IVF success rates, correctly estimating ZP elasticity may play a key role in optimizing embryo selection with the highest quality embryos as a selection criterion.
The objective of this paper is to study the mechanical properties of animal (mouse) and human ZP during fertilization. To determine Young's modulus of the ZP before and after fertilization, data from testing the ovum in a micropipette aspiration experiment have been evaluated with three different analytical elastic models of the ovum: half-space model, layered model and shell model. More specifically the study focuses on the mechanical change on the hardening of the ZP and mechanical properties of the ZP have been compared using the three models.
2. Material and methods
2.1. Preparation of human oocytes/embryos
The embryos were donated by infertile couples who did not wish to cryopreserve surplus embryos for future replacement. Female partners were first superovulated following downregulation, as described elsewhere (Porter et al. 1984). In brief, suppression of pituitary gonadotrophin secretion with the gonadotrophin-releasing hormone agonist (GnRHa) buserelin acetate (Superfact, Hoechst AG, Germany) at a dose of 500 µg d−1 by nasal spray was commenced in mid-luteal phase of the preceding ovarian cycle (day 21). Once ovarian suppression was confirmed (serum oestradiol ≤50 pg ml−1, LH ≤ 5 IU l−1), ovarian stimulation was initiated using a subcutaneous injection of 150 IU d−1 purified human menopausal gonadotrophin (HMG; Pergonal 500, Serono, Italy).
The dose was increased in tandem with ovarian follicular development as monitored by serial vaginal ultrasonography. When at least three follicles reached 18 mm in diameter, GnRHa and HMG were discontinued, and 10 000 IU of human chorionic gonadotrophin (HCG; Organon, The Netherlands) was administered. Oocyte retrieval was performed by ultrasound-guided follicle aspiration, 36–38 h after HCG administration. To remove cumuluse mass around the oocyte, the obtained cumulus–oocyte complexes were first denuded using Synvitro Cumulase (MediCult, Denmark) and gentle pipetting. Synvitro Cumulase is recombinant human hyaluronidase (80 U ml−1) in an animal produce-free mammalian expression system and allowed purification of the human glycoprotein to homogeneity with a purity 100-fold greater than the commonly used preparations. Synvitro Cumulase is therefore the safest and has no side effects on the oocyte with its ZP (MediCult, Product Catalogue 2008). The denuded oocytes underwent intracytoplasmic sperm injection (ICSI) and were cultured in G-1 v.3 (Vitrolife, Kungsbacka, Sweden) supplemented with 10 per cent recombinant human serum albumin (rHA; Vitrolife) for 24 h.
2.2. Retrieval of mouse oocytes/embryos
Six-to-eight-week-old female Naval Medical Research Institute (NMRI) mice were provided from Pasture Institute (Tehran, Iran). The mice were housed in an environmentally controlled room. Female mice were induced to superovulate by intraperitoneal injection of 7.5 IU pregnant mare's serum gonadotropin (PMSG; Intervet, The Netherlands) followed by 7.5 IU HCG (Intervet, The Netherlands) 48 h later. Oocytes were collected from oviducts approximately 14–16 h after HCG injection (48 h after the PMSG). The oocytes were treated with Synvitro Cumulase (MediCult) until the cumulus masses dissociated from the oocytes by gentle pipetting. They were rinsed and incubated at 37°C in T6 medium under 5 per cent CO2 in air before analysis. Some superovulated female mice were mated with males from the same strain and inspected for the presence of a vaginal plug the following day. Those with vaginal plugs were considered pregnant and were sacrificed 44–48 h post-HCG by cervical dislocation. Embryos were flushed from the oviduct with T6 medium supplemented with 4 mg ml−1 bovine serum albumin (BSA: Fraction V, Sigma). Morphologically normal embryos were washed and pooled in fresh G-1 v.3 (Vitrolife) with 10 per cent rHA (Vitrolife).
2.3. Instruments
The experimental system consisted of an inverted microscope (TE300, Nikon, Tokyo, Japan) equipped with Hoffman modulation optics (Nikon), a Micromanipulator 5173 (Eppendorf AG, Germany) for positioning the micropipette, a manual stage for positioning samples, a Transjector 5246 (Eppendorf AG) for generating well-controlled negative pressures, and a host computer for cell visual tracking and pressure data acquisition. The measurement resolution of the pressure system was 10 Pa. Sterilized glass micropipettes (borosilicate, B 100-75-10; Sutter Instrument Co., Novato, CA, USA) were pulled with heating (setting: ramp value, 500; heat = 750, pull = 55, vel. = 55, time = 200) using a micropipette puller (model P-97, Sutter Instrument Co.). The micropipette tip was cut on a microforge to obtain sharp edges. The system setup was mounted on a vibration isolation table for minimizing vibration.
2.4. Method of micropipette aspiration
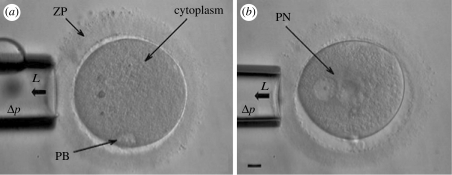
The ovum was brought into focus. The movement of the pipette was controlled using a micromanipulator, which allowed positioning of the pipette tip in the appropriate microscopic field. The ovum was held using a holding capillary and then was turned with the aid of a transfer capillary until the polar body (PB) came to rest either above (12.00) or below (06.00). Then the capillary and ovum were sharply focused. When the suction pressure in the pipette was generated, the surface of the ZP was aspirated into a glass pipette by using a controlled suction pressure and the resulting deformation was measured using video microscopy (figure 1). The test was carried out by applying a series of step increases in pressure (200 Pa) up to 2 kPa to the ZP and the ovum. A rest time of 30 s was considered in each step to allow for time-dependent recovery and cell equilibration. The length of the ZP projection inside the micropipette (L) was recorded at the end of each pressure interval (Δp).
Figure 1.
Micropipette aspiration of (a) human oocyte at MII stage and (b) human embryo at pronuclear (PN) stage. ZP, zona pellucida; PB, polar body; PN, pronuclei; L, aspiration length; Δp, applied negative pressure. Scale bar: 10 µm.
2.5. Equilibrium models
The micropipette aspiration (MA) technique can be used to quantify the elastic modulus of cells by pulling zona with a micropipette: when a pressure difference is applied, the membrane is put under tension and sucked inside the pipette (Evans & Yeung 1973, 1989; Hochmuth 2000). Here we applied the MA technique to quantify the elasticity of the ZP layer surrounding mammalian egg cells. Two different MA continuum models were used for characterizing the mechanical properties of solid-like (e.g. chondrocytes and endothelial cells) and liquid-like (e.g. neutrophils) cells (Needham & Hochmuth 1990; Ting-Beall et al. 1993; Discher et al. 1998; Jones et al. 1999). Nevertheless, the use of previous theories for determination of the ovum's mechanical properties suffers from significant limitations, especially regarding the treatment of sample layered condition which may bias the estimation of the sample stiffness in MA experiments.
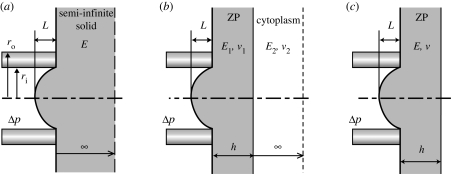
To determine Young's modulus of the ZP, experimental length–pressure data were analysed using three different theoretical models of the MA technique: a half-space model (Theret et al. 1988; Sato et al. 1990; Aoki et al. 1997; Haider & Guilak 2002), a layered half-space model and a shell model (Alexopoulos et al. 2003, 2005; figure 2). The half-space model, referred to as the punch model derived from Theret et al. (1988), assumes that the sample is an isotropic, incompressible, elastic half-space medium. With this model, Young's modulus of the ZP is given by
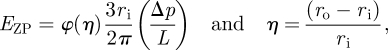 |
2.1 |
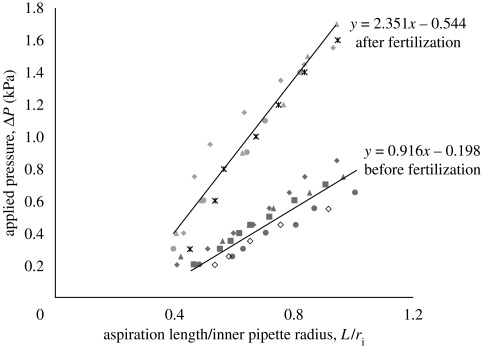
where φ(η) is defined as the wall function and is set equal to φ = 2.1; ri is the inner micropipette radius and (Δp/L) can be determined from the slope of the applied pressure versus the equilibrium length of ZP projection (figure 3).
Figure 2.
Mathematical models used for the quantification of mechanical properties of the ZP. (a) The half-space model assumes that the sample is an isotropic, incompressible, elastic (E) half-space medium. (b) The layered model represents the sample as a compressible (ν1), homogeneous, elastic layer (E1) with a finite thickness h (representing the ZP thickness) overlaying an isotropic homogeneous elastic half-space representing the cytoplasm (E2 and ν2). (c) In the final case, the ovum is modelled as a compressible (ν), layered half-space but under the assumption of zero stiffness for the cytoplasm (i.e. when EZP/Ecore→∞), resulting in a model of the ovum as an elastic (E) shell of thickness h. L, aspiration length; Δp, applied negative pressure; h, thickness of the ZP; ri, inner radius of micropipette; ro, outer radius of micropipette.
Figure 3.
Typical equilibrium response of the human ZP subjected to a series of step increases in aspiration pressure (Δp). Young's modulus of the ZP is determined from the slope of the length versus pressure curve, as described in the methods section. Each symbol represents an individual ovum for first donor (n = 5 cells). The second embryo was degenerated one day after injection then excluded from the study (p < 0.05). First donor, dark filled diamond, oocyte 1; dark filled square, oocyte 2; dark filled triangle, oocyte 3; dark filled circle, oocyte 4; open diamond, oocyte 5; light filled diamond, embryo 1; light filled triangle, embryo 3; light filled circle, embryo 4; asterisk, embryo 5.
To determine the influence of the layered structure of the ovum, it is represented by a compressible, homogeneous, elastic layer with a finite thickness h (representing the ZP thickness) overlaying an isotropic homogeneous elastic half-space which represents the cytoplasm. The primary advantage of this layered model is that it accounts for the thickness of the ZP and different mechanical properties in two distinct regions of the ovum. Young's modulus is again determined from the slope of the applied negative pressure (Δp) versus the normalized equilibrium length (L/ri) of ZP projection (figure 3), using the following equation:
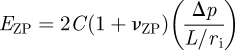 |
2.2 |
or
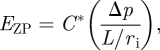 |
2.3 |
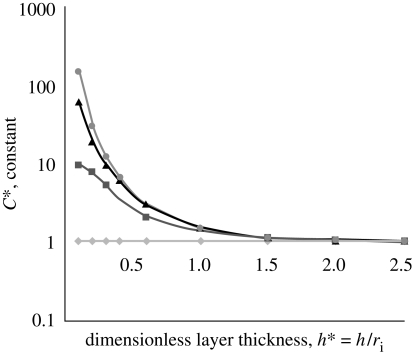
where C*=2C(1+νZP). In figure 4, the effect of the layer thickness h* and Young's moduli ratio EZP/Ecore, in the calculation of the constant C* for the Young's modulus of the ZP (equation (2.3)) by assuming incompressibility (ν1=ν2 = 0.5), are evaluated. If EZP/Ecore = 1 or h*→∞, we obtain the half-space solution (C* = 0.98). The most important parameters for the calculation of the constant C* are the dimensionless thickness of the layer h* = h/ri and Young's moduli ratio EZP/ECyt. It is also known that approximately 97 per cent of the ovum is water; therefore, it can be assumed that EZP/Ecore →∞, and thereby the layered model reduces to the aspiration of a shell (described as a shell model).
Figure 4.
Effect of the layer thickness h* and Young's moduli ratio EZP/Ecore in the calculation of the constant C* (filled diamond, 1 half space; filled square, 10; filled triangle, 100; filled circle, ∞).
In the final case, the ovum is modelled as described above for a compressible, layered half-space but under the assumption of zero stiffness for the cytoplasm (i.e. when EZP/ECyt→∞), resulting in a model of the ovum as a shell of thickness hZP. For calculation of the EZP, it is assumed that the properties of the substrate (cytoplasm) are similar to those of pure water (Saul & Wagner 1989; Smith et al. 2005). In addition, the Poisson ratio of the ZP is set equal to that of pericellular matrix (PCM; ν2 = 0.04; Alexopoulos et al. 2003, 2005).
Young's modulus of the ZP (EZP) is given by the equation
| 2.4 |
In this case, the constant C* is only a function of the dimensionless thickness h*=h/ri and can be approximated for h* > 0.1 by the following equation (see Alexopoulos et al. 2003, 2005):
 |
2.5 |
3. Results
3.1. Human case studies
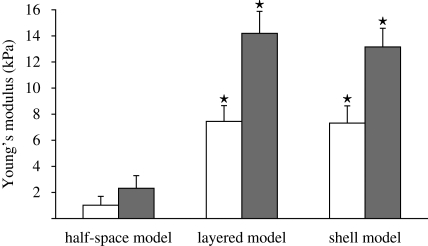
In this study, 15 surplus donated oocytes from three infertile couples were used. Oocytes did not exhibit major differences in shape or size based on the patients. Micropipette aspiration of the cells was carried out at metaphase-II (MII) and one day after insemination at the pronuclear (PN) stages. All ova exhibited linear deformation behaviour in response to a series of step increases in pressure (figure 3). The average value of ri for all micropipettes employed was 40 ± 5 µm, and the average value of hZP was 18.5 ± 4.5 µm. Using the layered model, embryos from donors exhibited a higher Young's modulus (EZP = 14.19 ± 1.18 kPa) than the oocyte (EZP = 7.47 ± 1.29 kPa). Both the shell and layered models predicted similar Young's moduli. Using the shell model, ZP of embryos have a mean Young's modulus equal to EZP = 13.18 ± 1.17 kPa, which was again higher than Young's modulus of ZP of the oocytes, EZP = 7.34 ± 1.36 kPa. The half-space model predicted a mean Young's modulus lower than that of the shell and layered models; ZP of embryos exhibited a mean Young's modulus EZP = 2.38 ± 1.24 kPa, which was higher than the Young's modulus of ZP of oocytes, EZP = 1.05 ± 0.51 kPa (figure 5).
Figure 5.
Young's modulus of the human ZP determined using the half-space model, the layered model and the shell model. Significant differences were found between the layered model and the half-space model (*p < 0.05 versus the half-space counterpart) as well as between the shell model and the half-space model (*p < 0.05 versus the half-space counterpart). Filled bar, embryo; unfilled bar, oocyte.
3.2. Animal (mouse) case studies
Mouse zona elasticity was measured at the MII (n = 22) and PN (n = 20) stages. The average value of ri for all micropipettes employed was 28 ± 5 µm, and the average value of hZP was 7.5 ± 1.3 µm. Using the shell model, Young's modulus of MII and PN stages was 11.8 ± 1.47 and 36.9 ± 2.34 kPa, respectively. The ZPs of all eggs showed significant hardening in the PN stage. The calculated hardening rates (3.1-fold) were almost the same as those characterized in previous studies.
4. Discussion
The objective of this study is to use an appropriate method to measure the mechanical properties of the ovum's extracellular envelope (ZP) and to determine whether these properties vary with fertilization. In this regard, we have used suitable mathematical models of micropipette aspiration to calculate the elastic properties of the ZP. Our findings support the hypothesis that there is a significant increase in Young's modulus of the ZP of the ovum after fertilization as compared to the non-fertilized ovum.
To calculate the mechanical properties of the ZP, we have used the available theoretical models of micropipette aspiration of an elastic layered structure, a half-space model, a layered half-space model and a shell model (Alexopoulos et al. 2003, 2005). The primary advantage of these models over previous models is that they account for different mechanical properties in two distinct regions of the ovum, a peripheral layer (ZP) and a substrate (cytoplasm). Young's modulus of the human embryo ZP at the PN stage (from the layered model EZP = 14.19 ± 1.18 kPa and from the shell model EZP = 13.18 ± 1.17 kPa) is found to be approximately 1.8-fold higher than that of Young's modulus of the oocyte ZP at MII stage (from the layered model EZP = 7.47 ± 1.29 kPa and from the shell model EZP = 7.34 ± 1.36 kPa). However, both the layered model and the shell model yield similar results. The important consideration in theoretical modelling of micropipette aspiration of the ovum is the incorporation of the ovum's layered geometry and compressibility of the ZP in the model. Significant differences have been found in the apparent modulus of the human ZP using different models, and the study suggests that neglecting ZP thickness and compressibility in an incompressible, elastic half-space model (PN: EZP = 2.38 ± 1.24 kPa versus MII: EZP = 1.05 ± 0.51 kPa) may lead to underestimation of the elastic modulus of the ZP by over 50 per cent. However, both the layered model and the shell model yielded close results. This study used human ovum ZP from different couples (n = 15 oocytes from the donors) but the ova were similar in terms of shape and size. Although the mechanical properties of the ZP might be influenced by genetics, age or other parameters (De Felice & Siracusa 1982; Cohen et al. 1990; Schiewe et al. 1995; Rankin & Dean 1996; Stanger et al. 2001), the study did not consider those parameters and left them for a future study.
To validate the animal (mouse) results, we compared our findings with previous attempts and observed a close agreement, as shown in table 1. In the first attempt, a quartz fibre method was used to measure the zona hardness of hamster ova by Green (1987, 1997), which concluded that sperm cannot penetrate the ZP only by force. The second attempt to measure zona hardness was made by Drobnis et al. (1988), who developed a ZP capillary suction apparatus. Hamster and mouse oocyte/embryos were deformed by decreasing pressure in a suction pipette and zona hardness was estimated from the zona deformation. The authors concluded that the ZP of mouse embryos was 1.8-fold harder than that of oocytes (Drobnis et al. 1988). The next attempt to measure zona hardness was made by Sun et al. (2003) with the benefit of recent developments in microelectromechanical system (MEMS) technology. Sun et al. (2003) developed a MEMS-based force sensor to measure forces applied to cells and concluded that mouse embryos were 2.4-fold harder than the oocytes. Although the previous two studies brought great advances in understanding zona hardening, the theories used improper models for the oocytes/embryos (the bilayer membrane was considered to be coupled with the ZP). Thus, in such a case, it was not theoretically suitable to calculate the elastic modulus of the ZP because the results should also relate to, for example: aspiration pressure, zona thickness and the entire cell size. More recently, Murayama et al. (2004, 2006, 2008) developed a noticeably less invasive tip, a microtactile sensor, to measure mouse zona hardness and concluded that the mouse embryos were 2.7-fold harder than oocytes.
Table 1.
Comparison of hardening rates of mouse ovum ZP studied by different techniques.
| cell types | technique | data | hardening rates | source |
|---|---|---|---|---|
| mouse oocyte | capillary suction apparatus | Lp/Δp = 41 (µm kPa−1) | 1.8 | Drobnis et al. (1988) |
| mouse embryo | Lp/Δp = 23 (µm kPa−1) | |||
| mouse oocyte | microinjection | E = 17.9 (kPa) | 2.4 | Sun et al. (2003) |
| mouse embryo | E = 42.2 (kPa) | |||
| mouse oocyte | microtactile sensor | E = 8.26 (kPa) | 2.7 | Murayama et al. (2006) |
| mouse embryo | E = 22.3 (kPa) | |||
| mouse oocyte | micropipette aspiration | E = 11.8 (kPa) | 3.1 | current study |
| mouse embryo | E = 36.9 (kPa) |
In this study, in order to determine the equilibrium elastic modulus of the mouse ZP, we used a theoretical model, the shell model. Young's modulus of the mouse embryo ZP (EZP = 36.9 ± 2.34 kPa) was found to be approximately 3.1-fold higher than that of the oocyte ZP (EZP = 11.8 ± 1.47 kPa). The observed discrepancy between results of different studies could be attributed to the differences in test techniques, the type and extent of deformation, the time that the deformation was applied and respective theoretical models.
After fertilization of mouse, hamster and human oocytes, CG exudates accumulate in the PVS and form a new coat, termed the CG envelope, around the fertilized eggs (Okada et al. 1993; Tahara et al. 1996). This envelope is possibly related to blocking polyspermic penetration at the level of the PVS or the oolemma by hindering gamete interaction or modifying an incoming sperm (Dandekar et al. 1995). CG release is dependent on Ca2+ rise and calcium-dependent proteins. A relatively low Ca2+ rise is sufficient to induce a partial cortical reaction, while a higher level of Ca2+ is required to complete the cortical reaction (Raz et al. 1998). Murayama et al. (2008) have examined the correlation between mechanical ZP hardening and the amount of CG release in mouse eggs. The results indicate that the ZP in the PN stage significantly hardened following natural fertilization as was shown in our results, and the partial CG release after partially activating (partial activation) slightly hardened the ZP. However, mechanical ZP hardening has been shown to be dependent on the amount of CG released into the PVS and oocyte activation (maturation, fertilization) may be evaluated by changes in ZP elasticity. Consequently, these findings may suggest that Young's modulus of the ZP can be applied in human assisted reproductive technology (ART) to embryo selection with the highest hatching and implantation potential as a selection criterion.
5. Conclusion
The outcome of this study may be used for better understanding of the biomechanical role of the ZP in the ovum. The study provides a method to examine mechanical changes on the ovum ZP during fertilization. Also the above approach demonstrates a step towards a more accurate estimation of the layered sample stiffness by using appropriate models. It is hoped that obtaining more realistic models will provide a good starting point for working towards a better understanding of ovum mechanics.
This study was approved by the local Ethics Committee at Royan Institute, Tehran, Iran, and was conducted at the institute's Assisted Conception Unit.
Acknowledgements
This work was supported by Royan Institute, Tehran, Iran. We would like to thank the Royan Department of Embryology for their assistance during this research.
References
- Abbott A. L., Ducibella T. 2001. Calcium and the control of mammalian cortical granule exocytosis. Front. Biosci. 6, 792–806. ( 10.2741/Abbott) [DOI] [PubMed] [Google Scholar]
- Alexopoulos L. G., Haider M. A., Vail T. P., Guilak F. 2003. Alterations in the mechanical properties of the human chondrocytes pericellular matrix with osteoarthritis. J. Biomech. Eng. 125, 323–333. ( 10.1115/1.1579047) [DOI] [PubMed] [Google Scholar]
- Alexopoulos L. G., Setton L. A., Guilak F. 2005. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 1, 317–325. ( 10.1016/j.actbio.2005.02.001) [DOI] [PubMed] [Google Scholar]
- Aoki T., Ohashi T., Matsumoto T., Sato M. 1997. The pipette aspiration applied to the local stiffness measurement of soft tissues. Ann. Biomed. Eng. 25, 581–587. ( 10.1007/BF02684197) [DOI] [PubMed] [Google Scholar]
- Braden A. W. H., Austin C. R., David H. A. 1954. The reaction of the zona pellucida to sperm penetration. Aust. J. Biol. Sci. 7, 391–409. [DOI] [PubMed] [Google Scholar]
- Cohen J., Elsner O., Kort H., Malter H., Massey J., Mayer M. P., Wiemer K. 1990. Impairment of the hatching process following IVF in the human and improvement of implantation by assisting hatching using micromanipulation. Hum. Reprod. 5, 7–13. [DOI] [PubMed] [Google Scholar]
- Dandekar P., Mate K. E., Talbot P. 1995. Perivitelline space of marsupial oocytes: extracellular matrix of the unfertilized oocytes and formation of a cortical granule envelope following the cortical reaction. Mol. Reprod. Dev. 41, 368–373. ( 10.1002/mrd.1080410313) [DOI] [PubMed] [Google Scholar]
- De Felice M., Siracusa G. 1982. Spontaneous hardening of mouse oocytes during in vitro culture. Gamete Res. 6, 107–115. ( 10.1002/mrd.1120060203) [DOI] [Google Scholar]
- Discher D. E., Boal D. H., Boey S. K. 1998. Simulations of the erythrocyte cytoskeleton at large deformation II: micropipette aspiration. Biophys. J. 75, 1584–1597. ( 10.1016/S0006-3495(98)74076-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobnis E., Andrew J., Katz D. 1988. Biophysical properties of the zona pellucida measured by capillary suction: is zona hardening a mechanical phenomenon? J. Exp. Zool. 245, 206–219. ( 10.1002/jez.1402450210) [DOI] [PubMed] [Google Scholar]
- Ducibella T., Kurasawa S., Duffy P., Kopf G. S., Schultz R. M. 1993. Regulation of the polyspermy block in the mouse egg: maturation-dependent differences in cortical granule exocytosis and zona pellucida modifications induced by inositol 1,4,5-triphosphate and an activator of protein kinase C. Biol. Reprod. 48, 1251–1257. ( 10.1095/biolreprod48.6.1251) [DOI] [PubMed] [Google Scholar]
- Evans E., Yeung A. 1973. New membrane concept applied to the analysis of fluid shear- and micropipette-deformed red blood cells. Biophys. J. 13, 941–954. ( 10.1016/S0006-3495(73)86036-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Yeung A. 1989. Apparent viscosity and cortical tension of blood granulocytes determined by micropipette aspiration. Biophys. J. 56, 151–160. ( 10.1016/S0006-3495(89)82660-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. P. 1987. Mammalian sperm cannot penetrate the zona pellucida solely by force. Exp. Cell Res. 169, 31–38. ( 10.1016/0014-4827(87)90221-7) [DOI] [PubMed] [Google Scholar]
- Green D. P. 1997. Three-dimensional structure of the zona pellucida. Rev. Reprod. 2, 147–156. ( 10.1530/ror.0.0020147) [DOI] [PubMed] [Google Scholar]
- Haider M. A., Guilak F. 2002. An axisymmetric boundary integral model for assessing elastic cell properties in the micropipette aspiration contact problem. ASME J. Biomech. Eng. 124, 586–595. ( 10.1115/1.1504444) [DOI] [PubMed] [Google Scholar]
- Hatanaka Y., Nagai T., Tobita T., Nakano M. 1992. Changes in the properties and composition of zona pellucida of pigs during fertilization in vitro. J. Reprod. Fertil. 95, 431–440. ( 10.1530/jrf.0.0950431) [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. 1967. Observations and measurements of sea urchin eggs with a centrifuge microscope. J. Cell. Physiol. 69, 219–230. ( 10.1002/jcp.1040690212) [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. 1976. Mechanical properties of starfish oocytes. Dev. Growth Differ. 18, 205–209. ( 10.1111/j.1440-169X.1976.00205.x) [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M. 2000. Micropipette aspiration of living cells. J. Biomech. 33, 15–22. ( 10.1016/S0021-9290(99)00175-X) [DOI] [PubMed] [Google Scholar]
- Iwamoto K., Ikeda K., Yonezawa N., Noguchi S., Kudo K., Hamano S., Kuwayama M., Nakano M. 1999. Disulfide formation in bovine zona pellucida glycoproteins during fertilization: evidence for the involvement of cystine cross-linkages in hardening of the zona pellucida. J. Reprod. Fertil. 117, 395–402. ( 10.1530/jrf.0.1170395) [DOI] [PubMed] [Google Scholar]
- Jones W. R., Beall H. P. T., Lee G. M., Kelley S. S., Hochmuth R. M., Guilak F. 1999. Alterations in the Young's modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J. Biomech. 32, 119–127. ( 10.1016/S0021-9290(98)00166-3) [DOI] [PubMed] [Google Scholar]
- Lim C. T., Zhou E. H., Li A., Vedula S. R. K., Fu H. X. 2006. Experimental techniques for single cell and single molecule biomechanics. Mater. Sci. Eng. 26, 1278–1288. ( 10.1016/j.msec.2005.08.022) [DOI] [Google Scholar]
- Mitchison J. M., Swann M. M. 1955. The mechanical properties of the cell surface: III. The sea-urchin egg from fertilization to cleavage. J. Exp. Biol. 32, 734–750. [Google Scholar]
- Murayama Y., Constantinou C. E., Omata S. 2004. Micro-mechanical sensing platform for the characterization of the elastic properties of the ovum via uniaxial measurement. J. Biomech. 37, 67–72. ( 10.1016/S0021-9290(03)00242-2) [DOI] [PubMed] [Google Scholar]
- Murayama Y., et al. 2006. Mouse zona pellucida dynamically changes its elasticity during oocyte maturation, fertilization and early embryo development. Human Cell 19, 119–125. ( 10.1111/j.1749-0774.2006.00019.x) [DOI] [PubMed] [Google Scholar]
- Murayama Y., et al. 2008. Elasticity measurement of zona pellucida using a micro tactile sensor to evaluate embryo quality. J. Mamm. Ova Res. 25, 18–16. ( 10.1274/jmor.25.8) [DOI] [Google Scholar]
- Nakamura S., Hiramoto Y. 1978. Mechanical properties of the cell surface in starfish eggs. Dev. Growth Differ. 20, 317–327. ( 10.1111/j.1440-169X.1978.00317.x) [DOI] [PubMed] [Google Scholar]
- Needham D., Hochmuth R. M. 1990. Rapid flow of passive neutrophils into a 4 µm pipet and measurement of cytoplasmic viscosity. J. Biomech. Eng. 112, 269–276. ( 10.1115/1.2891184) [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Hiramoto Y. 1985. Regional differences in mechanical properties of the cell surface in dividing echinoderm eggs. Dev. Growth Differ. 27, 371–383. ( 10.1111/j.1440-169X.1985.00371.x) [DOI] [PubMed] [Google Scholar]
- Okada A., Inomata K., Nagae T. 1993. Spontaneous cortical granule release and alteration of zona pellucida properties during and after meiotic maturation of mouse oocytes. Anat. Rec. 237, 518–526. ( 10.1002/ar.1092370412) [DOI] [PubMed] [Google Scholar]
- Porter R. N., Smith W., Craft I. L., Abdulwahid N. A., Jacobs H. S. 1984. Induction of ovulation for in vitro fertilization using buserelin and gonadotropins. Lancet 2, 1284–1285. ( 10.1016/S0140-6736(84)92840-X) [DOI] [PubMed] [Google Scholar]
- Rankin T., Dean J. 1996. The molecular genetics of the zona pellucida: mouse mutations and infertility. Hum. Reprod. 2, 889–894. ( 10.1093/molehr/2.11.889) [DOI] [PubMed] [Google Scholar]
- Raz T., Ben-Yosef D., Shalgi R. 1998. Segregation of the pathways leading to cortical reaction and cell cycle activation in the rat egg. Biol. Reprod. 58, 94–102. ( 10.1095/biolreprod58.1.94) [DOI] [PubMed] [Google Scholar]
- Sato M., Theret D. P., Wheeler L. T., Oshima N., Nerem R. M. 1990. Application of the micropipette technique to the measurement of cultured porcine aortic endothelial cell viscoelastic properties. ASME J. Biomech. Eng. 112, 263–268. ( 10.1115/1.2891183) [DOI] [PubMed] [Google Scholar]
- Saul A., Wagner W. 1989. A fundamental equation for water covering the range from the melting line to 1273 K at pressures up to 25000 MPa. J. Phys. Chem. Ref. Data 18, 1537–1564. [Google Scholar]
- Schiewe M. C., Araujo E., Asch R. H., Balmaceda J. P. 1995. Enzymatic characterization of zona pellucida hardening in human eggs and embryos. J. Assist. Reprod. Genet. 12, 2–7. ( 10.1007/BF02214120) [DOI] [PubMed] [Google Scholar]
- Schmell E. D., Gulyas B. J., Hedrick J. L. 1983. Egg surface changes during fertilization and the molecular mechanism of the block to polyspermy. In Mechanism and control of animal fertilization (ed. Hartmann J. F.), pp. 356–413. New York, NY: Academic Press. [Google Scholar]
- Smith B. A., Tolloczko B., Martin J. G., Grutter P. 2005. Probing the viscoelastic behavior of cultured airway smooth muscle cells with atomic force microscopy: stiffening induced by contractile agonist. Biophys. J. 88, 2994–3007. ( 10.1529/biophysj.104.046649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger J. D., Stevenson K., Lakmaker A., Woolcott R. 2001. Pregnancy following fertilization of zona-free, coronal cell intact human ova. Hum. Reprod. 16, 164–167. ( 10.1093/humrep/16.1.164) [DOI] [PubMed] [Google Scholar]
- Sun Q. Y. 2003. Cellular and molecular mechanisms leading to cortical reaction and polyspermy block in mammalian eggs. Microsc. Res. Tech. 61, 342–348. ( 10.1002/jemt.10347) [DOI] [PubMed] [Google Scholar]
- Sun Y., Wan K., Roverts K. P. 2003. Mechanical property characterization of mouse zona pellucida. IEEE Nanobiosci. 2, 279–286. ( 10.1109/TNB.2003.820273) [DOI] [PubMed] [Google Scholar]
- Tahara M., Tasaka K., Masumoto N., Mammato A., Ikebuchi Y., Miyake A. 1996. Dynamics of cortical granule exocytosis in living mouse eggs. Am. J. Physiol. 270, 1354–1361. [DOI] [PubMed] [Google Scholar]
- Theret D. P., Levesque M. J., Sato M., Nerem R. M., Wheeler L. T. 1988. The application of a homogeneous half-space model in the analysis of endothelial cell micropipette measurements. Trans. ASME 110, 190–199. [DOI] [PubMed] [Google Scholar]
- Ting-Beall H. P., Needham D., Hochmuth R. M. 1993. Volume and osmotic properties of human neutrophils. Blood 81, 2774–2780. [PubMed] [Google Scholar]
- Van Vliet K. J., Bao G., Suresh S. 2003. The biomechanics toolbox: experimental approaches for living cells and biomolecules. Acta Mater. 51, 5881–5905. ( 10.1016/j.actamat.2003.09.001) [DOI] [Google Scholar]
- Wassarman P. M., Jovine L., Litscher E. S. 2001. A profile of fertilization in mammals. Nat. Cell Biol. 3, 59–64. ( 10.1038/35055178) [DOI] [PubMed] [Google Scholar]
- Yoneda M., Dan K. 1972. Tension at the surface of the dividing sea-urchin egg. J. Exp. Biol. 57, 575–587. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Imura H., Murayama Y., Sakuma I., Tuji T., Omata S. 2000. Use of a piezo-electric ceramic tactile sensor to evaluate zona pellucida hardness at different stages of pig oocyte and embryo generated in vitro. Biol. Reprod. 66, 518. [Google Scholar]
- Zhao M., Dean J. 2002. The zona pellucida in follicullogenesis, fertilization and early development. Rev. Endocr. Metab. Disord. 3, 19–26. ( 10.1023/A:1012744617241) [DOI] [PubMed] [Google Scholar]