Abstract
While most of the world has enjoyed exponential economic growth, more than one-sixth of the world is today roughly as poor as their ancestors were many generations ago. Widely accepted general explanations for the persistence of such poverty have been elusive and are needed by the international development community. Building on a well-established model of human infectious diseases, we show how formally integrating simple economic and disease ecology models can naturally give rise to poverty traps, where initial economic and epidemiological conditions determine the long-term trajectory of the health and economic development of a society. This poverty trap may therefore be broken by improving health conditions of the population. More generally, we demonstrate that simple human ecological models can help explain broad patterns of modern economic organization.
Keywords: infectious disease, poverty, ecology, economics
1. Introduction
Over a billion people live in extreme poverty, defined as subsisting on less than 1 US dollar (USD) per day (UN Millennium Project 2005), and are roughly as poor today as their ancestors were thousands of years ago. There is extensive debate in both the scholarly and the popular literature over the potential causes of such poverty, which range from the relative roles of government, social institutions and infrastructure to the importance of biophysical factors broadly related to geography (Easterly & Levine 1997; Sachs 2005; Collier 2007b). An increasingly accepted explanation focuses on ‘poverty traps’, formed by the interaction between changes in per capita income over time and other economic variables, threshold levels of which are necessary for economic growth (Bowles et al. 2007). The most commonly considered threshold variable in the economics literature is household savings, but other (not necessarily inconsistent) predictors have been mooted, such as conflict (Collier et al. 2003), environmental degradation (Dasgupta et al. 2005) and infectious diseases (Gallup & Sachs 2001; Bloom et al. 2003).
In contrast to more conventional economic explanations of the persistence of poverty, the concept of a disease-driven poverty trap is especially provocative and potentially important because of the degree to which infectious diseases represent complex ecological agents. This implies that large-scale economic processes are coupled to nonlinear, potentially erratic biological phenomena. Indeed, infectious diseases are not only the leading killers of the poor (World Health Organization 2004), but have been argued to be the dominant predators of humans throughout history and thus have constituted an important selective force on human evolution (Black 1975; Cooke & Hill 2001). As is the case for many ecological systems, a preponderance of theory and evidence have shown that the dynamics of infectious diseases are typically nonlinear and have had significant and systematic impacts on the population dynamics of their hosts in the natural world as well as in laboratory settings (Hudson et al. 1990; Rohani et al. 2003). It is the recognition that infectious diseases may additionally influence economic development that makes the disease-driven poverty trap concept especially intriguing.
The intellectual basis for the disease-driven poverty trap stems from two substantial bodies of empirical fact. First, extreme poverty is indeed suffered disproportionately in areas where infectious diseases thrive. That poverty leads to an increased risk of disease is common sense and is well established in the epidemiology literature (World Health Organization 1998, 2001; Farmer 2001; Jong-Wook 2003). Second, there is a significant empirical literature on the effects of health on poverty (Strauss & Thomas 1998; Bloom & Canning 2000; World Health Organization 2001; Gallup & Sachs 2001; Sachs & Malaney 2002). Economic activity requires human resources—specifically, human capital—and therefore relies on basic biological processes for physical and cognitive development, which are compromised by infection (Nokes et al. 1992; Holding & Snow 2001; Glewwe 2002; Ezeamama et al. 2005; Fernando et al. 2006). So, while humans rely on their own resources for economic activity, they also provide direct biological resources to pathogens for their survival and transmission (Anderson & May 1992; Frank 1996). As the leading killers of the poor, and the cause of mortality of two-thirds of sub-Saharan Africans, this theory posits that infectious diseases may constitute fundamental barriers to economic development (World Health Organization 2004).
The combined causal effects of health on poverty and poverty on health implies a positive feedback system. Despite the importance of understanding such critical and systematic ecological interactions between humans and their most important natural enemies, and the anecdotal evidence that such poverty traps may indeed exist, we lack mechanistic frameworks of poverty traps that are rooted in the dynamics of disease. Here, we propose such a model. We find that a prototypical host–pathogen system, coupled with simple economic models, induces a poverty trap. More broadly, this model serves to illustrate how feedbacks between people and their environment can potentially give rise to major differences in human survival and economic welfare (Diamond 1997).
2. Our theory
The most significant economic impact on the poor comes through the cumulative detrimental health effects of infectious and parasitic diseases (World Health Organization 2001). Some pathogens (such as HIV/AIDS, TB, malaria, measles, pertussis and diarrhoeal diseases) have obvious, dramatic and high-profile effects on human capital, manifested via substantial morbidity and disease-induced mortality. Others (including macroparasites, such as parasitic worms) are chronic infections often with little outward sign of disease and yet are known to significantly impact nutrition and impair cognitive development (Nokes et al. 1992; Anderson & May 1992). Instead of attempting to explicitly incorporate the impact of possible infection with this diverse variety of pathogens, we illustrate our underlying concept using a general one-disease SIS (susceptible–infected–susceptible) model, where individuals can be serially reinfected over the course of their lifetime. This model is meant to serve as the simplest general way of representing the kind of repeated threats of infection faced by poor tropical communities. More specifically, the general model also resembles a typical malaria system (Gandon et al. 2001), which has high prevalence rates among the poor and has been especially implicated in hindering economic growth (Gallup & Sachs 2001).
The general SIS model is
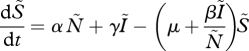 |
2.1 |
and
| 2.2 |
where S̃ and Ĩ represent the number of susceptible and infected individuals, respectively, and Ñ represents the total population size. The parameters α, μ and γ are respectively the rates of birth, natural death (i.e. in the absence of disease) and recovery. The transmission rate is β. The parameter ν is the additional death rate caused by disease. From equations (2.1) and (2.2), we can derive the equilibrium disease prevalence, I*, as a proportion of the total population, I = Ĩ/Ñ (for details, see electronic supplementary material):
| 2.3 |
To explore the implications of interactions between disease prevalence and income, we use the following method. First, we identify the parameters of the epidemiological model (equation (2.3)) that are functions of income, and then identify the corresponding equation for the equilibrium disease prevalence, I*(M). We define a simple model of income as a function of the disease prevalence, M*(I) separately. The consequences of these seemingly complex interactions are found by solving for income and disease prevalence simultaneously, M*(I*) and I*(M*).
(a). Disease prevalence as a function of income
In the standard epidemiological model, the natural death rate (μ), the recovery rate (γ) and the transmission rate (β) are fixed parameters (Anderson & May 1992; Keeling & Rohani 2007). In reality, these parameters vary with host nutrition, hygiene and healthy living conditions, which in turn vary with economic wealth (Gamage-Mendis et al. 1991; Wolday et al. 1995; Shankar 2000). To capture these basic interactions between economics and disease ecology we consider a model where μ, γ and β are functions of per capita income, which in turn is determined by individual health. We maintain the conventional assumption from the evolution of virulence literature that pathogen virulence, ν, is determined by the pathogen (Bremmermann & Pickering 1983; Frank 1996; Day 2001; Bonds et al. 2005; Delfino & Simmons 2005).
There is a range of functional forms for μ, γ and β that could be used here. Our priority is that these functions are simple and that they depict reasonable qualitative relationships between income and the relevant epidemiological parameters that can broadly explain how poverty results in greater disease burden. Hence, the functions for natural death and recovery rates that we use are
| 2.4 |
and
| 2.5 |
where h is a metric of nutrition and is determined by income M. The parameter μ̄ is the minimum death rate for a completely nourished individual, and ϱ and τ are exogenous parameters. Equations (2.4) and (2.5) are examples of simple functions that correspond to a system where the death rate falls and the recovery rate rises as levels of nutrition rise. To model nutrition as a function of income, we use a classic saturation function
| 2.6 |
where h̄ is the maximum level of nutrition attainable with unlimited income. Equation (2.6) is a simple way of depicting a system where nutrition levels asymptotically approach a maximum as income rises. This general functional form is used commonly in biological sciences to represent the process of organisms absorbing nutrients (Eppley et al. 1969). The κ is referred to as the half-saturation constant, which in this case represents the level of income necessary for an individual to be ‘half-nourished’ (i.e. where nutrition, h, is half of its maximum, h̄).
As mentioned above, the transmission rate, β, is also in part determined by income via access to transmission interventions (such as clothes, bed nets, screens and glass windows, housing conditions, etc.) as well as nutrition. For simplicity we assume a saturating effect of income on the transmission rate,
| 2.7 |
where β̄ represents the maximum transmission rate, which would be determined by factors outside the influence of human behaviour, and ϕ is the amount of income necessary to reduce the transmission rate by half.
In contrast with the standard model, equations (2.4)–(2.7) capture the effects of improved nutritional and living conditions in lowering death rates, increasing recovery rates from infection and reducing disease transmission. Combining equations (2.4)–(2.7) with equation (2.3) yields the equilibrium disease prevalence as a function of income,
| 2.8 |
illustrated in figures 1 and 2.
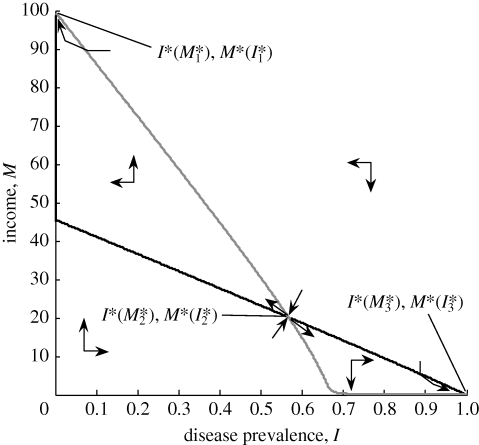
Figure 1.
Feedback between economics and the ecology of infectious diseases forms a poverty trap. The prevalence of infectious diseases, I*(M) (black line), falls as per capita income rises, while per capita income, M*(I) (grey line), falls as disease prevalence, I, rises. The disease and income functions are in equilibrium where these two curves intersect at (I*(M*), M*(I*)). Two of these equilibria (I*(M*1), M*(I*1) and I*(M*3), M*(I*3)) are stable, and one (I*(M*2), M*(I*2)) is unstable. The poverty trap is the basin of attraction around (I*(M*3), M*(I*3)). α = 0.06; β̄ = 40; μ̄ = 0.01; ν = 0.02; h̄ = 90; δ = 5; ϱ = 0.003; τ = 0.15; ϕ = 15; κ = 30.
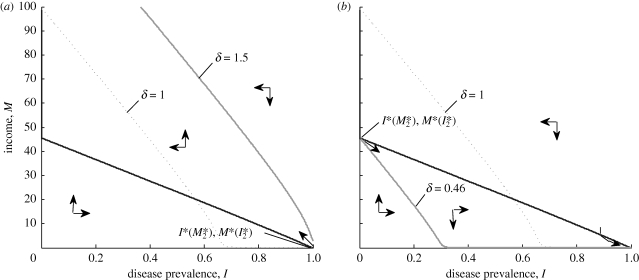
Figure 2.
(a) If labour productivity, δ, is sufficiently high, then all initial conditions lead to high income and low disease burden. (b) If δ is sufficiently low, then all initial conditions lead to low income and high disease burden. α = 0.06; β̄ = 40; μ̄ = 0.01; ν = 0.02; h̄ = 90; δ = 5; ϱ = 0.003; τ = 0.15; ϕ = 15; κ = 30. Black line, I*(M); grey line, M*(I); dotted grey line, M*(I).
(b). Income as a function of disease prevalence
Because infectious diseases impede children from acquiring human capital and adults from providing labour, the income necessary to protect individuals from diseases are themselves determined by disease prevalence (Nokes et al. 1992; Holding & Snow 2001; Ezeamama et al. 2005; Fernando et al. 2006; Bonds & Rohani in press). For simplicity, we model per capita income as proportional to the total time an individual spends uninfected, πS:
| 2.9 |
where δ is an exogenous parameter that determines the rate at which individuals produce income per unit time healthy (i.e. susceptible). The time spent susceptible, πS, can be represented as a Markov chain with a finite solution (see electronic supplementary material). Per capita income, M, can be expressed as
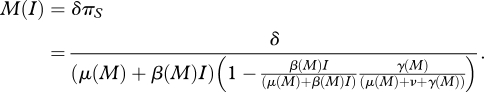 |
2.10 |
Note that among the determinants of per capita income represented in equation (2.10) are the disease transmission rate, β(M), the host recovery rate, γ(M), and the natural death rate, μ(M), which are themselves determined by income (equations (2.4)–(2.7)). To identify the equilibrium income as a function of only exogenous parameters and the disease prevalence, we solve equation (2.10) simultaneously for M, which yields an income function, M*(I). Note that the equilibrium M*(I) that we are referring to here is an equilibrium level of income determined by the feedback between income and transmission, income and recovery, and income and natural death. The equilibrium income as a function of the disease prevalence, M*(I), is too cumbersome to present here but is illustrated in figures 1 and 2.
3. The poverty trap
Because higher income affords some level of protection against infectious diseases, via nutrition and better sanitary conditions, disease prevalence, I*(M), in our model falls as income rises. However, because human health is required for economic productivity, per capita income, M*(I), falls as disease prevalence rises. To understand the implications of these feedbacks we overlay these functions in figures 1 and 2. The I*(M) curve represents disease prevalence as a function of income and the M*(I) curve represents income as a function of disease prevalence. The ultimate outcome of these interactions is where income and disease are simultaneously in equilibrium, which can be found where the curves intersect. These equilibria can also be found analytically by solving the equations for M*(I) and I*(M) simultaneously.
Figure 1 depicts a case where the feedbacks between income and the ecology of infectious diseases results in three equilibria, two of which are stable—(I*(M*1), M*(I*1)) and (I*(M*3), M*(I*3))—and one unstable, (I*(M*2), M*(I*2)). The basin of attraction around the third (stable) equilibrium, where disease prevalence is highest and income is lowest, is a poverty trap.
The defining feature of the poverty trap as a useful theoretical concept is the presence of the unstable equilibrium that implies important threshold effects. As we can see from figure 1, whether an equilibrium is stable or unstable depends on the relative effects of income and disease on each other (i.e. the relative slopes of the curves). Specifically, the unstable equilibrium is characterized by the product of the slopes of the two curves being greater than one; i.e.
| 3.1 |
If, for example, the slope of the income curve, M(I), was equal to 1 at equilibrium, then the stability of the equilibrium would require that the slope of the disease curve, I(M), be less than 1. If the slope of the income curve were instead greater than 1, then an increase in income by one unit would result in a decrease in disease prevalence by more than one unit, which would lead to a subsequent increase in income, creating a positive feedback between health and economic development.
In figure 1 we can see that multiple equilibria occur as a result of the nonlinearity of the income curve, M*(I). This is because of a combination of direct and indirect mechanisms by which greater disease prevalence results in greater time spent infected (and therefore lower income). The direct mechanism is simply an increase in the effective amount of contact between infected and uninfected individuals as disease prevalence rises. Indirectly, the higher disease prevalence also causes an increase in the transmission rate (which is a function of income) and a decrease in the recovery rate. Thus, the synergistic effects of higher disease prevalence on greater transmission and lower recovery results in an initial rapid decrease in income as disease prevalence rises. However, because the income curve is ultimately bounded at 0, as disease prevalence approaches 1, income approaches 0.
Whether the outcome of these feedbacks results in a population being stuck in a poverty trap depends on the initial conditions. If the initial per capita income is less than M*(I*2) and the disease prevalence is greater than I*(M*2), then the economy will shrink and the public health will diminish until they reach the poverty trap equilibrium, (I*(M*3), M*(I*3)). For a society to break free from this poverty trap, initial levels of economic productivity and public health must move beyond the threshold of (I*(M*2), M*(I*2)). Theoretically, this can occur through two mechanisms: (i) direct changes in the state variables of income or disease; or (ii) changes in the parameters of the system, such as changes in the labour productivity term, δ, which would correspond to investments in education or infrastructure.
This direct effect assumes that the parameters of the trap are constant and suggests that if, for example, health interventions such as bed nets or vaccine coverage were substantial and widespread enough to sustain a prevalence of infectious diseases below the threshold level, I*(M*2), then the trap could be broken and economic development would follow. Alternatively, it is possible to close the trap itself by affecting the economic parameters. For example, increases in labour productivity through education or higher levels of physical capital (e.g. through investments in infrastructure) could lead to a higher conversion rate of healthy labour to income and would be represented by an increase in δ in equation (2.10), corresponding to an upward shift of the M*(I) curve (figure 2a).
In figure 2a, labour productivity is sufficiently high that the threshold disease prevalence, I*(M*2), is 1 (i.e. there is no poverty trap), and all points in the variable space lead to a high stable equilibrium level of income and public health. Alternatively, if the labour productivity term, δ, were low enough, the opposite could be true: the threshold disease prevalence could be 0, and all initial conditions would fall within the trap, as depicted in figure 2b.
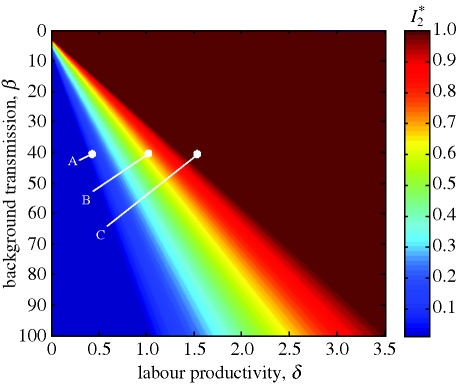
Figure 3 illustrates the sensitivity of this poverty trap model over the space of two different parameters, β̄ and δ, which respectively influence the disease transmission rate and per capita income. The z axis, I*(M*2), is simply the value of the unstable equilibrium at each combination of β̄ and δ. Notice that, for every value of β̄ presented, there is a range of values of δ for which the disease threshold, I*(M*2), is 0 (i.e. the whole economy is a poverty trap), 1 (i.e. there is no poverty trap) and between 0 and 1 (i.e. the initial conditions determine the long-term outcome of the population).
Figure 3.
Sensitivity analysis. The threshold level of disease prevalence beyond which an economy could grow out of poverty, I*2, is presented over a range of values for the transmission parameter, β̄, and the income parameter, δ. Notice that for every value of β̄ presented, there is a range of values of δ for which the entire economy is in a poverty trap (I*2 = 0; as in figure 2b), for which the poverty trap does not exist (I*2 = 1; as in figure 2a) and for which the initial conditions determine the long-term outcome of the economy (0 < I*2 < 1; as in figure 1). The points A, B, and C represent the system at three different values for δ, which are illustrated in figures 2b, 1 and 2a, respectively. α = 0.06; μ̄ = 0.01; ν = 0.02; h̄ = 90; ϱ = 0.003; τ = 0.15; ϕ = 15; κ = 30.
In such a model, with different mechanisms of feedbacks, it is difficult to make broad generalizations on how the functional forms of the different disease parameters affect the outcomes of this model (an analysis of many different models is beyond the scope of this article). It is easy to imagine a large range of ways in which the curves could theoretically intersect, with three of the possible outcomes represented in figures 1 and 2. The most important property of this system is that the curves slope in the same direction (i.e. that income lowers the disease prevalence and the disease prevalence lowers income), which would result from any reasonable functional relationship between income and the epidemiological parameters. This phenomenon alone implies important indirect consequences of health interventions. Whether the curves intersect in such a way as to generate qualitatively different outcomes of the model that depend on the initial conditions depends on the sensitivity of income and disease to each other (i.e. the relative slopes of the curves). The more sensitive the system is, the more likely there exists an unstable equilibrium. Such sensitivity would depend on the number of channels for positive feedback and the effects of those channels on the slopes of each of the curves. For the I(M) curve, the channels we consider here are recovery, transmission and the natural death rate, but other potential mechanisms exist as well. For the M(I) curve, we considered a simple direct relationship between healthy labour supply and income, but one could also explicitly model other feedback mechanisms, such as through human capital accumulation, fertility and death.
(a). Implications for the basic reproductive number, R0
One of the most informative parameters of any epidemiological model is its basic reproductive ratio, R0, which is equal to the number of secondary infections that result from a single infectious individual in a totally susceptible population. The ultimate objective of many disease-control strategies is to reduce the disease reproductive ratio to a value of less than 1, which would result in eradication. Many of the most dangerous diseases in resource-poor countries—such as malaria, hookworm, TB, polio and measles, among others—have, in fact, been virtually eliminated in wealthy countries by a suite of direct and indirect interventions that have reduced R0 to less than one.
The basic reproductive number that corresponds to equations (2.1) and (2.2) and accounts for income effects is:
| 3.2 |
where M* here represents the maximum income attainable in the absence of disease, and is equal to M* = (δh̄ − ϱκ)/(uūh̄ + ϱ). This basic reproductive ratio is therefore always lower than estimates of R0 that do not take into account the effect of economic feedbacks.
4. Empirical patterns
In the theoretical section above, we presented a system to explore the implications of feedbacks between income and the effects of infectious diseases. We showed that in our model, the consequences of cumulative infections have the potential to keep a population in a poverty trap. A direct empirical test of such a poverty trap would require high-resolution data at the household or, better still, the individual level to estimate the parameters of the system, or a way to inductively identify the existence of multiple equilibria with macro-level data. Unfortunately, given the absence of appropriate data, both approaches present formidable challenges for making an empirical test of our theory. Instead, we aim for a more accessible goal of determining plausibility: are the available population-level data consistent with the theoretical predictions above? Given data limitations, we consider these trends across countries.
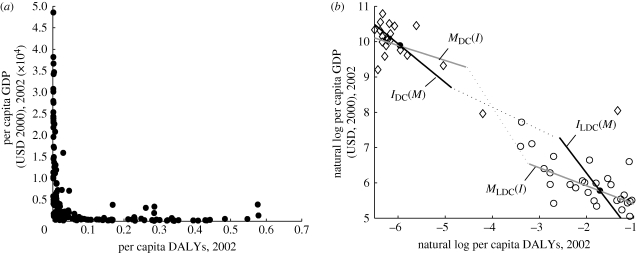
Figure 4a presents the per capita income and the total infectious disease burden measured by disability adjusted life years (DALYs; Murray & Lopez 1996; Lopez et al. 2006), for all the countries in the world. From a simple glance at the data, one can easily see that the correlation between income and infectious diseases is strongly negative and highly nonlinear, which could be considered suggestive of the positive feedback described above.
Figure 4.
Disease burden and income. (a) The correlation between per capita GDP (USD, 2000) in 2002 and the infectious disease burden (DALYs) in 2002 over 170 countries in the world is negative and highly nonlinear. (b) The natural log of the per capita DALYs and GDP are presented for least developed countries (LDCs, open circles) and developed countries (DCs, open diamonds) with the filled circles representing the average values for the LDCs and DCs. The slopes of the estimated effect of income on disease burden, IDC(M) and ILDC(M), and of disease burden on income, MDC(I) and MLDC(I), are represented by the solid lines. If the estimates of the stable equilibria are part of a continuous system, then there is an unstable equilibrium in-between, represented by the dotted lines. Data sources: Lopez et al. (2006); World Bank Development Data Group (2007).
We cannot directly observe an unstable equilibrium or empirically estimate the complete nonlinear functions for income, M(I), or disease burden, I(M), that would together generate an unstable equilibrium. Instead, we explore the empirical plausibility of this model by estimating linear approximations of these nonlinear equations around their observable stable equilibria. Specifically, at each stable equilibrium we estimate the impact of health on income and income on health via two-stage least squares (2SLS). 2SLS is a common technique used in econometrics to measure causal relationships between endogenous variables that simultaneously influence each other (Wooldridge 2002). The key feature of this method is the presence of instrumental variables (IVs), which occur in the structural specification of one of the two endogenous variables of interest, but not that of the other. The use of IVs therefore allows one to identify the effects of the one endogenous variable from the other (for more details, see the electronic supplementary material).
Accordingly, we estimate the linear approximations
| 4.1 |
and
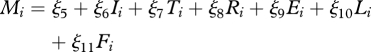 |
4.2 |
for ‘developed countries’ (DCs; CIA 2008), which are candidates for being in a stable equilibrium of high income and low disease, and separately for ‘least developed countries’ (LDCs; UN Office of the High Representative for the Least Developed Countries 2008), which are candidates for being trapped in a different stable equilibrium, one of poverty and disease (note that countries that are neither LDCs or DCs are likely to be in transition, and so, belonging to neither equilibrium, are used in neither estimation). Mi represents the natural log of the per capita income for country i, while Ii represents the natural log of the per capita DALYs from all infectious diseases. The IV for disease, Ii,row, is a weighted average of the disease burden of the ‘rest of the world’ from the perspective of country i, where the weight of the average DALYs of each other country decreases with distance from country i. Ii,row serves as a useful instrumental variable for Ii because infectious diseases are often spatially correlated, but the impact of the disease burden in country i on the ‘rest of the world’ is much smaller than the impact of the ‘rest of the world’ on country i (Grenfell et al. 2001). The variable E represents the natural log of the value of total energy production, L is a dummy variable for landlocked countries and F is an index of ethnolinguistic fractionalization, with all three serving as instrumental variables for income. Ti, the average temperature, and Ri, the total annual rainfall, are climate variables that are expected to impact both the disease burden and economic productivity. For more details, see electronic supplementary material.
As presented in table 1, the coefficient estimates are all negative, supporting the hypothesis that the disease burden lowers per capita income, whereas poverty is an underlying cause of disease. The product of these coefficients is less than one in both sets of estimations, implying that both equilibria are stable.
Table 1.
Coefficient estimates of health and income on each other in developed countries (DCs) and least developed countries (LDCs). The key results from the 2SLS regressions are below (for complete results, see electronic supplementary material). Note that triple asterisks (***) represent significance at the 5 per cent level, double asterisks (**) represent significance at the 10 per cent level and single asterisk (*) represents significance at the 15 per cent level. The R2 for the disease equation, I(M), is 0.559 for the DCs and 0.705 for the LDCs. The R2 for the income equation, M(I), is 0.744 for the DCs and 0.550 for the LDCs.
| estimates for DCs (n = 27) | estimates for LDCs (n = 38) | |
|---|---|---|
| I(M) impact of ln(DALYs) on ln(income), Mi | −0.425 (0.174)*** | −0.508 (0.192)*** |
| impact of ln(income), Mi, on ln(DALYs), Ii | −0.914 (0.602)* | −0.571 (0.302)** |
An illustration of the estimates of the infectious disease and income functions for the average DC, MDC(I) and IDC(M), and the average LDC, MLDC(I) and ILDC(M), are found in figure 4b. If we consider the LDC and DC equilibria to be part of a single continuous system, there must be an unstable equilibrium in-between. A hypothetical representation of the unstable equilibrium is found at the intersection of the dotted lines. The basin of attraction around the poor equilibrium would be the poverty trap. While all countries are naturally subject to different economic and epidemiological parameters and therefore can only be considered to be broadly subject to the same general forces, this evidence suggests that the data are at least consistent with the theoretical concepts presented above.
5. Discussion
Underlying the recent broad upsurge of interest in global health and economic development is an important over-riding question: why do people from some parts of the world enjoy continued exponential economic growth, while others, such as those in sub-Saharan Africa, suffer from the kind of extreme poverty their ancestors suffered many generations ago? Proposed explanations for such divergent economic trajectories have focused on a number of ways in which per capita income feeds back on other economically important variables, such as conflict, political institutions, land degradation, fertility and, notably, infectious diseases (Dasgupta & Ray 1987; Deaton 2003; Collier 2007a).
The literature on the interactions between income and disease tends to be unidirectional and to focus on either the effects of (i) income on health or (ii) health on income. In reality, the evidence suggests that both of the effects are important, if to different degrees. Our model here demonstrates that the feedbacks between income and disease have the potential to generate divergent trajectories of health and economic development that are dependent on the initial conditions. What may be most important in these debates is therefore not whether the effect of health on poverty is more significant than that of poverty on health, but whether the combined effect is powerful enough to generate self-perpetuating patterns of development or the persistence of poverty. If, for example, the effect of health on income is small, then the existence of a poverty trap would require that the effect of income on health be relatively large at one equilibrium point. This basic model suggests an important line of enquiry for those interested in socioeconomic relationships with infectious diseases. Instead of focusing on general relationships between health and income, it may be especially valuable to identify levels of income above which the burden of disease drops significantly. Such an outcome would imply that even small effects of health on income could result in significant long-term benefits of policy interventions that lower the burden of disease. What are needed are data on the nature of these feedbacks, as well as explicit models that can inform our understanding of these processes, and thereby the analysis of such data.
While we hope that our model framework can serve as a useful point of departure for exploring more complex relationships, the theoretical analysis we present here has significant implications: simply coupling economics with a well-established model of the ecology of infectious diseases can imply radically different levels of health and economic welfare (i.e. poverty traps) depending on initial conditions. The practical implications are also significant. Because the world's leading killers of the poor—malaria, HIV/AIDS, tuberculosis, diarrhoea and respiratory infections—are highly preventable and treatable, current global efforts to improve public health in areas of extreme poverty could theoretically pay long-term economic dividends. Furthermore, this analysis underscores that there are dramatic implications if economic activity is coupled with ecological processes that are well-known to behave in nonlinear ways.
Acknowledgments
The authors would like to thank Brenda Lin, Ulrich Wagner, Valerie Mueller, Helen Wearing, John Drake and the Earth Institute Fellows for comments on the manuscript. This project was supported by an Earth Institute Fellowship to M.H.B.
References
- Anderson R. M., May R. M.1992Infectious diseases of humans: dynamics and control. New York, NY: Oxford University Press [Google Scholar]
- Black F. L.1975Infectious diseases in primitive societies. Science 187, 515–518 (doi:10.1126/science.163483) [DOI] [PubMed] [Google Scholar]
- Bloom D. E., Canning D.2000The health and wealth of nations. Science 287, 1207–1209 (doi:10.1126/science.287.5456.1207) [DOI] [PubMed] [Google Scholar]
- Bloom D., Canning D., Sevilla J.2003Geography and poverty traps. J. Econ. Growth 8, 355–378 (doi:10.1023/A:1026294316581) [Google Scholar]
- Bonds M. H., Keenan D. C., Leidner A., Rohani P.2005Higher disease prevalence can induce greater sociality; a game-theoretic coevolutionary model. Evolution 59, 159–168 [PubMed] [Google Scholar]
- Bonds M. H., Rohani P.In press Herd immunity acquired indirectly from interactions between the ecology of infectious diseases, demography and economics. J. R. Soc. Interface (doi:10.1098/rsif.2009.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles S., Durlauf S. N., Hoff K.2007Poverty traps Princeton, NJ: Princeton University Press [Google Scholar]
- Bremmermann H. J., Pickering J.1983A game-theoretic model of parasite virulence. J. Theor. Biol. 100, 411–426 (doi:10.1016/0022-5193(83)90438-1) [DOI] [PubMed] [Google Scholar]
- CIA 2008The world factbook 2009 New York, NY: Skyhorse Publishing [Google Scholar]
- Collier P.2007aThe bottom billion New York, NY: Oxford University Press [Google Scholar]
- Collier P.2007bPoverty reduction in Africa. Proc. Natl Acad. Sci. 104, 16 763–16 768 (doi:10.1073/pnas.0611702104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier P., Hoeffler A., Elliot H., Hegre H., Reynal-Querol M., Sambanis N.2003Breaking the conflict trap: civil war and development policy Oxford and Washington, DC: Oxford University Press and World Bank [Google Scholar]
- Cooke G. S., Hill A. V.2001Genetics of susceptibility for human infectious diseases. Nat. Rev. Genet. 2, 967–977 (doi:10.1038/35103577) [DOI] [PubMed] [Google Scholar]
- Dasgupta P., Ray D.1987Inequality as a determinant of malnutrition and unemployment: policy. Econ. J. 97, 177–188 (doi:10.2307/2233329) [Google Scholar]
- Dasgupta S., Deichmann U., Meisner C., Wheeler D.2005Where is the poverty–environment nexus? Evidence from Cambodia, Lao PDR, and Vietnam. World Dev. 33, 617–638 (doi:10.1016/j.worlddev.2004.10.003) [Google Scholar]
- Day T.2001Parasite transmission modes and the evolution of virulence. Evolution 55, 2389–2400 [DOI] [PubMed] [Google Scholar]
- Deaton A.2003Health, inequality and economic development. J. Econ. Lit. 1, 113–158 (doi:10.1257/002205103321544710) [Google Scholar]
- Delfino D., Simmons P. J.2005Dynamics of tuberculosis and economic growth. Environ. Dev. Econ. 10, 719–743 (doi:10.1017/S1355770X05002500) [Google Scholar]
- Diamond J.1997Guns, germs, and steel: the fates of human societies New York, NY: W. W. Norton and Company [Google Scholar]
- Easterly W., Levine R.1997Africa's growth tragedy: policies and ethnic divisions. Q. J. Econ. 112, 1203–1250 (doi:10.1162/003355300555466) [Google Scholar]
- Eppley R. W., Rogers J. N., McCarthy J. J.1969Half-saturation constants for uptake of nitrate and ammonium by marine phytoplankton. Limnol. Oceanogr. 14, 912–920 [Google Scholar]
- Ezeamama A. E., Friedman J. F., Acosta L. P., Bellinger D. C., Langdon G. C., Manalo D. L., Olvedo R. M., Kurtis J. D., McGarvey S. T.2005Helminth infection and cognitive impairment among Filipino children. Am. J. Trop. Med. Hyg. 72, 540–548 [PMC free article] [PubMed] [Google Scholar]
- Farmer P.2001Infections and inequalities: the modern plagues Berkeley, CA: University of California Press [Google Scholar]
- Fernando D., De Silva D., Carter R., Mendis K. N., Wickremasinghe R.2006A randomized, double-blind, placebo-controlled, clinical trial of the impact of malaria prevention on the educational attainment of school children. Am. J. Trop. Med. Hyg. 74, 386–393 [PubMed] [Google Scholar]
- Frank S. A.1996Models of parasite virulence. Q. Rev. Biol. 71, 37–78 (doi:10.1086/419267) [DOI] [PubMed] [Google Scholar]
- Gallup J. L., Sachs J. D.2001The economic burden of malaria. Am. J. Trop. Med. Hyg. 64, 85–86 [DOI] [PubMed] [Google Scholar]
- Gamage-Mendis A. C., Carter R., Mendis C., De Zoysa A. P., Herath P. R., Mendis K. N.1991Clustering of malaria infections within an endemic population: risk of malaria associated with the type of housing construction. Am. J. Trop. Med. Hyg. 45, 77–85 [DOI] [PubMed] [Google Scholar]
- Gandon S., Mackinnon M. J., Nee S., Read A. F.2001Imperfect vaccines and the evolution of parasite virulence. Nature 414, 751–755 (doi:10.1038/414751a) [DOI] [PubMed] [Google Scholar]
- Glewwe P.2002Schools and skills in developing countries: education policies and socioeconomic outcomes. J. Econ. Lit. 40, 436–482 (doi:10.1257/002205102320161258) [Google Scholar]
- Grenfell B. T., Bjørnstad O. N., Kappey J.2001Traveling waves and spatial hierarchies in measles epidemics. Nature 414, 716–723 (doi:10.1038/414716a) [DOI] [PubMed] [Google Scholar]
- Holding P. A., Snow R. W.2001Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence. Am. J. Trop. Med. Hyg. 64, 68–75 [DOI] [PubMed] [Google Scholar]
- Hudson P. J., Dobson A. P., Newborn D.1990Prevention of population cycles by parasite removal. Science 282, 2256–2258 (doi:10.1126/science.282.5397.2256) [DOI] [PubMed] [Google Scholar]
- Jong-Wook L.2003Global health improvement and WHO: shaping the future. Lancet 362, 2083–2088 (doi:10.1016/S0140-6736(03)15107-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling M. J., Rohani P.2007Modelling infectious diseases Princeton, NJ: Princeton University Press [Google Scholar]
- Lopez A. D., Mather C. D., Ezzati M., Jamison D. T., Murray C. J. L.2006Global burden of disease and risk factors New York, NY: Oxford University Press; (doi:10.1596/978-0-8213-6262-4) [Google Scholar]
- Murray C. J. L., Lopez A. D.1996Global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020 Cambridge, MA: Harvard University Press; (on behalf of the World Health Organization and the World Bank) [Google Scholar]
- Nokes C., Grantham-McGregor S. M., Sawyer A. W., Cooper E. S., Bundy D. A. P.1992Parasitic helminth infection and cognitive function in school children. Proc. R. Soc. Lond. B 247, 77–81 (doi:10.1098/rspb.1992.0011) [DOI] [PubMed] [Google Scholar]
- Rohani P., Earn D. J. D., Grenfell B. T.2003Natural enemy specialization and the period of population cycles. Ecol. Lett. 6, 381–384 (doi:10.1046/j.1461-0248.2003.00437.x) [Google Scholar]
- Sachs J.2005End of poverty: economic possibilities of our time New York, NY: Penguin Press [Google Scholar]
- Sachs J., Malaney P.2002The economic and social burden of malaria. Nature 415, 680–685 (doi:10.1038/415680a) [DOI] [PubMed] [Google Scholar]
- Shankar A. H.2000Nutritional modulation of malaria morbidity and mortality. J. Infect. Dis. 182, S37–S53 [DOI] [PubMed] [Google Scholar]
- Strauss J., Thomas D.1998Health, nutrition and economic development. J. Econ. Lit. 36, 766–817 [Google Scholar]
- UN Millennium Project 2005Investing in development: a practical plan to achieve the Millennium Development Goals. New York, NY: UN Millennium Project [Google Scholar]
- UN Office of the High Representative for the Least Developed Countries 2008List of least developed countries. See http://www.un.org/special-rep/ohrlls/ldc/list.htm [Google Scholar]
- Wolday D., Kibreab T., Bukenva D., Kodes R.1995Sensitivity of Plasmodium falciparum in vivo to chloroquine and pyrimethamine-sulfadoxine in Rwandan patients in a refugee camp in Zaire. Trans. R. Soc. Trop. Med. Hyg. 89, 654–656 (doi:10.1016/0035-9203(95)90431-X) [DOI] [PubMed] [Google Scholar]
- Wooldridge J. M.2002Econometric analysis of cross section and panel data Cambridge, MA: MIT Press [Google Scholar]
- World Bank Development Data Group 2007World development indicators online Washington, DC: The World Bank; See http://go.worldbank.org/3JU2HA60D0 [Google Scholar]
- World Health Organization 1998Health for all in the twenty-first century. Geneva, Switzerland: World Health Organization [Google Scholar]
- World Health Organization 2001Macroeconomics and health: investing in health for development—report of the Commission on Macroeconomics and Health Geneva, Switzerland: World Health Organization [Google Scholar]
- World Health Organization 2004World health report: changing history. Geneva, Switzerland: World Health Organization [Google Scholar]