Abstract
One consequence of climate change is an increasing mismatch between timing of food requirements and food availability. Such a mismatch is primarily expected in avian long-distance migrants because of their complex annual cycle, and in habitats with a seasonal food peak. Here we show that insectivorous long-distance migrant species in The Netherlands declined strongly (1984–2004) in forests, a habitat characterized by a short spring food peak, but that they did not decline in less seasonal marshes. Also, within generalist long-distance migrant species, populations declined more strongly in forests than in marshes. Forest-inhabiting migrant species arriving latest in spring declined most sharply, probably because their mismatch with the peak in food supply is greatest. Residents and short-distance migrants had non-declining populations in both habitats, suggesting that habitat quality did not deteriorate. Habitat-related differences in trends were most probably caused by climate change because at a European scale, long-distance migrants in forests declined more severely in western Europe, where springs have become considerably warmer, when compared with northern Europe, where temperatures during spring arrival and breeding have increased less. Our results suggest that trophic mismatches may have become a major cause for population declines in long-distance migrants in highly seasonal habitats.
Keywords: climate change, population trend, migration, habitat seasonality, trophic mismatch, geographical variation
1. Introduction
Climate change has led to general advances in the timing of organismal life-history events (phenology), but responses at different trophic levels are often dissimilar, leading to a mismatch between the timing of predators and their prey (Stenseth et al. 2002; Parmesan & Yohe 2003; Visser & Both 2005; Both et al. 2009). This mismatch has resulted in population consequences in a long-distance migratory bird, the pied flycatcher, Ficedula hypoleuca; populations declined strongest in forests with an early and narrow food peak (Both et al. 2006). These declines were owing to a limited reaction of breeding date to increased spring temperatures, possibly because arrival from the African wintering grounds has not advanced (Both & Visser 2001; Hüppop & Winkel 2006). Recently, it was shown that across Europe, migrant species with the least temporal advance in spring arrival date declined most during the last two decades (Møller et al. 2008), suggesting that the problem of insufficient adjustment of arrival phenology to climate change has recently become a more general cause of population declines in long-distance migrants. However, we do not expect the problem of a mismatch to be prevalent in all habitats, because habitats are likely to differ in the penalties of being late depending on the seasonality of food availability. Furthermore, not all areas in Europe have experienced the same amount of spring warming during the pre-laying period of migrant birds (Both et al. 2004; Both & te Marvelde 2007), and consequently the detrimental effect of an increased mismatch is only expected in areas with an advanced phenology. Here, we aim to address the generality of an increased trophic mismatch between food availability and requirements as a consequence of climate change and examine whether this could be one of the causes of the widespread population declines of long-distance migrants in Europe (Sanderson et al. 2006; Heldbjerg & Fox 2008).
We predict that long-distance migrants are more vulnerable to climate change than residents and short-distance migrants, because long-distance migrants—while on their distant wintering grounds—cannot predict when spring starts on their breeding grounds (Gwinner 1996). Long-distance migrants have changed their spring arrival times to a lesser extent than short-distance migrants (Strode 2003; Lehikoinen et al. 2004; Rubolini et al. 2007; Miller-Rushing et al. 2008), probably because their departure from the wintering grounds is less plastic. Furthermore, we predict that a trophic mismatch will lead to the largest population declines in habitats with relatively narrow peaks in food availability compared with less seasonal habitats, because in seasonal habitats fitness consequences of missing the food peak will be more severe (Both et al. 2006).
As seasonal habitats we chose temperate forests, because these have a short burst of mainly herbivorous insects that forage on young leaves of deciduous trees before the production of secondary plant compounds starts (Feeny 1970; Buse & Good 1996; Southwood et al. 2004, see also the electronic supplementary material). Breeding of forest birds has been shown to be highly synchronized with this food peak (Perrins & McCleery 1989; Charmantier et al. 2008; Both et al. 2009), and failure to adjust to directional changes in the food peak date can lead to population declines (Both et al. 2006). We chose Phragmites-dominated marshlands as less seasonal habitats because these are known to have more extended periods of food abundance, as reflected in the long breeding season of marsh-inhabiting passerines (e.g. Schaefer et al. 2006; Halupka et al. 2008; Dyrcz & Halupka 2009). The reason for the longer period of insect abundance is probably because reed (Phragmites australis) continues growing during spring and summer (Dykyjova et al. 1970) and the biomass of herbivorous insects is consequently less peaked (Halupka et al. 2008; see the electronic supplementary material for seasonal changes in insect abundance in Dutch marshes and forests). Additionally, insects emerge from the water over an extended period in spring and summer (Ward 2005). In conclusion, (deciduous) forests have a stronger seasonality in insect availability than marshes (Ostendorf 1993; Schaefer et al. 2006), and because the forest insect peak has advanced owing to climate change (Buse et al. 1999; Visser et al. 2006), we expect that forest birds suffer more from climate change than marshland birds if they fail to adapt to the advanced phenology of their habitat.
We have a clear functional hypothesis of how climate change would affect population trends in different habitats, but we also aim to specifically address the question of whether climate change is the likely cause of part of the population declines by comparing regions within Europe with a stronger and weaker degree of spring warming. In contrast to western and central Europe, spring temperatures in northern Europe have not increased, or increased only mildly at the time long-distance migrants arrive and lay their eggs (Both & te Marvelde 2007), and as a result laying dates of resident tit species Paridae (Visser et al. 2003) and migratory Ficedula flycatchers (Both et al. 2004) have not advanced in northern Europe. Moreover, arrival dates of many migrant species, including pied flycatchers, on their northern European breeding grounds have advanced (Ahola et al. 2004; Jonzen et al. 2006; Rubolini et al. 2007), possibly owing to milder conditions during migration (Ahola et al. 2004; Both & te Marvelde 2007), which may allow them to anticipate earlier food phenology. Furthermore, northern forest habitats are characterized by a greater proportion of coniferous trees compared with more southern forests, and conifers have later and less peaked caterpillar abundance than decidous trees (Gibb & Betts 1963; Van Balen 1973; Eeva et al. 2000), making northern habitats less seasonal in this aspect of food availability. If the increased mismatch hypothesis owing to spring temperature increases were true, we thus expect that forest-breeding long-distance migrants would decline less severely in northern than in western Europe.
Population trends of individual species are probably caused by multiple factors, which could act during the breeding and/or the non-breeding season, and for each species a different set of factors could be responsible depending on their specific ecology (Newton 1998). We do not aim to explain all variance in population trends owing to species-specific factors, but aim to study whether there is support for the hypothesis that increased mismatches with food availability as a result of climate change are a more general cause of population decline in highly seasonal habitats and in long-distance migrants. If trophic mismatches increase as a consequence of climate change and hence contribute to population declines, we expect (i) that the effect is stronger in habitats with a stronger seasonality in food availability, (ii) that the effect is stronger in species that are less able to advance their breeding, i.e. long-distance migrants, and (iii) the effect to be stronger in areas with more warming during the laying period of long-distance migrants. We realize that the presented evidence for a trophic mismatch as a general cause of the declines is indirect. We will therefore consider two alternative hypotheses to explain the stronger declines of long-distance migrants. The first is that the declines are owing to changes at the wintering grounds or during migration, and for this we compare population trends within species for two habitats within the same geographical region and for two geographical regions. If the decline is driven by factors on the wintering grounds or during migration, we do not expect that the decline is stronger in the seasonal compared with the less seasonal environment, nor in the area with more than with less spring warming. The alternative hypothesis is that residents benefit from milder winters and outcompete the migrants (Berthold et al. 1998; Lemoine & Bohning-Gaese 2003; Ahola et al. 2007). Under this hypothesis, we expect that long-distance migrants decline stronger in areas with a larger increase in numbers of resident species.
2. Material and methods
(a). Breeding bird surveys
We compared population trends of birds species between different habitats and regions to test the different hypotheses, using different datasets and methodologies. (i) We analysed differences in trends between two habitats in The Netherlands (marshes and forests) in relation to the migratory strategy of bird species. In this country, spring temperature has increased considerably during the sampling period, the food peak in forests (herbivorous caterpillars) has advanced (Visser et al. 2006) and, for some bird species, a clear advance in laying date has been demonstrated (Both et al. 2009). (ii) We used the fact that trends in spring temperatures differ across Europe to address why forest species have declined more in geographical regions where spring temperatures increased during the period of arrival and laying of long-distance migrants than in regions where temperatures increased less (Both & te Marvelde 2007).
For the analysis of differences in trends between marshes and forests in The Netherlands, we used data from the Dutch Breeding Bird Monitoring Programme (BMP), which has been running since 1984. The data are collected mainly by volunteers and the project is coordinated by SOVON Dutch Center for Field Ornithology. It is based on territory mapping in fixed study plots (Bibby et al. 1997). All common and scarce breeding bird species in The Netherlands are covered. Fieldwork and interpretation methods are highly standardized (Van Dijk 2004). Between March and July, all plots (10–500 ha each) are visited five to 10 times. Size of study plots, as well as number, timing and duration of visits depend on habitat type and species selection. All birds showing breeding behaviour (e.g. song, display, alarm-calling, food transportations, fledglings) are mapped. Species-specific interpretation criteria are used to determine the number of territories per species at the end of the season (Van Dijk 2004). Interpretation criteria focus on the type of behaviour observed, the number of observations required (depending on species-specific detection probabilities) and the period of observations (to exclude non-breeding migrants). Observers interpret their own field data and submit the results on standard forms. After a first check by SOVON, Statistics Netherlands performs standardized checks by computer routines to detect possible errors. Observers check and if necessary correct these errors. Between 1984 and 2005, a total of 3671 different study plots were covered in at least 2 years, ranging from around 300 per year in 1984 to a maximum of almost 1900 in 2002 (on average 158 (s.e. 6.5) forest, and 84 (s.e. 6.1) marshland plots per year). These data thus give an estimate of the number of breeding pairs per species per plot per year.
Yearly abundance indices were calculated using Poisson regression (log-linear models; McCullagh & Nelder 1989), as implemented in TRIM software (TRends and Indices for Monitoring data; Gregory et al. 2005; Pannekoek & Van Strien 2005). TRIM is a widely used freeware program with an efficient implementation of Poisson regression to analyse time-series counts (log-linear models) (Gregory et al. 2005; Van Dyck et al. 2009). Poisson regression is also available in the generalized linear model modules of many statistical packages. The estimation method in TRIM is based on generalized estimating equations (GEE; Liang & Zeger 1986; McCullagh & Nelder 1989), thereby taking into account serial correlation and over-dispersion from Poisson distribution. The models are run for each species, and the estimated number of breeding pairs per plot are used as the dependent variable. Time series within the same plots rarely covered the entire study period. Before calculating population trends, data from the missing counts were estimated, based on a GEE model with plot identity, year, and the interactions between year and habitat and year and geographical regions within The Netherlands. We thus estimated the population numbers for the missing counts on the basis of the average numbers within the plot when it was counted, and on the trends over the years observed in other plots with similar habitat and within the same region. On the basis of this dataset with both the observed and estimated counts, habitat-specific trends were calculated. These trends were calculated based on the yearly indices computed, taking into account their uncertainty, and expressed as ratios of the population present in 2004 compared with 1984. The estimates of the trends are expected to be normally distributed and were treated as dependent variables in a further generalized linear model (GLM) with identity link and normal errors. The population trends mostly reflect changes in density within plots, rather than changes in the amount of habitat available within The Netherlands, and are thus hardly influenced by habitat destruction or regeneration.
European trends were analysed for two regions that differ in the extent of spring warming and its subsequent effect on the phenology of the species’ breeding seasons (Both & te Marvelde 2007). We consider the temperature during the period when long-distance migrants arrive on their breeding grounds as the most relevant measure of temperature change. This period differs markedly between latitudes, being relatively late in the north. Temperatures in this time window have changed differently between western/central Europe (hereafter called western Europe) and northern Europe, which is strongly reflected in trends in the laying date of at least one migratory bird: the pied flycatcher (Both et al. 2004). Interestingly, bud-burst phenology of some tree species has advanced in northern Europe (Nordli et al. 2008), which is most likely caused by increasing temperatures in early spring. Since temperatures during arrival and laying have not strongly increased (Both & te Marvelde 2007), it is likely that insect food peaks have not advanced greatly. Western Europe (clear spring warming) comprises the countries Austria, Belgium, Denmark, former West Germany, Ireland, The Netherlands, Switzerland, UK and France. Northern Europe (less spring warming, although some variation exists within this large area) compromises Finland, Norway and Sweden. Information on trends of bird species comes from annual breeding bird monitoring schemes in European countries, collated by the Pan-European Common Bird Monitoring scheme (PECBM: http://www.ebcc.info/pecbm.html). National trend data, obtained via spot mapping, territory mapping, line transects or point counts (Gregory et al. 2005), are used to produce yearly indices and scheme totals (with standard errors and covariances between years) for each species for each country, using TRIM (see above). Species-specific trends for western and northern Europe were produced by combining national results for the selected species, weighted for national population size (Van Strien et al. 2001; Gregory et al. 2005). A problem is that the time series per country differ in length and, again, that not all study sites are covered in all years within the study periods. TRIM was used in a similar way to cope with missing values as described for the Dutch trends (Van Strien et al. 2001).
(b). Species selection
For the analysis of Dutch data, we selected all insectivorous passerine species for which we could calculate a population trend for either one habitat or both habitats separately (electronic supplementary material, table S1). Species were classified as residents, short-distance migrants (not crossing the Sahara) and long-distance migrants, based on data available for The Netherlands (see the electronic supplementary material for species data). Some species are clearly habitat specialists, but other species are more generalist. For the generalist species occurring in both habitats, we also compared within-species differences in population trends between the habitats.
For the analyses of European data, we selected all species being (i) widespread, (ii) forest specialist, (iii) small passerine, and (iv) insectivorous. Furthermore, we selected species occurring in both western and northern Europe (see the electronic supplementary material for the species selected). This resulted in six long-distance migrants and nine resident/short-distance migrant species for which we have population trends in both regions.
(c). Bird arrival data
One of us (R.G.B.) recorded in every year during the study period the first arriving three males of all migrant species that do not winter in the area and breed in the forests of Drenthe (northern Netherlands, 6°17′ E, 52°52′ N). The area was visited on a daily basis during spring and summer (from late February onwards). The study area is forested with conifers and interspersed with heaths and deciduous woodland. Arrival dates of males was monitored by observing singing birds, and given the intensity of the observer's presence, are probably accurate. For instance, when birds were seen before any song was heard, singing was almost always recorded later the same day.
3. Results
(a). Comparing Dutch population trends between marshes and forests
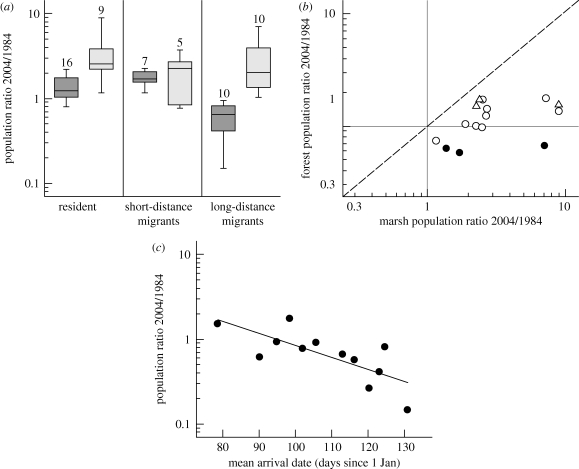
Between 1984 and 2004, all species of long-distance migrants in forests declined (on average by 38%), whereas no systematic declines were found in marsh-inhabiting long-distance migrants (average 158% increase), nor in short-distance migrants or residents in both habitats (figure 1a; see the electronic supplementary material for species-specific data). Intraspecific trends for generalist species living in both marshland and forest gave a similar pattern: long-distance migrants showed a larger decline in forests than in marshes, whereas populations increased in residents and short-distance migrants in both habitats, although stronger in marshes than in forests (figure 1b). Furthermore, population trends correlated with average spring arrival date of migrant species in forests: species with late spring arrival (such as wood warbler Phylloscopus sibilatrix, icterine warbler Hippolais icterina and spotted flycatcher Muscicapa striata) showed a stronger decline (up to 85%) than earlier arriving species (figure 1c). Our data are thus consistent with the hypothesis that long-distance migrants declined as a result of climate change because they have adapted insufficiently to maintain the synchrony with the advanced food peak in a seasonal habitat.
Figure 1.
(a) Population trends of passerines in Dutch forests and marshlands between 1984 and 2004 for species with different migration behaviour. Results GLM: interaction habitat * migration status: F2,51 = 6.16, p = 0.004 (shaded boxes, forest; open boxes, marsh). (b) Within-species comparison of population trends in forests and marshes, showing that within species long-distance migrants decline stronger in forests than in marshes (open triangles, residents; open circles, short-distance migrants; filled circles, long-distance migrants). GLM: dependent: forest growth rate, explanatory variables: marsh growth rate: F1,11 = 7.08, p = 0.022, migration status: F2,11 = 18.49, p < 0.001, interaction: F2,9 = 0.82, p = 0.47. (c) Population trends of migratory passerines living in forests and their spring arrival date on the breeding grounds. Later arriving species declined most (GLM: mean arrival date: F1,10 = 12.41, p = 0.006). Population trends are expressed as the ratio of the densities present in 2004 relative to 1984, which is based on the annual population growth rates (1 = stable, 0.1 is a 90% decline, 10 is a 10-fold increase). Population trends are from the Dutch Breeding BMP (see the electronic supplementary material for details). Arrival data are based on the first three males arriving annually in a study site in Drenthe (northern Netherlands).
(b). Comparing population trends between western and northern Europe
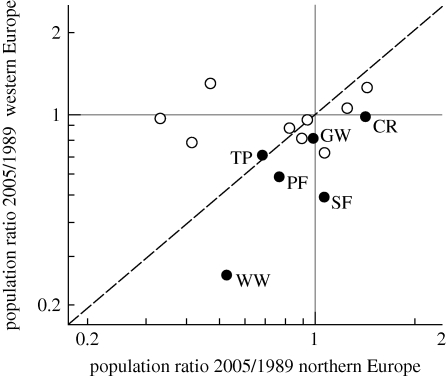
We found that for five out of six species of forest-breeding long-distance migratory passerines, the decline in numbers was greater in western than in northern Europe (figure 2; paired t-test: t5 = 3.11, p = 0.027). The average decline in western Europe was 35 per cent, and in northern Europe 9 per cent. Analysis of trends within species showed significant interactions of area * year in four out of the six species (electronic supplementary material, table S2: stronger decline in western Europe: common redstart Phoenicurus phoenicurus, wood warbler, garden warbler Sylvia borin, pied flycatcher, spotted flycatcher). The reason is probably not that wintering grounds differ largely between northern and western European breeding populations for these species: recovery positions largely overlap in Africa for common redstarts, garden warblers and pied flycatchers, but less so for spotted flycatchers; for tree pipit and wood warbler, recovery data from sub-Saharan Africa are too few to outline migratory connectivity (Zwarts et al. 2009). By contrast, for resident and short-distance migrant species, we found no difference in population trends between western and northern Europe (paired t-test: t8 = −0.95, p = 0.37). The average decline in western Europe was 3 per cent, in northern Europe 15 per cent, suggesting that the forest habitat did not deteriorate to a greater extent in western than in northern Europe over this period of time (see the electronic supplementary material for individual species trends).
Figure 2.
Population trends (1989–2005) of 15 species of forest-breeding passerines in northern and western Europe, separated for long-distance migrants (filled circles) and residents and short-distance migrants (open circles). Only species are used for which we had trends in both regions, and each dot is a pair of species’ population trends. The x = y line is dotted and species that fall below this line fare worse in western compared with northern Europe. Population trends are from the PECBM (see the electronic supplementary material for details). For migrants, common species names are given in abbreviations: TP, tree pipit; CR, common redstart; WW, wood warbler; GW, garden warbler; PF, pied flycatcher; SF, spotted flycatcher.
4. Discussion
Long-distance migrants are relatively inflexible to respond to advances in spring phenology of their breeding habitat (Gwinner 1996). Therefore, climate change is expected to lead to increased trophic mismatches, resulting in declining population sizes (Møller et al. 2008). We indeed found that in The Netherlands, long-distance migrants in seasonal forests declined much stronger than in less seasonal marshes (both within and between species), whereas no difference in trends between habitats was found for residents and short-distance migrants. Consistent with the mismatch hypothesis, the effect was strongest in species arriving latest in spring. Additional indications that temperature changes in spring are a likely explanation comes from the comparison of population trends between European regions that differ in spring temperature change: long-distance migrants declined stronger in western Europe, where spring warming is prevalent, than in northern Europe, where temperatures around arrival and laying increased only mildly (Visser et al. 2003; Both et al. 2004). Apart from the weaker advance in the onset of spring in northern compared with western Europe, some northern long-distance migrants have managed to advance their spring arrival to a greater extent than western European birds (Hüppop & Winkel 2006). They may profit from increased temperatures during migration in Europe, whereas birds breeding at more southern latitudes migrate earlier and temperatures during migration for these populations have not increased (Both & te Marvelde 2007). Furthermore, forest habitats at higher latitudes are likely to have a broader food peak because they contain higher proportions of coniferous trees, which have lower, but more extended food peaks (Gibb & Betts 1963; Eeva et al. 2000). The stronger declines of long-distance migrant populations in the region with more spring warming and more narrow food peaks thus strengthen the conclusion that climate change is the underlying cause.
In contrast to our analyses, Jones & Cresswell (2010) concluded that trophic mismatches on the breeding grounds could explain population declines of long-distance migrants in the nearctic, but not in the palearctic. The apparent contrast between these analyses most probably originates from the fact that these authors did not distinguish between habitats of different seasonality, nor did they acknowledge the spatial variation in the strength of spring warming within continents.
The difference between forests and marshes was partly owing to an increase in marsh-inhabiting long-distance migrants, which may be a direct consequence of climate change: reed warblers Acrocephalus scirpaceus advanced the start, but also extended the length of their breeding season during the last decades, allowing more pairs to raise two successful broods during the season (Halupka et al. 2008; see also Dyrcz & Halupka 2009). By contrast, some forest-breeding passerines shortened their breeding season in response to climate change, partly owing to less birds producing second broods (Visser et al. 2003; Husby et al. 2009) and also because the laying date distribution of first broods became narrower (Both et al. 2009). This may not only be owing to a stronger advance of the food peak date relative to the bird breeding dates, but also owing to caterpillar peaks becoming narrower at high temperatures (Buse et al. 1999). If seasonal habitats therefore become even more seasonal with narrower food peaks, this may seriously negatively affect insectivorous species, and may explain why especially late arriving species suffered most.
Two other, not mutually exclusive, hypotheses have been put forward to explain the vulnerability of long-distance migrants to climate change: (i) migrants face stronger competition from residents because resident populations increase owing to milder winters (Berthold et al. 1998; Lemoine & Bohning-Gaese 2003; Ahola et al. 2007), and (ii) climate change leads to a deterioration of wintering habitats (Peach et al. 1991; Sillett et al. 2000). The data do not support the first hypothesis because residents increased in both marshes and forests, whereas migrants only declined in forests. Furthermore, at the European scale, we did not find that the resident populations increased more in the region with a stronger decline in long-distance migrants. More support exists for the second hypothesis: migrant population sizes are often tightly correlated with climate-related ecological conditions at the wintering sites (Newton 2004). Also in our data we found some support for this because population trends within the six long-distance migrant species tended to be positively correlated between northern and western Europe (r = 0.752, n = 6, p = 0.085, figure 2). This suggests that there may be a common cause determining the between-species correlation in population trends, which may well be habitat degradation and/or climate-related habitat change at the shared wintering grounds (Sanderson et al. 2006).
That effects in Africa have a large impact on breeding population numbers in Europe has been shown especially for species wintering in the Sahel, of which the numbers plummeted during the severe droughts in the 1970s and 1980s (Baillie & Peach 1992; Foppen et al. 1999; Zwarts et al. 2009). The start of the monitoring programme in the Netherlands coincided with the end of this drought-related population crash, affecting initial population growth rates in several species. The subsequent partial recoveries can be attributed to the improvement of rainfall figures in the Sahel, as recorded in common whitethroat Sylvia communis and sedge warbler Acrocephalus schoenobaenus. Also, common redstart populations crashed as a result of the droughts (Zwarts et al. 2009), but interestingly their partial recovery since then in northern Europe is not mirrored by western European populations (see the electronic supplementary material).
Our data support the hypothesis that during the last two decades climate change has contributed to the decline of long-distance migrant bird species inhabiting highly seasonal habitats. Does this mean that these species will continue to suffer while resident species will be unaffected by climate change? Long-distance migrants may adjust their migratory timing, by either phenotypic plasticity and/or an evolutionary response, allowing them to restore the synchrony with their breeding environment (Jonzen et al. 2006). Until now, there is little evidence for an evolutionary response, but it is likely to happen in the future, although it may still be insufficient to track the advancement of spring. In the past, these species have been able to survive drastic climatic fluctuations, but now, habitat loss at the wintering grounds and during migration has put long-distance migrants under pressure (Sanderson et al. 2006), which may reduce their capacity to respond to the ongoing effects of climate change. Insufficient adjustment to the advanced food peak for raising offspring is not restricted to long-distance migrants, but is also observed in one resident great tit Parus major population (Visser et al. 1998), although another great tit population adjusted sufficiently (Charmantier et al. 2008). Reduced reproduction in residents is probably compensated by higher survival owing to milder winters and density-dependent feedbacks. Therefore, population sizes of these species remain rather stable or even increase. However, a further advance of the food peak may reduce reproduction to such an extent that resident populations will also decline, especially if the food peak narrows further owing to climate change (Buse et al. 1999). More, in general, we expect that all habitats characterized by a short burst of food availability, such as temperate meadows (Schekkerman & Beintema 2007) and tundras (Tulp & Schekkerman 2008), are probably inhabited by species that require a good temporal match between food requirements and abundance, and hence are susceptible to climate change.
Acknowledgements
This work would not have been possible without the efforts of many thousands of volunteer birdwatchers across Europe, who gathered the trend data. SOVON coordinators were responsible for processing and analysing the Dutch data, of whom we want to mention Arend J. Van Dijk in particular. The authors wish to thank the European Bird Census Council for providing the data on European trends: international coordinators Petr Voříšek and Alena Klvaňová and the national coordinators for Austria (Norbert Teufelbauer and Michael Dvorak), Belgium (Jean-Paul Jacob and Anne Weiserbs), Denmark (Henning Heldbjerg and Michael Grell), Finland (Risto Väisänen), France (Frédéric Jiguet), Germany (Martin Flade and Johannes Schwarz), Ireland (Olivia Crowe), Norway (Magne Husby), Sweden (Åke Lindström), Switzerland (Hans Schmid and Verena Keller) and the UK (David Noble). Adriaan Gmelig Meyling helped computing the trend data. Sandra Bouwhuis collected the forest insect samples. The authors have declared that no competing interests exist. C.A.M.T. was involved in the Dutch Breeding Bird Monitoring Programme and calculated the Dutch trend data. R.P.B.F. was involved in the Pan-European Common Bird Monitoring scheme. A.J.S. calculated the Pan-European trend data. H.S. collected the insect data in marshlands. R.G.B. collected the long-term data on the arrival of forest birds. C.B. collected the insect data in forests, analysed the data and wrote the paper. C.B. and R.G.B. were supported by a VIDI grant from the Dutch Foundation for Scientific Research (N.W.O) to C.B. C.A.M.T. and R.P.B.F. were supported by the core funding of SOVON Dutch Center for Field Ornithology. A.J.S. was supported by core funding of Statistics Netherlands, whereas H.S. was supported by core funding of Alterra and Wageningen University and Research Center.
References
- Ahola M., Laaksonen T., Sippola K., Eeva T., Rainio K., Lehikoinen E.2004Variation in climate warming along the migration route uncouples arrival and breeding date. Global Change Biol. 10, 1–8 [Google Scholar]
- Ahola M. P., Laaksonen T., Eeva T., Lehikoinen E.2007Climate change can alter competitive relationships between resident and migratory birds. J. Anim. Ecol. 76, 1045–1052 (doi:10.1111/j.1365-2656.2007.01294.x) [DOI] [PubMed] [Google Scholar]
- Baillie S. R., Peach W. J.1992Population limitation in Palaearctic–African migrant passerines. Ibis 134, 120–132 [Google Scholar]
- Berthold P., Fiedler W., Schlenker R., Querner U.199825-year study of the population development of Central European songbirds: a general decline, most evident in long-distance migrants. Naturwissenschaften 85, 350–353 (doi:10.1007/s001140050514) [Google Scholar]
- Bibby C. J., Burgess N. D., Hill D. A.1997Bird census techniques London, UK: Academic Press [Google Scholar]
- Both C., te Marvelde L.2007Climate change and timing of avian breeding and migration throughout Europe. Climate Res. 35, 93–105 (doi:10.3354/cr00716) [Google Scholar]
- Both C., Visser M. E.2001Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 (doi:10.1038/35077063) [DOI] [PubMed] [Google Scholar]
- Both C., et al. 2004Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. Lond. B 271, 1657–1662 (doi:10.1098/rspb.2004.2770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both C., Bouwhuis S., Lessells C. M., Visser M. E.2006Climate change and population declines in a long distance migratory bird. Nature 441, 81–83 (doi:10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- Both C., Van Asch M., Bijlsma R. G., Van den Burg A. B., Visser M. E.2009Climate change and unequal phenological changes across four trophic levels: constraints or adaptations. J. Anim. Ecol. 78, 73–83 (doi:10.1111/j.1365-2656.2008.01458.x) [DOI] [PubMed] [Google Scholar]
- Buse A., Good J. E. G.1996Synchronisation of larval emergence of winter moth (Operophtera brumata L) and budburst in pedunculate oak (Quercus robur L) under simulated climate change. Ecol. Entomol. 21, 335–343 (doi:10.1046/j.1365-2311.1996.t01-1-00001.x) [Google Scholar]
- Buse A., Dury S. J., Woodburn R. J. W., Perrins C. M., Good J. E. G.1999Effects of elevated temperature on multi-species interactions: the case of pedunculate oak, winter moth and tits. Funct. Ecol. 13(Suppl. 1), 74–82 [Google Scholar]
- Charmantier A., McCleery R. H., Cole L. R., Perrins C., Kruuk L. E. B., Sheldon B. C.2008Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- Dykyjova D., Ondok J. P., Priban K.1970Seasonal changes in productivity and vertical structure of reed-stands (Phragmites communis Trin). Photosynthetica 4, 280–287 [Google Scholar]
- Dyrcz A., Halupka L.2009The response of the Great Reed Warbler Acrocephalus arundinaceus to climate change. J. Ornithol. 150, 39–44 (doi:10.1007/s10336-008-0315-9) [Google Scholar]
- Eeva T., Veistola S., Lehikoinen E.2000Timing of breeding in subarctic passerines in relation to food availability. Can. J. Zool. 78, 67–78 (doi:10.1139/cjz-78-1-67) [Google Scholar]
- Feeny P.1970Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51, 565–581 (doi:10.2307/1934037) [Google Scholar]
- Foppen R., Ter Braak C. J. F., Verboom J., Reijnen R.1999Dutch sedge warblers Acrocephalus schoenobaenus and West-African rainfall: empirical data and simulation modelling show low population resilience in fragmented marshlands. Ardea 87, 113–127 [Google Scholar]
- Gibb J. A., Betts M. M.1963Food and food-supply of nestling tits (Paridae) in Breckland pine. J. Anim. Ecol. 32, 489–533 [Google Scholar]
- Gregory R. D., Van Strien A., Vorisek P., Gmelig Meyling A. W., Noble D. G., Foppen R. P. B., Gibbons D. W.2005Developing indicators for European birds. Phil. Trans. R. Soc. B 360, 269–288 (doi:10.1098/rstb.2004.1602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinner E.1996Circannual clocks in avian reproduction and migration. Ibis 138, 47–63 [Google Scholar]
- Halupka L., Dyrcz A., Borowiec M.2008Climate change affects breeding of reed warblers Acrocephalus scirpaceus. J. Avian Biol. 39, 95–100 (doi:10.1111/j.0908-8857.2008.04047.x) [Google Scholar]
- Heldbjerg H., Fox T.2008Long-term population declines in Danish trans-Saharan migrant birds. Bird Study 55, 267–279 (doi:10.1080/00063650809461532) [Google Scholar]
- Hüppop O., Winkel W.2006Climate change and timing of spring migration in the long-distance migrant Ficedula hypoleuca in central Europe: the role of spatially different temperature changes along migration routes. J. Ornithol. 147, 326–343 [Google Scholar]
- Husby A., Kruuk L. E. B., Visser M. E.2009Decline in the frequency and benefits of multiple brooding in great tits as a consequence of a changing environment. Proc. R. Soc. B 276, 1845–1854 (doi:10.1098/rspb.2008.1937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T., Cresswell W.2010The phenology mismatch hypothesis: are declines of migrant birds linked to uneven global climate change? J. Anim. Ecol. 79, 98–108 [DOI] [PubMed] [Google Scholar]
- Jonzen N., et al. 2006Rapid advance of spring arrival dates in long-distance migratory birds. Science 312, 1959–1961 (doi:10.1126/science.1126119) [DOI] [PubMed] [Google Scholar]
- Lehikoinen E., Sparks T. H., Zalakevicius M.2004Arrival and departure dates. Adv. Ecol. Res. 35, 1–31 (doi:10.1016/S0065-2504(04)35001-4) [Google Scholar]
- Lemoine N., Bohning-Gaese K.2003Potential impact of global climate change on species richness of long-distance migrants. Conserv. Biol. 17, 577–586 (doi:10.1046/j.1523-1739.2003.01389.x) [Google Scholar]
- Liang K. Y., Zeger S. L.1986Longitudinal data analysis using generalized linear models. Biometrika 73, 13–22 (doi:10.1093/biomet/73.1.13) [Google Scholar]
- McCullagh P., Nelder J. A.1989Generalized linear models, 2nd edn.London, UK: Chapman and Hall [Google Scholar]
- Miller-Rushing A. J., Lloyd-Evans T. L., Primack R. B., Satzinger P.2008Bird migration times, climate change, and changing population sizes. Global Change Biol. 14, 1959–1972 (doi:10.1111/j.1365-2486.2008.01619.x) [Google Scholar]
- Møller A. P., Rubolini D., Lehikoinen A.2008Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl Acad. Sci. USA 105, 16 195–16 200 (doi:10.1073/pnas.0803825105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton I.1998Population limitation in birds New York, NY: Academic Press [Google Scholar]
- Newton I.2004Population limitation in migrants. Ibis 146, 197–226 (doi:10.1111/j.1474-919X.2004.00293.x) [Google Scholar]
- Nordli O., Wielgolaski F. E., Bakken A. K., Hjeltnes S. H., Mage F., Sivle A., Skre O.2008Regional trends for bud burst and flowering of woody plants in Norway as related to climate change. Int. J. Biometeorol. 52, 625–639 (doi:10.1007/s00484-008-0156-5) [DOI] [PubMed] [Google Scholar]
- Ostendorf W.1993Schilf als lebensraum. Beih. Veroff. Naturschutz Landschaftspflege Bad. Wurtt. 68, 173–280 [Google Scholar]
- Pannekoek J., Van Strien A.2005TRIM 3 Manual. Trends and indices for monitoring data. Research paper 0102 Voorburg, The Netherlands: Statitics Netherlands, CBS; See http://www.ebcc.info/index.php?ID=13 [Google Scholar]
- Parmesan C., Yohe G.2003A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- Peach W. J., Baillie S. R., Underhill L. G.1991Survival of British sedge warblers Acrocephalus schoenobaenus in relation to West African rainfall. Ibis 133, 300–305 (doi:10.1111/j.1474-919X.1991.tb04573.x) [Google Scholar]
- Perrins C. M., McCleery R. H.1989Laying dates and clutch size in the great tit. Wilson Bull. 101, 236–253 [Google Scholar]
- Rubolini D., Møller A. P., Rainio K., Lehikoinen E.2007Assessing intraspecific consistency and geographic variability in temporal trends of spring migration phenology among European bird species. Climate Res. 35, 135–146 (doi:10.3354/cr00720) [Google Scholar]
- Sanderson F. J., Donald P. F., Pain D. J., Burfield I. J., Van Bommel F. P. J.2006Long-term population declines in Afro-Palearctic migrant birds. Biol. Conserv. 131, 93–105 (doi:10.1016/j.biocon.2006.02.008) [Google Scholar]
- Schaefer T., Ledebur G., Beier J., Leisler B.2006Reproductive responses of two related coexisting songbird species to environmental changes: global warming, competition, and population sizes. J. Ornithol. 147, 47–56 (doi:10.1007/s10336-005-0011-y) [Google Scholar]
- Schekkerman H., Beintema A. J.2007Abundance of invertebrates and foraging success of black-tailed godwit Limosa limosa chicks in relation to agricultural grassland management. Ardea 95, 39–54 [Google Scholar]
- Sillett T. S., Holmes R. T., Sherry T. W.2000Impacts of a global climate cycle on population dynamics of a migratory songbird. Science 288, 2040–2042 (doi:10.1126/science.288.5473.2040) [DOI] [PubMed] [Google Scholar]
- Southwood T. R. E., Wint G. R. W., Kennedy C. E. J., Greenwood S. R.2004Seasonality, abundance, species richness and specificity of the phytophagous guild of insects on oak (Quercus) canopies. Eur. J. Entomol. 101, 43–50 [Google Scholar]
- Stenseth N. C., Mysterud A., Ottersen G., Hurrell J. W., Chan K.-S., Lima M.2002Ecological effects of climate fluctuations. Science 297, 1292–1296 (doi:10.1126/science.1071281) [DOI] [PubMed] [Google Scholar]
- Strode P. K.2003Implications of climate change for North American wood warblers (Parulidae). Global Change Biol. 9, 1137–1144 (doi:10.1046/j.1365-2486.2003.00664.x) [Google Scholar]
- Tulp I., Schekkerman H.2008Has prey availability for arctic birds advanced with climate change? Hindcasting the abundance of tundra arthropods using weather and seasonal variation. Arctic 61, 48–60 [Google Scholar]
- Van Balen J. H.1973A comparative study of the breeding ecology of the great tit Parus major in different habitats. Ardea 61, 1–93 [Google Scholar]
- Van Dijk A. J.2004Handleiding Broedvogel Monitoring Project Beek, Ubbergen, The Netherlands: SOVON Vogelonderzoek Nederland [Google Scholar]
- Van Dyck H., Van Strien A. J., Maes D., Van Swaay C. A. M.2009Declines in common, widespread butterflies in a landscape under intense human use. Conserv. Biol. 23, 957–965 [DOI] [PubMed] [Google Scholar]
- Van Strien A. J., Pannekoek J., Gibbons D. W.2001Indexing European bird population trends using results of national monitoring schemes: a trial of a new method. Bird Study 48, 200–213 (doi:10.1080/00063650109461219) [Google Scholar]
- Visser M. E., Both C.2005Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2560 (doi:10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., Van Noordwijk A. J., Tinbergen J. M., Lessells C. M.1998Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870 [Google Scholar]
- Visser M. E., et al. 2003Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. Lond. B 270, 367–372 (doi:10.1098/rspb.2002.2244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., Holleman L. J. M., Gienapp P.2006Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 167–172 [DOI] [PubMed] [Google Scholar]
- Ward M. P.2005Habitat selection by dispersing yellow-headed blackbirds: evidence of prospecting and the use of public information. Oecologia 145, 650–657 (doi:10.1007/s00442-005-0179-0) [DOI] [PubMed] [Google Scholar]
- Zwarts L., Bijlsma R. G., Van der Kamp J., Wymenga E.2009Living on the edge: wetlands and birds in a changing Sahel Zeist, Utrecht, The Netherlands: KNNV Uitgeverij [Google Scholar]