Abstract
How do flying insects monitor foraging efficiency? Honeybees (Apis mellifera) use optic flow information as an odometer to estimate distance travelled, but here we tested whether optic flow informs estimation of foraging costs also. Bees were trained to feeders in flight tunnels such that bees experienced the greatest optic flow en route to the feeder closest to the hive. Analyses of dance communication showed that, as expected, bees indicated the close feeder as being further, but they also indicated this feeder as the more profitable, and preferentially visited this feeder when given a choice. We show that honeybee estimates of foraging cost are not reliant on optic flow information. Rather, bees can assess distance and profitability independently and signal these aspects as separate elements of their dances. The optic flow signal is sensitive to the nature of the environment travelled by the bee, and is therefore not a good index of flight energetic costs, but it provides a good indication of distance travelled for purpose of navigation and communication, as long as the dancer and recruit travel similar routes. This study suggests an adaptive dual processing system in honeybees for communicating and navigating distance flown and for evaluating its energetic costs.
Keywords: Apis mellifera, waggle dance, odometer, foraging energetics, choice behaviour
1. Introduction
To benefit their colony, forager honeybees must make an energetic profit. There is clear evidence that forager bees adjust their foraging behaviour to maximize the energetic efficiency of their foraging trips (Seeley 1994, 1995). Honeybees are also social foragers that use symbolic dance communication to recruit nest mates to the most profitable resources (Frisch 1967; Seeley 1995). Analyses of dance language and foraging behaviour have shown that bees can signal the location of food sources up to several kilometres away (Frisch 1967). The duration of the waggle phase of the waggle dance indicates the distance to the food source, whereas the number of dance circuits performed correlates with the relative value of the food source in order to recruit the greatest number of bees to the most valuable resources (Dyer 2002).
Walking (Wittlinger et al. 2006) and flying insects need to gauge distance. There has been a long debate regarding the manner in which bees evaluate distance flown. Frisch (1967) suggested two reasonable hypotheses: (i) by assessing the energy consumption of flying that distance and (ii) by integrating optic flow during flight, which is the total amount the image of the environment moves across the eye as the bee moves through space. Whereas earlier studies seemed to support the former hypothesis, it is now clear that both the distance signal of the waggle dance and bee navigation are informed by visual odometric information (Srinivasan et al. 2000; Esch et al. 2001; Dacke & Srinivasan 2008). But do bees also use visual odometric information to estimate foraging energetics?
As there would normally be a strong correlation between distance travelled while foraging and foraging costs, perhaps the simplest hypothesis is that bees would use the optic flow signal (their principle index of distance travelled) to also inform their estimates of foraging cost and foraging energetic efficiency. We tested this hypothesis by analysing the dance behaviour and foraging choices of forager bees using two sucrose feeders located within flight tunnels that manipulated the amount of optic flow to experimentally separate the size of the optic flow signal from actual distance travelled.
The two flight tunnels were lined with checked material (see §2). The amount of optic flow generated by the walls of the flight tunnels is proportional to the distance between the tunnel walls and the flying bee: as a consequence of this, a bee flying in a narrow tunnel experiences greater optic flow than a bee flying the same distance in a wider tunnel (Esch & Burns 1995; Srinivasan et al. 2000). The ‘long tunnel’ (LT) in our study was twice as long and four times as wide as the ‘short tunnel’ (ST). We expected that bees flying 1 m in the ST would experience four times the optic flow of bees flying 1 m in the LT. Therefore, a bee travelling through the ST to the feeder would experience greater optic flow than a bee using the LT.
2. Material and methods
(a). Bees
Honeybees were a commercial stock available in Australia and derived principally from Apis mellifera ligustica. A colony of approximately 6000 bees was housed in a four-frame glass-walled observation hive located in a controlled-temperature room held at 29°C.
(b). Flight enclosure and tunnels
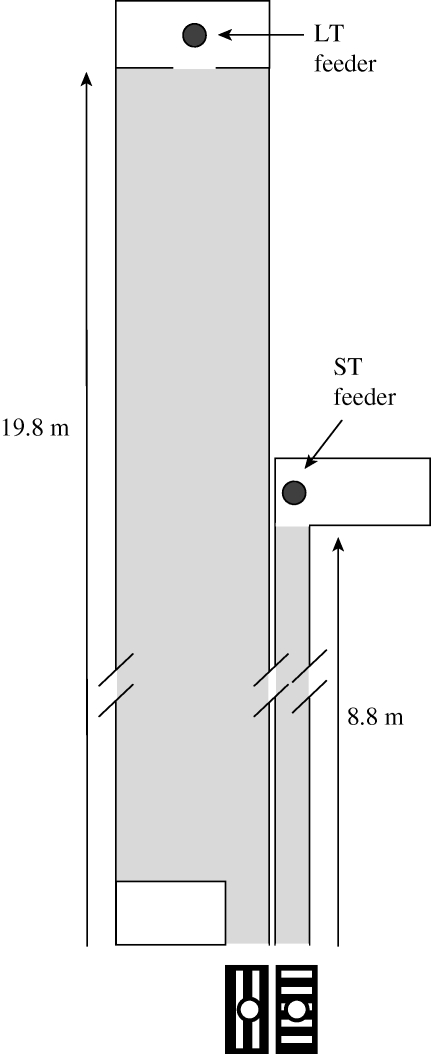
Individually marked bees from a glass-walled observation hive were trained to ad libitum 2 M sucrose feeders placed at the distal ends of two flight tunnels lined with green/white checked material (each square of area 6.25 cm2). The LT was in total 19.8 m long and 40 cm wide. The ST was 8.8 m long and 10 cm wide. Both tunnels had side walls 20 cm high, and the roof was covered with insect mesh (figure 1).
Figure 1.
Top view of the experimental tunnels, and front view of the horizontal and vertical grids marking the entrance to the tunnels. The long tunnel (LT) was 40 cm wide and the short tunnel (ST) was 10 cm wide (not drawn to scale). See text for details.
Our experiments were all conducted in a large flight enclosure 25 m by 8 m, with a 5 m high ceiling. The observation hive was mounted to the wall of the controlled-temperature room so that bees exited the observation hive directly into the flight enclosure. Within the flight enclosure were the two flight tunnels aligned side by side.
Bees could enter each of the two tunnels at the end closest to the observation hive (approx. 5 m) through a 5 cm diameter circular hole. To facilitate bees distinguishing the two tunnels, these entrance holes were surrounded by either a horizontal or a vertical grid, 10 × 20 cm, of alternating black and white stripes 2 cm wide. The tunnel entrances could be blocked by a white plug. Because of shade cast by the walls, the ST was darker than the LT. So that bee tunnel choice would not be biased by this difference prior to them having learned the features of both tunnels, the first 1.17 m of the LT was 10 cm wide so that when viewed from the perspective of the entrance hole, both LT and ST appeared identical in width and light level.
The distal end of each tunnel terminated at a Perspex cube of volume 64 l. All side walls of each cube were covered with a black and white grid pattern to match that surrounding the tunnel entrance. The roof of the cube was covered with black insect mesh, which was removable so that bees could be released. An ad libitum 2 M sucrose feeder was placed on the floor in the middle of each cube. When bees had completed feeding, they almost always flew upwards and could be released from the cube to fly back to the observation hive.
(c). Bee training
Bees with individually distinctive paint marks to their thorax and abdomen were trained to enter and fly through the flight tunnels to the 2 M sucrose feeder placed at the distal end. Bees were first trained to a sucrose feeder placed close to the entrances of the tunnels. To begin tunnel training, one tunnel was opened, the entrance to the other tunnel was blocked. The feeder was first moved to a location just inside the entrance of the open tunnel and placed so it was still visible to bees viewing the tunnel entrance from outside. Bees that entered the tunnel and fed were released from the tunnel by lifting the mesh roof. After 10 min, the tunnel was closed, the alternative tunnel opened and the feeder moved into it. Every 10 min, the feeder was swapped between the two tunnels and the bees constrained to only visit the tunnel containing the feeder. With each move, the feeder was placed deeper into each tunnel. In this way, bees were trained all the way to the end of both tunnels.
Once trained to both tunnels, bees were encouraged to visit both tunnels by constraining tunnel availability (no-choice phase). This was so that each individual bee could learn the features of both tunnels to inform their behaviour in the later tunnel-choice phase of the experiment. Only one tunnel was open at any one time, and every 10 min the available tunnel was swapped. Each tunnel was open for five to 10 min periods.
Up to 10 bees were successfully trained to visit both tunnels on any one day. Our experiment pooled data gathered over 16 days. The left–right arrangement of the tunnels, the grid pattern associated with each tunnel and whether the no-choice phase began with exposure to the LT or ST were all varied systematically over the 16 days of the experiment so that these possible confounds of tunnel choice were balanced across our experimental period.
During the ‘no-choice’ phase, we recorded the identity of each visiting bee, and also video recorded any dances performed when they returned to the hive. Bees made a similar number of visits to both tunnels during this period (mean ± s.e.) visits to ST = 3.9 (0.2) and LT = 3.7 (0.2), paired t-test, t = 0.95, d.f. = 135, p = 0.344).
Both tunnels were then opened for 1 h (choice phase), and the number of visits by each bee to each feeder was recorded. When possible, we also recorded the flight time of bees from entering a tunnel to alighting at the feeder.
(d). Dance and ground speed observations
A baffle at the entrance to the observation hive constrained bees to walk up one side of the frame only, and this ensured that the vast majority of dances occurred on a single side of the frame closest to the hive entrance. This frame was observed and video recorded throughout the no-choice phase using a SONY digital video recorder. Dances recorded on tape were later analysed frame-by-frame. For each dance, we scored the duration of the waggle phase of each dance circuit and the total number of circuits.
To confirm that bees responded to the different optical environments of the LT and ST, we recorded their ground speed in these two tunnels. A digital camera was mounted above the tunnels pointing down and we recorded bee flights. From later frame-by-frame analyses of the tapes for both the LT and ST, we calculated the time taken for bees to cover a distance of 1 m in unbroken linear flight.
(e). Statistical analysis
Two-tailed Student's t-tests were used to analyse the significance of differences in mean waggle duration between the two tunnels. We used paired t-tests for data from the same individuals for both tunnels. When data did not conform to ANOVA requirements, we used the non-parametric Wilcoxon signed-rank-sum test. This test was used for comparing flight velocity, tunnel navigation time and mean total number of dance circuits for the two tunnels. We used the χ2 likelihood ratio test to compare choice proportions.
3. Results
(a). Flight velocity
Flying bees regulate their ground speed by optic flow (Barron & Srinivasan 2006). As evidence that bees responded to the different optic flow signals in the LT and ST, we measured their ground speeds in both tunnels. As expected, bees flew significantly faster in the LT than the ST (median (and interquartile range) linear ground speed in ST = 0.25 m s−1 (0.21–0.27), n = 18; LT = 0.42 s−1 (0.33–0.52), n = 35. Wilcoxon signed-rank-sum test: S = 206.5, p < 0.0001).
(b). Tunnel navigation time
The ST feeder was closer to the hive than the LT feeder, and bees were significantly faster navigating the ST than the LT (median (and interquartile range) flight time from entering the tunnel to alighting at the feeder: ST = 47.12 s (34.05–69.84), n = 52 bees; LT = 70.27 s (54.82–95.25), n = 43 bees. Wilcoxon signed-rank-sum test: S = 2613, p < 0.0001. To avoid pseudoreplication, for those bees for which we had several timings in the same tunnel, we used the mean flight time to represent each bee once only for each tunnel).
(c). Dance analyses and tunnel choice
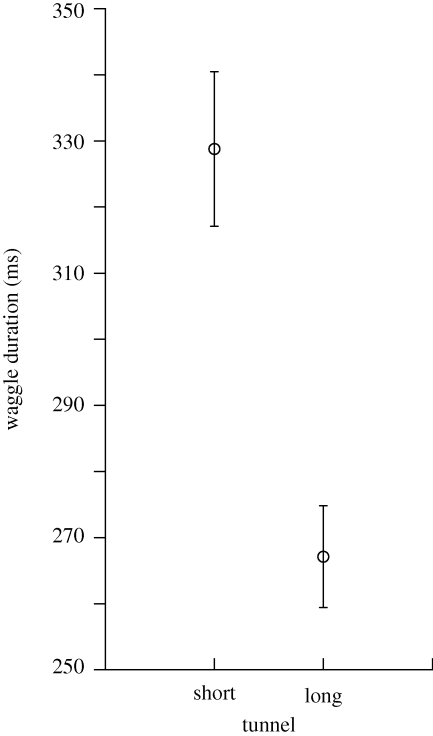
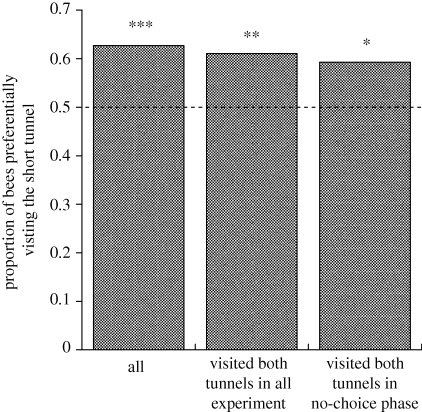
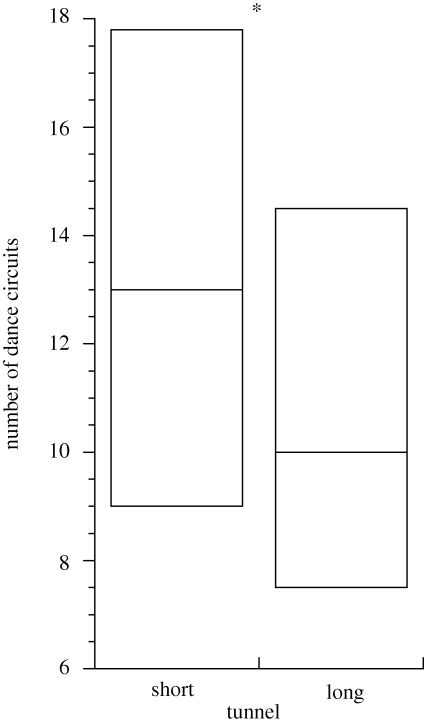
Dance analyses indicated that bees signalled the ST feeder further from the hive than the LT feeder. Dance waggle phases were significantly longer for the ST than the LT (figure 2). This result is consistent with bees estimating distance by the amount of optic flow experienced en route to the feeder (Esch et al. 2001). However, despite signalling the ST to be further away than the LT, foragers preferred to visit the ST (figure 3). Further, bees performed a greater number of dance circuits when dancing for the ST than for the LT (figure 4), indicating that bees considered the ST feeder to be the more profitable.
Figure 2.
Mean waggle duration (±s.e.) of dances for the short (n = 38 bees) and long (n = 41 bees) tunnels (t-test, t = 4.47, d.f. = 77, p < 0.0001). For 29 bees, we had data from both ST and LT. Considering these data only, waggle duration was significantly longer for the ST than for the LT (paired t-test: t = 4.7, d.f. = 28, p < 0.0001).
Figure 3.
Proportion of bees that visited the ST more often than the LT in the choice phase. Of the 136 individual bees in the experiment, 10 visited both tunnels equally. Of the remaining 126 bees, 63% visited the ST more than the long one during the choice phase (***: χ2 likelihood ratio = 8.2, d.f. = 1, p = 0.004, n = 126). Considering just the 113 bees that had visited both tunnels during either the no-choice or choice phase 61% preferred the ST during the choice phase (**: χ2 = 5.6, d.f. = 1, p = 0.018, n = 113). Considering just the 108 that had visited both tunnels during the no-choice phase, 59% preferred the ST during the choice phase (*: χ2 = 3.7, d.f. = 1, p = 0.054, n = 108).
Figure 4.
We measured mean total number of dance circuits in the first bout of uninterrupted dancing for 65 bees. Boxes represent median and interquartile range for the ST (n = 49) and LT (n = 53). Asterisk: Wilcoxon signed-rank-sum test: S = 2872, p = 0.0196. For 37 bees, we had data from both ST and LT. Considering just these data, total number of dance circuits in all dance bouts measured was greater for the ST than for the LT (paired t-test: t = 2.38, d.f. = 36, p = 0.0226; Wilcoxon signed-rank-sum test: S = 131, p = 0.0296).
4. Discussion
It has been shown unequivocally that optic flow affects the perception of distance for purpose of communication and navigation (Srinivasan et al. 2000; Esch et al. 2001; Dacke & Srinivasan 2008). Distance is communicated by the waggle duration during the straight run of the waggle dance (Frisch 1967). Our results support this conclusion; waggle duration was greater in dances for the ST, which generated greater overall optic flow than the LT.
The number of dance circuits is an index of the dancer's subjective evaluation of the profitability of the food source (Frisch 1967). The bee's evaluation of profitability can also be assessed in choice experiments, in which bees are given a choice between near and far feeders. From dance analyses and choice experiments, it is clear that flight distance affects the evaluation of profitability, which is often according to energetic efficiency (Marden & Waddington 1981; Seeley 1994). However, farther flights tend to be energetically more costly, take a longer time and to generate greater optic flow than shorter flights. It is not known which of these (or other) factors is used by bees to evaluate the energetic cost of distant flight. The most parsimonious hypothesis would be that as bees evaluate total optic flow during flight, and use this information to guide their navigation and communication of distance, they would also use this information to evaluate profitability.
Our data demonstrate that while bees clearly use optic flow information for signalling feeder distance in their dances, they do not use this information to determine either their foraging choices or their dance signals of feeder profitability. Despite flying faster in the LT (owing to the lower optic flow it generated) than in the ST, it took bees longer to navigate the LT and reach the feeder. Therefore, in this study, bee foraging choices and the number of dance circuits were more related to time in flight than optic flow information. Time in flight would have been confounded with several stimuli, any of which may inform bees’ foraging preferences and profitability calculations. These would include the total energetic costs of the foraging trip, and also non-energetic costs, such as the time exposed to risk outside the hive. Any or all of these parameters may be informing bees’ profitability assessments.
The ‘visual odometer’ (Srinivasan et al. 2000) provides a good indication of distance travelled for purpose of navigation and communication, as long as the dancer and recruit travel similar routes (Barron et al. 2005). As an index of distance for the calculation of foraging costs and energetic efficiency, optic flow is less useful as the magnitude of the optic flow signal will vary with the nature of the environment travelled by the bee (Barron et al. 2005). The colony allocates recruits to different sources in proportion to the intensity of several dances that may be performed concurrently on the dance floor (Seeley 1995). For such allocation to be efficient, dance intensity must rely on cues directly associated with the profitability of the source, and not on cues associated with visual properties of the terrain en route to each source.
Honeybees exhibit an adaptive dual system solution: they clearly use optic flow information as a navigation cue only, but rely on other information to assess foraging energetics. Despite their very small brains, bees have the capacity to monitor optic flow and other information throughout their foraging trip to independently calculate distance travelled and foraging costs, which are then signalled as separate elements of the waggle dance.
Acknowledgements
S.S. thanks the Department of Brain, Behaviour and Evolution of Macquarie University for hosting him while he was on sabbatical.
References
- Barron A., Srinivasan M. V.2006Visual regulation of ground speed and headwind compensation in freely flying honeybees (Apis mellifera L.). J. Exp. Biol. 209, 978–984 (doi:10.1242/jeb.02085) [DOI] [PubMed] [Google Scholar]
- Barron A. B., Zhu H., Robinson G. E., Srinivasan M. V.2005Influence of flight time and flight environment on distance communication by dancing honey bees. Insect. Soc. 52, 402–407 (doi:10.1007/s00040-005-0827-8) [Google Scholar]
- Dacke M., Srinivasan M. V.2008Two odometers in honeybees? J. Exp. Biol. 211, 3281–3286 (doi:10.1242/jeb.021022) [DOI] [PubMed] [Google Scholar]
- Dyer F. C.2002The biology of the dance language. Annu. Rev. Entomol. 47, 917–949 (doi:10.1146/annurev.ento.47.091201.145306) [DOI] [PubMed] [Google Scholar]
- Esch H. E., Burns J. E.1995Honeybees use optic flow to measure the distance of a food source. Naturwissenschaften 82, 38–40 (doi:10.1007/BF01167870) [Google Scholar]
- Esch H. E., Zhang S., Srinivasan M. V., Tautz J.2001Honeybee dances communicate distances measured by optic flow. Nature 411, 581 (doi:10.1038/35079072) [DOI] [PubMed] [Google Scholar]
- Frisch K. V.1967The dance language and orientation of bees Cambridge, MA: Harvard University Press [Google Scholar]
- Marden J. H., Waddington K. D.1981Floral choices by honeybees in relation to the relative distances to flowers. Physiol. Entomol. 6, 431–435 (doi:10.1111/j.1365-3032.1981.tb00658.x) [Google Scholar]
- Seeley T. D.1994Honey bee foragers as sensory units of their colonies. Behav. Ecol. Sociobiol. 34, 51–62 (doi:10.1007/BF00175458) [Google Scholar]
- Seeley T. D.1995The wisdom of the hive Cambridge, MA: Harvard University Press [Google Scholar]
- Srinivasan M. V., Zhang S. W., Altwein M., Tautz J.2000Honeybee navigation: nature and calibration of the ‘odometer’. Science 287, 851–853 (doi:10.1126/science.287.5454.851) [DOI] [PubMed] [Google Scholar]
- Wittlinger M., Wehner R., Wolf H.2006The ant odometer: stepping on stilts and stumps. Science 312, 1965–1967 (doi:10.1126/science.1126912) [DOI] [PubMed] [Google Scholar]