Abstract
Nesting behaviour is critical for reproductive success in oviparous organisms with no parental care. In organisms where sex is determined by incubation temperature, nesting behaviour may be a prime target of selection in response to unbalanced sex ratios. To produce an evolutionary change in response to sex-ratio selection, components of nesting behaviour must be heritable. We estimated the field heritability of two key components of nesting behaviour in a population of painted turtles (Chrysemys picta) with temperature-dependent sex determination by applying the ‘animal model’ to a pedigree reconstructed from genotype data. We obtained estimates of low to non-detectable heritability using repeated records across all environments. We then determined environment-specific heritability by grouping records with similar temperatures for the winter preceding the nesting season, a variable known to be highly associated with our two traits of interest, nest vegetation cover and Julian date of nesting. The heritability estimates of nest vegetation cover and Julian date of nesting were qualitatively highest and significant, or nearly so, after hot winters. Additive genetic variance for these traits was not detectable after cold winters. Our analysis suggests that the potential for evolutionary change of nesting behaviour may be dependent on the thermal conditions of the preceding winter, a season that is predicted to be especially subject to climate change.
Keywords: Chrysemys, phenology, G × E, climate change, animal model, heritability
1. Introduction
Nesting behaviour is a major factor in determining maternal fitness in oviparous species (Weisrock & Janzen 1999; Reguera & Gomendio 2002). Poor nest-site choice can result in increased predation (Sargent & Gebler 1980; Hatchwell et al. 1996; Downes & Shine 1999; Kolbe & Janzen 2001), reduced hatching success (Cagle et al. 1993; Wilson 1998; Warner & Andrews 2002) and reduced offspring fitness (Shine & Brown 2002; Patterson & Blouin-Demers 2008). Thus, for species with no maternal care after oviposition, finding a suitable nest site is especially critical (Kolbe & Janzen 2001; Blouin-Demers et al. 2004; Hughes & Brooks 2006).
Still, nesting behaviour probably represents a compromise that balances the cost of finding an appropriate oviposition site while balancing with factors affecting maternal and offspring fitness (Thompson 1988; Tucker et al. 1999; Spencer 2002; Spencer & Thompson 2003). As a result, selection may act on multiple components of nesting behaviour, yet the genetic architecture of this complex trait has received little attention (but see Singer et al. 1988; Fox et al. 2004).
Maternal nest-site choice is central in theoretical explanations of the evolution, adaptive value and maintenance of temperature-dependent sex determination (TSD; Bulmer & Bull 1982; Bull et al. 1988; Roosenburg 1996; Reinhold 1998; Roosenburg & Niewiarowski 1998; but see Valenzuela & Janzen 2001; Morjan & Janzen 2003). With TSD, the thermal environment of the nest during incubation determines sex, rather than a genotypic cue at conception (Janzen & Paukstis 1991). This form of sex determination is widely distributed among reptile lineages and has been maintained throughout rapid climatic upheavals such as the Cretaceous–Tertiary boundary (Rage 1998; Janzen & Krenz 2004; Organ & Janes 2008). The relative responses to selection in restoring equilibrium sex ratios during these periods (Fisher 1930) are unknown for specific traits in wild populations of TSD species (Bulmer & Bull 1982). Nest-site choice in TSD species has been hypothesized to have a more dominant role in the evolutionary response to sex-ratio bias than the thermal sensitivity of the sex determination pathway (Bulmer & Bull 1982; Bull et al. 1988; Doody et al. 2006), as maternal nest placement in extreme microclimates may override variation for the thermal sensitivity of the actual sex determination pathway (Bull et al. 1982). Previous research has documented that geographical variation in nest site microclimate occurs, indicating that local adaptation is possible (Ewert et al. 2005; Doody et al. 2006). Further, much phenotypic variation exists for nest placement, as females can also alter the incubation temperature experienced by embryos by digging deeper or shallower nests or nesting at different times during the season (Georges 1992; Morjan 2003b; Doody et al. 2006; Schwanz & Janzen 2008).
An important corollary to the hypothesis that nesting behaviour plays a role in the response to selection against sex-ratio bias in TSD species is that nest-site choice has a heritable basis (Bulmer & Bull 1982; Bull et al. 1988), but few studies have tested this possibility. In the laboratory, nest-site choice in the leopard gecko (Eublepharis macularius) exhibited a repeatability of less than 0.20 (i.e. upper bound of heritability; Bull et al. 1988). In the field, overstorey vegetation cover provides a stable cue for nesting turtles that is predictive of nest sex ratio (Janzen 1994b). Measures of repeatability of nest overstorey vegetation cover in the painted turtle (Chrysemys picta) and hawksbill turtle (Eretmochelys imbricata) are different but significant in both cases (repeatability = 0.18–0.20 and 0.7, respectively; no standard errors given in either reference; Janzen & Morjan 2001; Kamel & Mrosovsky 2005). Moreover, adjusting nesting phenology could permit a female to regulate the sex ratio of a clutch, although this trait is not known to be repeatable (repeatability = 0.03 for C. picta; Schwanz & Janzen 2008). While these studies provide some insight into the inheritance of nesting behaviour in TSD species, estimates of the heritability for temporal or spatial nest-site choice in the field are lacking.
Estimating the additive genetic variation underlying nesting behaviour over repeated measures for a single female is potentially complicated by individual plasticity and changes in additive genetic and environmental variance across different years (Charmantier & Garant 2005; Nussey et al. 2007; Brommer et al. 2008). Indeed, the average date of first nesting in a population of painted turtles is correlated with September–April temperatures prior to the nesting season, and individual linear reaction norms vary significantly in slope (significant individual-by-environment interaction (I × E); Schwanz & Janzen 2008). Traits of this nature, with among-individual variability in response to an environmental variable, are excellent systems for investigating environment-specific heritability and genotype-by-environment interactions (G × E), which are essential to more accurately assess the microevolutionary response to climate change (Via et al. 1995; Nussey et al. 2007; Gienapp et al. 2008; Uller 2008).
We estimated the field heritability of Julian date of nesting and nest vegetation cover for a wild population of painted turtles by applying the ‘animal model’. The animal model is a mixed model that estimates the contribution of additive genetic and environmental variance components of a trait's phenotypic variance with restricted maximum likelihood (Lynch & Walsh 1998). This approach is especially well suited for estimating variance components in natural populations because it can use incomplete (i.e. unbalanced) datasets, information across generations without a breeding design and can incorporate repeated measures from the same individuals (Kruuk 2004). We determined the heritability of nesting behaviour following different winter environments to elucidate potential genotype-by-environment interactions for a long-studied population of turtles with TSD (e.g. Via et al. 1995; Nussey et al. 2007).
2. Material and methods
(a). Field data collection
Chrysemys picta ranges from southern Canada to northern Mexico (Ernst et al. 1994; Starkey et al. 2003). Data were collected from a well-characterized population of painted turtles at the Thomson Causeway Recreation Area (TCRA) along the Mississippi River near Thomson, IL, USA (Janzen 1994a,b; Schwanz & Janzen 2008). We focused on the southeast portion of the island where the nesting beach is a level grassy area, soil moisture is relatively uniform (Janzen 1994b) and variable levels of overstorey vegetation cover are present (Morjan 2003a). In this population, clutch sex ratios have been evaluated over the past 20 years (Janzen 1994b; F. J. Janzen 2009, unpublished data). Females in this population mature in 5–7 years (Morjan 2003a) and oviposit one to three clutches from late May to early July.
From 1995 to 2008, the nesting grounds were monitored from 06.00 to 20.00 during the May–July nesting season. Turtles in this population typically emerge from the water, nest and return to the water within 2 h, and nearly all nesting events were observed. Nesting turtles were marked uniquely, and a blood sample was collected from the post-cranial sinus using a 28 ga insulin syringe. The sample was preserved in lysis buffer and stored in liquid nitrogen or at −20°C.
(b). Molecular markers
Genotypes of five microsatellite loci (GmuD21, GmuD28, GmuD62, GmuD70, GmuD79; King & Julian 2004) were obtained from 435 nesting females using standard molecular techniques (electronic supplementary material). All homozygotes and any ambiguous alleles were rerun for confirmation, resulting in greater than half of the dataset being evaluated at least twice to confirm the genotype. Each of the amplified loci contained a four base-pair repeat motif and was hypervariable (number of alleles: GmuD21, 17; GmuD28, 18; GmuD62, 22; GmuD70, 60; GmuD79, 27). The dataset contained no missing data. GenAlex v. 6.0 determined that these five loci provided exclusionary probability of greater than 0.9999 when neither parent is known (Jamieson & Taylor 1997; Peakall & Smouse 2006).
Analysis using GenePop v. 4.0 with the default parameters (Raymond & Rousset 1995) indicated that GmuD28 and GmuD70 significantly deviated from Hardy–Weinberg equilibrium and linkage between GmuD28 and several loci was evident (tables S1 and S2 in the electronic supplementary material). We interpreted the heterozygote deficiencies and high error rates in Gmu28 and GmuD70 (electronic supplementary material) to reflect the presence of null alleles and specified that these loci had null alleles when reconstructing genealogies. Reconstruction of relationships omitting GmuD28 resulted in more relationships being identified; therefore, we took the conservative approach to our pedigree reconstruction and left this locus in the analysis to provide higher exclusion power.
(c). Pedigree reconstruction
All pedigree links were inferred mainly from genotypic data in this study for two reasons. First, chelonian reproductive biology (e.g. sperm storage across years and multiple paternity within clutches; Pearse et al. 2001, 2002) makes pedigree reconstruction solely from field observations nearly impossible. Second, high mortality from hatching to reproductive maturity (estimated annual juvenile survivorship is 21–51%; Ernst et al. 1994) renders uniquely marking individuals at the neonate stage time- and cost-inefficient. For these reasons, relationships cannot be derived solely from field observations.
The parent–offspring (PO) and sibling relationships were determined as the most likely relationship between a pair of individuals as deduced from genotype data by maximum likelihood with ML-Relate (Kalinowski et al. 2006; Wagner et al. 2006). We refined PO pairs initially identified by ML-Relate by creating an enriched dataset that contained only female pairs with 5 years (the shortest time to maturity for females in this population) or more between each of their first recorded nesting events. Requiring this time between first recorded nesting events may have removed some full-sib (FS) or half-sib (HS) pairs that were misclassified as PO. These field observations also provided unambiguous assignment of which individuals were the parent and which were the offspring. Any offspring with multiple individuals classified as being their parent were removed from the final pedigree (n = 39), as these probably represent false positives. For females with multiple offspring, the putative siblings were confirmed to be FS or HS by examining their most likely relationship from ML-Relate. If these relationships were not concordant, the offspring was removed (n = 3). In all, 77 PO links (58 females with 77 offspring) were identified. Nineteen females had multiple offspring resulting in 10 FS links and 15 HS links included in the pedigree. All relationship designations were consistent with the genetic data at a 0.05 significance level in ML-Relate. Likelihood-ratio tests (LRTs) indicated that each PO and FS designation was significant compared with the null hypothesis of no relationship (p < 0.005 in all PO links, p < 0.040 in all FS links). While LRT indicated that only 10 HS designations were significant at α = 0.05, all shared a maternal link that was significant by LRT and consistent with the genetic data at a 0.05 significance level.
(d). Traits of interest
We evaluated both onset of nesting and nest vegetation cover as crucial measures of nesting behaviour. Onset of nesting was measured by recording the Julian date for the first nesting event of the season for each female from 1995–2008. Our total dataset for onset of nesting contained 1965 first nesting events of the season from 631 females (mean nesting events per female = 3.11, range = 1–12).
Nest vegetation cover was determined using a spherical densitometer (Janzen 1994b; Weisrock & Janzen 1999). The percentage of south and west cover was summed to obtain a single vegetation cover measurement for each nest. This measure is used here, as opposed to total vegetation cover from all cardinal directions, because it is more strongly correlated with the nest sex ratio (Janzen 1994b). Our total dataset for nest vegetation cover contained 2212 (1676 first nests of the season, 536 second nests of the season) nesting events from 631 females (mean nesting events per female = 3.51, range = 1–18). Vegetation cover was collected from 1995–2008, excluding 2004 and 2005 because a different measure of vegetation cover was used in those years.
To measure winter environment, heating degree days (HDD) for September through April preceding the nesting season were used (for HDD calculation, see Schwanz & Janzen 2008). Climate data for Clinton, Iowa (10 km from the TCRA), were obtained from the National Climate Data Center (NCDC; http://www.ncdc.noaa.gov). HDD values are the sum of the difference in Fahrenheit between each daily mean temperature and the base temperature (base temperature 65°F for NCDC data, or approximately 18°C) for days where the mean is less than the base temperature. Low HDD values represent warmer winters because less heating was required to reach the base temperature.
Julian nest date and vegetation cover deviated from a normal distribution (all Shapiro–Wilks p < 0.050). However, the deviations were minimal (figures S1 and S2 in the electronic supplementary material), so both traits were used without transformation in statistical genetic analyses because the animal model is fairly robust to slight deviations from normality (Kruuk 2004). For both traits, the only genetic data included were pedigree links mentioned in the above section.
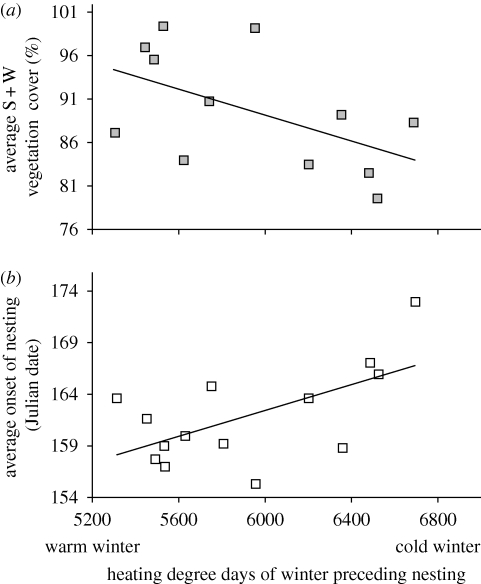
The average first nesting date of the season and the average nest vegetation cover in this population of painted turtles are significantly correlated, or nearly so, with the temperature of the winter preceding the nesting season (figure 1; date: r = 0.60, d.f. = 13, pdate < 0.02, r = −0.54, d.f. = 11, pveg < 0.07), and the relative influence of winter temperatures on nesting date is variable across females (Schwanz & Janzen 2008). Given that this population has significant individual-by-environment interactions for date (I × E; Schwanz & Janzen 2008), we explored whether heritability changed across winter environments by binning winter environments and treating each binned environment as a separate trait. Specifically, measurements from the four coldest, four hottest and four mid-temperature years of data collection were lumped as separate traits so that the new traits consisted of date and vegetation cover measures for each of the three individual environments. This binning strategy allowed the finest scale subsetting of the environment that preserved enough power to estimate heritability and maintained a consistent number of winters in each bin. Halving the data into only two bins did not improve the power for estimating heritability. These six traits are hereafter referred to as ‘environment traits’. Table 1 lists the number of individuals and records used to estimate heritability for these environment traits.
Figure 1.
Relationship between mean nesting trait values in each nesting season of the painted turtle, C. picta, in Illinois, USA, and the HDD from September to April preceding the nesting season (Schwanz & Janzen 2008). (a) average percentage south + west vegetation cover of first nest laid by females in different years in response to HDD (y = −0.0075x + 134.52, R2 = 0.289, p < 0.07). (b) Average Julian nest date of the first nest laid by females in different years in response to HDD (y = 0.0063x + 124.94, R2 = 0.364, p < 0.02).
Table 1.
Repeatability compared with heritability calculated by a univariate animal model for onset of nesting and south + west vegetation cover over a nest for female painted turtles, C. picta, from Illinois, USA. Reconstruction of PO pairs, FS and HS links by the maximum-likelihood analysis of genotypes was used as a pedigree. Each row represents a separate univariate analysis. All records available were fitted by the animal model for the ‘total’ dataset and other measures represent environment-specific analyses. The number of individuals (n) for which records were available is subscripted to the number of records. The subscript number in the ‘mean’ column is the raw phenotypic variance for that trait. For vegetation cover after mid-temperature winters, additive genetic variance had to be constrained to be positive, and so this analysis was essentially uninformative (denoted by ‘n.a.’). χ2 represents twice the likelihood difference between a model that contained only the individual effect and the one that also contained an additive genetic effect. The p-value is the result of the LRT and is corrected via the method suggested by Visscher (2006).
| records | mean | VA | repeatability | heritability | χ2 | p | |
|---|---|---|---|---|---|---|---|
| Julian date of the first nest of the season | |||||||
| total dataset | 1965(n = 631) | 161.55(86.04) | 0 | 0.060 (0.026, 0.095) | 0 | 0 | n.a. |
| cold winters only | 395(n = 277) | 166.67(51.62) | 0.038 (−0.078, 0.154) | 0.022 (−0.080, 0.124) | 0.060 (−0.131, 0.250) | 0.220 | 0.320 |
| mid winters only | 531(n = 373) | 158.95(95.57) | 0.084 (−0.047, 0.215) | 0.057 (−0.083, 0.197) | 0.103 (−0.073, 0.278) | 1.054 | 0.152 |
| hot winters only | 693(n = 405) | 160.72(83.07) | 0.131 (0.029, 0.236) | 0.118 (0.014, 0.222) | 0.166 (0.020, 0.313) | 2.138 | 0.072 |
| south + west vegetation cover of the nest | |||||||
| total dataset | 2212(n = 631) | 89.46(1834.375) | 0.043 (−0.126, 0.212) | 0.140 (0.097, 0.182) | 0.043 (−0.126, 0.211) | 0.180 | 0.336 |
| cold winters only | 531(n = 298) | 83.60(1799.968) | n.a. | 0.260 (0.146, 0.374) | n.a. | 0 | 1 |
| mid winters only | 792(n = 444) | 89.28(1807.729) | n.a. | 0.072 (−0.021, 0.166) | n.a. | 0 | 1 |
| hot winters only | 889(n = 421) | 93.12(1848.79) | 0.189 (0.098, 0.280) | 0.181 (0.097, 0.266) | 0.188 (0.104, 0.271) | 2.916 | 0.044 |
(e). Heritability calculations
The animal model was run in ASReml v. 2.0 to determine the heritability of nesting behaviour (Kruuk 2004; Gilmour et al. 2006). In all models, random effects included year, ‘animal’ (which is referenced back to the pedigree) and a term accounting for permanent environmental effects (pe). Permanent environmental effects represent non-additive genetic between-individual variation accompanying multiple measures per individual (Lynch & Walsh 1998). Clutch order in the season for each nest was included as a fixed effect in the model for vegetation cover (Wald test, p = 0.61–0.85 depending on animal model variation used). In all ASReml runs, starting values for variance and covariance were calculated from the original dataset and refined by using output values from runs as new starting values. Residual plots were examined for normality in ASReml.
Two variations of the animal model were used to evaluate the quantitative genetics of nesting date and vegetation cover. (i) Two univariate animal models (one for date and one for vegetation cover) used a pedigree reconstructed from molecular marker data and field observations to estimate the overall heritability of date and vegetation cover from all available data points (Kruuk 2004). In this model, random effects included year of nesting and additive genetic and permanent environmental terms. The permanent environmental effect for the analysis of date was constrained to be positive, as it was otherwise estimated by ASReml to be negative. For vegetation cover only, clutch order was included as a fixed effect. LRTs evaluated whether a model with the additive genetic effect fit the data significantly better than the one that contained only the effect of individual identity. LRTs are conservative when the additional model parameter is bound by zero (e.g. additive genetic variance); thus, the p-values of the LRTs were halved as the distribution of the LRTs should be an equal mix of χ2 distributions with one degree of freedom and zero degrees of freedom (Visscher 2006). (ii) The measurements for date and vegetation cover were binned by similar winter environments, as described above, and treated as separate traits. These environment traits were separately used as response variables in univariate animal models with the same random and fixed effects as in the first model. In these environment-specific analyses, permanent environmental effects were constrained to be positive if they were estimated as negative regardless of the starting values. As above, LRTs, implemented as in Visscher (2006), evaluated whether a model that contained the additive genetic effect fit the data significantly better than one that did not. Attempts to fit multivariate animal models using two environment traits (Via et al. 1995) or sophisticated models, such as the random regression animal model or character process models, to estimate G × E (reviewed in Jaffrézic & Pletcher 2000; Nussey et al. 2007) were unsuccessful owing to the low power of the pedigree or the lack of detectable additive genetic variance for one or more environment traits (table S3 in the electronic supplementary material).
Because our estimates of heritability are based purely on a pedigree reconstructed from genotypes, we also calculated repeatability for each of the six environment traits and for the total datasets of date and vegetation cover (Lessells & Boag 1987). Repeatability is simply a measure of the degree of self-similarity for nesting behaviour, and this measure is independent of any pedigree designations (Lessells & Boag 1987; Dohm 2002). We used mixed models identical to models described above, except they did not include the additive genetic effect. Repeatability was measured here as the sum of the individual variance components (i.e. pe + additive genetic) over the sum of all variance components as calculated by ASReml (i.e. pe + additive genetic + year + residuals all conditioned on the fixed effect of clutch in the vegetation cover analysis). Concordance between environment-specific heritability and environment-specific repeatability in direction and magnitude would bolster our conclusions from the animal model analyses.
3. Results
LRTs indicated that, in most cases, models including an additive genetic effect did not fit the data significantly better than analogous models only including individual identity (table 1). For the univariate analyses of date and nest vegetation cover, including all records, heritability estimates were very small (h2date = 0, h2veg = 0.043, 95% CI = −0.126, 0.211). But much of the repeatability for date (date = 0.060, 95% CI = 0.026, 0.095) and vegetation cover (vegetation cover = 0.140, 95% CI = 0.097, 0.182) was due to permanent environmental effects (σ2 pe/σ2 total; date = 0.0604, 95% CI = 0.026, 0.095; vegetation cover = 0.0972, 95% CI = −0.075, 0.270).
Heritability of both nesting date and vegetation cover was qualitatively highest following hot winters (date h2=0.166, 95% CI = 0.020, 0.313; vegetation cover h2=0.188, 95% CI = 0.104, 0.271), but nearly undetectable after cold winters (table 1). A model including additive genetic variance for nest vegetation cover after hot winters was significantly more likely than the one that did not (table 1; χ2 = 2.916, p = 0.044). The additive genetic variance for the onset of nesting after hot winters was marginally non-significant (table 1; χ2 = 2.138, p = 0.072). The confidence intervals for environment-specific heritability estimates overlapped with the overall analysis and other environment-specific analyses (table 1); thus, there are no statistically significant differences in additive genetic variance across environment traits. The repeatability estimates were generally concordant with environment-specific heritability estimates (table 1).
4. Discussion
Nesting behaviour is a key component of individual fitness in oviparous organisms, yet little is known about its inheritance in free-ranging animals. Our study sought to quantify the additive genetic variance underlying aspects of nesting behaviour in a natural population of turtles with TSD. By applying a reconstructed pedigree to the animal model, we estimated heritability for female preference for Julian date of nesting (i.e. date) and south + west overstorey vegetation cover. Our results revealed that, when measured over many different winter environments, both onset of nesting and nest-site vegetation cover have low or undetectable heritability and that heritability may be environment-specific in this system. Our study also reflects the difficulty of achieving sufficient statistical power when studying quantitative genetics of traits in wild populations, and much of our interpretation is tentative.
Our assessment, using all records across all winter environments, detected very low levels of heritability for first nesting date and vegetation cover. Likewise, traits associated with oviposition behaviour in other systems have been estimated to have low heritability (e.g. brood mass weight in dung beetles (Hunt & Simmons 2002); oviposition behaviour in crickets (Réale & Roff 2002); oviposition preference in seed beetles (Fox et al. 2004)). Upon closer inspection, environment-specific analyses revealed that winter temperature prior to the nesting season might influence the potential for the evolutionary change of nesting behaviour. Although future years of data and additional pedigree links may refine these estimates, preliminarily these data suggest that more additive genetic variance for onset of nesting and nest vegetation cover may exist after hot winters than after cold winters (figure 1, table 1). In fact, we detected significant, or nearly significant, heritability for nest vegetation cover and onset of nesting after hot winters (table 1). Thus, after hot winters, there is substantial additive genetic variance for early nesting and high amounts of nest vegetation cover (figure 1).
Our study contributes to a broader body of work on the response of nesting date to climatic conditions. Some evidence of G × E of nesting date across different climatic conditions has been found in the collared flycatcher (Ficedula albicollis), a Dutch population of great tits (Parus major), and the common gull (Larus canus) (Brommer et al. 2005, 2008; Nussey et al. 2005). Yet, no individual variation in laying date response to temperature was found in a UK population of great tits or in the common guillemot (Uria aalge) (Reed et al. 2006; Charmantier et al. 2008). Two factors hypothesized to influence G × E in these systems include: (i) stabilizing selection on the correspondence of time of highest food provisioning to young to the season's highest food abundance (Nussey et al. 2005; Charmantier et al. 2008) and (ii) maintaining population-level breeding synchrony. In our system, however, timing of resource abundance and reproductive synchrony most probably do not apply, as females do not provision offspring after oviposition, and hatchlings overwinter in the nest without feeding and emerge the following spring (Weisrock & Janzen 1999). Earlier nesting turtles, however, do have a higher probability of laying subsequent clutches in the season than late nesters (Schwanz & Janzen 2008; Tucker et al. 2008). So, by different means, advancing nesting date may confer a fitness advantage at least equivalent to that for the bird populations.
Overall, our study suggests that past theoretical work that predicted the relative roles of nest-site choice and thermal sensitivity of the sex determination pathway in the response of TSD to sex-ratio biases may have insufficiently appreciated the complexity of inheritance in this system (Bull et al. 1982; Bulmer & Bull 1982; Bull et al. 1988; Morjan 2003a). If environment-specific heritability exists in this system, as suggested by our quantitative genetic analyses, nest-site choice and thermal sensitivity of sex determination may each respond more efficiently to sex-ratio bias in different situations. For instance, under predicted climate warming, warmer nests may overproduce females (e.g. Janzen 1994a; Rage 1998; Morjan 2003a; Doody et al. 2006). Substantial heritability in nesting date after hotter winters may allow a greater evolutionary response to selection. However, simply advancing nesting date is unlikely to correct sex-ratio bias (Schwanz & Janzen 2008), thus it is unlikely that selection on offspring sex would manifest solely as selection on nesting date. Alternatively, we have detected significant additive genetic variance for increased levels of nest vegetation cover after hot winters, suggesting that female vegetation cover preference may respond to selection in a climate-warming scenario. The relative lack of additive genetic variance detected after cold winters indicates that selection on nest-site choice would not be efficient in a climate-cooling scenario, and we hypothesize that selection on the thermal sensitivity of the sex-determination pathway may be more capable of a response to sex-ratio bias in that context (sensu Morjan 2003a).
Our study must be interpreted with several considerations. First, if unaccounted for factors that contribute to phenotypic variance, such as non-additive maternal or common environment effects, the model might yield inaccurate additive genetic variance estimates. Since our pedigree analysis consisted mainly of PO relationships, teasing apart the relative contributions of imprinting and maternal genetic effects remains difficult (Kruuk 2004; Kruuk & Hadfield 2007). Imprinting in this population, though, was previously dismissed as an explanation for repeatable nesting behaviour (Janzen & Morjan 2001; Morjan 2003a). We also expect any common environmental effects to be negligible, as the probability of any two individuals sharing common nest environments (i.e. the frequency of siblings and half-siblings in the dataset) is small. Further, owing to sperm storage and multiple paternity (Pearse et al. 2001, 2002), even these molecularly identified siblings may have been laid in different nests within or even between years. Second, our study employed a relatively low number of microsatellite loci, and two of these loci exhibited problems with null alleles. Yet, the number of alleles per locus was high, the pedigree reconstruction methods accounted for null alleles and no genotypes were missing in the dataset, which allowed utilization of the full power indicated by the exclusion analysis. We generated a fairly conservative pedigree by using field data and significance testing to validate the relationship designations. Lastly, datasets for each environment trait contained a different number of phenotypic records per animal and our heritability estimates may simply reflect different variance partitioning by the animal model in response to these varied data structures (e.g. allocation of variance to permanent environmental effects rather than additive genetic variance, or vice versa). If this scenario were true, it is likely that our conclusions regarding the high heritability for nesting behaviour after hot winters and practically non-existent heritability calculated from the total dataset would remain valid.
Importantly, the repeatability estimates, which are independent of the pedigree, exhibited a relatively similar magnitude and pattern of difference across environments. Thus, our conclusions concerning the potential significance of the heritability of nesting behaviour are strengthened. In conclusion, it is likely that future predictive models of the microevolution of TSD in response to sex-ratio bias, including those relevant to conservation actions involving the many imperilled species with TSD, may be more informative if the complexity of environment-specific heritability and G × E were more thoroughly investigated.
Acknowledgements
D. Garrick furnished invaluable guidance. K. Meyer, J. Dekkers, F. Frentiu, G. Page, J. Brommer, L. Rönnegård and L. Flagel provided bioinformatics assistance and useful discussion for earlier versions of this manuscript. H. Gao, X. Liu and K. Wikstrom helped genotype turtles. The Janzen and Bronikowski labs, Alastair Wilson and an anonymous reviewer provided comments on earlier versions of the manuscript. Special thanks to the Turtle Camp crews over many years for essential field data collection. This work was supported by NSF grants DEB-9629529, DEB-0089680 and DEB-0640932 to F.J.J. and IBN-0212935 to F.J.J. and R.M.B. Collections were made with permission from the US Army Corps of Engineers, under annual scientific collecting permits from the Illinois Department of Natural Resources, and annually approved IACUC protocols from Iowa State University to F.J.J. S.E.M. was supported by a Graduate Research Fellowship from the NSF.
References
- Blouin-Demers G., Weatherhead P. J., Row J. R.2004Phenotypic consequences of nest-site selection in black rat snakes (Elaphe obsoleta). Can. J. Zool. 82, 449–456 (doi:10.1139/z04-014) [Google Scholar]
- Brommer J. E., Merilä J., Sheldon B. C., Gustafsson L.2005Natural selection and genetic variation for reproductive reaction norms in a wild bird population. Evolution 59, 1362–1371 (doi:10.1111/j.0014-3820.2005.tb01785.x) [PubMed] [Google Scholar]
- Brommer J. E., Rattiste K., Wilson A. J.2008Exploring plasticity in the wild: laying date–temperature reaction norms in the common gull Larus canus. Proc. R. Soc. B. 275, 687–693 (doi:10.1098/rspb.2007.0951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull J. J., Vogt R. C., Bulmer M. G.1982Heritability of sex ratio in turtles with environmental sex determination. Evolution 36, 333–341 (doi:10.2307/2408052) [DOI] [PubMed] [Google Scholar]
- Bull J. J., Gutzke W. H. N., Bulmer M. G.1988Nest choice in a captive lizard with temperature-dependent sex determination. J. Evol. Biol. 2, 177–184 (doi:10.1046/j.1420-9101.1988.1020177.x) [Google Scholar]
- Bulmer M. G., Bull J. J.1982Models of polygenic sex determination and sex ratio control. Evolution 36, 13–26 (doi:10.2307/2407962) [DOI] [PubMed] [Google Scholar]
- Cagle K., Packard G., Miller K., Packard M.1993Effects of the microclimate in natural nests on development of embryonic painted turtles, Chrysemys picta. Funct. Ecol. 7, 653–660 (doi:10.2307/2390185) [Google Scholar]
- Charmantier A., Garant D.2005Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B. 272, 1415–1425 (doi:10.1098/rspb.2005.3117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmantier A., McCleery R., Cole L. R., Perrins C., Kruuk L. E. B., Sheldon B. C.2008Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- Dohm M. R.2002Repeatability estimates do not always set an upper limit to heritability. Funct. Ecol 16, 273–280 [Google Scholar]
- Doody J. S., Guarino E., Georges A., Corey B., Murray G., Ewert M.2006Nest site choice compensates for climate effects on sex ratios in a lizard with environmental sex determination. Evol. Ecol. 20, 307–330 (doi:10.1007/s10682-006-0003-2) [Google Scholar]
- Downes S. J., Shine R.1999Do incubation-induced changes in a lizard's phenotype influence its vulnerability to predators? Oecologia 120, 9–18 (doi:10.1007/s004420050827) [DOI] [PubMed] [Google Scholar]
- Ernst C. H., Lovich J. E., Barbour R. W.1994Turtles of the United States and Canada. Washington, DC: Smithsonian Institution Press [Google Scholar]
- Ewert M. A., Lang J. W., Nelson C. E.2005Geographic variation in the pattern of temperature-dependent sex determination in the American snapping turtle (Chelydra serpentina). J. Zool. Lond. 265, 81–95 (doi:10.1017/S0952836904006120) [Google Scholar]
- Fisher R. A.1930The genetical theory of natural selection Oxford, UK: Clarendon Press [Google Scholar]
- Fox C. W., Stillwell R. C., Amarillo-S A. R., Czesak M. E., Messina F. J.2004Genetic architecture of population differences in oviposition behaviour of the seed beetle Callosobruchus maculatus. J. Evol. Biol. 17, 1141–1151 (doi:10.1111/j.1420-9101.2004.00719.x) [DOI] [PubMed] [Google Scholar]
- Georges A.1992Thermal-characteristics and sex determination in field nests of the pig-nosed turtle, Carettochelys insculpta (Chelonia, Carettochelydidae), from northern Australia. Aust. J. Zool. 40, 511–521 (doi:10.1071/ZO9920511) [Google Scholar]
- Gienapp P., Teplitsky C., Alho J. S., Mills J. A., Merilä J.2008Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178 (doi:10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- Gilmour A. R., Gogel B. J., Cullis B. R., Thompson R.2006ASReml user guide release 2.0 Hemel Hempstead, UK: VSN International Ltd [Google Scholar]
- Hatchwell B. J., Chamberlain D. E., Perrins C. M.1996The reproductive success of blackbirds Turdus merula in relation to habitat structure and choice of nest site. Ibis 138, 256–262 (doi:10.1111/j.1474-919X.1996.tb04337.x) [Google Scholar]
- Hughes E. J., Brooks R. J.2006The good mother: does nest-site selection constitute parental investment in turtles? Can. J. Zool. 84, 1545–1554 (doi:10.1139/Z06-148) [Google Scholar]
- Hunt J., Simmons L. W.2002The genetics of maternal care: direct and indirect genetic effects on phenotype in the dung beetle Onthophagus taurus. Proc. Natl Acad. Sci. USA 99, 6828–6832 (doi:10.1073/pnas.092676199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrézic F., Pletcher S. D.2000Statistical models for estimating the genetic basis of repeated measures and other function-valued traits. Genetics 156, 913–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A., Taylor C. S., St1997Comparisons of three probability formulae for parentage exclusion. Anim. Genet. 28, 397–400 (doi:10.1111/j.1365-2052.1997.00186.x) [DOI] [PubMed] [Google Scholar]
- Janzen F. J.1994aClimate change and temperature-dependent sex determination in reptiles. Proc. Natl Acad. Sci. USA 91, 7487–7490 (doi:10.1073/pnas.91.16.7487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen F. J.1994bVegetational cover predicts the sex ratio of hatchling turtles in natural nests. Ecology 75, 1593–1599 (doi:10.2307/1939620) [Google Scholar]
- Janzen F. J., Krenz J. G.2004Which was first, TSD or GSD? In Temperature-dependent sex determination in vertebrates (eds Valenzuela N., Lance V. A.), pp. 121–130 Washington, DC: Smithsonian Books [Google Scholar]
- Janzen F. J., Morjan C. L.2001Repeatability of microenvironment-specific nesting behaviour in a turtle with environmental sex determination. Anim. Behav. 62, 73–82 (doi:10.1006/anbe.2000.1732) [Google Scholar]
- Janzen F. J., Paukstis G. L.1991Environmental sex determination in reptiles: ecology, evolution, and experimental design. Q. Rev. Biol. 66, 149–179 (doi:10.1086/417143) [DOI] [PubMed] [Google Scholar]
- Kalinowski S. T., Wagner A. P., Taper M. L.2006ML-Relate: a computer program for maximum likelihood estimation of relatedness and relationship. Mol. Ecol. Notes 6, 576–579 (doi:10.1111/j.1471-8286.2006.01256.x) [Google Scholar]
- Kamel S. J., Mrosovsky N.2005Repeatability of nesting preferences in the hawksbill sea turtle, Eretmochelys imbricata, and their fitness consequences. Anim. Behav. 70, 819–828 (doi:10.1016/j.anbehav.2005.01.006) [Google Scholar]
- King T. L., Julian S.2004Conservation of microsatellite DNA flanking sequence across 13 emydid genera assayed with novel bog turtle (Glyptemys muhlenbergii) loci. Conserv. Genet. 5, 719–725 (doi:10.1007/s10592-004-1854-0) [Google Scholar]
- Kolbe J. J., Janzen F. J.2001The influence of propagule size and maternal nest-site selection on survival and behaviour of neonate turtles. Funct. Ecol. 15, 772–781 (doi:10.1046/j.0269-8463.2001.00587.x) [Google Scholar]
- Kruuk L. E. B.2004Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. Lond. B 359, 873–890 (doi:10.1098/rstb.2003.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk L. E. B., Hadfield J. D.2007How to separate genetic and environmental causes of similarity between relatives. J. Evol. Biol. 20, 1890–1903 (doi:10.1111/j.1420-9101.2007.01377.x) [DOI] [PubMed] [Google Scholar]
- Lessells C. M., Boag P. T.1987Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121 [Google Scholar]
- Lynch M., Walsh B.1998Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer [Google Scholar]
- Morjan C. L.2003aHow rapidly can maternal behaviour affecting primary sex ratio evolve in a reptile with environmental sex determination? Am. Nat. 162, 205–219 (doi:10.1086/376583) [DOI] [PubMed] [Google Scholar]
- Morjan C. L.2003bVariation in nesting patterns affecting nest temperatures in two populations painted turtles (Chrysemys picta) with temperature-dependent sex determination. Behav. Ecol. Sociobiol. 53, 254–261 (doi:10.1007/s00265-002-0570-3) [Google Scholar]
- Morjan C. L., Janzen F. J.2003Nest temperature is not related to egg size in a turtle with temperature-dependent sex determination. Copeia 2003, 366–372 (doi:10.1643/0045-8511(2003)003[0366:NTINRT]2.0.CO;2) [Google Scholar]
- Nussey D. H., Postma E., Gienapp P., Visser M. E.2005Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- Nussey D. H., Wilson A. J., Brommer J. E.2007The evolutionary ecology of plasticity in the wild. J. Evol. Biol. 20, 831–844 (doi:10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- Organ C. L., Janes D. E.2008Evolution of sex chromosomes in Sauropsida. Integr. Comp. Biol. 48, 512–519 (doi:10.1093/icb/icn041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson L. D., Blouin-Demers G.2008The effect of constant and fluctuating incubation temperatures of the phenotype of black rat snakes (Elaphe obsoleta). Can. J. Zool. 86, 882–889 (doi:10.1139/Z08-067) [Google Scholar]
- Peakall R., Smouse P. E.2006Genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6, 288–295 (doi:10.1111/j.1471-8286.2005.01155.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse D. E., Janzen F. J., Avise J. C.2001Genetic markers substantiate long-term storage and utilization of sperm by female painted turtles. Heredity 86, 378–384 (doi:10.1046/j.1365-2540.2001.00841.x) [DOI] [PubMed] [Google Scholar]
- Pearse D. E., Janzen F. J., Avise J. C.2002Multiple paternity, sperm storage, and reproductive success of female and male painted turtles (Chrysemys picta) in nature. Behav. Ecol. Sociobiol. 51, 164–171 (doi:10.1007/s00265-001-0421-7) [Google Scholar]
- Rage C.1998Latest Cretaceous extinctions and environmental sex determination in reptiles. Bull. Soc. Geol. France 169, 479–483 [Google Scholar]
- Raymond M., Rousset F.1995Genepop v. 1.2: population genetics software for exact tests and ecumenicism. J. Hered. 86, 248–249 [Google Scholar]
- Réale D., Roff D. A.2002Quantitative genetics of oviposition behaviour and interactions among oviposition traits in the sand cricket. Anim. Behav. 64, 397–406 (doi:10.1006/anbe.2002.3084) [Google Scholar]
- Reed T. E., Wanless S., Harris M. P., Frederiksen M., Kruuk L. E. B., Cunningham E.2006Responding to environmental change: plastic responses vary little in a synchronous breeder. Proc. R. Soc. B 273, 2713–2719 (doi:10.1098/rspb.2006.3631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera P., Gomendio M.2002Flexible oviposition behaviour in the golden egg bug (Phyllomorpha laciniata) and its implications for offspring survival. Behav. Ecol. 13, 70–74 (doi:10.1093/beheco/13.1.70) [Google Scholar]
- Reinhold K.1998Nest-site philopatry and selection for environmental sex determination. Evol. Ecol. 12, 245–250 (doi:10.1023/A:1006591914859) [Google Scholar]
- Roosenburg W. M.1996Maternal condition and nest site choice: an alternative for the maintenance of environmental sex determination? Am. Zool. 36, 157–168 (doi:10.1093/icb/36.2.157) [Google Scholar]
- Roosenburg W. M., Niewiarowski P.1998Maternal effects and the maintenance of environmental sex determination. In Maternal effects as adaptations (eds Mousseau T. A., Fox C. W.), pp. 307–322 Oxford, UK: Oxford University Press [Google Scholar]
- Sargent R. C., Gebler J. B.1980Effects of nest site concealment on hatching success, reproductive success, and paternal behaviour of the threespine stickleback, Gasterosteus aculeatus. Behav. Ecol. Sociobiol. 7, 137–142 (doi:10.1007/BF00299519) [Google Scholar]
- Schwanz L. E., Janzen F. J.2008Climate change and temperature-dependent sex determination: can individual plasticity in nesting phenology prevent extreme sex ratios? Physiol. Biochem. Zool. 81, 826–834 (doi:10.1086/590220) [DOI] [PubMed] [Google Scholar]
- Shine R., Brown G. P.2002Effects of seasonally varying hydric conditions on hatchling phenotypes of keelback snakes (Tropidonophis mairii, Colubridae) from the Australian wet–dry tropics. Biol. J. Linn. Soc. 76, 339–347 (doi:10.1046/j.1095-8312.2002.00068.x) [Google Scholar]
- Singer M. C., Ng D., Thomas C. D.1988Heritability of oviposition preference and its relationship to offspring performance within a single insect population. Evolution 42, 977–985 (doi:10.2307/2408913) [DOI] [PubMed] [Google Scholar]
- Spencer R.-J.2002Experimentally testing nest site selection fitness trade-offs and predation risk in turtles. Ecology 83, 2136–2144 (doi:10.1890/0012-9658(2002)083[2136:ETNSSF]2.0) [Google Scholar]
- Spencer R.-J., Thompson M. B.2003The significance of predation in nest site selection of turtles: an experimental consideration of macro- and microhabitat preferences. Oikos 102, 592–600 (doi:10.1034/j.1600-0706.2003.12436.x) [Google Scholar]
- Starkey D. E., Shaffer H. B., Burke R. L., Forstner M. R. J., Iverson J. B., Janzen F. J., Rhodin A. G. J., Ultsch G. R.2003Molecular systematics, phylogeography, and the effects of Pleistocene glaciation in the painted turtle (Chrysemys picta) complex. Evolution 57, 119–128 (doi:10.1111/j.0014-3820.2003.tb00220.x) [DOI] [PubMed] [Google Scholar]
- Thompson J. N.1988Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl. 47, 3–14 (doi:10.1007/BF00186709) [Google Scholar]
- Tucker J. K., Filoramo N. I., Janzen F. J.1999Size-biased mortality due to predation in a nesting freshwater turtle, Trachemys scripta. Am. Midl. Nat. 141, 198–203 (doi:10.1674/0003-0031(1999)141[0198:SBMDTP]2.0.CO;2) [Google Scholar]
- Tucker J. K., Paukstis G. L., Janzen F. J.2008Does predator swamping promote synchronous emergence of turtle hatchlings among nests? Behav. Ecol 19, 35–40 (doi:10.1093/beheco/arm097) [Google Scholar]
- Uller T.2008Developmental plasticity and the evolution of paternal effects. Trends Ecol. Evol. 23, 432–438 (doi:10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- Valenzuela N., Janzen F. J.2001Nest-site philopatry and the evolution of temperature-dependent sex determination. Evol. Ecol. Res. 3, 779–794 [Google Scholar]
- Via S., Gomulkiewicz R., de Jong G., Scheiner S. M., Schlichting C. D., Van Tienderen P. H.1995Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212–217 (doi:10.1016/S0169-5347(00)89061-8) [DOI] [PubMed] [Google Scholar]
- Visscher P. M.2006A note on the asymptotic distribution of likelihood ratio tests to test variance components. Twin Res. Hum. Genet. 9, 490–495 (doi:10.1375/twin.9.4.490) [DOI] [PubMed] [Google Scholar]
- Wagner A., Creel S., Kalinowski S.2006Estimating relatedness and relationships using microsatellite loci with null alleles. Heredity 97, 336–345 (doi:10.1038/sj.hdy.6800865) [DOI] [PubMed] [Google Scholar]
- Warner D. A., Andrews R. M.2002Nest-site selection in relation to temperature and moisture by the lizard Sceloporus undulatus. Herpetologica 58, 399–407 (doi:10.1655/0018-0831(2002)058[0399:NSIRTT]2.0.CO;2) [Google Scholar]
- Weisrock D. W., Janzen F. J.1999Thermal and fitness-related consequences of nest location in painted turtles (Chrysemys picta). Funct. Ecol. 13, 94–101 (doi:10.1046/j.1365-2435.1999.00288.x) [Google Scholar]
- Wilson D. S.1998Nest-site selection: microhabitat variation and its effects on the survival of turtle embryos. Ecology 79, 1884–1892 (doi:10.1890/0012-9658(1998)079[1884:NSSMVA]2.0.CO;2) [Google Scholar]