Abstract
Membrane protein function is regulated by the host lipid bilayer composition. This regulation may depend on specific chemical interactions between proteins and individual molecules in the bilayer, as well as on non-specific interactions between proteins and the bilayer behaving as a physical entity with collective physical properties (e.g. thickness, intrinsic monolayer curvature or elastic moduli). Studies in physico-chemical model systems have demonstrated that changes in bilayer physical properties can regulate membrane protein function by altering the energetic cost of the bilayer deformation associated with a protein conformational change. This type of regulation is well characterized, and its mechanistic elucidation is an interdisciplinary field bordering on physics, chemistry and biology. Changes in lipid composition that alter bilayer physical properties (including cholesterol, polyunsaturated fatty acids, other lipid metabolites and amphiphiles) regulate a wide range of membrane proteins in a seemingly non-specific manner. The commonality of the changes in protein function suggests an underlying physical mechanism, and recent studies show that at least some of the changes are caused by altered bilayer physical properties. This advance is because of the introduction of new tools for studying lipid bilayer regulation of protein function. The present review provides an introduction to the regulation of membrane protein function by the bilayer physical properties. We further describe the use of gramicidin channels as molecular force probes for studying this mechanism, with a unique ability to discriminate between consequences of changes in monolayer curvature and bilayer elastic moduli.
Keywords: hydrophobic coupling, lipid bilayer elasticity, gramicidin channels
1. Introduction
Cell membranes are dynamically regulated composites of many different types of lipids and proteins, with the overall membrane organization being a lipid bilayer ‘backbone’ plus membrane-spanning and peripherally adsorbed proteins. The importance of having a variety of membrane proteins can be understood with reference to the number of functions that any given membrane supports. The importance of having a variety of membrane lipids similarly reflects their roles in many biological functions. A key lipid bilayer function, being a barrier to unregulated solute movement, can be provided by just one, or a few, lipid species. Cell membranes, however, are composed of more than 200 different lipid species (Myher et al. 1989), and many cell and membrane protein functions are regulated by changes in membrane lipid composition (Spector & Yorek 1985).
The regulation of membrane protein function by membrane lipids may occur through mechanisms ranging from specific chemical interactions between proteins and individual lipid molecules, to non-specific interactions between proteins and the bilayer behaving as a macrostructure with collective physical properties (e.g. thickness, intrinsic monolayer curvature or elastic moduli; Lee 2005; Lundbæk 2006, 2008; Andersen & Koeppe 2007; Marsh 2008). Specific regulation may arise from phospholipid head group interactions with specific proteins—as in the case of the polyphosphoinositides (Hilgemann et al. 2001; McLaughlin & Murray 2005; Suh & Hille 2008)—or because lipids are structural elements in membrane proteins (e.g. Lee 2003). Specific regulation also arises when membrane lipids are precursors for cell signalling molecules—such as arachidonic acid in prostaglandin biosynthesis (Schmitz & Ecker 2008) or the hydrolysis of phosphatidylinositol-4,5-bisphosphate to inositol-1,4,5-triphosphate and diacylglycerol (Suh & Hille 2008). Such specific mechanisms can be investigated using well-known concepts from ligand–receptor interactions; they will not be discussed further here.
The focus of this review is the non-specific regulation of membrane protein function by the host bilayer, which arises from the hydrophobic coupling between the protein and the bilayer. Membrane proteins perturb the surrounding lipids (Seelig et al. 1981; Jähnig et al. 1982; Nezil & Bloom 1992; Marsh 2008), and this perturbation will incur an energetic cost (Marcelja 1976; Kirk et al. 1984; Mouritsen & Bloom 1984; Huang 1986; Fattal & Ben-Shaul 1993). Protein conformational changes that involve the protein–bilayer boundary will alter the packing and dynamics of the adjacent lipids to locally deform the bilayer—and the ensuing bilayer deformation energy contributes to the free energy difference between the protein conformations (Huang 1986; Nielsen et al. 1998). The bilayer deformation energy varies as a function of bilayer physical properties, such as thickness, intrinsic curvature and the bilayer compression and bending moduli (Huang 1986; Nielsen et al. 1998; Nielsen & Andersen 2000), meaning that changes in bilayer physical properties can alter membrane protein conformational distribution and function (Sackmann 1984).
Physico-chemical studies on peptide–bilayer interactions have provided mechanistic insight into how bilayer physical properties can be determinants of membrane protein function. Studies on the bilayer regulation of membrane protein function in systems with defined lipid composition have shown that changes in bilayer properties, such as bilayer thickness or intrinsic lipid curvature, for example, lead to changes in protein function (Andersen & Koeppe 2007). The interpretation of such experiments is not straightforward, however, because experimental manoeuvres designed to alter a desired bilayer property, such as intrinsic curvature, are likely to alter other bilayer properties also, such as thickness (Rostovtseva et al. 2008). That is, though the general principles are well understood, the relative importance of the different contributions to the bilayer deformation energy may be difficult to assess, and it becomes important to have methods to determine the overall energetic consequences of changes in bilayer composition.
Lipids and lipid metabolites that alter bilayer physical properties (such as lysophospholipids, cholesterol and polyunsaturated fatty acids; Lundbæk & Andersen 1994; Lundbæk et al. 1996, 2004; Bruno et al. 2007) regulate membrane proteins in a seemingly non-specific manner (see references in Lundbæk & Andersen 1994; Lundbæk et al. 1996, 2004; Bruno et al. 2007), but it is not clear to what extent the altered bilayer physical properties play a causal role in these effects. Similarly, many drugs that regulate membrane protein function are amphiphiles that adsorb to lipid bilayers and alter bilayer physical properties at concentrations used in cell physiological studies, e.g. genistein, capsaicin or curcumin (Hwang et al. 2003; Lundbæk et al. 2005; Ingólfsson et al. 2007; Lundbæk 2008). Again, the contributions from altered bilayer properties have, with a few exceptions (e.g. Lundbæk et al. 2004, 2005), not been established—and are often not even considered.
The aim of this review is to provide an introduction to bilayer mechanics and the energetics of protein-induced bilayer deformations, and to introduce the use of gramicidin channels as molecular force probes to examine how changes in bilayer composition, or the adsorption of amphiphiles, alter the bilayer deformation energy. Section 2 describes the core concepts, the basis for the regulation of membrane function by bilayer physical properties and the use of gramicidin channels as probes for changes in these properties. Section 3 provides a more quantitative analysis of the underlying principles, with emphasis on the gramicidin channel-based approach, which allows for discrimination between the consequences of changes in monolayer curvature versus bilayer elastic moduli, as experienced by bilayer-spanning proteins.
2. Lipid Bilayer Regulation of Protein Function
2.1. The hydrophobic coupling mechanism
The organization of a cell membrane reflects the need to optimize hydrophobic interactions among membrane lipids and proteins (Singer & Nicolson 1972; Mouritsen & Andersen 1998). The energetic penalty for exposing hydrophobic surfaces to water, approximately 5 kcal (mol nm2)−1 (Sharp et al. 1991), causes lipid molecules to be organized as a bilayer, in which the hydrophilic lipid head groups are exposed to the aqueous solution and the acyl chains are sequestered in the bilayer hydrophobic core. In proteins, correspondingly, the side chains that are exposed to the aqueous solution are hydrophilic, whereas those at the exterior surface of the proteins' trans-membrane domains (TMDs) are hydrophobic.
A mismatch between a TMD's hydrophobic length (length of the protein exterior facing the bilayer core) and the bilayer's hydrophobic thickness (thickness of the acyl chain core) would—unless the bilayer and protein adapt to each other—incur a considerable energetic cost owing to increased contact between hydrophobic and polar residues (including water). For a protein with a 2 nm radius, the energetic penalty for a 0.15 nm mismatch, corresponding to one amino acid in a trans-membrane α-helix, would be approximately 9 kcal mol−1. (For comparison, the energy released by covalent bond breaking during hydrolysis of ATP to ADP is approx. 13 kcal mol−1 (Veech et al. 1979).) Therefore, a protein-bilayer mismatch will cause a local change in bilayer thickness as well as a change in protein length (Mouritsen & Bloom 1984), whether it is by minor adjustments of the amino acid side chains or by a shift in the balance between different protein conformations. Focusing on the bilayer, a local thickness change has an associated energetic cost, the bilayer deformation energy (ΔGdef) (Mouritsen & Bloom 1984; Huang 1986). The function of membrane proteins whose conformational changes involve the boundary between the TMD and the bilayer core thus will be influenced by changes in ΔGdef.
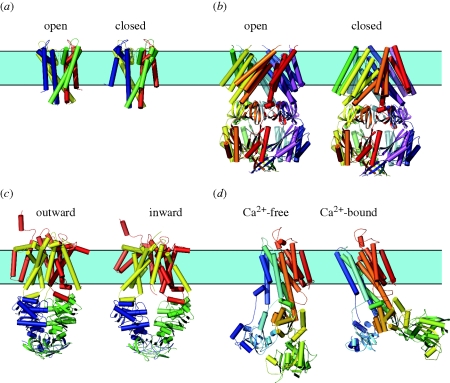
Figure 1 shows examples of membrane proteins for which there is structural information about more than one conformation: the KcsA channel in a closed and an open state; the mechano-sensitive MscS channel in a closed and an open state; the maltose transporter MalFGK2 in an outward- and an inward-facing state; and the sarcoplasmic reticulum Ca2+-ATPase in a Ca2+-bound E1 state and a Ca2+-free E2 state. In all four cases, the protein conformational changes involve the TMD/bilayer boundary. Table 1 lists other membrane proteins for which there is structural information about more than one conformation. Except in two cases (the β-barrel outer-membrane proteins (OmpG; Yildiz et al. 2006) and the colicin I receptor (Buchanan et al. 2007)), the structural transitions involve the TMD/bilayer boundary. Similar conclusions have been drawn for other proteins based on chemical cross-linking, spectroscopic and cysteine accessibility studies, summarized in Lundbæk (2006). The available evidence suggests that it is the norm, rather than the exception, that conformational changes underlying membrane protein function involve the TMD/bilayer boundary.
Figure 1.
Conformational changes in membrane proteins. Note the complex structural changes in the bilayer-spanning part of the proteins. (a) KcsA channel in an open and closed conformation. The former structure is based on a truncated form of the channel lacking the C-terminal domain (PDB: 1K4C, Morais-Cabral et al. 2001). For comparison, the latter structure (PDB: 3EFF, Uysal et al. 2009) is shown without this domain. (b) Mechano-sensitive MscS channel in an open and closed conformation (PDB: 2OAU, 2VV5, Bass et al. 2002; Wang et al. 2008). (c) Maltose transporter (MalFGK2) in an inward- and outward-facing state. The former structure is based on a truncated form lacking the TM1 helix (PDB: 3FH6, Khare et al. 2009). For comparison the latter (PDB: 2R6G, Oldham et al. 2007) is shown without the TM1. (d) Sarcoplasmic reticulum Ca2+–ATPase in the Ca2+-loaded E1 and the thapsigargin-stabilized Ca2+-free E2 conformations (PDB: 1SU4, 1IWO, Toyoshima et al. 2000; Toyoshima & Nomura 2002). Figure prepared using Pymol.
Table 1.
Membrane proteins with structural information about more than one conformation.
| protein | PDB ID | reference |
|---|---|---|
| KcsA | 1BL8, 1JQ2 | Doyle et al. (1998); Perozo et al. (1999) |
| NaK channel | 2AHY, 3E86 | Shi et al. (2006); Alam & Jiang (2009) |
| M2 proton channel | 2RLF | Schnell & Chou (2008) |
| mechano-sensitive MscS channel | 2OAU, 2VV5 | Bass et al. (2002); Wang et al. (2008) |
| mechano-sensitive MscL channel | 2OAR | Chang et al. (1998); Perozo et al. (2002) |
| nicotinic acetylcholine receptor | Toyoshima & Unwin (1988) | |
| prokaryotic pentameric ligand-gated ion channel pLGIC versus GLIC | 2VL0, 3EHZ, 3EAM | Hilf & Dutzler (2008, 2009); Bocquet et al. (2009) |
| gap junction channel | 2ZW3 | Unwin & Ennis (1984); Maeda et al. (2009) |
| OmpG | 2IWV, 2IWW | Yildiz et al. (2006) |
| colicin I receptor | 2HDF 2HDI | Buchanan et al. (2007) |
| rhodopsin | 3CAP, 1U19 | Park et al. (2008); Okada et al. (2004) |
| bacteriorhodopsin | 1C8R, 1C8S | Luecke et al. (1999); Vonck (2000) |
| intramembrane protease, GlpG | 2IC8, 2O7L | Wang et al. (2006); Wang & Ha (2007) |
| Ca2+-ATPase | 1SU4, 1IWO | Toyoshima et al. (2000); Toyoshima & Nomura (2002) |
| maltose transporter, MalFG2 | 2R6G, 3FH6 | Oldham et al. (2007); Khare et al. (2009) |
| multidrug transporter | 2DHH, 2DRD, 2DR6 | Murakami et al. (2006) |
| LeuT leucine transporter | 2A65, 3F3A | Yamashita et al. (2005); Singh et al. (2008) |
| NhaA Na+/H+ antiporter | 1ZCD, 3FI1 | Hunte et al. (2005); Appel et al. (2009) |
| BtuCD ABC transporter | 2QI9 | Hvorup et al. (2007); Gerber et al. (2008) |
| ModBC ABC transporter | 3D31 | Gerber et al. (2008) |
| MsbA lipid ‘flippase’ | 3B5W, 3B60 | Ward et al. (2007, 2009) |
| P-glycoprotein | 3G5U, 3G60, 3G61 | Aller et al. (2009) |
| DsbB–Fab complex | 2ZUQ | Inaba et al. (2009) |
| Mhp1 benzyl-hydantoin transporter | 2JLN | Weyand et al. (2008) |
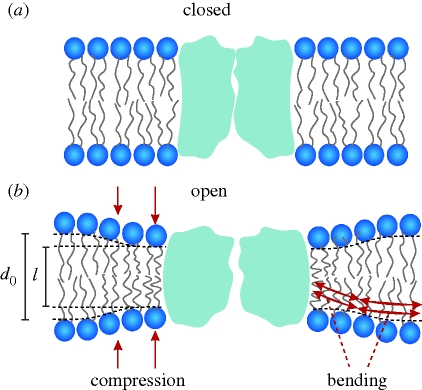
Protein conformational changes at the TMD/bilayer boundary will alter lipid packing in the immediate surrounding bilayer. Changes in TMD hydrophobic length, in particular, will induce a local change in bilayer thickness, as illustrated schematically in figure 2.
Figure 2.
Hydrophobic coupling between a bilayer-spanning membrane protein (ion channel) and host lipid bilayer. A protein conformational change causes a local bilayer deformation; for simplicity, there is no hydrophobic mismatch in the closed state. Modified from Andersen & Koeppe (2007).
Because bilayer deformations incur an energetic cost (Huang 1986), the difference in bilayer deformation energy associated with two protein conformations A and B (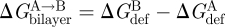 ) will contribute to the total energetic cost of the protein conformational change (
) will contribute to the total energetic cost of the protein conformational change (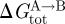 ):
):
| 2.1 |
where 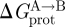 denotes energetic contributions due to the protein (contributions other than those due to the bilayer deformation per se). The equilibrium distribution between the protein conformations (in the case of an ion channel, figure 2, between open and closed states) is given by
denotes energetic contributions due to the protein (contributions other than those due to the bilayer deformation per se). The equilibrium distribution between the protein conformations (in the case of an ion channel, figure 2, between open and closed states) is given by
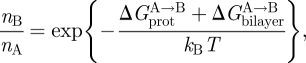 |
2.2 |
where nA and nB denote the number of proteins (per unit area) in each of the two states, and kB and T are Boltzmann's constant and temperature in kelvin. The protein–bilayer hydrophobic interactions thus provide for an energetic coupling between the protein conformational preference and the cost of deforming the bilayer. This hydrophobic coupling provides a means by which changes in bilayer composition can regulate membrane protein function (Lundbæk et al. 2004).
It is non-refutable that protein function, in principle, could be regulated by the hydrophobic coupling mechanism, as described by equations (2.1) and (2.2). For this mechanism to be an important regulator of protein function, however, changes in bilayer composition should alter 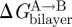 , the bilayer contribution to
, the bilayer contribution to 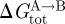 , by more than kBT. It thus becomes important to understand what determines the magnitude of
, by more than kBT. It thus becomes important to understand what determines the magnitude of 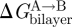 , and how
, and how 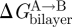 is altered by changes in bilayer composition.
is altered by changes in bilayer composition.
2.2. Theory of elastic bilayer deformations
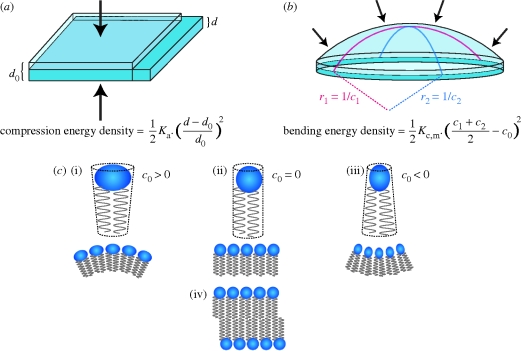
The energetic cost of adapting the bilayer hydrophobic thickness to match the hydrophobic length of an embedded protein has been analysed using continuum theories of elastic bilayer deformations. We follow Huang (1986) and later studies (Helfrich & Jakobsson 1990; Dan & Safran 1998; Nielsen et al. 1998; Nielsen & Andersen 2000; Partenskii & Jordan 2002), in which the bilayer deformation is decomposed as the sum of two major contributions (cf. figures 2 and 3): bilayer compression (originally proposed by Mouritsen & Bloom (1984)); and monolayer bending (originally proposed by Gruner (1985)); the energetic consequences related to the bending of the bilayer per se have been considered by Phillips et al. (2009). Energetic contributions related to Gaussian curvature and bilayer interfacial tension should be negligible (Nielsen et al. 1998) and will not be considered here.
Figure 3.
Bilayer and monolayer deformations. (a) Bilayer compression and associated energy density; d0 and d denote the thickness of the unperturbed and compressed bilayer, respectively. (Lipid bilayers have much lower volume compressibility than area compressibility (Evans & Hochmuth 1978), and a bilayer thinning is associated with an increase in bilayer area such that the product of thickness and area is approximately constant.) (b) Monolayer bending and associated energy density; c0 is the curvature of the relaxed monolayer, c1 and c2 are the principal curvatures of the deformed monolayer. (c) Lipid molecules with a cylindrical molecular ‘shape’ (indicated by the stippled lines) form monolayers with c0 = 0 (ii). Cone-shaped molecules form non-planar monolayers with, depending on the orientation of the cone section relative to the interface, c0 > 0 (i) or c0 < 0 (iii). In all three cases, two apposed monolayers having similar curvature form planar bilayers (iv).
Lipid bilayer compression/expansion is associated with a compression energy density (CED):
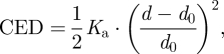 |
2.3 |
where Ka is the bilayer compression modulus (figure 3a), d0 the equilibrium thickness of the unperturbed bilayer and d the thickness of the perturbed bilayer. (Lipid bilayers are relatively soft, and the bilayer–solution interface undergoes thermal fluctuations (Wiener & White 1992), which involve local movement of individual phospholipid molecules and more global bilayer undulations and peristaltic motions (Wiener & White 1992; Lindahl & Edholm 2000). The bilayer–solution interface is thus ‘fuzzy’; but the average bilayer thickness, which is relevant here, is a well-defined parameter.)
Monolayer bending is similarly associated with a bending energy density (BED), which can be approximated as
| 2.4 |
where Kc,m is the monolayer bending modulus, c1 and c2 are the principal curvatures and c0 the intrinsic monolayer curvature (figure 3b).
The intrinsic curvature of a lipid monolayer is determined by the variation of intermolecular interactions across the monolayer (Helfrich 1981; Seddon 1990; Szeiler et al. 1990; Petrov 1999), which is often expressed in terms of the effective ‘shape’ of the lipids in the monolayer (Israelachvili 1977; Cullis & de Kruijff 1979). Cylindrical molecules will form planar and cone-shaped molecules will form non-planar monolayers (figure 3c). Though the intrinsic monolayer curvature may differ from zero, a bilayer formed by the apposition of two such monolayer leaflets of equal composition will be planar—and the associated change in the average molecular shape, from cone-shaped to cylindrical, causes a stress with an energy density 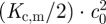 in each monolayer.
in each monolayer.
The energetic cost of the bilayer deformation needed to match the local thickness of a lipid bilayer to the length of a cylindrical bilayer-spanning protein of radius r0 thus becomes (cf. equations (2.3) and (2.4))
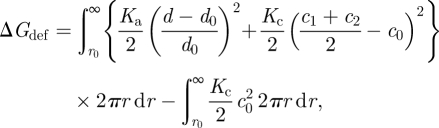 |
2.5 |
where d denotes the bilayer thickness as a function of r (d = l, the protein hydrophobic length, at r0), and Kc the bilayer bending modulus (=2Kc,m). The second integral denotes the curvature frustration energy of the unperturbed (flat) bilayer.
Equation (2.5) can be solved analytically (Goulian et al. 1998; Nielsen et al. 1998; Nielsen & Andersen 2000) and can be expressed as a biquadratic function of the channel–bilayer mismatch (l − d0) and c0 (cf. Nielsen & Andersen 2000; Lundbæk et al. 2004; note that we have changed the convention for the hydrophobic mismatch, from d0 − l to l − d0, such that HX and HC are positive):
| 2.6 |
where HB, HX and HC are elastic coefficients that are functions of Ka, Kc, d0 and r0. HB, HX and HC increase with increasing elastic moduli (and vice versa). Quantitative estimates of the coefficients can be obtained as described in Nielsen & Andersen (2000).
In the above treatment, equation (2.6) is derived from equation (2.5), and the evaluation of H coefficients depends on a physical model similar to that underlying equation (2.5). The biquadratic relation between ΔGdef and (l − d0) and c0 should apply quite generally, however, because equation (2.6) can be derived as a serial expansion of ΔGdef in terms of (l − d0) and c0 (Andersen & Koeppe 2007). That is, though one may question how well a continuum description applies at molecular length scales, the simplicity of equation (2.6) should be a general feature of models of elastic bilayer deformations in which the deformation varies as a function of bilayer thickness and intrinsic lipid curvature.
The energetic cost of the hydrophobic adaptation between a bilayer-spanning protein and the host bilayer varies as a function of protein–bilayer mismatch, intrinsic monolayer curvature and the bilayer elastic moduli (equations (2.5) and (2.6)). Table 2 summarizes the direction of changes in ΔGdef, as a function of thickness, curvature and the elastic moduli.
Table 2.
Direction of changes in ΔGdef as a function changes in bilayer physical properties.
| bilayer physical property that is varied | long protein (l > d0) | short protein (l < d0) |
|---|---|---|
| increasing bilayer thickness (d0) | ΔGdef ↓ | ΔGdef ↑ |
| more negative intrinsic curvature (c0) | ΔGdef ↓ | ΔGdef ↑ |
| decreasing elastic moduli (Ka and Kc) | ΔGdef ↓ | ΔGdef ↓ |
The effects of changes in bilayer composition on d0, c0, Ka and Kc can be measured in protein-free systems (Helfrich 1973; Evans & Hochmuth 1978; Schneider et al. 1984; Gruner 1985; Rand & Parsegian 1997; Nagle & Tristram-Nagle 2000; Rawicz et al. 2000; Liu & Nagle 2004). So, in principle, one should be able to use equations (2.5) and (2.6) to predict the effects of changes in bilayer composition on the bilayer contribution, 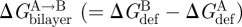 , to the free energy difference between two protein conformations, A and B, that differ in hydrophobic length, l. In practice, this is not yet possible.
, to the free energy difference between two protein conformations, A and B, that differ in hydrophobic length, l. In practice, this is not yet possible.
2.3. Determining ΔGdef
Protein conformational changes at the TMD/bilayer boundary are likely to be complex (cf. figure 1) and the associated bilayer deformations remain largely unknown. Even if a protein conformational change involved a simple change in l—associated with a local adjustment of bilayer thickness—the effects of altered bilayer composition on ΔGdef (and therefore the bilayer contribution to the free energy difference for the conformation change) may be difficult to estimate. This difficulty arises, in part, because d0, c0, Ka and Kc are treated as separable parameters in theories of elastic bilayer deformations (Rawicz et al. 2000), though they all are determined by the profile of intermolecular interactions across the bilayer (Helfrich 1981; Seddon 1990; Szeiler et al. 1990; Petrov 1999). In practice, therefore, these parameters are inter-related: Ka and Kc are related through scaling relations (Evans 1974); and the bilayer thickness varies with changes in phospholipid head group structure (Nagle & Wiener 1988; Kučerka et al. 2005; Rostovtseva et al. 2008) or acyl chain unsaturation (Lewis & Engelman 1983; Separovic & Gawrisch 1996), manipulations that are used to alter c0. Changes in bilayer composition thus are likely to alter more than one of the parameters that determine ΔGdef. In addition, the local elastic properties adjacent to a protein may differ from the bulk properties (Partenskii & Jordan 2002), though the structure of equation (2.6) remains valid—and it remains unclear to what extent global bilayer properties determined in (protein-free) lipid vesicles apply to biological membranes with their diversity of lipid species and approximately 20 per cent of the membrane area being occupied by proteins (Dupuy & Engelman 2008).
The consequences of these ‘uncertainties’ can be considerable. Even the sign of ΔGdef, and thus 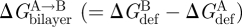 for a membrane protein conformational change, may be affected. In Lundbæk et al. (2005) and Bruno et al. (2007) for example, changes in c0 did not cause the predicted changes in
for a membrane protein conformational change, may be affected. In Lundbæk et al. (2005) and Bruno et al. (2007) for example, changes in c0 did not cause the predicted changes in 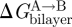 because of concomitant changes in the bilayer elasticity.
because of concomitant changes in the bilayer elasticity.
That the net change in ΔGdef may be difficult to estimate becomes a particular problem when the changes in different parameters, in isolation, should have opposite effects on ΔGdef. For example, in a bilayer–protein system with l < d0, addition of capsaicin (Lundbæk et al. 2005) or docosahexanoic acid (Bruno et al. 2007) would be expected to increase ΔGdef by causing a negative-going change in c0—and to decrease ΔGdef by decreasing Ka and Kc as both compounds are reversibly adsorbing amphiphiles (cf. Evans et al. 1995; Zhelev 1998). The quantitative consequences of an altered bilayer composition, in terms of ΔGdef, may be more complex than suggested in table 2.
To explore the bilayer–protein energetic coupling, one needs systems where the consequences of changes in bilayer composition on ΔGdef can be measured directly—and where specific lipid–protein interactions should be of little importance (Gruner 1991). Moreover, the reporter protein should preferably be an ion-conducting channel or receptor (Andersen et al. 1998) where one can examine the equilibrium distribution between different conformations and
— protein functional transitions can be studied at single molecule resolution,
—the transitions involve simple, well-described changes in protein ‘shape’, e.g. changes in the hydrophobic length, l, and
— the associated bilayer deformations are understood.
Channels formed by the linear gramicidins represent such a system. Gramicidin A (gA) is a mini protein that forms cation-conducting channels, and has been extensively used to explore how changes in bilayer properties alter protein function. Moreover, a methodology using gA channels as molecular force probes for in situ measurements of changes in ΔGdef has been developed (Lundbæk 2006; Andersen et al. 2007a), which can be used to predict the effects of changes in bilayer molecular composition on membrane protein function in living cells (Lundbæk et al. 2005).
2.4. Gramicidin channels as molecular force probes
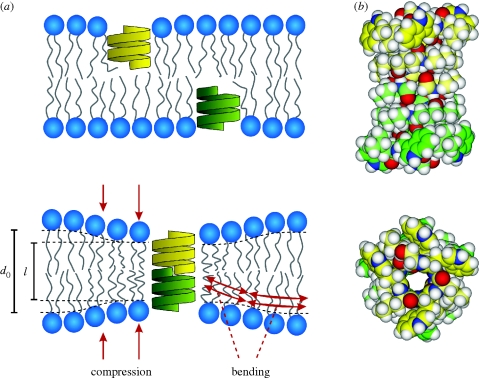
Cation-selective gA channels are mini proteins formed by trans-bilayer association (O'Connell et al. 1990) of β6.3-helical subunits from each leaflet (monolayer) in a lipid bilayer (figure 4a).
Figure 4.
Gramicidin channel formation. (a) Gramicidin channels form by the trans-bilayer dimerization of two subunits, one from each bilayer leaflet. Channel formation is associated with a local bilayer deformation. Modified from Andersen & Koeppe (2007). (b) Side and end views of a bilayer-spanning gramicidin channel, in which the carbon atoms of the two subunits are indicated in yellew and green, respectively. Energy minimized structure representing a composite of structures determined using solid-state and solution NMR (Arseniev et al. 1986; Ketchem et al. 1997; Townsley et al. 2001). We thank Dr Roger E. Koeppe II for the coordinates.
The sequence of the native [Val1]gA is (Sarges & Witkop 1965):
Formyl-l-Val-(d)-Gly-l-Ala-d-Leu-l-Ala-
-d-Val-l-Val-d-Val-l-Trp-d-Leu-l-Trp-
-d-Leu-l-Trp-d-Leu-l-Trp-ethanolamide.
Bilayer-incorporated gA dimers are ion-conducting channels (Bamberg & Läuger 1973; Veatch et al. 1975), and gA channel function can be studied at single-molecule resolution using electrophysiological methods; for a recent review see Andersen et al. (2007b). The channel structure, a single-stranded, right-handed β6.3-helical dimer, is known at atomic resolution (Arseniev et al. 1986; Ketchem et al. 1997; Townsley et al. 2001; Allen et al. 2003; figure 4b). The structure is remarkably invariant with respect to changes in the bilayer environment; minor differences between the structure in sodium dodecyl sulphate micelles (Arseniev et al. 1986; Townsley et al. 2001) and in dimyristoylphosphatidylcholine lipid bilayers (Ketchem et al. 1997) can be reconciled by molecular dynamics simulations (Allen et al. 2003). (In contrast to the situation in lipid bilayers, gA is conformationally polymorphic in organic solvents, where it exists in various double-stranded structures that are quite different from the single-stranded channel structure in bilayers or bilayer-mimetic environments (Bystrov & Arseniev 1988; Abdul-Manan & Hinton 1994); see also Andersen et al. (2007b)).
For not too extreme changes in phospholipid acyl chain length (10 ≤ nC ≤ 20, where nC denotes the number of carbon atoms in the acyl chain), the channel structure is insensitive to the channel–bilayer hydrophobic mismatch (Wallace et al. 1981; Greathouse et al. 1994; Galbraith & Wallace 1998). Nor does the channel's single-channel conductance (Mobashery et al. 1997; Bruno et al. 2007) or helical pitch (Katsaras et al. 1992) vary as a function of the hydrophobic mismatch, indicating that the average orientation of interfacial tryptophan residues varies little with changes in hydrophobic mismatch. Moreover, the channel function does not depend on the chirality of the channel-forming subunits or the bilayer-forming lipids (Providence et al. 1995), indicating that specific channel–phospholipid interactions are likely to be unimportant. Though the structure of the bilayer-embedded, non-conducting monomers has not been determined, it appears to be similar to that of the subunits in the conducting dimer (He et al. 1994).
The gA channels' structural and functional features make the channels well suited to probe changes in lipid bilayer properties—as sensed by a bilayer-spanning channel. Channel formation in a lipid bilayer with a hydrophobic thickness, d0 (typically 3–5 nm), that exceeds the channel hydrophobic length, l (approx. 2.2 nm; Elliott et al. 1983; Huang 1986), involves a local decrease in bilayer thickness to match the channel (Harroun et al. 1999) as illustrated in figure 4 (cf. figure 2). The ensuing bilayer deformation energy contributes to the energetic cost of channel formation (Huang 1986).
Changes in 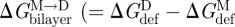 , where
, where 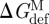 and
and 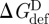 denote the bilayer deformation energies for the monomer and dimer states, respectively) are reflected in gA channel behaviour. An increase in
denote the bilayer deformation energies for the monomer and dimer states, respectively) are reflected in gA channel behaviour. An increase in 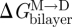 is reported as
is reported as
— decreased channel appearance rate (f),
— decreased channel lifetime (τ), and
— decreased channel activity (time-averaged number of conducting channels, n = fτ).
A decrease in 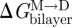 has opposite effects. gA channels thus provide for in situ measurements of the bilayer response to a conformational change in an embedded protein.
has opposite effects. gA channels thus provide for in situ measurements of the bilayer response to a conformational change in an embedded protein.
2.5. Gramicidin single-channel experiments
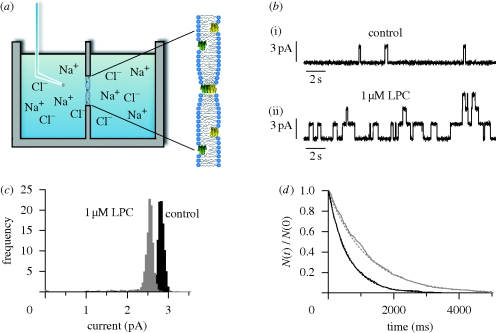
Figure 5a shows an experimental setup used in gA single-channel experiments (see also Ingólfsson et al. 2008; Kapoor et al. 2008).
Figure 5.
Gramicidin single-channel experiments. (a) Experimental setup, with a Teflon chamber separated into two halves by a partition with a hole that allows for contact between the two phases. The bilayer is formed across a hole in the partition. gA is added to the electrolyte solution on both sides of the membrane and adsorbs to the bilayer–solution interface, as illustrated in the expanded view to the right. (b) Effects of lysophosphatidylcholine (LPC) on gA channel behaviour in a diphytanoylphosphatidylcholine (DPhPC)/n-decane bilayer. Current traces recorded from bilayer patches isolated from the same large membrane before (i) and after (ii) addition of 1 µM LPC to the electrolyte solution. (c,d) Current transition amplitude histograms and lifetime distributions for gA channels in the absence or presence of LPC. The lifetime distributions are plotted as survivor curves and fitted by single exponential distributions, N(t)/N(0) = exp{ − t/τ}, where N(t) is the number of channels with lifetimes longer than time t, and τ the average single-channel lifetime. Black line, control; grey line, 1 µM LPC. Modified from Lundbæk & Andersen (1994). 1.0 M NaCl, 200 mV, 25°C.
A lipid bilayer is formed across a hole (diameter approx. 1 mm) in a Teflon partition separating two electrolyte solutions in a Teflon chamber. gA (1–100 pM, depending on gA analogue and bilayer composition) is added to the electrolyte solutions on both sides of the bilayer (making sure to stir the solution). The hydrophobic gA subunits adsorb to the bilayer, where they fold into channel-forming β6.3-helices. To observe single channels, it is necessary to have a good signal/noise ratio, which can be accomplished by isolating a small bilayer area using a glass pipette (diameter approx. 30 µm; Andersen 1983). The formation and disappearance of conducting gA channels are observed as rectangular current transitions when a trans-bilayer potential difference is applied; figure 5b shows a current trace from an experiment on the effects of the lipid metabolite lysophosphatidylcholine (LPC) on gA channels in a planar lipid bilayer (discussed further below). During subsequent computer-assisted analysis, the single channel conductance (g) is calculated from current-transition amplitude histograms (figure 5c), whereas τ is determined from single-channel lifetime distributions (figure 5d). The channel appearance rate, f, is determined by manual or computer-assisted assignment from the number of current transitions per unit time.
The effects of changes in lipid bilayer composition on gA channel function can be understood using the framework provided by the continuum theory of elastic bilayer deformations described above (equations (2.5) and (2.6)). This approach has been validated by examining the relation between changes in gA channel lifetime and bilayer thickness (Huang 1986; Lundbæk & Andersen 1999) and bilayer tension (Goulian et al. 1998), where changes in tension (σ) alter gA channel function because of the associated changes in bilayer thickness (Evans & Needham 1987; Goulian et al. 1998).
Because gA channels form (and disappear) by trans-bilayer association/dissociation (O'Connell et al. 1990), it becomes useful to introduce the so-called disjoining force (Fdis) that acts on the bilayer-spanning channel, tending to break it apart. Fdis is obtained by differentiating ΔGdef (equation (2.6)) with respect to (l − d0) (Lundbæk et al. 2005; Andersen & Koeppe 2007):
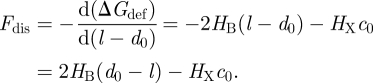 |
2.7 |
As is the case for ΔGdef (cf. table 2), Fdis is increased and f, τ and n are decreased by
— increasing bilayer–channel mismatch (d0 − l),
— more negative c0, and
— increasing bilayer elastic moduli.
These predictions have been validated experimentally, as will be described in the following.
2.6. Channel–bilayer mismatch
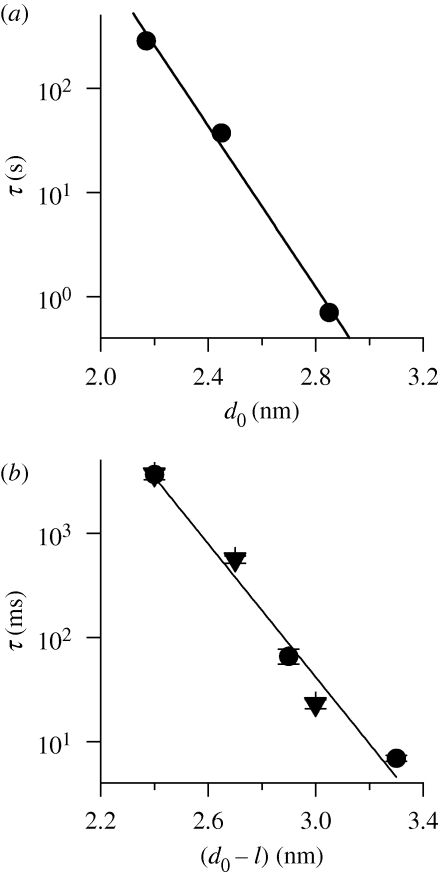
Figure 6a shows the relation between gA single-channel lifetime (τ) and the thickness of monoacylglyceride/squalene bilayers (Elliott et al. 1983; from Lundbæk & Andersen 1999). (Bilayers formed using squalene in the bilayer-forming solution are virtually hydrocarbon-free (Simon et al. 1977; White 1978), and squalene seems to be a universal component of living cells, even red blood cells (Liu et al. 1976).)
Figure 6.
gA channel lifetime (τ) as function of the channel–bilayer hydrophobic mismatch. (a) Relation between τ and hydrophobic thickness (d0) of monoacylglyceride/squalene bilayers. Based on these results, the slope of the ln{τ}–d0 relation is −8.9 nm−1 (r = 0.99), corresponding to HB = 69 kJ mol−1 nm−2. After Lundbæk & Andersen (1999). Lifetime results from Elliott et al. (1983); bilayer hydrophobic thickness determined from bilayer capacitance measurements (Benz et al. 1975). (b) Relation between channel lifetime and channel–bilayer hydrophobic mismatch for phosphatidylcholine/n-decane bilayers. The mismatch was altered using either a sequence-extended 17 amino acid long gA analogue (endo-Gly-D-Ala-gA) in monounsaturated phosphatidylcholine/n-decane bilayers having varying acyl chain length (18, 20 and 22 carbon atoms; filled circles), or using gA channels having varying amino acid sequence length (13, 15 and 17) in dioleoylphosphatidylcholine/n-decane bilayers (filled triangles); bilayer hydrophobic thickness determined from bilayer capacitance measurements (Benz et al. 1975). Based on these results, the slope of the relation between ln{τ} and (d0 − l) is −7.2 nm−1 (r = 0.98), corresponding to HB = 56 kJ mol−1 nm−2. Experimental results from Hwang et al. (2003).
When the bilayer thickness, d0, is altered by changing the lipid acyl chain length, ln{τ} decreases as a linear function of d0. This decrease is expected because a larger channel–bilayer mismatch should increase the magnitude of Fdis (cf. equation (2.7)) and thereby lower the activation energy for channel dissociation. The linearity of the ln{τ}–d0 relation conforms to the continuum theory of elastic bilayer deformations: Fdis varies as a linear function of the channel–bilayer hydrophobic mismatch, d0 − l, meaning that ΔGdef should vary as (d0 − l)2 (cf. equation (2.6)). Moreover, the slope of the ln{τ}–d0 relation conforms to predictions based on the continuum theory using experimentally determined elastic moduli (Lundbæk & Andersen 1999; see also §3). (The agreement between observed and predicted slopes of the ln{τ}–d0 relations is gratifying, but should not be overinterpreted. It is most likely another reflection of the surprising ability of continuum theories to provide reasonable predictions at the molecular level, as evident in the use of the Stokes–Einstein to estimate ionic radii (e.g. Robinson & Stokes 1965, pp. 43–44), or the Born model to estimate ionic solvation energies (e.g. Bockris & Reddy 1970, pp. 48–72).)
The relation between channel lifetime and channel–bilayer mismatch has also been studied using n-decane-containing phospholipid bilayers. Figure 6b shows results obtained when the hydrophobic mismatch was varied by altering either the lipid acyl chain length (bilayer thickness) or the channel length. In either case, ln{τ} decreases as an approximately linear function of (d0 − l) and, though the single-channel lifetime does depend on the specific sequence at the subunit interface (Mattice et al. 1995), the slope of the relation is similar whether (d0 − l) is altered by changing d0 or l. n-Decane increases the bilayer thickness (Benz et al. 1975) compared with nominally hydrocarbon-free bilayers, and makes the bilayers softer (Helfrich & Jakobsson 1990; Lundbæk & Andersen 1999), but the linear relation between ln{τ} and (d0 − l) conforms to the continuum theory of elastic bilayer deformations as embodied in equations (2.6) and (2.7) (Lundbæk & Andersen 1999).
2.7. Intrinsic monolayer curvature
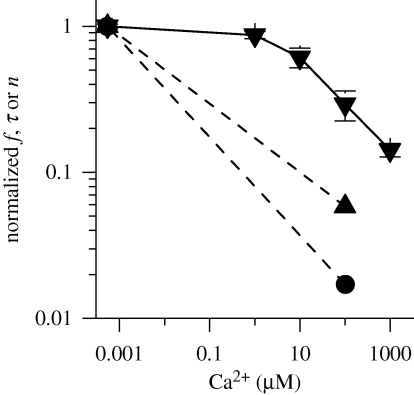
The relation between gA channel function and the intrinsic monolayer curvature, c0, has been studied by varying the ‘shape’ of the bilayer-forming lipids (Lundbæk et al. 1997; Maer et al. submitted). A decrease in lipid head group size promotes a more negative c0 (cf. figure 3c), as well as an increase in bilayer thickness (Nagle & Wiener 1988; Kučerka et al. 2005; Rostovtseva et al. 2008). Figure 7 shows results from experiments in which c0 of dioleoylphosphatidylserine/n-decane bilayers was varied by altering the electrostatic repulsion among the charged serine head groups and thus the ‘effective’ head group size (Lundbæk et al. 1997). (Because the experiments were performed in n-decane-containing bilayers, the bilayer thickness did not vary as the head group interactions were altered by the addition of Ca2+ (Lundbæk et al. 1997) and the results can be interpreted in terms of changes in c0.)
Figure 7.
Concentration-dependent effects of Ca2+ on gA channel appearance rate (f; dashed triangle line), lifetime (τ; solid triangle line) and activity (the time-averaged number of conducting channels, n; dashed circle line). By increasing [Ca2+], c0 is changed in the negative direction, and the gA channels are increasingly destabilized. The dashed lines connecting the results for the normalized changes in f and n are meant to guide the eye. Experimental results from Lundbæk et al. (1997).
When the electrostatic repulsion is reduced, by increasing the aqueous [Ca2+], the more negative c0 is associated with a decrease in f, τ and therefore n. This is expected because a negative-going c0 should increase the magnitude of Fdis (cf. equation (2.7)). Similar results were obtained when c0 was altered by varying the ratios of dioleoylphosphatidylcholine (DOPC, with a relatively large head group) and dioleoylphosphatidylethanolamine (DOPE, with a relatively small head group) in n-decane-containing bilayers (Maer et al. submitted). As noted by Rostovtseva et al. (2008), increasing the mole-fraction of DOPE also will increase bilayer thickness, d0, and it may be difficult to separate the consequences of the changes in c0 and d0. This could be done in the experiments by Maer et al. (submitted) because the thickness changes are small in n-decane-containing bilayers.
It is instructive to compare the phospholipid head group-dependent changes in gA channel function, summarized above, with the changes in alamethicin channel function in bilayers of similar composition (Keller et al. 1993; Bezrukov et al. 1998). Alamethicin channels are formed by bilayer-spanning α-helices (He et al. 1996) that are organized in a barrel-stave arrangement (Qian et al. 2008). The channel structure has not been determined at molecular resolution, but it is believed that the channels can exist in different oligomeric assemblies that correspond to different conductance levels in the multistate single-channel behaviour (Woolley & Wallace 1992). Like gA channels, alamethicin channels also sense changes in lipid bilayer properties (Bezrukov 2000), and the changes in alamethicin and gA channel are related: manoeuvres that shift the gA monomer ↔ dimer equilibrium towards the dimeric state reduce the probability of observing the higher conductance states in gA channels (Lundbæk et al. 1997; Bezrukov 2000). That is, though the two channels form by different mechanisms, they have correlated responses to changes in bilayer physical properties—suggestive of a common regulatory mechanism.
2.8. Water-soluble amphiphiles
As noted above, changes in lipid bilayer composition often alter the bilayer physical properties in a manner that cannot be described by a change in just one of the parameters that determine ΔGdef. Water-soluble amphiphiles, for example, may not only alter c0—though their effects are often ascribed to changes in c0. By reversibly adsorbing to lipid bilayers, they also tend to decrease the bilayer elastic moduli (Evans et al. 1995; Zhelev 1998; Ly & Longo 2004; Lundbæk et al. 2005; Zhou & Raphael 2005; Bruno et al. 2007) leading to a bilayer softening. In such cases, the net effect on Fdis becomes difficult to predict.
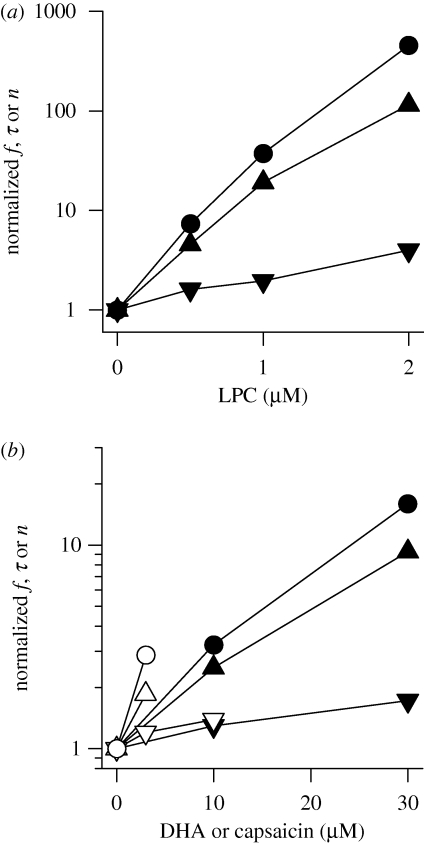
Micelle-forming amphiphiles, owing to their molecular cone ‘shape’, tend to promote a positive-going change in c0 (cf. figure 3c). Both the more positive c0 and the decrease in elastic moduli will tend to decrease ΔGdef and Fdis—and micelle-forming amphiphiles increase f, τ and n (Lundbæk & Andersen 1994). Figure 8a shows the concentration-dependent effects of micelle-forming LPC using an experimental setup as in figure 5. (The actual aqueous amphiphile concentration may be orders of magnitude less than the nominal concentration owing to partitioning into the non-polar phase (e.g. Bruno et al. 2007; Ingólfsson et al. 2007); the depletion will depend on the lipid/water volume ratio (Lundbæk 2008).)
Figure 8.
Concentration dependence of the effects of small amphiphiles on gA channel appearance rate (f), lifetime (τ) and activity (n). (a) Results for LPC from Lundbæk & Andersen (1994). Filled triangle, f; filled inverted triangle, τ; filled circle, n. (b) Results for capsaicin from Lundbæk et al. (2005), for DHA from Bruno et al. (2007). Filled triangle: capsaicin, f; filled inverted triangle: capsaicin, τ; filled circle: capsaicin, n; open triangle: DHA, f; open inverted triangle: DHA, τ; open circle: DHA, n.
In the case of amphiphiles that promote a more negative c0 (e.g. capsaicin or polyunsaturated fatty acids), the decrease in the elastic moduli is opposed by the negative-going c0. What, then, is the net effect on ΔGdef and Fdis? Both capsaicin and docosahexaenoic acid (DHA) increase f, τ and n (Lundbæk et al. 2005), meaning that Fdis and ΔGdef (Bruno et al. 2007) are decreased (figure 8b). Similar results were obtained with curcumin (Ingólfsson et al. 2007), which also promotes a more negative c0 (Barry et al. 2009). In these cases the decrease in Ka and Kc trumps the negative-going change in c0!
It is a general observation that small amphiphiles alter ΔGdef and Fdis. Except for a few examples—such as halothane (Bradley et al. 1981; Weinrich et al. 2009), long-chain alcohols (Pope et al. 1982), chloroform, benzene (Sawyer et al. 1990) and limonene (Andersen et al. 1999)—the predominant effects are that f and τ are increased (ΔGdef and Fdis are decreased). Table 3 summarizes results from a number of studies.
Table 3.
Effects of reversibly adsorbing amphiphiles on Fdis.a
| class | name | effect on Fdis (concentration)b | reference |
|---|---|---|---|
| surfactants | Triton X-100 | ↓ (10 µM) | Sawyer et al. (1989) |
| reduced Triton X-100 | ↓ (10 µM) | Sawyer et al. (1989) | |
| Genapol X-100 | ↓ (10 µM) | Sawyer et al. (1989) | |
| β-octyl glucoside | ↓ (1 mM) | Sawyer et al. (1989) | |
| Zwittergent 3-12 | ↓ (100 µM) | Sawyer et al. (1989) | |
| lipid metabolites | platelet-activating factor | ↓ (1 µM) | Sawyer & Andersen (1989) |
| lysophosphatidylcholine | ↓ (1 µM) | Lundbæk & Andersen (1994) | |
| lysophosphatidylethanolamine | ↓ (1 µM) | Lundbæk & Andersen (1994) | |
| lysophosphatidylinositol | ↓ (1 µM) | Lundbæk & Andersen (1994) | |
| lysophosphatidylserine | ↓ (1 µM) | Lundbæk & Andersen (1994) | |
| docosahexaenoic acid | ↓ (3 µM) | Bruno et al. (2007) | |
| eicosapentaenoic acid | ↓ (3 µM) | Andersen et al. (2007a) | |
| oleic acid | − (10 µM)c | Bruno et al. (2007) | |
| alcohols | methanol | − (50 mM) | Sawyer et al. (1990) |
| ethanol | ↓ (30 mM) | Sawyer et al. (1990) | |
| trifluoroethanol | ↓ (30 mM) | Sawyer et al. (1990) | |
| n-hexanol | ↑d | Pope et al. (1982) | |
| n-octanol | ↑d | Pope et al. (1982) | |
| n-octanol-(oxyethylene)3-ol | ↓ (0.5 mM) | Elliott et al. (1985) | |
| solvents | dimethylsulphoxide | −(30 mM) | Sawyer et al. (1990) |
| dioxane | −(25 mM) | Sawyer et al. (1990) | |
| ethylacetate | −(20 mM) | Sawyer et al. (1990) | |
| benzene | ↑ (25 mM) | Sawyer et al. (1990) | |
| chloroform | ↑ (25 mM) | Sawyer et al. (1990) | |
| phytochemicals | phloretin | ↓ (10 µM) | Andersen et al. (1976) |
| forskolin | ↓ (30 µM) | Andersen et al. (1992) | |
| genistein | ↓ (10 µM) | Hwang et al. (2003) | |
| genistin | − (40 µM) | Hwang et al. (2003) | |
| daidzein | ↓ (30 µM) | Hwang et al. (2003) | |
| capsaicin | ↓ (30 µM) | Lundbæk et al. (2005) | |
| ECGC | ↓ (1 µM) | Adachi et al. (2007) | |
| EC | − (10 µM) | Adachi et al. (2007) | |
| curcumin | ↓ (1 µM) | Ingólfsson et al. (2007) | |
| drugs | capsazepine | ↓ (10 µM) | Lundbæk et al. (2005) |
| 2,3-butanedione monoxime | ↓ (10 mM) | Artigas et al. (2006) | |
| halothane | ↑ (5 mM) | Bradley et al. (1981) | |
| olfactants | limonene | ↑ (1 mM) | Andersen et al. (1999) |
| cyclic nucleotides | 8-Br-cAMP | − (250 µM) | Liu et al. (2004) |
| CPT-cGMP | − (100 µM) | Liu et al. (2004) | |
| anti-fusion peptides | Z-Gly-Phe | ↓ (300 µM) | Ashrafuzzaman et al. (2006) |
| Z-Gly-d-Phe | ↓ (300 µM) | Ashrafuzzaman et al. (2006) | |
| Z-Phe-Tyr | ↓ (30 µM) | Ashrafuzzaman et al. (2006) | |
| Z-d-Phe-Phe-Gly | ↓ (30 µM) | Ashrafuzzaman et al. (2006) | |
| neurotoxins | GsMTx-4 | ↓ (1 µM) | Suchyna et al. (2004) |
aExcept for the results with n-hexanol and n-octanol, which are based on single-channel lifetime measurements in glycerolmonoolate/squalene membrane, the table is based on results obtained in diphytanoylphosphatidylcholine/n-decane or dioleoylphosphatidylcholine/n-decane bilayers.
bThe concentrations listed in column 3 denote the nominal aqueous concentrations at which changes in Fdis were observed.
c− no effect at the concentration indicated.
dAdded through the lipid phase; similar results were obtained in diphytanoylphosphatidylcholine/n-decane when added to the aqueous solution (A. D. Laskey & O. S. Andersen 1995, unpublished data).
At the concentrations tested, some compounds (e.g. dimethylsulphoxide) do not alter bilayer ΔGdef and Fdis. This does not imply that these compounds are truly inert, just that the changes in ΔGdef and Fdis are too small to be measured. Though some compounds, such as long-chain alcohols, cause changes that conform to predictions based on changes in curvature (i.e. that τ is decreased), this seems to be an exception rather than a rule.
2.9. Regulation of gA channel function by the hydrophobic coupling mechanism
The conclusion of studies on gA channels is that a bilayer-embedded protein can be regulated by the hydrophobic coupling between membrane proteins and their host bilayer. The equilibrium distribution between non-conducting monomers and conducting dimers (the number of conducting channels) can be altered several hundredfold by manoeuvres that alter c0 or d0 (Lundbæk et al. 1997; Mobashery et al. 1997). Similarly, the monomer ↔ dimer equilibrium can be altered by amphiphiles or other manoeuvres that change the bilayer elastic moduli, as demonstrated by the differential effects on gA channels of different lengths (Lundbæk & Andersen 1994; Lundbæk et al. 2004; Bruno et al. 2007; Ingólfsson et al. 2007). The changes in channel activity correspond to changes in ΔGdef of about ±5kBT or ±3 kcal mol−1 (see §3). Because the energetic cost of the deformation induced by a bilayer-spanning inclusion scales as an approximately linear function of the channel radius (Nielsen & Andersen 2000), the corresponding changes in ΔGdef could be ±6 kcal mol−1 for a typical membrane protein with a radius of 2 nm (rather than 1 nm for the gA channel; cf. Andersen & Koeppe 2007).
2.10. Going from model system to membrane proteins in living cells—bilayer stiffness
The results on gA channels illustrate how changes in bilayer composition can alter the conformational preferences of membrane proteins—and that changes in channel behaviour, in simple cases, conform to predictions based on the continuum theory of elastic bilayer deformations. They also demonstrate the difficulties that arise when the changes in each of the bilayer parameters, per se, have opposite effects on ΔGdef—and illustrate the perils of focusing on just a single parameter, such as curvature. Though the 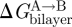 contribution to
contribution to 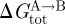 (equations (2.1) and (2.2)) may differ among proteins embedded in the same bilayer, with some proteins being more sensitive to changes in curvature, c0, and others to changes in hydrophobic mismatch (l − d0), it is those changes in
(equations (2.1) and (2.2)) may differ among proteins embedded in the same bilayer, with some proteins being more sensitive to changes in curvature, c0, and others to changes in hydrophobic mismatch (l − d0), it is those changes in 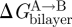 that are important for protein function.
that are important for protein function.
How can one determine whether amphiphiles, for example, regulate membrane protein function in biological systems by altering bilayer physical properties? It is possible to do so by using gA channels as molecular force transducers to measure the net effects of amphiphiles on the bilayer elastic response to a change in hydrophobic length of an embedded protein. Given the underlying complexities, it is useful to summarize such changes as changes in bilayer stiffness.
Operationally, we define a change in bilayer stiffness as a change in bilayer physical properties that, at constant bilayer thickness, alters Fdis (the bilayer disjoining force acting on a gA channel; Lundbæk et al. 1996, 2005). A decrease in stiffness means that Fdis (and 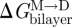 ) is decreased. This is observed as increases in both f and τ (provided other factors affecting the monomer ↔ dimer kinetics remain constant). Conversely, an increase in stiffness means that Fdis (and
) is decreased. This is observed as increases in both f and τ (provided other factors affecting the monomer ↔ dimer kinetics remain constant). Conversely, an increase in stiffness means that Fdis (and 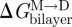 ) is increased, which is observed as decreases in both f and τ.
) is increased, which is observed as decreases in both f and τ.
The use of bilayer stiffness as a descriptor of the changes in bilayer properties that regulate protein function implies that the regulation is an energetic question that to a first approximation can be addressed using the continuum theory of elastic bilayer deformations with minimal chemical specificity (Andersen et al. 1998). The situation is similar to that for electrified interfaces, where the Gouy–Chapman theory of the diffuse double layer serves as a major organizing principle (e.g. McLaughlin 1989), with the Debye length being a major descriptor of the electrostatic interactions in the system.
2.11. Bilayer stiffness and membrane protein function in living cells
The functions of N-type calcium channels (NCCs) and voltage-dependent sodium channels (VDSCs) in mammalian cells have been studied using whole-cell voltage clamp (Lundbæk et al. 1996, 2004, 2005). Amphiphiles that decrease bilayer stiffness also, reversibly, promote NCC and VDSC inactivation. This is observed as a hyperpolarizing shift in the membrane potential causing 50 per cent channel inactivation (Vin). Figure 9 shows the relation between changes in Vin for VDSCs and gA channel lifetime (where ln{τ/τref} is a measure of the change in Fdis) for five structurally different amphiphiles.
Figure 9.
Relation between the amphiphile-induced shifts in VDSC inactivation and gA channel lifetime. The shift in membrane potential for 50 per cent VDSC inactivation (Vin), plotted as Vin − Vin,ref versus the changes in gA channel lifetime (ln{τ/τref}) determined in dioleoylphosphatidylcholine/n-decane bilayers. Black triangle, Triton X-100; red triangle, β-octyl glucoside; green triangle, reduced Triton X-100; blue diamond, Genapol X-100; light blue triangle, capsaicin. For the VDSC experiments, human muscle VDSC, NaV1.4, was expressed in HEK293 cells; the cells were depolarized to +20 mV following 300 ms prepulses to potentials varying from −130 to +50 mV. The gA experiments were done in diphytanoylphosphatidylcholine/n-decane bilayers using 1.0 M NaCl, pH 7 (10 mM HEPES), ±200 mV, 25°C. Experimental results from Lundbæk et al. (2004, 2005).
At low amphiphile concentrations, the Vin–ln{τ/τref} relations for the different compounds are very similar, indicating that both membrane environments respond similarly to the adsorption of the amphiphiles. Whereas manoeuvres that decrease bilayer stiffness promote inactivation of both NCCs and VDSCs, cholesterol, which increases bilayer stiffness, inhibits NCC inactivation (Lundbæk et al. 1996)—and cholesterol removal promotes VDSC inactivation (Lundbæk et al. 2004). Similarly, amphiphiles that decrease bilayer stiffness promote GABAA receptor desensitization and muscimol binding, whereas cholesterol promotes muscimol binding (Søgaard et al. 2006).
The correlation between amphiphile-induced changes in bilayer stiffness and protein function can be extended to other membrane proteins. Table 4 shows results obtained with six different channel types. Morris & Juranka (2007) provide additional information about the modulation of VDSCs by bilayer-modifying agents. In all cases, the nominal amphiphile concentrations used in the gA-based bilayer stiffness measurements are equal to or lower than those that regulate the biological channels.
Table 4.
Effects of amphiphiles on bilayer stiffness and ion channel function. The effects of structurally different amphiphiles on bilayer stiffness measured using gA channels and on VDSCs; NCCs; Ca2+-activated potassium channels (BKCa); nicotinic acetylcholine receptors (nAChR); and GABAA receptors. Modified from Søgaard et al. (2006).
| voltage-dependent channels |
Cys-loop receptors |
|||||||
|---|---|---|---|---|---|---|---|---|
| VDSC |
NCC |
BKCa |
nAChR |
GABAA |
||||
| amphiphile | bilayer stiffness (using gA channels) | activation | inactivation | activation | inactivation | activation | desensitization | muscimol binding |
| positive c0 | ||||||||
| Triton X-100 | ↓b | 0b | ↑b | 0c | ↑c | ↑d,e | ↑f | |
| β-octyl glucoside | ↓b | 0b | ↑b | 0c | ↑c | ↑e | ↑f | |
| pentobarbital | ↓g | 0h | ↑h | 0i | ↑i | ↑j | ↑k | |
| negative c0 | ||||||||
| capsaicin | ↓l | 0l | ↑l | ↑m | ↑f | |||
| docosahexaenoic acid | ↓n | 0 | ↑o | ↑p | ↑q,a | ↑f,r | ||
| arachidonic acid | ↓s | 0t | ↑t | ↑u | ↑u | ↑v | ↑q,a | ↑r |
| oleic acid | 0n | 0o | 0o | 0u | (↑)v | ↑q,a | ↑r | |
| cholesterol | ↑c | ↓b | ↓b | 0c | ↓c | ↓w | ↓x | ↓f |
aFatty acids decrease the single-channel lifetimes and shorten burst durations, it is not clear whether these changes reflect enhanced desensitization.
bLundbæk et al. (2004); cLundbæk et al. (1996); dAnwyl & Narahashi (1980); eMcCarthy & Moore (1992); fSøgaard et al. (2006); gSun et al. (2002); hRehberg et al. (1995); iGundersen et al. (1988); jFirestone et al. (1994); kQuast & Brenner (1983); lLundbæk et al. (2005); mEllis et al. (1997); nBruno et al. (2007); oVreugdenhil et al. (1996); pYe et al. (2002); qBouzat & Barrantes (1993); rWitt et al. (1999); sBruno et al. (2005); tLee et al. (2002); uLiu et al. (2001); vDenson et al. (2000); wChang et al. (1995); xBaenziger et al. (2000).
In the case of Triton X-100 and β-octyl glucoside, the changes in channel function occur at concentrations that are one to two orders of magnitude less than their critical micellar concentrations, indicating that the changes in function occur at amphiphile mole-fractions in the membrane of 0.01–0.1. (Amphiphile mole-fractions in lipid bilayers scale as the aqueous concentration relative to the critical micellar concentration (Heerklotz & Seelig 2000).) There is less information about membrane concentrations of the other compounds, and one cannot exclude that specific mechanisms, such as displacement of boundary lipids, may be involved in some cases. Indeed, the fatty acid effects on ligand-activated channels may reflect, in part, the adsorption (or accumulation) of fluid-phase fatty acids at the protein–bilayer boundary (Andreasen & McNamee 1980; Antollini & Barrantes 2002). The overall results, however, are unlikely to reflect solely specific interactions. It will be a challenge to determine the relative importance of specific versus non-specific interactions, and the separation is unlikely to be straightforward because a protein-induced bilayer deformation in its own right may alter the local amphiphile concentration near the protein (cf. Bruno et al. 2007).
2.12. Bilayer stiffness, fluidity, monolayer curvature and lateral pressure profile
The correlation between amphiphile-induced changes in bilayer stiffness measured in planar lipid bilayers and membrane protein function in living cells is surprisingly simple—and likely to break down at some point. Nevertheless, the correlations observed in figure 9 and table 4 strongly indicate that bilayer stiffness measurements report properties of lipid bilayers that are of general importance for membrane protein function. It further suggests that gA-based measurements of bilayer stiffness provide reliable information about the net effect of changes in bilayer composition on the bilayer elastic response to membrane protein conformational changes.
Historically, a number of descriptors have been used to describe (and explain) non-specific regulation of membrane protein function by the lipid bilayer (table 5); for recent reviews, see Lundbæk (2006) and Andersen & Koeppe (2007).
Table 5.
Descriptors of bilayer regulation of membrane protein function.
| bilayer fluidity (Linden et al. 1973) |
| bilayer coupling (Sheetz & Singer 1974) |
| hydrophobic mismatch/bilayer compression (Mouritsen & Bloom 1984) |
| intrinsic lipid curvature (Gruner 1985) |
| bilayer deformation energy (Huang 1986) |
| bilayer free volume (Mitchell et al. 1990) |
| acyl chain packing (Fattal & Ben-Shaul 1993) |
| bilayer stiffness (Lundbæk et al. 1996) |
| curvature frustration (Starling et al. 1996) |
| lateral pressure profile (Cantor 1997) |
| lipid packing stress (Bezrukov 2000) |
The first such descriptor was ‘bilayer fluidity’ (Linden et al. 1973). The consequences of altered bilayer composition continue to be ascribed to changes in bilayer ‘fluidity’, as evaluated using electron spin resonance probes, such as TEMPO (2,2,6,6-tetramethylpiperidine-1-oxy1; Shimshick & McConnell 1973), or fluorescent probes, such as diphenylhexatriene (DPH; Shinitzky & Barenholz 1978). But, though a liquid-crystalline bilayer organization is likely to be required for membrane protein function (to allow for protein conformation changes), the causal relation between bilayer ‘fluidity’ and protein function has never been clear—in part because probes such as DPH report a combination of acyl chain dynamics and order (Lentz 1993). Moreover, the equilibrium distribution between conformational states of a (bilayer-embedded) protein cannot be altered by a change in ‘fluidity’, per se (Schurr 1970; Lee 1991).
Changes in membrane protein function often correlate with changes in lipid intrinsic curvature, c0 (cf. Andersen & Koeppe 2007). Lipid bilayer regulation of function therefore is often interpreted in terms of changes in c0, or changes in bilayer lateral pressure profile (Cantor 1997, 1999), which have equivalent effects on the conformation of an embedded protein (Andersen & Koeppe 2007; Marsh 2007). Such unimodal interpretations, however, do not encompass the consequences of changes in bilayer molecular composition that also alter the bilayer elastic moduli. Nor do they fully describe the interplay between changes in hydrophobic mismatch and curvature, or the curvature-dependent changes in bilayer thickness. Neither the effects of amphiphiles on gA channels (figure 8), nor on a number of other ion channels (table 4), correlate with changes in just c0.
2.13. Amphiphile-regulation of membrane proteins—detecting bilayer-mediated effects
Studies on membrane protein function in cells may involve manipulations that alter the elastic properties of lipid bilayers. In cases where protein functional changes are induced by high nanomolar to micromolar (and higher) amphiphile concentrations, for example, it becomes prudent to consider whether some (or all) of the effects could result from the hydrophobic coupling between bilayers and their embedded proteins. It is therefore important to have experimental tests to determine whether amphiphile-induced changes in membrane protein function could be bilayer-mediated. Several such tests have been developed.
— Can the changes in protein function be reproduced by structurally very different amphiphiles known to alter bilayer stiffness, e.g. capsaicin or Triton X-100 (Smith & Proks 1998; Lundbæk et al. 2004, 2005; Søgaard et al. 2006; Matta et al. 2007)?
— At the concentrations used, does the amphiphile regulate the function of other, structurally unrelated bilayer-embedded proteins (including gA channels), suggesting that they may alter bilayer properties (Liu et al. 2004; Lundbæk et al. 2004, 2005; Søgaard et al. 2006)?
— Can the quantitative relation between the amphiphile's effects on the protein of interest and on gA channel lifetime be reproduced by structurally diverse amphiphiles (cf. figure 9; Lundbæk et al. 2004, 2005; Søgaard et al. 2006)?
The first of these approaches, in particular, offers a simple test for whether amphiphile-induced modulation of a membrane protein function could be bilayer-mediated.
3. Gramicidin channels as molecular force probes—a quantitative description
To obtain a more thorough understanding of gA channels as tools to explore changes in bilayer properties, it is necessary to develop a more physical/mathematical description. In the following, we describe the relations between channel energetics, kinetics and function, and the consequences of changes in bilayer physical properties. For more complete descriptions, the reader should consult Lundbæk (2006) and Andersen et al. (2007a).
3.1. Channel energetics, kinetics and function
The kinetics of gA channel formation/dissociation can be described as
where M and D denote gA monomers (one in each leaflet) and bilayer-spanning dimers, conducting channels (Bamberg & Läuger 1973; Veatch et al. 1975), and k1 and k−1 are the association and dissociation rate constants.
The channel dimerization constant (KD) is given by
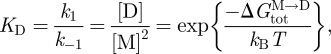 |
3.1 |
where the bilayer concentrations of M and D are in moles per unit area, and 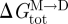 is the free energy difference for channel formation. All gA dimers seem to be conducting channels (Veatch et al. 1975); therefore, changes in n, the time-averaged number of channels per unit membrane area (cf. equation (2.2)), can be determined from changes in the (time-averaged) membrane conductance.
is the free energy difference for channel formation. All gA dimers seem to be conducting channels (Veatch et al. 1975); therefore, changes in n, the time-averaged number of channels per unit membrane area (cf. equation (2.2)), can be determined from changes in the (time-averaged) membrane conductance.
The relation between k1, f and the activation energy for subunit association (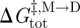 ) becomes
) becomes
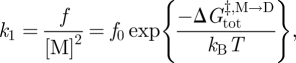 |
3.2 |
where f0 is a frequency factor for the reaction. The corresponding relation between k−1, τ and the activation energy for dimer dissociation (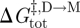 ) is given by
) is given by
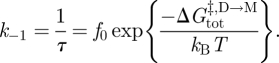 |
3.3 |
3.2. Channel energetics and bilayer deformation energy
The contribution from the bilayer deformation energy to the energetics and kinetics of channel formation can then be expressed using the relations (cf. equation (2.1))
| 3.4 |
and
| 3.5 |
where
| 3.6 |
| 3.7 |
such that
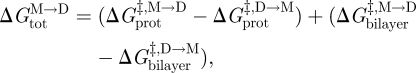 |
3.8 |
where 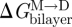 ,
, 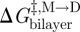 and
and 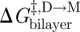 denote the changes in bilayer deformation energy associated with adjusting the bilayer thickness from d0 (the unperturbed thickness) to l (the channel length), from d0 to (l + δ), and from l to (l + δ), respectively, where δ (approx. 0.16 nm; Durkin et al. 1993; Lundbæk & Andersen 1999; Miloshevsky & Jordan 2004) is the subunit separation at the transition state for channel formation/dissociation.
denote the changes in bilayer deformation energy associated with adjusting the bilayer thickness from d0 (the unperturbed thickness) to l (the channel length), from d0 to (l + δ), and from l to (l + δ), respectively, where δ (approx. 0.16 nm; Durkin et al. 1993; Lundbæk & Andersen 1999; Miloshevsky & Jordan 2004) is the subunit separation at the transition state for channel formation/dissociation.
3.3. Bilayer deformation energy and channel energetics, kinetics and function
Changes in bilayer physical properties that alter the bilayer contribution to 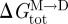 can be evaluated by combining equations (3.1) and (3.4) (assuming that the experimental manipulation does not alter
can be evaluated by combining equations (3.1) and (3.4) (assuming that the experimental manipulation does not alter 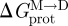 ):
):
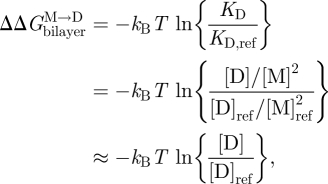 |
3.9 |
where the subscript ‘ref’ denotes the reference state. The last relation becomes exact in the limit when [M]ref ≈ [M] (Lundbæk & Andersen 1994). It thus becomes possible to estimate 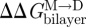 from [D]/[D]ref, which can be estimated from the relative changes in membrane conductance, or time-averaged channel activity (n = fτ).
from [D]/[D]ref, which can be estimated from the relative changes in membrane conductance, or time-averaged channel activity (n = fτ).
Similarly for 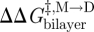 and
and 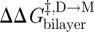 :
:
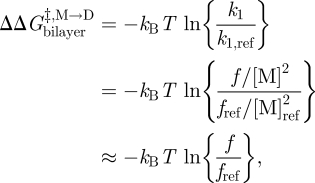 |
3.10 |
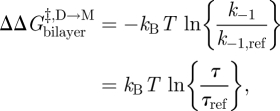 |
3.11 |
and using equation (3.5):
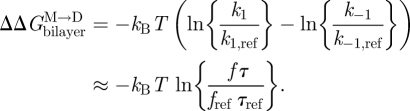 |
3.12 |
One can thus estimate 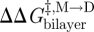 ,
, 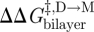 and
and 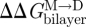 from changes in ln{f} and ln{τ}. Similar monitoring of the energetics of elastic bilayer deformations for well-described changes in a bilayer-spanning inclusion (protein) is, at present, not possible for other systems.
from changes in ln{f} and ln{τ}. Similar monitoring of the energetics of elastic bilayer deformations for well-described changes in a bilayer-spanning inclusion (protein) is, at present, not possible for other systems.
3.4. Channel function and channel–bilayer mismatch
We are now in a position where we can compare experimental relations between lipid bilayer physical properties and gA channel function with quantitative predictions based on the continuum theory of elastic bilayer deformations. We first address the relation between changes in bilayer physical properties and the monomer ↔ dimer equilibrium (scheme 1). The relation between 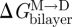 and the bilayer physical properties is given by (cf. equation (2.6))
and the bilayer physical properties is given by (cf. equation (2.6))
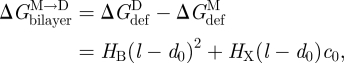 |
3.13 |
where we use that 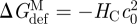 .
. 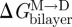 thus is a function of HB and HX, as well as (l − d0) and c0. We would expect that effects of changes in bilayer elasticity (reflected in HB and HX) or c0—as reported by changes in [D]—will depend on the hydrophobic mismatch (l−d0), which can be manipulated in experiments using thicker bilayers (increasing d0) or shorter channels (decreasing l). That is, the change in [D] should be larger for larger values of |l − d0|. This has been observed in most (Lundbæk et al. 2005; Artigas et al. 2006; Bruno et al. 2007), but not all (Ashrafuzzaman et al. 2006) systems investigated so far.
thus is a function of HB and HX, as well as (l − d0) and c0. We would expect that effects of changes in bilayer elasticity (reflected in HB and HX) or c0—as reported by changes in [D]—will depend on the hydrophobic mismatch (l−d0), which can be manipulated in experiments using thicker bilayers (increasing d0) or shorter channels (decreasing l). That is, the change in [D] should be larger for larger values of |l − d0|. This has been observed in most (Lundbæk et al. 2005; Artigas et al. 2006; Bruno et al. 2007), but not all (Ashrafuzzaman et al. 2006) systems investigated so far.
The relation between 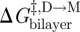 , as reported by τ, and d0 (or rather d0 − l) is then obtained by noting that gA channel dissociation involves the separation of the two subunits by a distance δ (approx. 0.16 nm; Durkin et al. 1993; Lundbæk & Andersen 1999; Miloshevsky & Jordan 2004). Using equation (2.6), we find that
, as reported by τ, and d0 (or rather d0 − l) is then obtained by noting that gA channel dissociation involves the separation of the two subunits by a distance δ (approx. 0.16 nm; Durkin et al. 1993; Lundbæk & Andersen 1999; Miloshevsky & Jordan 2004). Using equation (2.6), we find that 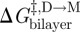 , the difference in bilayer deformation energy in the transition state relative to the conducting dimer state is
, the difference in bilayer deformation energy in the transition state relative to the conducting dimer state is
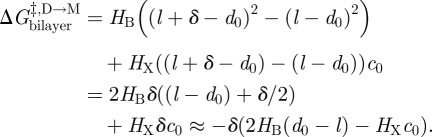 |
3.14 |
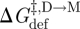 , and thus ln{τ}, therefore should be linear functions of d0 − l (cf. equations (3.7) and (3.11)), which is the case (Lundbæk & Andersen 1999; see also figure 6). Moreover, in nominally hydrocarbon-free bilayers, the slope of the ln{τ}–d0 relation is remarkably close to predictions based on the theory of elastic bilayer deformations (equation (2.5)) using independently measured values of the bilayer elastic moduli (Lundbæk & Andersen 1999). Though one should question the range of validity of continuum models when analysing molecular events, the continuum description of elastic bilayer deformations seems to provide a useful foundation for analysing the energetic coupling between membrane proteins and their host bilayers.
, and thus ln{τ}, therefore should be linear functions of d0 − l (cf. equations (3.7) and (3.11)), which is the case (Lundbæk & Andersen 1999; see also figure 6). Moreover, in nominally hydrocarbon-free bilayers, the slope of the ln{τ}–d0 relation is remarkably close to predictions based on the theory of elastic bilayer deformations (equation (2.5)) using independently measured values of the bilayer elastic moduli (Lundbæk & Andersen 1999). Though one should question the range of validity of continuum models when analysing molecular events, the continuum description of elastic bilayer deformations seems to provide a useful foundation for analysing the energetic coupling between membrane proteins and their host bilayers.
3.5. Channel function and c0
The relative clarity that pertains to the consequences of changing d0 extends also to changes in c0, as long as c0 is altered by changes in head group interactions of the bilayer-forming lipid. That is, a negative-going change in c0 increases Fdis and 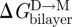 (Lundbæk et al. 1997; Maer et al. submitted; see also Andersen & Koeppe 2007). If the changes in c0 arise from changes in the bilayer-forming lipids that involve the bilayer hydrophobic core, such as increasing acyl chain unsaturation, the situation becomes murky. In this case, the lifetime changes tend to be in a direction opposite to that predicted from the expected changes in c0 (Girshman et al. 1997), maybe because increasing acyl unsaturation will tend to thin lipid bilayers (Lewis & Engelman 1983) and reduce the bilayer bending moduli (Rawicz et al. 2000)—changes that in their own right will tend to reduce
(Lundbæk et al. 1997; Maer et al. submitted; see also Andersen & Koeppe 2007). If the changes in c0 arise from changes in the bilayer-forming lipids that involve the bilayer hydrophobic core, such as increasing acyl chain unsaturation, the situation becomes murky. In this case, the lifetime changes tend to be in a direction opposite to that predicted from the expected changes in c0 (Girshman et al. 1997), maybe because increasing acyl unsaturation will tend to thin lipid bilayers (Lewis & Engelman 1983) and reduce the bilayer bending moduli (Rawicz et al. 2000)—changes that in their own right will tend to reduce 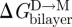 , and that may dominate over the c0-dependent increase.
, and that may dominate over the c0-dependent increase.
If c0 is altered by small amphiphiles that adsorb to lipid bilayers, rather than by changing the bilayer-forming lipid, the curvature-dependent effects also tend to be less important (Lundbæk et al. 2005; Bruno et al. 2007), as would be expected from the thermodynamics of solute adsorption to the bilayer–solution interface (Evans et al. 1995; Zhelev 1998). Compounds that cause either negative or positive changes in c0 therefore may have similar effects on gA channel function, as well as on the function of integral membrane protein channels (table 4). As noted earlier, this is because reversibly adsorbing amphiphiles decrease Ka and Kc (and thereby HB and HX; cf. Evans et al. 1995; Zhelev 1998; Ly & Longo 2004; Zhou & Raphael 2005), which would tend to decrease 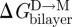 and Fdis. If the decrease in the HB(l − d0)2 term in equation (3.13) dominates the curvature-dependent increase in HX(l − d0)c0 term, the changes in (membrane protein) function will deviate from predictions based solely on changes in c0.
and Fdis. If the decrease in the HB(l − d0)2 term in equation (3.13) dominates the curvature-dependent increase in HX(l − d0)c0 term, the changes in (membrane protein) function will deviate from predictions based solely on changes in c0.
3.6. Separating effects of changes in c0 versus changes in elastic moduli
The regulation of membrane protein function by changes in lipid bilayer physical properties has been extensively investigated—usually in terms of isolated changes in c0, thickness or, maybe, elastic moduli. As noted previously, these unimodal interpretations may be misleading; it is important to have methods that discriminate between consequences of changes in c0 versus bilayer elastic moduli.
gA channels can be used for this purpose because, when the bilayer physical properties are altered, the resulting changes in τ can be separated into a term that depends on (d0 − l) and one that depends on c0. Using equation (3.14) (and assuming that 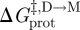 remains invariant):
remains invariant):
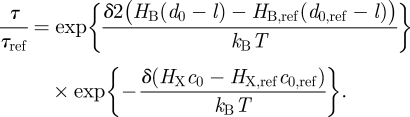 |
3.15 |
Changes in τ that are owing to altered bilayer elasticity (HB and HX) thus should vary as a function of (d0 − l), whereas changes owing to altered c0 should be impervious to changes in (d0 − l) (Maer et al. submitted). This distinction can be observed experimentally by comparing the effects of altered bilayer composition on τ for gA channels formed by analogues of different length (e.g. 13 and 15 amino acids; Maer et al. submitted). In cases where HB is decreased, τ/τref will be larger for the shorter channels relative to the longer channels (Lundbæk et al. 2005; Bruno et al. 2007; Ingólfsson et al. 2007; Maer et al. submitted). When only c0 is altered, τ/τref should be the same for the longer and shorter channels (Artigas et al. 2006; Ashrafuzzaman et al. 2006; Maer et al. submitted).
The quantitative relation between difference in channel length and changes in τ and HB may be expressed as
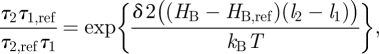 |
3.16 |
where the subscripts ‘1’ and ‘2’ refer to the two different gA channels (Maer et al. submitted). It thus becomes possible to evaluate whether an experimental manoeuvre alters bilayer elasticity, as expressed through HB, in experiments with gA channels of different lengths. This approach thus provides a means for resolving some of the persisting uncertainties in the bilayer regulation of membrane protein function.
4. Conclusion
This review has focused on non-specific regulation of membrane protein function by bilayer physical properties. The chemical heterogeneity of biological membranes and the complexity of protein–bilayer boundaries continue to pose barriers to a mechanistic understanding, but the hydrophobic coupling mechanism provides an organizing principle for exploring the energetic coupling between membrane proteins and their host bilayer. Moreover, the continuum theory of elastic bilayer deformation provides a foundation for how to relate events at the molecular level to changes in bilayer physical properties.
In many cases the regulation of protein function also depends on more specific lipid–protein interactions (Lee 2005; Marsh 2008; Suh & Hille 2008), and it may be difficult to determine the relative importance of lipid–protein and bilayer–protein interactions. Even when the regulation is due to bilayer–protein interactions, the lipid composition near a protein may differ from the bulk composition owing to constraints on the lipid packing around the protein (Sperotto & Mouritsen 1993). The bilayer control of membrane protein function, however, is an energetic question that to a first approximation can be approached using the continuum theory of elastic bilayer deformations.
One must be wary of using continuum arguments at the molecular level, but they provide a surprising ability to predict the direction and even magnitude of changes in function. In this context, gA channels have proven useful because they are sufficiently simple to allow for a quantitative analysis of the channel–bilayer coupling. gA channels also have proven useful as probes for changes in bilayer properties, expressed as changes in bilayer stiffness. Reducing the complexity of the lipid bilayer, and the bilayer–protein interactions, to just one descriptor is a major simplification (a sort of ultimate ‘coarse graining’ in molecular modelling terms).
This simplicity is both a strength, because it is a robust measure, and a weakness, because of the limitations of the continuum description. To explore these limitations, it is important to push the gA system to the point where these limitations become evident, as already has been done in the case of the reversibly adsorbing amphiphiles. It also will be important to develop systems with more complex functions, which allow for a similar level of quantitative analysis and thereby allow further explorations of the limitations of the continuum description. Yet, the intuitive simplicity provided by the theory of elastic bilayer deformations is likely to continue to serve as an organizing principle for understanding the energetic coupling between membrane proteins and their host bilayer.
Acknowledgements
This work was supported in part by the National Institutes of Health grant GM21342 (O.S.A.) and the Josiah Macy Jr Foundation (O.S.A.). We thank Kevin Lum, Radda Rusinova and an anonymous reviewer for incisive comments on the manuscript.
References
- Abdul-Manan N., Hinton J. F. 1994. Conformation states of gramicidin A along the pathway to the formation of channels in model membranes determined by 2D NMR and circular dichroism spectroscopy. Biochemistry 33, 6773–6783. ( 10.1021/bi00188a005) [DOI] [PubMed] [Google Scholar]
- Adachi S., Nagao T., Ingólfsson H. I., Maxfield F. R., Andersen O. S., Kopelovich L., Weinstein I. B. 2007. The inhibitory effect of (-)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 67, 6493–6501. ( 10.1158/0008-5472.CAN-07-0411) [DOI] [PubMed] [Google Scholar]
- Alam A., Jiang Y. 2009. High-resolution structure of the open NaK channel. Nature Struct. Mol. Biol. 16, 30–34. ( 10.1038/nsmb.1531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. W., Andersen O. S., Roux B. 2003. The structure of gramicidin A in a lipid bilayer environment determined using molecular dynamics simulations and solid-state NMR data. J. Am. Chem. Soc. 125, 9868–9878. ( 10.1021/ja029317k) [DOI] [PubMed] [Google Scholar]
- Aller S. G., et al. 2009. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323, 1718–1722. ( 10.1126/science.1168750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S. 1983. Ion movement through gramicidin A channels. Single-channel measurements at very high potentials. Biophys. J. 41, 119–133. ( 10.1016/S0006-3495(83)84414-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Koeppe R. E., II 2007. Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 36, 107–130. ( 10.1146/annurev.biophys.36.040306.132643) [DOI] [PubMed] [Google Scholar]
- Andersen O. S., Finkelstein A., Katz I., Cass A. 1976. Effect of phloretin on the permeability of thin lipid membranes. J. Gen. Physiol. 67, 749–771. ( 10.1085/jgp.67.6.749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Sawyer D. B., Koeppe R. E., II 1992. Modulation of channel function by the host bilayer. In Biomembrane structure and function (eds Easwaran K. R. K., Gaber B.), pp. 227–244. Schenectady, NY: Adenine Press. [Google Scholar]
- Andersen O. S., Nielsen C., Maer A. M., Lundbæk J. A., Goulian M., Koeppe R. E., II 1998. Gramicidin channels: molecular force transducers in lipid bilayers. Biol. Skr. Dan. Vid. Selsk. 49, 75–82. [Google Scholar]
- Andersen O. S., Nielsen C., Maer A. M., Lundbæk J. A., Goulian M., Koeppe R. E., II 1999. Ion channels as tools to monitor lipid bilayer-membrane protein interactions: gramicidin channels as molecular force transducers. Meth. Enzymol. 294, 208–224. ( 10.1016/S0076-6879(99)94013-2) [DOI] [PubMed] [Google Scholar]
- Andersen O. S., Bruno M. J., Sun H., Koeppe R. E., II 2007a. Single-molecule methods for monitoring changes in bilayer elastic properties. Methods Mol. Biol. 400, 543–570. ( 10.1007/978-1-59745-519-0_37) [DOI] [PubMed] [Google Scholar]
- Andersen O. S., Koeppe R. E., II, Roux B. 2007b. Gramicidin channels. Versatile tools. In Biological membrane ion channels: dynamics, structure, and applications (eds Chung S.-H., Andersen O. S., Krishnamurthy V.), pp. 33–80. New York, NY: Springer Verlag. [Google Scholar]
- Andreasen T. J., McNamee M. G. 1980. Inhibition of ion permeability control properties of acetylcholine receptor from Torpedo californica by long-chain fatty acids. Biochemistry 19, 4719–4726. ( 10.1021/bi00561a027) [DOI] [PubMed] [Google Scholar]
- Antollini S. S., Barrantes F. J. 2002. Unique effects of different fatty acid species on the physical properties of the torpedo acetylcholine receptor membrane. J. Biol. Chem. 277, 1249–1254. ( 10.1074/jbc.M106618200) [DOI] [PubMed] [Google Scholar]
- Anwyl R., Narahashi T. 1980. Comparison of desensitization and time-dependent block of the acetylcholine receptor responses by chlorpromazine, cytochalasin B, triton X-100 and other agents. Br. J. Pharmacol. 69, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel M., Hizlan D., Vinothkumar K. R., Ziegler C., Kühlbrandt W. 2009. Conformations of NhaA, the Na+/H+ exchanger from Escherichia coli, in the pH-activated and ion-translocating states. J. Mol. Biol. 388, 659–672. ( 10.1016/j.jmb.2009.03.010) [DOI] [PubMed] [Google Scholar]
- Arseniev A. S., Lomize A. L., Barsukov I. L., Bystrov V. F. 1986. Gramicidin A transmembrane ion-channel. Three-dimensional structure reconstruction based on NMR spectroscopy and energy refinement. Biol. Membr. 3, 1077–1104. [Google Scholar]
- Artigas P., Al'aref S. J., Hobart E. A., Diaz L. F., Sakaguchi M., Straw S., Andersen O. S. 2006. 2,3-Butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: the role of bilayer material properties. Mol. Pharmacol. 70, 2015–2026. ( 10.1124/mol.106.026070) [DOI] [PubMed] [Google Scholar]
- Ashrafuzzaman Md., Lampson M. A., Greathouse D. V., Koeppe R. E., II, Andersen O. S. 2006. Manipulating lipid bilayer material properties using biologically active amphipathic molecules. J. Phys.: Condens. Matter 18, S1235–S1255. ( 10.1088/0953-8984/18/28/S08) [DOI] [Google Scholar]
- Baenziger J. E., Morris M. L., Darsaut T. E., Ryan S. E. 2000. Effect of membrane lipid composition on the conformational equilibria of the nicotinic acetylcholine receptor. J. Biol. Chem. 275, 777–784. ( 10.1074/jbc.275.2.777) [DOI] [PubMed] [Google Scholar]
- Bamberg E., Läuger P. 1973. Channel formation kinetics of gramicidin A in lipid bilayer membranes. J. Membr. Biol. 11, 177–194. ( 10.1007/BF01869820) [DOI] [PubMed] [Google Scholar]
- Barry J., Fritz M., Brender J. R., Smith P. E., Lee D. K., Ramamoorthy A. 2009. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin. J. Am. Chem. Soc. 131, 4490–4498. ( 10.1021/ja809217u) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass R. B., Strop P., Barclay M., Rees D. C. 2002. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298, 1582–1587. ( 10.1126/science.1077945) [DOI] [PubMed] [Google Scholar]
- Benz R., Fröhlich O., Läuger P., Montal M. 1975. Electrical capacity of black lipid films and of lipid bilayers made from monolayers. Biochim. Biophys. Acta 394, 323–334. [DOI] [PubMed] [Google Scholar]
- Bezrukov S. M. 2000. Functional consequences of lipid packing stress. Curr. Opin. Coll. Interface Sci. 5, 237–243. ( 10.1016/S1359-0294(00)00061-3) [DOI] [Google Scholar]
- Bezrukov S. M., Rand R. P., Vodyanoy I., Parsegian V. A. 1998. Lipid packing stress and polypeptide aggregation: alamethicin channel probed by proton titration of lipid charge. Faraday Discuss. 111, 173–183. Discussion 225–246 ( 10.1039/a806579i) [DOI] [PubMed] [Google Scholar]
- Bockris J. O. M., Reddy A. K. N. 1970. Modern electrochemistry, vol. 1 New York, NY: Plenum. [Google Scholar]
- Bocquet N., Nury H., Baaden M., Le Poupon C., Changeux J. P., Delarue M., Corringer P. J. 2009. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114. ( 10.1038/nature07462) [DOI] [PubMed] [Google Scholar]
- Bouzat C. B., Barrantes F. J. 1993. Effects of long-chain fatty acids on the channel activity of the nicotinic acetylcholine receptor. Receptors Channels 1, 251–258. [PubMed] [Google Scholar]
- Bradley R. J., Urry D. W., Parenti-Castelli G., Lenaz G. 1981. Effects of halothane on channel activity of N-acetyl gramicidin. Biochem. Biophys. Res. Commun. 101, 963–969. ( 10.1016/0006-291X(81)91843-X) [DOI] [PubMed] [Google Scholar]
- Bruno M. J., Koeppe R. E., II, Andersen O. S. 2005. Modification of gramicidin channel function by PUFAs depends on double-bond structure (abstract). Biophys. J. 88, 575A. [Google Scholar]
- Bruno M. J., Koeppe R. E., II, Andersen O. S. 2007. Docosahexaenoic acid alters bilayer elastic properties. Proc. Natl Acad. Sci. USA 104, 9638–9643. ( 10.1073/pnas.0701015104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan S. K., Lukacik P., Grizot S., Ghirlando R., Ali M. M., Barnard T. J., Jakes K. S., Kienker P. K., Esser L. 2007. Structure of colicin I receptor bound to the R-domain of colicin Ia: implications for protein import. EMBO J. 26, 2594–2604. ( 10.1038/sj.emboj.7601693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrov V. F., Arseniev A. S. 1988. Diversity of the gramicidin A spatial structure: two-dimensional proton NMR study in solution. Tetrahedron 44, 925–940. ( 10.1016/S0040-4020(01)86126-3) [DOI] [Google Scholar]
- Cantor R. S. 1997. Lateral pressures in cell membranes: a mechanism for modulation of protein function. J. Phys. Chem. B 101, 1723–1725. ( 10.1021/jp963911x) [DOI] [Google Scholar]
- Cantor R. S. 1999. The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem. Phys. Lipids 101, 45–56. ( 10.1016/S0009-3084(99)00054-7) [DOI] [PubMed] [Google Scholar]
- Chang G., Spencer R. H., Lee A. T., Barclay M. T., Rees D. C. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282, 2220–2226. ( 10.1126/science.282.5397.2220) [DOI] [PubMed] [Google Scholar]
- Chang H. M., Reitstetter R., Mason R. P., Gruener R. 1995. Attenuation of channel kinetics and conductance by cholesterol: an interpretation using structural stress as a unifying concept. J. Membrane Biol. 143, 51–63. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. 1979. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta 559, 399–420. [DOI] [PubMed] [Google Scholar]
- Dan N., Safran S. A. 1998. Effect of lipid characteristics on the structure of transmembrane proteins. Biophys. J. 75, 1410–1414. ( 10.1016/S0006-3495(98)74059-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson D. D., Wang X., Worrell R. T., Eaton D. C. 2000. Effects of fatty acids on BK channels in GH3 cells. Am. J. Physiol. Cell Physiol. 279, C1211–C1219. [DOI] [PubMed] [Google Scholar]
- Doyle D. A., Morais Cabral J., Pfuetzner R. A., Kuo A., Gulbis J. M., Cohen S. L., Chait B. T., MacKinnon R. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77. ( 10.1126/science.280.5360.69) [DOI] [PubMed] [Google Scholar]
- Dupuy A. D., Engelman D. M. 2008. Protein area occupancy at the center of the red blood cell membrane. Proc. Natl Acad. Sci. USA 105, 2848–2852. ( 10.1073/pnas.0712379105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin J. T., Providence L. L., Koeppe R. E., II, Andersen O. S. 1993. Energetics of heterodimer formation among gramicidin analogues with an NH2-terminal addition or deletion. Consequences of a missing residue at the join in channel. J. Mol. Biol. 231, 1102–1121. ( 10.1006/jmbi.1993.1355) [DOI] [PubMed] [Google Scholar]
- Elliott J. R., Needham D., Dilger J. P., Haydon D. A. 1983. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim. Biophys. Acta 735, 95–103. [DOI] [PubMed] [Google Scholar]
- Elliott J. R., Needham D., Dilger J. P., Brandt O., Haydon D. A. 1985. A quantitative explanation of the effects of some alcohols on gramicidin single-channel lifetime. Biochim. Biophys. Acta 814, 401–404. [DOI] [PubMed] [Google Scholar]
- Ellis J. L., Sham J. S., Undem B. J. 1997. Tachykinin-independent effects of capsaicin on smooth muscle in human isolated bronchi. Am. J. Resp. Crit. Care Med. 155, 751–755. [DOI] [PubMed] [Google Scholar]
- Evans E. A. 1974. Bending resistance and chemically induced moments in membrane bilayers. Biophys. J. 14, 923–931. ( 10.1016/S0006-3495(74)85959-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. 1978. Mechanochemical properties of membranes. Curr. Top. Membr. Transp. 10, 1–64. ( 10.1016/S0070-2161(08)60833-3) [DOI] [Google Scholar]
- Evans E., Needham D. 1987. Physical properties of surfactant bilayer membranes: thermal transitions, elasticity, rigidity, cohesion, and colloidal interactions. J. Phys. Chem. 91, 4219–4228. ( 10.1021/j100300a003) [DOI] [Google Scholar]
- Evans E., Rawicz W., Hofmann A. F. 1995. Lipid bilayer expansion and mechanical disruption in solutions of water-soluble bile acid. In Bile acids in gastroenterology: basic and clinical advances (eds Hofmann A. F., Paumgartner G., Stiehl A.), pp. 59–68. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Fattal D. R., Ben-Shaul A. 1993. A molecular model for lipid-protein interaction in membranes: the role of hydrophobic mismatch. Biophys. J. 65, 1795–1809. ( 10.1016/S0006-3495(93)81249-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone L. L., Alifimoff J. K., Miller K. W. 1994. Does general anesthetic-induced desensitization of the Torpedo acetylcholine receptor correlate with lipid disordering? Mol. Pharmacol. 46, 508–515. [PubMed] [Google Scholar]
- Galbraith T. P., Wallace B. A. 1998. Phospholipid chain length alters the equilibrium between pore and channel forms of gramicidin. Faraday Discuss. 111, 159–164. Discussion 225–246 ( 10.1039/a808270g) [DOI] [PubMed] [Google Scholar]
- Gerber S., Comellas-Bigler M., Goetz B. A., Locher K. P. 2008. Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science 321, 246–250. ( 10.1126/science.1156213) [DOI] [PubMed] [Google Scholar]
- Girshman J., Greathouse J. V., Koeppe R. E., II, Andersen O. S. 1997. Gramicidin channels in phospholipid bilayers having unsaturated acyl chains. Biophys. J. 73, 1310–1319. ( 10.1016/S0006-3495(97)78164-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M., Mesquita O. N., Fygenson D. K., Nielsen C., Andersen O. S., Libchaber A. 1998. Gramicidin channel kinetics under tension. Biophys. J. 74, 328–337. ( 10.1016/S0006-3495(98)77790-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greathouse D. V., Hinton J. F., Kim K. S., Koeppe R. E., II 1994. Gramicidin A/short-chain phospholipid dispersions: chain length dependence of gramicidin conformation and lipid organization. Biochemistry 33, 4291–4299. ( 10.1021/bi00180a025) [DOI] [PubMed] [Google Scholar]
- Gruner S. M. 1985. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc. Natl Acad. Sci. USA 82, 3665–3669. ( 10.1073/pnas.82.11.3665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner S. M. 1991. Lipid membrane curvature elasticity and protein function. In Biologically inspired physics (ed. Peliti L.), pp. 127–135. New York, NY: Plenum Press. [Google Scholar]
- Gundersen C. B., Umbach J. A., Swartz B. E. 1988. Barbiturates depress currents through human brain calcium channels studied in Xenopus oocytes. J. Pharmacol. Exp. Ther. 247, 824–829. [PubMed] [Google Scholar]
- Harroun T. A., Heller W. T., Weiss T. M., Yang L., Huang H. W. 1999. Experimental evidence for hydrophobic matching and membrane-mediated interactions in lipid bilayers containing gramicidin. Biophys. J. 76, 937–945. ( 10.1016/S0006-3495(99)77257-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Ludtke S. J., Wu Y., Huang H. W., Andersen O. S., Greathouse D., Koeppe R. E., II 1994. Closed state of gramicidin channel detected by X-ray in-plane scattering. Biophys. Chem. 49, 83–89. ( 10.1016/0301-4622(93)E0085-J) [DOI] [PubMed] [Google Scholar]
- He K., Ludtke S. J., Heller W. T., Huang H. W. 1996. Mechanism of alamethicin insertion into lipid bilayers. Biophys. J. 71, 2669–2679. ( 10.1016/S0006-3495(96)79458-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerklotz H., Seelig J. 2000. Correlation of membrane/water partition coefficients of detergents with the critical micelle concentration. Biophys. J. 78, 2435–2440. ( 10.1016/S0006-3495(00)76787-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich P., Jakobsson E. 1990. Calculation of deformation energies and conformations in lipid membranes containing gramicidin channels. Biophys. J. 57, 1075–1084. ( 10.1016/S0006-3495(90)82625-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich W. 1973. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. 28C, 693–703. [DOI] [PubMed] [Google Scholar]
- Helfrich W. 1981. Amphiphilic mesophases made of defects. In Physique des défauts (Physics of defects) (eds Balian R., Kléman M., Poirier J.-P.), pp. 716–755. New York, NY: North-Holland Publishing Company. [Google Scholar]
- Hilf R. J., Dutzler R. 2008. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379. ( 10.1038/nature06717) [DOI] [PubMed] [Google Scholar]
- Hilf R. J., Dutzler R. 2009. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118. ( 10.1038/nature07461) [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W., Feng S., Nasuhoglu C. 2001. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE 2001, RE19. [DOI] [PubMed] [Google Scholar]
- Huang H. W. 1986. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys. J. 50, 1061–1070. ( 10.1016/S0006-3495(86)83550-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunte C., Screpanti E., Venturi M., Rimon A., Padan E., Michel H. 2005. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 435, 1197–1202. ( 10.1038/nature03692) [DOI] [PubMed] [Google Scholar]
- Hvorup R. N., Goetz B. A., Niederer M., Hollenstein K., Perozo E., Locher K. P. 2007. Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD-BtuF. Science 317, 1387–1390. ( 10.1126/science.1145950) [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Koeppe R. E., II, Andersen O. S. 2003. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry 42, 13 646–13 658. ( 10.1021/bi034887y) [DOI] [PubMed] [Google Scholar]
- Inaba K., Murakami S., Nakagawa A., Iida H., Kinjo M., Ito K., Suzuki M. 2009. Dynamic nature of disulphide bond formation catalysts revealed by crystal structures of DsbB. EMBO J. 28, 779–791. ( 10.1038/emboj.2009.21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingólfsson H. I., Koeppe R. E., II, Andersen O. S. 2007. Curcumin is a modulator of bilayer material properties. Biochemistry 46, 10 384–10 391. ( 10.1021/bi701013n) [DOI] [PubMed] [Google Scholar]
- Ingólfsson H. I., Kapoor R., Collingwood S. A., Andersen O. S. 2008. Single-molecule methods for monitoring changes in bilayer elastic properties. J. Vis. Exp. 21, http://www.jove.com/index/Details.stp?ID=1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. N. 1977. Refinement of the fluid-mosaic model of membrane structure. Biochim. Biophys. Acta 469, 221–225. [DOI] [PubMed] [Google Scholar]
- Jähnig F., Vogel H., Best L. 1982. Unifying description of the effect of membrane proteins on lipid order. Verification for the melittin/dimyristoylphosphatidylcholine system. Biochemistry 21, 6790–6798. ( 10.1021/bi00269a027) [DOI] [PubMed] [Google Scholar]
- Kapoor R., Kim J. H., Ingólfsson H. I., Andersen O. S. 2008. Preparation of artificial bilayers for electrophysiology experiments. J. Vis. Exp. 20, http://www.jove.com/index/Details.stp?ID=1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsaras J., Prosser R. S., Stinson R. H., Davis J. H. 1992. Constant helical pitch of the gramicidin channel in phospholipid bilayers. Biophys. J. 61, 827–830. ( 10.1016/S0006-3495(92)81888-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S. L., Bezrukov S. M., Gruner S. M., Tate M. W., Vodyanoy I., Parsegian V. A. 1993. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys. J. 65, 23–27. ( 10.1016/S0006-3495(93)81040-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchem R. R., Roux B., Cross T. A. 1997. High-resolution polypeptide structure in a lamellar phase lipid environment from solid state NMR derived orientational constraints. Structure 5, 1655–1669. ( 10.1016/S0969-2126(97)00312-2) [DOI] [PubMed] [Google Scholar]
- Khare D., Oldham M. L., Orelle C., Davidson A. L., Chen J. 2009. Alternating access in maltose transporter mediated by rigid-body rotations. Mol. Cell 33, 528–536. ( 10.1016/j.molcel.2009.01.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk G. L., Gruner S. M., Stein D. L. 1984. A thermodynamic model of the lamellar to inverse hexagonal phase transition of lipid membrane-water systems. Biochemistry 23, 1093–1102. ( 10.1021/bi00301a009) [DOI] [Google Scholar]
- Kučerka N., Liu Y., Chu N., Petrache H. I., Tristram-Nagle S., Nagle J. F. 2005. Structure of fully hydrated fluid phase DMPC and DLPC lipid bilayers using X-ray scattering from oriented multilamellar arrays and from unilamellar vesicles. Biophys. J. 88, 2626–2637. ( 10.1529/biophysj.104.056606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. G. 1991. Lipids and their effects on membrane proteins: evidence against a role for fluidity. Prog. Lipid Res. 30, 323–348. ( 10.1016/0163-7827(91)90002-M) [DOI] [PubMed] [Google Scholar]
- Lee A. G. 2003. Lipid–protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta 1612, 1–40. [DOI] [PubMed] [Google Scholar]
- Lee A. G. 2005. How lipids and proteins interact in a membrane: a molecular approach. Mol. Biosyst. 1, 203–212. ( 10.1039/b504527d) [DOI] [PubMed] [Google Scholar]
- Lee G. Y., Shin Y. K., Lee C. S., Song J. H. 2002. Effects of arachidonic acid on sodium currents in rat dorsal root ganglion neurons. Brain Res. 950, 95–102. ( 10.1016/S0006-8993(02)03008-1) [DOI] [PubMed] [Google Scholar]
- Lentz B. R. 1993. Use of fluorescent probes to monitor molecular order and motions within liposome bilayers. Chem. Phys. Lipids 64, 99–116. ( 10.1016/0009-3084(93)90060-G) [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. 1983. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J. Mol. Biol. 166, 211–217. ( 10.1016/S0022-2836(83)80007-2) [DOI] [PubMed] [Google Scholar]
- Lindahl E., Edholm O. 2000. Mesoscopic undulations and thickness fluctuations in lipid bilayers from molecular dynamics simulations. Biophys. J. 79, 426 ( 10.1016/S0006-3495(00)76304-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. 1973. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc. Natl Acad. Sci. USA 70, 2271–2275. ( 10.1073/pnas.70.8.2271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. C., Ahrens E. H., Jr, Schreibman P. H., Crouse J. R. 1976. Measurement of squalene in human tissues and plasma: validation and application. J. Lipid Res. 17, 38–45. [PubMed] [Google Scholar]
- Liu L., Barrett C. F., Rittenhouse A. R. 2001. Arachidonic acid both inhibits and enhances whole cell calcium currents in rat sympathetic neurons. Am. J. Physiol. Cell Physiol. 280, C1293–C1305. [DOI] [PubMed] [Google Scholar]
- Liu L., Yang T., Bruno M. J., Andersen O. S., Simon S. A. 2004. Voltage-gated ion channels in nociceptors: modulation by cGMP. J. Neurophysiol. 92, 2323–2332. ( 10.1152/jn.00355.2004) [DOI] [PubMed] [Google Scholar]
- Liu Y., Nagle J. F. 2004. Diffuse scattering provides material parameters and electron density profiles of biomembranes. Phys. Rev. E 69, 040 901 ( 10.1103/PhysRevE.69.040901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecke H., Schobert B., Richter H. T., Cartailler J. P., Lanyi J. K. 1999. Structural changes in bacteriorhodopsin during ion transport at 2 Å resolution. Science 286, 255–261. ( 10.1126/science.286.5438.255) [DOI] [PubMed] [Google Scholar]
- Lundbæk J. A. 2006. Regulation of membrane protein function by lipid bilayer elasticity: a single molecule technology to measure the bilayer properties experienced by an embedded protein. J. Phys.: Condens. Matter 18, S1305–S1344. ( 10.1088/0953-8984/18/28/S13) [DOI] [PubMed] [Google Scholar]
- Lundbæk J. A. 2008. Lipid bilayer-mediated regulation of ion channel function by amphiphilic drugs. J. Gen. Physiol. 131, 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J. A., Andersen O. S. 1994. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104, 645–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J. A., Andersen O. S. 1999. Spring constants for channel-induced lipid bilayer deformations—estimates using gramicidin channels. Biophys. J. 76, 889–895. ( 10.1016/S0006-3495(99)77252-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J. A., Birn P., Girshman J., Hansen A. J., Andersen O. S. 1996. Membrane stiffness and channel function. Biochemistry 35, 3825–3830. ( 10.1021/bi952250b) [DOI] [PubMed] [Google Scholar]
- Lundbæk J. A., Maer A. M., Andersen O. S. 1997. Lipid bilayer electrostatic energy, curvature stress, and assembly of gramicidin channels. Biochemistry 36, 5695–5701. ( 10.1021/bi9619841) [DOI] [PubMed] [Google Scholar]
- Lundbæk J. A., et al. 2004. Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling: effects of micelle-forming amphiphiles and cholesterol. J. Gen. Physiol. 123, 599–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J. A., Birn P., Tape S. E., Toombes G. E., Søgaard R., Koeppe R. E., II, Gruner S. M., Hansen A. J., Andersen O. S. 2005. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol. Pharmacol. 68, 680–689. [DOI] [PubMed] [Google Scholar]
- Ly H. V., Longo M. L. 2004. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys. J. 87, 1013–1033. ( 10.1529/biophysj.103.034280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Nakagawa S., Suga M., Yamashita E., Oshima A., Fujiyoshi Y., Tsukihara T. 2009. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature 458, 597–602. ( 10.1038/nature07869) [DOI] [PubMed] [Google Scholar]
- Maer M. A., Providence L. P., Ingólfsson H. I., Collingwood S. A., Lundbæk J. A., Andersen O. S. Submitted Regulation of gramicidin channel function by phospholipid head group interactions. [Google Scholar]
- Marcelja S. 1976. Lipid-mediated protein interaction in membranes. Biochim. Biophys. Acta 455, 1–7. [DOI] [PubMed] [Google Scholar]
- Marsh D. 2007. Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys. J. 93, 3884–3899. ( 10.1529/biophysj.107.107938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. 2008. Protein modulation of lipids, and vice-versa, in membranes. Biochim. Biophys. Acta 1778, 1545–1575. [DOI] [PubMed] [Google Scholar]
- Matta J. A., Miyares R. L., Ahern G. P. 2007. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J. Physiol. 578, 397–411. ( 10.1113/jphysiol.2006.121988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattice G. L., Koeppe R. E., II, Providence L. L., Andersen O. S. 1995. Stabilizing effect of D-alanine2 in gramicidin channels. Biochemistry 34, 6827–6837. ( 10.1021/bi00020a029) [DOI] [PubMed] [Google Scholar]
- McCarthy M. P., Moore M. A. 1992. Effects of lipids and detergents on the conformation of the nicotinic acetylcholine receptor from Torpedo californica. J. Biol. Chem. 267, 7655–7663. [PubMed] [Google Scholar]
- McLaughlin S. 1989. The electrostatic properties of membranes. Ann. Rev. Biophys. Biophys. Chem. 18, 113–136. ( 10.1146/annurev.bb.18.060189.000553) [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Murray D. 2005. Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–611. ( 10.1038/nature04398) [DOI] [PubMed] [Google Scholar]
- Miloshevsky G. V., Jordan P. C. 2004. Gating gramicidin channels in lipid bilayers: reaction coordinates and the mechanism of dissociation. Biophys. J. 86, 92–104. ( 10.1016/S0006-3495(04)74087-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. C., Straume M., Miller J. L., Litman B. J. 1990. Modulation of metarhodopsin formation by cholesterol-induced ordering of bilayer lipids. Biochemistry 29, 9143–9149. ( 10.1021/bi00491a007) [DOI] [PubMed] [Google Scholar]
- Mobashery N., Nielsen C., Andersen O. S. 1997. The conformational preference of gramicidin channels is a function of lipid bilayer thickness. FEBS Lett. 412, 15–20. ( 10.1016/S0014-5793(97)00709-6) [DOI] [PubMed] [Google Scholar]
- Morais-Cabral J., Zhou Y., MacKinnon R. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature 414, 37–42. ( 10.1038/35102000) [DOI] [PubMed] [Google Scholar]
- Morris C. E., Juranka P. F. 2007. Nav channel mechanosensitivity: activation and inactivation accelerate reversibly with stretch. Biophys. J. 93, 822–833. ( 10.1529/biophysj.106.101246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen O. G., Andersen O. S. 1998. Do we need a new biomembrane model. Biol. Skr. Dan. Vid. Selsk. 49, 7–12. [Google Scholar]
- Mouritsen O. G., Bloom M. 1984. Mattress model of lipid–protein interactions in membranes. Biophys. J. 46, 141–153. ( 10.1016/S0006-3495(84)84007-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Nakashima R., Yamashita E., Matsumoto T., Yamaguchi A. 2006. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443, 173–179. ( 10.1038/nature05076) [DOI] [PubMed] [Google Scholar]
- Myher J. J., Kuksis A., Pind S. 1989. Molecular species of glycerophospholipids and sphingomyelins of human erythrocytes: improved method of analysis. Lipids 24, 396–407. ( 10.1007/BF02535147) [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Tristram-Nagle S. 2000. Structure of lipid bilayers. Biochim. Biophys. Acta 1469, 159–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Wiener M. C. 1988. Structure of fully hydrated bilayer dispersions. Biochim. Biophys. Acta 942, 1–10. [DOI] [PubMed] [Google Scholar]
- Nezil F. A., Bloom M. 1992. Combined influence of cholesterol and synthetic amphiphillic peptides upon bilayer thickness in model membranes. Biophys. J. 61, 1176–1183. ( 10.1016/S0006-3495(92)81926-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C., Andersen O. S. 2000. Inclusion-induced bilayer deformations: effects of monolayer equilibrium curvature. Biophys. J. 79, 2583–2604. ( 10.1016/S0006-3495(00)76498-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C., Goulian M., Andersen O. S. 1998. Energetics of inclusion-induced bilayer deformations. Biophys. J. 74, 1966–1983. ( 10.1016/S0006-3495(98)77904-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell A. M., Koeppe R. E., II, Andersen O. S. 1990. Kinetics of gramicidin channel formation in lipid bilayers: transmembrane monomer association. Science 250, 1256–1259. ( 10.1126/science.1700867) [DOI] [PubMed] [Google Scholar]
- Okada T., Sugihara M., Bondar A. N., Elstner M., Entel P., Buss V. 2004. The retinal conformation and its environment in rhodopsin in light of a new 2.2 Å crystal structure. J. Mol. Biol. 342, 571–583. ( 10.1016/j.jmb.2004.07.044) [DOI] [PubMed] [Google Scholar]
- Oldham M. L., Khare D., Quiocho F. A., Davidson A. L., Chen J. 2007. Crystal structure of a catalytic intermediate of the maltose transporter. Nature 450, 515–521. ( 10.1038/nature06264) [DOI] [PubMed] [Google Scholar]
- Park J. H., Scheerer P., Hofmann K. P., Choe H. W., Ernst O. P. 2008. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 454, 183–188. ( 10.1038/nature07063) [DOI] [PubMed] [Google Scholar]
- Partenskii M. B., Jordan P. C. 2002. Membrane deformation and the elastic energy of insertion: perturbation of membrane elastic constants due to peptide insertion. J. Chem. Phys. 117, 10 768–10 776. ( 10.1063/1.1519840) [DOI] [Google Scholar]
- Perozo E., Cortes D. M., Cuello L. G. 1999. Structural rearrangements underlying K+-channel activation gating. Science 285, 73–78. ( 10.1126/science.285.5424.73) [DOI] [PubMed] [Google Scholar]
- Perozo E., Cortes D. M., Sompornpisut P., Kloda A., Martinac B. 2002. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418, 942–948. ( 10.1038/nature00992) [DOI] [PubMed] [Google Scholar]
- Petrov A. G. 1999. The lyotropic state of matter: molecular physics and living matter physics. New York, NY: Gordon & Breach. [Google Scholar]
- Phillips R., Ursell T., Wiggins P., Sens P. 2009. Emerging roles for lipids in shaping membrane-protein function. Nature 459, 379–385. ( 10.1038/nature08147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. G., Urban B. W., Haydon D. A. 1982. The influence of n-alkanols and cholesterol on the duration and conductance of gramicidin single channels in monoolein bilayers. Biochim. Biophys. Acta 688, 279–283. [DOI] [PubMed] [Google Scholar]
- Providence L. L., Andersen O. S., Greathouse D. V., Koeppe R. E., II, Bittman R. 1995. Gramicidin channel function does not depend on phospholipid chirality. Biochemistry 34, 16 404–16 411. ( 10.1021/bi00050a022) [DOI] [PubMed] [Google Scholar]
- Qian S., Wang W., Yang L., Huang H. W. 2008. Structure of the alamethicin pore reconstructed by x-ray diffraction analysis. Biophys. J. 94, 3512–3522. ( 10.1529/biophysj.107.126474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast U., Brenner O. 1983. Modulation of [3H]muscimol binding in rat cerebellar and cerebral cortical membranes by picrotoxin, pentobarbitone, and etomidate. J. Neurochem. 41, 418–425. ( 10.1111/j.1471-4159.1983.tb04758.x) [DOI] [PubMed] [Google Scholar]
- Rand R. P., Parsegian V. A. 1997. Hydration, curvature, and bending elasticity of phospholipid monolayers. Curr. Topics Mem. Transp. 44, 167–189. [Google Scholar]
- Rawicz W., Olbrich K. C., McIntosh T., Needham D., Evans E. 2000. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79, 328–339. ( 10.1016/S0006-3495(00)76295-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg B., Bennett E., Xiao Y. H., Levinson S. R., Duch D. S. 1995. Voltage- and frequency-dependent pentobarbital suppression of brain and muscle sodium channels expressed in a mammalian cell line. Mol. Pharmacol. 48, 89–97. [PubMed] [Google Scholar]
- Robinson R. A., Stokes R. H. 1965. Electrolyte solutions, 2nd edn. London, UK: Butterworth. [Google Scholar]
- Rostovtseva T. K., Petrache H. I., Kazemi N., Hassanzadeh E., Bezrukov S. M. 2008. Interfacial polar interactions affect gramicidin channel kinetics. Biophys. J. 94, L23–L25. ( 10.1529/biophysj.107.120261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann E. 1984. Physical basis of trigger processes and membrane structures. In Biological membranes (ed. Chapman D.), pp. 105–143. London, UK: Academic Press. [Google Scholar]
- Sarges R., Witkop B., Gramicidin A. V. 1965. The structure of valine- and isoleucine-gramicidin A. J. Am. Chem. Soc. 87, 2011–2019. ( 10.1021/ja01087a027) [DOI] [PubMed] [Google Scholar]
- Sawyer D. B., Andersen O. S. 1989. Platelet-activating factor is a general membrane perturbant. Biochim. Biophys. Acta 987, 129–132. [DOI] [PubMed] [Google Scholar]
- Sawyer D. B., Koeppe R. E., II, Andersen O. S. 1989. Induction of conductance heterogeneity in gramicidin channels. Biochemistry 28, 6571–6583. ( 10.1021/bi00442a007) [DOI] [PubMed] [Google Scholar]
- Sawyer D. B., Koeppe R. E., II, Andersen O. S. 1990. Gramicidin single-channel properties show no solvent-history dependence. Biophys. J. 57, 515–523. ( 10.1016/S0006-3495(90)82567-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G., Ecker J. 2008. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 47, 147–155. ( 10.1016/j.plipres.2007.12.004) [DOI] [PubMed] [Google Scholar]
- Schneider M. B., Jenkins J. T., Webb W. W. 1984. Thermal fluctuations of large cylindrical phospholipid vesicles. Biophys. J. 45, 891–899. ( 10.1016/S0006-3495(84)84235-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell J. R., Chou J. J. 2008. Structure and mechanism of the M2 proton channel of influenza A virus. Nature 451, 591–595. ( 10.1038/nature06531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr J. M. 1970. The role of diffusion in enzyme kinetics. Biophys. J. 10, 717–727. ( 10.1016/S0006-3495(70)86331-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J. M. 1990. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim. Biophys. Acta 1031, 1–69. [DOI] [PubMed] [Google Scholar]
- Seelig J., Tamm L., Hymel L., Fleischer S. 1981. Deuterium and phosphorus nuclear magnetic resonance and fluorescence depolarization studies of functional reconstituted sarcoplasmic reticulum membrane vesicles. Biochemistry 20, 3922–3932. ( 10.1021/bi00516a040) [DOI] [PubMed] [Google Scholar]
- Separovic F., Gawrisch K. 1996. Effect of unsaturation on the chain order of phosphatidylcholines in a dioleoylphosphatidylethanolamine matrix. Biophys. J. 71, 274–282. ( 10.1016/S0006-3495(96)79223-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp K. A., Nicholls A., Fine R. F., Honig B. 1991. Reconciling the magnitude of the microscopic and macroscopic hydrophobic effects. Science 252, 106–109. ( 10.1126/science.2011744) [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. 1974. Biological membranes as bilayer couples. A molecular mechanism of drug–erythrocyte interactions. Proc. Natl Acad. Sci. USA 71, 4457–4461. ( 10.1073/pnas.71.11.4457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N., Ye S., Alam A., Chen L., Jiang Y. 2006. Atomic structure of a Na+- and K+-conducting channel. Nature 440, 570–574. ( 10.1038/nature04508) [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. 1973. Lateral phase separation in phospholipid membranes. Biochemistry 12, 2351–2360. ( 10.1021/bi00736a026) [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. 1978. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim. Biophys. Acta 515, 367–394. [DOI] [PubMed] [Google Scholar]
- Simon S. A., Lis L. J., MacDonald R. C., Kauffman J. W. 1977. The noneffect of a large linear hydrocarbon, squalene, on the phosphatidylcholine packing structure. Biophys. J. 19, 83–90. ( 10.1016/S0006-3495(77)85570-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. 1972. The fluid mosaic model of the structure of cell membranes. Science 175, 720–731. ( 10.1126/science.175.4023.720) [DOI] [PubMed] [Google Scholar]
- Singh S. K., Piscitelli C. L., Yamashita A., Gouaux E. 2008. A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322, 1655–1661. ( 10.1126/science.1166777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A., Proks P. 1998. Inhibition of the ATP-sensitive potassium channel from mouse pancreatic beta-cells by surfactants. Br. J. Pharmacol. 124, 529–539. ( 10.1038/sj.bjp.0701858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard R., Werge T. M., Bertelsen C., Lundbye C., Madsen K. L., Nielsen C. H., Lundbæk J. A. 2006. GABAA receptor function is regulated by lipid bilayer elasticity. Biochemistry 45, 13 118–13 129. ( 10.1021/bi060734+) [DOI] [PubMed] [Google Scholar]
- Spector A. A., Yorek M. A. 1985. Membrane lipid composition and cellular function. J. Lipid Res. 26, 1015–1035. [PubMed] [Google Scholar]
- Sperotto M. M., Mouritsen O. G. 1993. Lipid enrichment and selectivity of integral membrane proteins in two-component lipid bilayers. Eur. Biophys. J. 22, 323–328. ( 10.1007/BF00213555) [DOI] [PubMed] [Google Scholar]
- Starling A. P., Dalton K. A., East J. M., Oliver S., Lee A. G. 1996. Effects of phosphatidylethanolamines on the activity of the Ca2+-ATPase of sarcoplasmic reticulum. Biochem. J. 320, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna T. M., Tape S. E., Koeppe R. E., II, Andersen O. S., Sachs F., Gottlieb P. A. 2004. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430, 235–240. ( 10.1038/nature02743) [DOI] [PubMed] [Google Scholar]
- Suh B. C., Hille B. 2008. PIP2 is a necessary cofactor for ion channel function: how and why? Annu. Rev. Biophys. 37, 175–195. ( 10.1146/annurev.biophys.37.032807.125859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Jogini V., Andersen O. S. 2002. Pharmacological modification of gramicidin channel function. Biophys. J. 82, 550a. [Google Scholar]
- Szeiler I., Kramer D., Ben-Shaul A., Gelbart W. M., Safran S. A. 1990. Molecular theory of curvature elasticity in surfactant films. J. Chem. Phys. 92, 6800–6817. ( 10.1063/1.458267) [DOI] [Google Scholar]
- Townsley L. E., Tucker W. A., Sham S., Hinton J. F. 2001. Structures of gramicidins A, B, and C incorporated into sodium dodecyl sulfate micelles. Biochemistry 40, 11 676–11 686. ( 10.1021/bi010942w) [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Nomura H. 2002. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418, 605–611. ( 10.1038/nature00944) [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Unwin N. 1988. Ion channel of acetylcholine receptor reconstructed from images of postsynaptic membranes. Nature 336, 247–250. ( 10.1038/336247a0) [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Nakasako M., Nomura H., Ogawa H. 2000. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6Å resolution. Nature 405, 647–655. ( 10.1038/35015017) [DOI] [PubMed] [Google Scholar]
- Unwin P. N. T., Ennis P. D. 1984. Two configurations of a channel-forming membrane protein. Nature 307, 609–613. ( 10.1038/307609a0) [DOI] [PubMed] [Google Scholar]
- Uysal S., Vasquez V., Tereshko V., Esaki K., Fellouse F. A., Sidhu S. S., Koide S., Perozo E., Kossiakoff A. 2009. Crystal structure of full-length KcsA in its closed conformation. Proc. Natl Acad. Sci. USA 106, 6644–6649. ( 10.1073/pnas.0810663106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch W. R., Mathies R., Eisenberg M., Stryer L. 1975. Simultaneous fluorescence and conductance studies of planar bilayer membranes containing a highly active and fluorescent analog of gramicidin A. J. Mol. Biol. 99, 75–92. ( 10.1016/S0022-2836(75)80160-4) [DOI] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. 1979. Cytosolic phosphorylation potential. J. Biol. Chem. 254, 6538–6547. [PubMed] [Google Scholar]
- Vonck J. 2000. Structure of the bacteriorhodopsin mutant F219L N intermediate revealed by electron crystallography. EMBO J. 19, 2152–2160. ( 10.1093/emboj/19.10.2152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil M., Bruehl C., Voskuyl R. A., Kang J. X., Leaf A., Wadman W. J. 1996. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc. Natl Acad. Sci. USA 93, 12 559–12 563. ( 10.1073/pnas.93.22.12559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. A., Veatch W. R., Blout E. R. 1981. Conformation of gramicidin A in phospholipid vesicles: circular dichroism studies of effects of ion binding, chemical modification, and lipid structure. Biochemistry 20, 5754–5760. ( 10.1021/bi00523a018) [DOI] [PubMed] [Google Scholar]
- Wang W., Black S. S., Edwards M. D., Miller S., Morrison E. L., Bartlett W., Dong C., Naismith J. H., Booth I. R. 2008. The structure of an open form of an E. coli mechanosensitive channel at 3.45 Å resolution. Science 321, 1179–1183. ( 10.1126/science.1159262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Ha Y. 2007. Open-cap conformation of intramembrane protease GlpG. Proc. Natl Acad. Sci. USA 104, 2098–2102. ( 10.1073/pnas.0611080104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang Y., Ha Y. 2006. Crystal structure of a rhomboid family intramembrane protease. Nature 444, 179–180. ( 10.1038/nature05255) [DOI] [PubMed] [Google Scholar]
- Ward A., Reyes C. L., Yu J., Roth C. B., Chang G. 2007. Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc. Natl Acad. Sci. USA 104, 19 005–19 010. ( 10.1073/pnas.0709388104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A., Mulligan S., Carragher B., Chang G., Milligan R. A. 2009. Nucleotide dependent packing differences in helical crystals of the ABC transporter MsbA. J. Struct. Biol. 165, 169–175. ( 10.1016/j.jsb.2008.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich M., Rostovtseva T. K., Bezrukov S. M. 2009. Lipid-dependent effects of halothane on gramicidin channel kinetics: a new role for lipid packing stress. Biochemistry 48, 5501–5503. ( 10.1021/bi900494y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand S., et al. 2008. Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science 322, 709–713. ( 10.1126/science.1164440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H. 1978. Formation of ‘solvent-free’ black lipid bilayer membranes from glyceryl monooleate dispersed in squalene. Biophys. J. 23, 337–347. ( 10.1016/S0006-3495(78)85453-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., White S. H. 1992. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and neutron diffraction data. III. Complete structure. Biophys. J. 61, 434–447. ( 10.1016/S0006-3495(92)81849-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt M. R., Poulsen C. F., Lukensmejer B., Westh-Hansen S. E., Nabekura J., Akaike N., Nielsen M. 1999. Structural requirements for the interaction of unsaturated free fatty acids with recombinant human GABAA receptor complexes. Ann. NY Acad. Sci. 868, 697–700. ( 10.1111/j.1749-6632.1999.tb11349.x) [DOI] [PubMed] [Google Scholar]
- Woolley G. A., Wallace B. A. 1992. Model ion channels: gramicidin and alamethicin. J. Membr. Biol. 129, 109–136. [DOI] [PubMed] [Google Scholar]
- Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. 2005. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 437, 215–223. ( 10.1038/nature03978) [DOI] [PubMed] [Google Scholar]
- Ye D., Zhang D., Oltman C., Dellsperger K., Lee H. C., VanRollins M. 2002. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 303, 768–776. ( 10.1124/jpet.303.2.768) [DOI] [PubMed] [Google Scholar]
- Yildiz O., Vinothkumar K. R., Goswami P., Kuhlbrandt W. 2006. Structure of the monomeric outer-membrane porin OmpG in the open and closed conformation. EMBO J. 25, 3702–3713. ( 10.1038/sj.emboj.7601237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelev D. V. 1998. Material property characteristics for lipid bilayers containing lysolipid. Biophys. J. 75, 321–330. ( 10.1016/S0006-3495(98)77516-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Raphael R. M. 2005. Effect of salicylate on the elasticity, bending stiffness, and strength of SOPC membranes. Biophys. J. 89, 1789–1801. ( 10.1529/biophysj.104.054510) [DOI] [PMC free article] [PubMed] [Google Scholar]