Abstract
As animals travel through the environment, powerful reflexes help stabilize their gaze by actively maintaining head and eyes in a level orientation. Gaze stabilization reduces motion blur and prevents image rotations. It also assists in depth perception based on translational optic flow. Here we describe side-to-side flight manoeuvres in honeybees and investigate how the bees’ gaze is stabilized against rotations during these movements. We used high-speed video equipment to record flight paths and head movements in honeybees visiting a feeder. We show that during their approach, bees generate lateral movements with a median amplitude of about 20 mm. These movements occur with a frequency of up to 7 Hz and are generated by periodic roll movements of the thorax with amplitudes of up to ±60°. During such thorax roll oscillations, the head is held close to horizontal, thereby minimizing rotational optic flow. By having bees fly through an oscillating, patterned drum, we show that head stabilization is based mainly on visual motion cues. Bees exposed to a continuously rotating drum, however, hold their head fixed at an oblique angle. This result shows that although gaze stabilization is driven by visual motion cues, it is limited by other mechanisms, such as the dorsal light response or gravity reception.
Keywords: vision, gaze stabilization, behaviour, visuomotor control, bee, flight control
1. Introduction
In order to safely find their way, animals need to acquire information about the three-dimensional layout of their environment. During translatory motion, visual motion signals can provide such depth information because images of close objects move faster across the retina than those of more distant objects. Insects are known to use the apparent velocity of nearby surfaces to detect objects during locomotion (Collett 1988; Lehrer et al. 1988; Pfaff & Varjú 1991; Kimmerle et al. 1996), and honeybees can even be trained to distinguish camouflaged figures by using motion parallax as a cue (Zhang et al. 1995). The specific pattern of retinal motion signals a moving animal experiences is determined by both the layout of the environment and the animal's behaviour (e.g. Gibson 1950; Lappe 2000; Dahmen et al. 2001). Therefore, seeing involves not only the passive take-up of information, or ‘vision while moving’ (Land & Collett 1997), but also the active generation and acquisition of visual information through highly structured movements, as has been shown for several insects that produce image motion patterns carrying motion parallax information (e.g. Wallace 1959; Horridge 1986; Sobel 1990; Collett & Paterson 1991; Lehrer 1991; Zeil et al. 1996; Kral & Poteser 1997; Voss & Zeil 1998).
The processing of depth information from motion parallax depends on the availability of relatively pure translational optic flow. Precise gaze stabilization is therefore crucial, as has been shown in locusts (Collett 1978) and blowflies (Kern et al. 2006). Although gaze stabilization has been studied in great detail using tethered insects (e.g. Goodman 1965; Land 1973; Stange 1981; Hengstenberg 1988; Gilbert et al. 1995; Pix et al. 2000; Maksimovic et al. 2007), only a few studies have done so in freely flying insects (Wehner & Flatt 1977; van Hateren & Schilstra 1999). Because insects cannot move their eyes within the head capsule, their gaze is determined by the orientation of the head relative to the external world. Blowflies have been shown to compensate roll and pitch movements of the thorax in flight by counter-rotations of the head relative to the thorax and to rapidly shift gaze by very fast saccadic head movements (Schilstra & van Hateren 1998). In dipteran flies, fast gaze stabilization is mainly achieved by mechanosensory input from halteres that act as gyroscopes (Sandeman & Markl 1980; Hengstenberg 1988). Honeybees, however, like many other insects, lack such specialized inertial sensors. How do they control gaze direction in flight? We describe here an optomotor reflex that uses visual motion to stabilize the head with respect to the visual environment.
2. Material and methods
(a). Experimental procedure and set-up
Our experimental honeybee colony (Apis mellifera L.) was housed in a hive mounted on the wall of a modified glasshouse (beehouse) in which the internal temperature was maintained at 24 ± 5°C during the day and 17 ± 3°C at night. The hive had two entrances that allowed bees to access both the outside and inside of the beehouse, where we performed our experiments. For each experiment, up to 20 bees were trained to collect sugar water (1 M) from a piece of cotton wool (feeder) inside the experimental apparatus (indicated by the star in figure 1a). Bees that continued to visit the feeder regularly were individually marked with non-toxic acrylic paint on thorax and abdomen. The ambient light intensity in the beehouse was continuously monitored by means of an upward-pointing luxmeter that integrates light over one hemisphere. Light intensity ranged between 15 000 and 20 000 lux in all experiments. During periods of low natural light intensity (mainly late in the afternoon), six DC-powered halogen lamps (50 W each) were switched on to keep the light level in the required range. The lamps were placed centrally above the set-up, providing a natural light gradient.
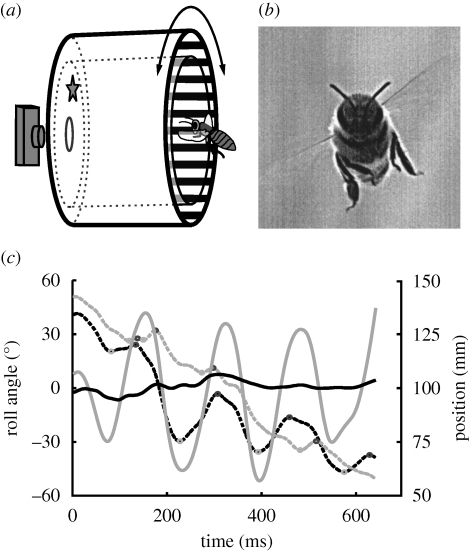
Figure 1.
Experimental set-up and example. (a) Schematic diagram of the experimental set-up: honeybees collected sugar water from a feeder (position indicated by a star) inside the experimental apparatus, which consisted of two nested 150-mm-long clear perspex drums (not drawn to scale). The inner drum (diameter: 115 mm) was stationary and prevented air currents that might have been generated by the movable outer drum from affecting the bees. The outer drum (diameter: 150 mm) was attached to a variable-speed electric motor and had a black and white striped pattern on the inside (18 mm stripe width, which corresponds to an apparent size of about 14° as viewed from the centre of the drum). (b) Frontal view of a honeybee entering the apparatus filmed with a high-speed digital video camera (1024 × 1024 pixels at 500 Hz) through the hole in the centre of the back panel of the apparatus. We analysed only sequences with bees flying in the depth of field of the camera, which was 90 mm deep, and reached from about 50 mm in front of the feeder to the end of the perspex drum. (c) Example of a 640 ms flight sequence in the stationary drum. In this example, the bee performs about four oscillations of the regular thorax roll movements we observed in most flights. These oscillations are linked to side-to-side peering flight manoeuvres changing the lateral position of the bee in the apparatus (dashed black line). The bee's vertical position (dashed grey line) increases with time as the bee is approaching the goal from below. Grey and black circles mark local maxima and minima in the bee's position. The head is held almost perfectly horizontal (solid black line) during roll movements of the thorax (solid grey line).
The bees had access to the feeder by flying through two 150-mm-long perspex drums. The two drums were mounted horizontally, one inside the other, on a heavy stand. The inner drum (diameter: 115 mm) was stationary, whereas the outer drum (diameter: 150 mm) was attached to a variable-speed electrically controlled AC motor. The inner drum was either transparent or lined with paper and prevented air currents that might have been generated by the motion of the outer drum from reaching the flight corridor. The outer drum had a black and white striped pattern, with a stripe width of 18 mm on the inside. The apparent size of a stripe was about 14° as viewed from the centre of the drum. The drum was either continuously rotating at a rate of 0.7 rotations per second, which corresponds to a temporal frequency of 18 Hz, or sinusoidally oscillating back and forth at a frequency of 3.1 Hz with 38° peak-to-peak amplitude. To create the continuous motion, the motor was linked to the drum by a loop of strong monofilament line. To reverse the direction of rotation, the loop was crossed such that it formed a figure of eight. For the sinusoidal movement, a metal lever connected the drum and the motor such that every full rotation of the motor resulted in one period of sinusoidal drum motion. A 2-cm-long piece of toothpick attached to the entrance of the drum was used to reconstruct drum orientation from video footage. Once the bees had entered the experimental apparatus, this small indicator was consistently located in their posterior visual field, where it was partly occluded by the bee's thorax. It is therefore unlikely to have influenced the bee's behaviour, as evidenced by the results of our experiments with the continuously rotating drum, where the bees ignored the moving indicator, but stabilized their heads relative to the stationary side pattern. Frontal views (figure 1b) of honeybees entering the apparatus were filmed through a hole in the centre of the back panel of the apparatus (figure 1a) with a carefully levelled high-speed digital video camera (Redlake Motion Pro 10 000). The camera was equipped with a 20 mm Sill Optics macro lens (S5LPJ9150) that provided a field of view of 40° and a depth of field of about 90 mm with the aperture set to f5.6. We analysed only sequences that had clear and sharp images of bees flying in the region defined by the depth of field, which reached from 50 mm in front of the feeder to the end the of the perspex drum. The camera was connected to a Sterling portable computer (Aztec-ATX4) running Redlake MIDAS software for capture at 500 frames s−1 with a spatial resolution of 1024 × 1024 pixels. The high-speed system recorded images into a circular memory buffer until triggered by the experimenter. The size of this memory buffer (2 GB) limited the maximal length of video sequences to 4.09 s. Video sequences were stored as uncompressed 8-bit AVI files on computer hard disks for offline processing. Each experiment was conducted over three different days with different sets of bees. All conditions of an experiment (pattern on different parts of the walls of the drum and different types of drum motion) were tested in random order on a single day. Data was pooled from different days and no repeated measurements were included from individual bees.
(b). Data analysis
We developed MATLAB (The MathWorks, Inc.) code to analyse the position and the orientation of the bee's head and thorax angle, using a combination of automatic tracking algorithms and a custom-built interactive graphic user interface for manual measurements. Templates for automatic tracking were made by cutting out two 40 × 80 pixel pieces from an image of the sequence where the head was oriented horizontally and the eyes were clearly visible, such that the templates contained images of the left and right part of the bee's head. In every image of the video sequence, the pixel coordinates of the two eyes were then determined automatically by shifting and rotating the templates of the two eyes until the best match was found, determined by cross-correlation analysis of the template with the whole image. A similar procedure was used to generate templates for the hind legs (size: 20 × 30 pixels), which we used to determine the thorax roll angle. We seldom observed bees moving their legs relative to the thorax during approach flights (see also figure 1b) and if this happened we excluded such flights from our analysis. A fifth template was used to measure the position and orientation of the wooden stick that indicated the orientation of the inner drum. The orientation of the line connecting the top of the two eyes was used to estimate the bee's head angle. The centre of this line was used as a measure of head position. The line connecting the end of the legs was used to determine thorax roll angle. All positions and orientations were manually checked and corrected using the following method. A region of interest (ROI; size 90 × 90 pixels) was defined around the automatically determined centre of the head (see above) and a second ROI around the central point of the line connecting the legs. Then new images were generated by counter-rotating the ROI by the automatically measured angle. The new images contained straightened portraits of the bee's head and thorax. By watching these images as a movie sequence, orientation errors were easily detected and corrected. The inverse of the angle that was used to straighten the image then gave the corrected roll orientations of the bee's head and thorax. All data points were manually checked this way. We could not use a static pixel-to-mm correspondence to transform the two-dimensional image coordinates into three-dimensional world coordinates, as this conversion factor depends on the bee's distance from the camera. We therefore calculated the appropriate pixel-to-mm ratio for each frame using the width of the bee's head as reference. The ratio between the size of the head and the number of pixels subtended by the head's image is a direct measure of the conversion factor. In experiments with the oscillating drum configuration, we recorded 10 flights from different bees per experimental condition and determined the bee's orientation and position in every image at 2 ms intervals, resulting in a total of 5774 data points. In the experiments with the continuously rotating pattern, we recorded 10 flights per condition and the orientation of the bee's head was determined every 10th frame, which resulted in a total of 1781 analysed frames. In order to check whether this temporal down-sampling degraded the quality of our data, we analysed one sequence at the full temporal resolution (2 ms interframe interval) and found that the average head orientation did not change between the low and high temporal resolution. To reduce noise, all data taken from 500 fps movies were smoothed by convolution with a Gaussian window (σ = 4 ms), which did not noticeably alter the shape of the time series. As the drum oscillated in a predictable manner we were able to check the precision of our methods using the wooden indicator for drum position. The maximum of potential orientation errors is in the order of 1° and the position error below 1 mm.
(i). Data pooling
To make sure that each bee contributed equally to the final mean, we normalized the data of each flight by its total length before pooling data from different bees. Probability density graphs show means and standard errors in the same colour, with the area indicating the standard errors using a lighter colour. Pooling power spectra from different bees required resampling of the data, since the sample base of the power spectrum depends on the length of the individual sequence. We therefore linearly interpolated individual power spectra between 0.1 and 20 Hz with a sample base of 0.1 Hz before averaging the data from different bees.
3. Results
(a). Stationary drum
Close-up, high-speed recordings of honeybees flying in the stationary drum reveal a consistent temporal fine structure of their flight. While approaching the feeder, the bees consistently make fast side-to-side movements (figure 1c, dashed black line). In the example shown in figure 1c, about four of these lateral movements are performed within a period of 600 ms. As we will show below, they are probably caused by regular changes in thorax roll orientation (figure 1c, solid grey line), which change the stroke plane of the wings and thus the direction of the force produced by the flight motor. The vertical position of the bee (figure 1c, dashed grey line) does not show periodic variation but rather changes continuously, which is because the bee is approaching the feeder from below. Despite the large (greater than ±45°) and fast roll oscillations of the thorax, the head is held horizontal to within ±6° throughout the whole sequence (figure 1c, solid black line). Pooled data for the flight paths of 10 different bees flying in a stationary drum (figure 2a) confirm this observation: deviations of the head roll angle (figure 2a, black line) from the horizontal are much smaller than deviations of the thorax roll angle (figure 2a, grey line). In fact, the head roll orientation never deviates more than about 14° from the horizontal.
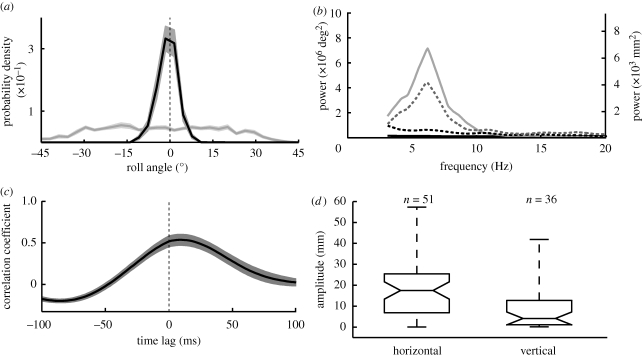
Figure 2.
Flight behaviour in a stationary drum. (a) Probability densities of the bee's head and thorax roll angle. Deviations of the head roll angle (black line) from the horizontal are much smaller than deviations of the thorax roll angle (grey line) from the horizontal. The grey areas indicate the standard error of the mean (s.e.m.). Pooled data of 10 individual bees that approached the feeder in the stationary drum (2374 data points). (b) Mean power spectra of head roll (black line) and thorax roll angles (grey line); vertical (dashed black line) and horizontal position (dashed grey line) of bees averaged over 10 different individuals flying in the stationary drum (same flights as in (a)). The dominant frequency of thorax oscillations and horizontal peering movements is around 6 Hz. (c) Changes in thorax roll orientation are accompanied by changes in the bee's lateral position. Mean across 20 cross-covariance functions where the lateral position of the bee in the drum was correlated with thorax roll angle. Data taken from 10 flights in the stationary and 10 flights in the oscillating visual environment. The grey area indicates the s.e.m. (d) Box-and-whisker plot of the amplitudes of vertical and horizontal movements. The amplitude of every oscillation of the bee's position is calculated as the difference between subsequent local maxima and minima in the bee's position (grey and black circles in figure 1c). The box has horizontal lines at the lower quartile, median and upper quartile values. The lines extending from each end of the box show the extent of the remaining data. The medians (central lines) of the two box-and-whisker plots are significantly different (p < 0.05).
The dominant frequency of thorax oscillations, averaged over 10 different bees, is around 6 Hz (figure 2b, solid grey line). The power spectrum of the head roll orientation does not reveal prominent frequencies (figure 2b, solid black line) because the bee's head remains horizontal despite fast thorax rotations. A cross-covariance analysis of the lateral position of the bee in the drum with thorax roll angle (figure 2c) shows a positive correlation with a time lag of around 10 ms. This indicates that changes in thorax roll orientation precede changes in the bee's lateral position (see also figure 1c), suggesting that the fast side-to-side movements shown in figure 1c are caused by these thorax roll oscillations. In order to compare vertical and lateral movement amplitudes, we estimated movement amplitude by calculating differences between subsequent local maxima and minima (grey and black circles in figure 1c). The median lateral amplitudes (18 mm) are much larger than the vertical amplitudes (4 mm; figure 2d). It is likely that the vertical estimate is an overestimation as we counted any reversal in direction and not just those that are synchronized with thorax rolls. The dominant frequency of the bee's horizontal movements, averaged over 10 different bees, is around 6 Hz (figure 2b, dashed grey line), whereas the power spectrum of vertical movements (figure 2b, dashed black line) shows not a single peak at this frequency.
(b). Oscillating drum
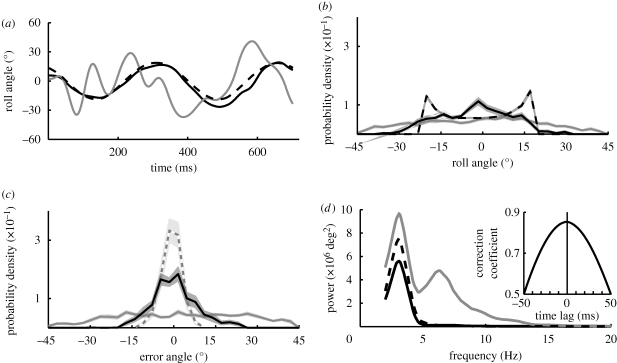
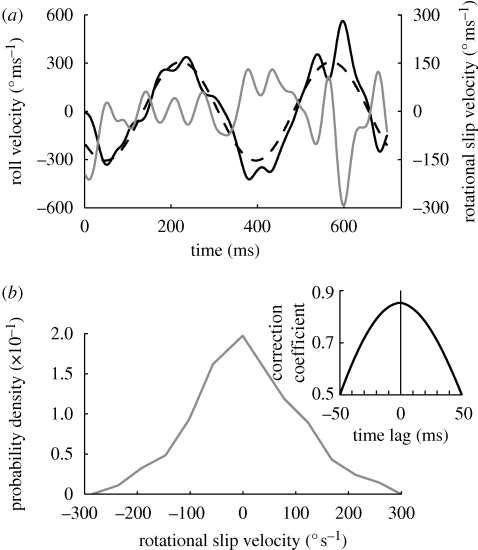
To test whether head stabilization is based on visual or non-visual control signals or passive mechanisms, we periodically oscillated the drum (§2) while the bees were flying through it. Under these conditions, bees still approach the feeder, even though large parts of the visual environment keep changing orientation (figure 3a, dashed black line). The bees still make fast thorax roll oscillations (figure 3a, grey line), similar to those seen in the experiment with the stationary drum. Instead of stabilizing the head horizontally with respect to gravity, however, the roll orientation of the head (figure 3a, black line) now follows the motion of the drum (figure 3a, dashed black line). Compared to the stationary case, this leads to a flattening of the probability density function of head orientation relative to the horizontal (compare figures 2a and 3b). Head and drum orientation vary over approximately the same range (figure 3b, black and grey line), indicating that head orientation is accurately stabilized with respect to the visual environment, leading to a small error angle (difference between head and drum orientation; figure 3c). The probability density function of head orientation relative to the oscillating drum has now a narrower peak around 0° and is similar to what we observed under stationary conditions (figure 2a). The power spectra for head and drum orientation (figure 3d) both peak at the same frequency (3.1 Hz, stimulus frequency). This again demonstrates that this stabilization reflex is based on visual information. While the head is clamped to the rotating drum, the thorax orientation power spectrum (figure 3d, grey line) has two distinct peaks. In addition to the peak around 6 Hz, which is very similar to the spontaneous thorax roll oscillations in a stationary drum, there is now also a peak at 3.1 Hz (stimulus frequency). This indicates that the coherent pattern motion of the drum affects both the neck motor and flight motor systems. Head stabilization is very fast; the cross-covariance analysis shows no pronounced time lag between the orientation of the head and the orientation of the drum (inset, figure 3d). Close inspection of individual trajectories indicates that the bee's head orientation does in some instances indeed lag behind the orientation of the drum but in other instances it also overshoots (e.g. at 400 ms in figure 3a). The same is true for the relationship between the drum and the head roll velocity (figure 4). The bees’ head roll velocity clearly fluctuates around the drum velocity, sometimes lagging behind and sometimes overshooting. The rotational slip velocity, which is the difference between the drum and the head velocity, thus oscillates about 0° s−1 (figure 4a, grey line). The retinal rotational slip velocity probability density function for all 10 flights in the oscillating drum peaks at about 0° s−1 (figure 4b). There is again no significant time lag between the drum roll velocity and the head roll velocity.
Figure 3.
Flight behaviour in an oscillating drum. The outer drum sinusoidally oscillated back and forth at a frequency of 3.1 Hz with 38° peak-to-peak amplitude. (a) The roll angle of the bee's head (solid black line) follows pattern movement (dashed black line). Bees still make fast thorax roll oscillations (solid grey line), similar to those seen in the experiments with the stationary drum (figure 2). (b) Probability density of head orientation relative to the horizontal (3400 data points). Head and drum orientation vary over approximately the same range (black and black dashed line, respectively), indicating that head orientation is stabilized with respect to the visual environment (compare with figure 2a). (c) Difference between head and drum orientation (black) and between thorax and drum orientation (grey). The probability density function of head orientation relative to the oscillating drum (black) has a narrow peak around 0°, which is similar to the peak obtained for the stationary condition (dashed grey lines, taken from figure 2a). (d) The power spectra for head (black) and drum orientation (dashed black line) both have a peak at the same frequency (3.1 Hz), indicating that this stabilization reflex is based on visual information. While the head is clamped to the rotating drum, the thorax orientation power spectrum (grey line) has two distinct peaks. In addition to the peak around 6 Hz, which is the same frequency as for the spontaneous thorax roll oscillations in a stationary drum (figure 2b), there is also a peak at 3.1 Hz (stimulus frequency). Inset: head stabilization is very fast; the cross-covariation between head movement and pattern movement shows no pronounced time lag.
Figure 4.
Head roll velocity in the oscillating drum. (a) The relationship between pattern and head velocity. The bees' head roll velocity (black line) fluctuates around the drum velocity (dashed black line), sometimes lagging behind and sometimes overshooting. The rotational slip velocity (grey line), which is the difference between the drum and the head velocity, oscillates around 0° s−1. (b) The rotational slip velocity probability density function for all 10 flights in the oscillating drum has its peak at about 0° s−1. For most of the time, the magnitude of the rotational slip velocity is well below 200°. Inset: cross-covariance analysis between the drum roll velocity and the head roll velocity.
(c). Continuously rotating drum
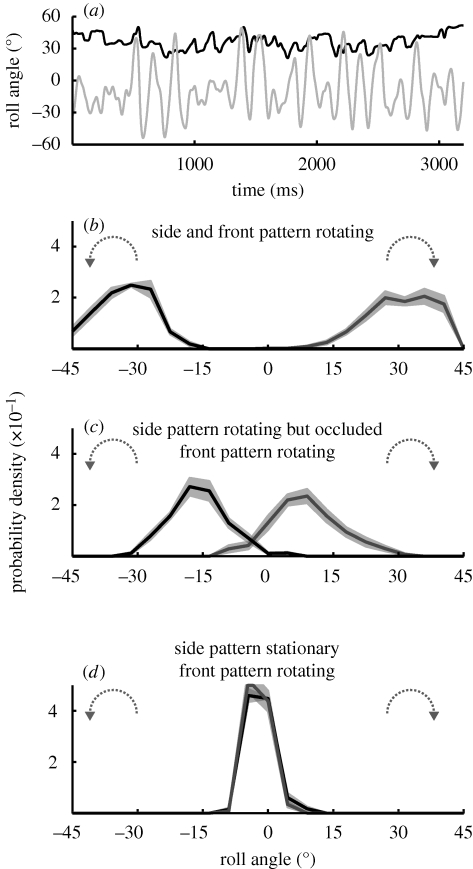
We explored the limits of the head roll control system by flying bees through a continuously rotating drum. We employed different pattern combinations in the bees’ frontal and lateral visual fields. When the whole visual environment rotates continuously in one direction, bees never reach the feeder in flight. Immediately after entering the apparatus, they hover at the far end of the drum, occasionally crashing into the side wall. Apart from this, the most striking feature of this experiment is that the bees hold their heads at a constant oblique orientation. The example in figure 5a shows the time course of head and thorax orientation. While the head is held at a mean angle of 36° (s.d. ± 7°), the bees’ thorax orientation fluctuates in both directions around 0°. Figure 5b shows the probability density function of head orientation for clockwise (grey) and counterclockwise (black) drum rotation for all 10 flights in this experimental condition. Depending on the direction of drum rotation, the bees hold their heads at roll angles around +35° or −35°. These results not only emphasize the importance of visual motion cues for head stabilization, but also show that there must be an additional mechanism that constrains the response. We never observed bees that completely followed the rotation of the drum, which would have turned them upside down.
Figure 5.
Flight behaviour in a continuously rotating drum. The outer drum is continuously rotating in one direction. This stimulus condition prevented all bees from reaching the feeder in flight. (a) The head is held at an oblique roll orientation, depending on drum rotation direction. The example shows the time course of head (black line) and thorax orientation (grey line). The bee's thorax orientation fluctuates in both directions around 0°, while the head is held at a mean angle of 36° (s.d. ± 7°). (b) Data from all 10 flights for this experimental condition. Depending on the direction of drum rotation, the bee head's offset angle is around +35° or −35°. (c) In this experiment, a white piece of paper covers the side wall of the inner drum entirely but leaves the frontal part uncovered (§2). Bees approaching the feeder (n = 10) thus saw a rotating pattern in the front visual field, but no high-contrast pattern elements in the lateral field of view. Under these conditions, they hold their heads at an oblique angle, but clearly less so than when confronted with a rotating panorama (compare figure 5b). (d) The inner drum is lined with a striped pattern. Head orientation is close to normal, i.e. the head is held close to horizontal (compare with figure 2a), although there are still rotating pattern elements in their frontal visual field (compare with figure 5c).
In the next experiment, a white piece of paper covered the side wall of the inner drum entirely (§2) but left the frontal part uncovered. Bees approaching the feeder see a rotating pattern in their frontal visual field but no high-contrast pattern elements on the side. In this situation, bees hold their heads at an oblique angle (figure 5c; n = 10; data processing as in figure 5b), but clearly less so than when confronted with a rotating panorama (compare figure 5b and c). When the inner drum is lined with a striped pattern, thus filling the bee's lateral visual field with stationary high-contrast pattern, the head orientation returns back to normal (i.e. the head is held close to horizontal; figure 5d; n = 10; data processing as in figure 5b). Even though bees still see the rotating pattern in their frontal visual field, the otherwise high-contrast stationary environment enables them to keep their head orientation stable (compare figure 5c and d).
4. Discussion
We found that honeybees visually stabilize their heads against rotation while performing fast lateral movements that are caused by periodic roll movements of the thorax. These side-to-side movements have not been described before, probably because they are hardly visible without the use of high-speed recording equipment. We suggest that their function is very similar to the much slower peering movements in locusts and mantids, where it was shown that peering aids range estimation by generating visual motion parallax (reviewed in Kral & Poteser 1997). We hypothesize that the bees’ lateral movement indicates an active vision strategy that helps bees judge the distance of objects. This would overcome the bees' limited range for stereovision (Srinivasan 1993). In bees, depth perception through stereopsis is restricted to distances of a few centimetres since the spatial resolution of their eyes is low and the distance between the eyes is short (reviewed in Collett & Harkness 1982). It has been shown previously that bees can use depth information extracted from self-induced image motion (reviewed in Lehrer 1996; Srinivasan & Zhang 2004). Depth information is generated during translational movements as the pattern of optic flow depends on the distance of objects. Peering movements of flying bees may thus serve to facilitate the detection of objects ahead as they do for a monocular robot that employs a zigzag locomotion strategy (Sobey 1994). It will be particularly interesting to see how this behaviour is modified, if at all, when bees fly through scenes with a different depth structure.
Our experiments that employed an oscillating pattern clearly showed that vision plays a dominant role in the control of head roll. Bees exposed to this oscillating pattern aligned their heads with respect to their visual environment, causing head orientation to diverge from the horizontal (figure 3a,b). Other cues like the direction of gravity and the light gradient were constant throughout the experiments but did not noticeably help the bees to stabilize their gaze. We can exclude that the bee's head orientation was influenced by artefacts, such as air currents generated by the moving drum. When we prevented the bees from seeing the rotating outer drum by lining the inner drum with a stationary pattern, the bees' head orientation was identical to the orientation in a static environment (compare figures 2a and 5d).
From figure 5d we can see that the lateral part of the visual field is dominant for head stabilization as the rotating radial grating in the front of the bees does not cause the bees to change head orientation if there is a stationary pattern on the side walls. In the absence of strong contrast on the stationary sidewalls, however, the visual motion stimulus in front of the bee elicits a smaller but clear change in head orientation (figure 5c). This demonstrates that the frontal visual field is used to stabilize vision if no other cues are available. It remains unclear, however, whether and how the different parts of the visual field interact during visual head stabilization (figure 5b). It has been shown previously that a visual mechanism that aligns head orientation with pattern contours plays a comparatively weak role in the roll control system of the blowfly Calliphora (e.g. Hengstenberg 1993). We cannot preclude that such a visual mechanism also has some impact in our current experiments.
Bees clearly have some absolute reference for head and thorax orientation that prevented them from turning upside down (figure 5b) in the continuously ‘rolling drum’. The dorsal light response and gravity reception are potential mechanisms. In tethered flies, Hengstenberg et al. (1986) found a very similar head roll response to a continuously rotating drum, with the difference that flies turned their head by ±90°, which is close to the mechanical limits of the fly's neck joint. In these experiments, however, the authors eliminated gravity and light gradients as an orienting vector for roll movements by mounting the fly vertically in a homogeneously illuminated striped drum. In our free-flight experiments, in contrast, both gravity and light gradient cues were available to the bees.
An interesting question is how the bees were able to stably use visuomotor control for head stabilization without noticeable time lags (figure 3d, inset). Visual motion stimuli evoke neural activity in the brains of flies with a delay of about 30 ms (Warzecha & Egelhaaf 2000), much of which is due to the slow process of visual transduction in photoreceptors (reviewed in Hardie 1986). Mechanosensory control loops, in contrast, can be very fast because the structure of mechanoreceptors allows for a direct transduction of the stimulus into changes of the receptor potential. In blowflies, the latency measured in neck motor neurons from haltere deflection is only about 3 ms (Sandeman & Markl 1980). Experiments by Hengstenberg (1993) and Sherman & Dickinson (2003, 2004) show that in flies the visual system is tuned to relatively slow rotations whereas the haltere-mediated response to mechanical rotation increases with increasing angular velocity. Honeybees, however, lack specialized inertial sensors like halteres. The minimal time lag between pattern velocity and compensatory head rotations found in figure 3d could be due to overshooting head movements. Whether this indicates some form of predictive behaviour or a mere sign of high gain (i.e. close to the stability limits) in a velocity feedback control system that uses visual motion to clamp rotational slip velocity to zero (Land 1992) is unclear. Experiments on rabbits and salamanders show that such anticipation can be explained on the basis of spatially extended receptive fields and other known mechanisms of retinal processing, like nonlinear contrast-gain control (Berry et al. 1999). This issue cannot be resolved without further experiments with different stimulus dynamics. In addition, a detailed analysis of the temporal resolving power, time lags and speed tuning in the motion vision pathway of bees is needed to understand how visuomotor control for head stabilization is performed stably without noticeable time lags.
The experiments with the oscillating pattern revealed not only that the head is stabilized relative to the visual environment, but that the bees' high-frequency thorax roll rotations are also influenced by pattern movement. One possible explanation is that head orientation acts as the set point for thorax orientation. The two peaks in the power spectrum of the thorax orientation in the oscillating drum (figure 3d) support this hypothesis. The first peak, around 6 Hz, corresponds to the frequency we found for the spontaneous thorax roll oscillations in a stationary environment (figure 2b). The second peak, at 3.1 Hz, corresponds to the stimulus frequency. The faster, spontaneous body rolls are thus superimposed on the slower, stimulus-driven oscillations (see also figure 3a). A similar relationship was also observed in tethered flying locusts, flies and dragonflies, where a turn of the head evokes active thorax rotation (Mittelstaedt 1950; Goodman 1965; Liske 1977). The control systems that coordinate head and thorax orientation (recently reviewed in Taylor & Krapp 2007) break down after deafferentation of cervical mechanosensors, demonstrating the essential role of proprioceptive information for this posture reflex (Mittelstaedt 1950; Goodman 1965; Preuss & Hengstenberg 1992; Gilbert & Bauer 1998; Gilbert & Kim 2007). Bees do possess proprioceptive hair plates in the neck region, which could be used to measure the relative orientation between the head and the thorax (Lindauer & Nedel 1959; Markl 1962). Such a control mechanism would explain why bees cannot reach the feeder under the continuous roll condition. As they roll their thoraxes about an orientation determined by the head (approx. 35°), their flight becomes very unstable and they often sink and bang against the wall. Those bees who manage to stay airborne for a while, despite their oblique head orientation, never reach the feeder on the wing, illustrating the ultimate importance of gaze stabilization for successful navigation.
Acknowledgements
We thank M. V. Srinivasan for lending us the high-speed camera used in this study and for discussions on many of the topics raised in this paper. A special thank you goes to Jochen Zeil for his helpful comments, for motivating discussions in all phases of the project and for his thorough criticism of the manuscript. Supported by DFG to N.B. and by the ARC Center of Excellence programme. We are grateful to the two anonymous reviewers for their comments and helpful suggestions.
References
- Berry M. J., Brivanlou I. H., Jordan T. A., Meister M.1999Anticipation of moving stimuli by the retina. Nature 398, 334 (doi:10.1038/18678) [DOI] [PubMed] [Google Scholar]
- Collett T. S.1978Peering—a locust behaviour pattern for obtaining motion parallax information. J. Exp. Biol. 76, 237–241 [Google Scholar]
- Collett T. S.1988How ladybirds approach nearby stalks: a study of visual selectivity and attention. J. Comp. Physiol. A 163, 355 (doi:10.1007/BF00604011) [Google Scholar]
- Collett T. S., Harkness L. I. K.1982Depth vision in animals. In Analysis of visual behavior (eds Ingle D. J., Goodale M. A., Mansfield R. J. W.), pp. 111–176 Cambridge, MA: The MIT Press [Google Scholar]
- Collett T. S., Paterson C. J.1991Relative motion parallax and target localization in the locust, Schistocerca gregaria. J. Comp. Physiol. A 169, 615–621 [Google Scholar]
- Dahmen H., Franz M. O., Krapp H. G.2001Extracting egomotion from optic flow: limits of accuracy and neural matched filters. In Motion vision: computational, neural, and ecological constraints (eds Zanker J. M., Zeil J.), pp. 143–168 Berlin, Germany: Springer [Google Scholar]
- Gibson J. J.1950The perception of the visual world Boston, MA: Houghton Mifflin [Google Scholar]
- Gilbert C., Bauer E.1998Resistance reflex that maintains upright head posture in the flesh fly Neobellieria bullata (Sarcophagidae). J. Exp. Biol. 201, 2735–2744 [DOI] [PubMed] [Google Scholar]
- Gilbert C., Kim M.2007Effects of male age and cervical proprioceptors on sexual aerial pursuit by male flesh flies, Neobellieria bullata (Diptera: Sarcophagidae). J. Insect Behav. 20, 427–435 (doi:10.1007/s10905-007-9088-x) [Google Scholar]
- Gilbert C., Gronenberg W., Strausfeld N. J.1995Oculomotor control in Calliphorid flies: head movements during activation and inhibitions of neck motor neurons corroborate neuroanatomical predictions. J. Comp. Neurol. 361, 285–297 (doi:10.1002/cne.903610207) [DOI] [PubMed] [Google Scholar]
- Goodman L. J.1965The role of certain optomotor reactions in regulating stability in the rolling plane during flight in the desert locust, Schistocerca Gregaria. J. Exp. Biol. 42, 385–407 [Google Scholar]
- Hardie R. C.1986The photoreceptor array of the dipteran retina. Trends Neurosci. 9, 419–423 (doi:10.1016/0166-2236(86)90136-0) [Google Scholar]
- Hengstenberg R.1988Mechanosensory control of compensatory head roll during flight in the blowfly Calliphora erythrocephala Meig. J. Comp. Physiol. A 163, 151–165 (doi:10.1007/BF00612425) [Google Scholar]
- Hengstenberg R.1993Multisensory control in insect oculomotor systems. In Visual motion and its role in the stabilization of gaze (eds Miles F. A., Wallman J.), pp. 285–298 Amsterdam, The Netherlands: Elsevier; [PubMed] [Google Scholar]
- Hengstenberg R., Sandeman D. C., Hengstenberg B.1986Compensatory head roll in the blowfly Calliphora during flight. Proc. R. Soc. Lond. B 227, 455–482 (doi:10.1098/rspb.1986.0034) [Google Scholar]
- Horridge G. A.1986A theory of insect vision: velocity parallax. Proc. R. Soc. Lond. B 229, 13–27 (doi:10.1098/rspb.1986.0071) [Google Scholar]
- Kern R., van Hateren J. H., Egelhaaf M.2006Representation of behaviourally relevant information by blowfly motion-sensitive visual interneurons requires precise compensatory head movements. J. Exp. Biol. 209, 1251–1260 (doi:10.1242/jeb.02127) [DOI] [PubMed] [Google Scholar]
- Kimmerle B., Srinivasan M. V., Egelhaaf M.1996Object detection by relative motion in freely flying flies. Naturwissenschaften 83, 380–381 (doi:10.1007/BF01142005) [Google Scholar]
- Kral K., Poteser M.1997Motion parallax as a source of distance information in locusts and mantids. J. Insect Behav. 10, 145–163 (doi:10.1007/BF02765480) [Google Scholar]
- Land M. F.1973Head movements of flies during visually guided flight. Nature 243, 299–300 (doi:10.1038/243299a0) [Google Scholar]
- Land M. F.1992Visual tracking and pursuit: humans and arthropods compared. J. Insect Physiol. 38, 939–951 (doi:10.1016/0022-1910(92)90002-U) [Google Scholar]
- Land M. F., Collett T. S.1997A survey of active vision in invertebrates. In From living eyes to seeing machines (eds Srinivasan M. V., Venkatesh S.), pp. 16–36 Oxford, UK: Oxford University Press [Google Scholar]
- Lappe M.2000Neuronal processing of optic flow New York, NY: Academic Press [Google Scholar]
- Lehrer M.1991Bees which turn back and look. Naturwissenschaften 78, 274–276 (doi:10.1007/BF01134357) [Google Scholar]
- Lehrer M.1996Small-scale navigation in the honeybee: active acquisition of visual information about the goal. J. Exp. Biol. 199, 253–261 [DOI] [PubMed] [Google Scholar]
- Lehrer M., Srinivasan M. V., Zhang S. W., Horridge G. A.1988Motion cues provide the bee's visual world with a third dimension. Nature 332, 356–357 (doi:10.1038/332356a0) [Google Scholar]
- Lindauer M., Nedel J. O.1959Ein Schweresinnesorgan der Honigbiene. Z. vergl. Physiol. 42, 334–364 (doi:10.1007/BF00298125) [Google Scholar]
- Liske E.1977The influence of head position on the flight behaviour of the fly, Calliphora erythrocephala. J. Insect Physiol. 23, 375–379 (doi:10.1016/0022-1910(77)90276-1) [Google Scholar]
- Maksimovic S., Layne J. E., Buschbeck E. K.2007Behavioral evidence for within-eyelet resolution in twisted-winged insects (Strepsiptera). J. Exp. Biol. 210, 2819–2828 (doi:10.1242/jeb.004697) [DOI] [PubMed] [Google Scholar]
- Markl H.1962Borstenfelder an den Gelenken als Schweresinnesorgane bei Ameisen und anderen Hymenopteren. Z. vergl. Physiol. 45, 475 (doi:10.1007/BF00342998) [Google Scholar]
- Mittelstaedt H.1950Physiologie des Gleichgewichtssinnes bei fliegenden Libellen. J. Comp. Physiol. A 32, 422. [PubMed] [Google Scholar]
- Pfaff M., Varjú D.1991Mechanisms of visual distance perception in the hawk moth Macroglossum stellatarum. Zool. Jahrb. Physiol. 95, 315–321 [Google Scholar]
- Pix W., Zanker J. M., Zeil J.2000The optomotor response and spatial resolution of the visual system in male Xenos vesparum (Strepsiptera). J. Exp. Biol. 203, 3397–3409 [DOI] [PubMed] [Google Scholar]
- Preuss T., Hengstenberg R.1992Structure and kinematics of the prosternal organs and their influence on head position in the blowfly Calliphora erythrocephala. Meig. J. Comp. Physiol. A 171, 483–493 [Google Scholar]
- Sandeman D. C., Markl H.1980Head movements in flies (Calliphora) produced by deflexion of the halteres. J. Exp. Biol. 85, 43–60 [Google Scholar]
- Schilstra C., van Hateren J. H.1998Stabilizing gaze in flying blowflies. Nature 395, 654 (doi:10.1038/27114) [DOI] [PubMed] [Google Scholar]
- Sherman A., Dickinson M. H.2003A comparison of visual and haltere-mediated equilibrium reflexes in the fruit fly Drosophila melanogaster. J. Exp. Biol. 206, 295–302 (doi:10.1242/jeb.00075) [DOI] [PubMed] [Google Scholar]
- Sherman A., Dickinson M. H.2004Summation of visual and mechanosensory feedback in Drosophila flight control. J. Exp. Biol. 207, 133–142 (doi:10.1242/jeb.00731) [DOI] [PubMed] [Google Scholar]
- Sobel E. C.1990The locust's use of motion parallax to measure distance. J. Comp. Physiol. A 167, 579–588 [DOI] [PubMed] [Google Scholar]
- Sobey P.1994Active navigation with a monocular robot. Biol. Cybern. 71, 433 (doi:10.1007/BF00198919) [Google Scholar]
- Srinivasan M. V.1993How insects infer range from visual motion. In Visual motion and its role in the stabilization of gaze (eds Miles F. A., Wallman J.), pp. 139–156 Amsterdam, The Netherlands: Elsevier; [PubMed] [Google Scholar]
- Srinivasan M. V., Zhang S.2004Visual motor computations in insects. Annu. Rev. Neurosci. 27, 679–696 (doi:10.1146/annurev.neuro.27.070203.144343) [DOI] [PubMed] [Google Scholar]
- Stange G.1981The ocellar component of flight equilibrium control in dragonflies. J. Comp. Physiol. A 141, 335–347 [Google Scholar]
- Taylor G. K., Krapp H. G.2007Sensory systems and flight stability: what do insects measure and why? Adv. Insect Physiol. 34, 231–316 (doi:10.1016/S0065-2806(07)34005-8) [Google Scholar]
- van Hateren J. H., Schilstra C.1999Blowfly flight and optic flow. II. Head movements during flight. J. Exp. Biol. 202, 1491–1500 [DOI] [PubMed] [Google Scholar]
- Voss R., Zeil J.1998Active vision in insects: an analysis of object-directed zig-zag flights in wasps (Odynerus spinipes, Eumenidae). J. Comp. Physiol. A 182, 377–387 (doi:10.1007/s003590050187) [Google Scholar]
- Wallace G. K.1959Visual scanning in the desert locust Schistocerca gregaria Forskal. J. Exp. Biol. 36, 512–525 [Google Scholar]
- Warzecha A.-K., Egelhaaf M.2000Response latency of a motion-sensitive neuron in the fly visual system: dependence on stimulus parameters and physiological conditions. Vis. Res. 40, 2973 (doi:10.1016/S0042-6989(00)00147-4) [DOI] [PubMed] [Google Scholar]
- Wehner R., Flatt I.1977Visual fixation in freely flying bees. Z. Naturforsch. 32, 469–471 [Google Scholar]
- Zeil J., Kelber A., Voss R.1996Structure and function of learning flights in bees and wasps. J. Exp. Biol. 199, 245–252 [DOI] [PubMed] [Google Scholar]
- Zhang S. W., Srinivasan M. V., Collett T.1995Convergent processing in honeybee vision: multiple channels for the recognition of shape. Proc. Natl Acad. Sci. USA 92, 3029–3031 (doi:10.1073/pnas.92.7.3029) [DOI] [PMC free article] [PubMed] [Google Scholar]