Abstract
It has been hypothesized that most morphological evolution occurs by allometric differentiation. Because rodents encapsulate a phenomenal amount of taxonomic diversity and, among several clades, contrasting levels of morphological diversity, they represent an excellent subject to address the question: how variable are allometric patterns during evolution? We investigated the influence of phylogenetic relations and ecological factors on the results of the first quantification of allometric disparity among rodents by exploring allometric space, a multivariate morphospace here derived from, and encapsulating all, the ontogenetic trajectories of 34 rodent species from two parallel phylogenetic radiations. Disparity was quantified using angles between ontogenetic trajectories for different species and clades. We found an overlapping occupation of allometric space by muroid and hystricognath species, revealing both clades possess similar abilities to evolve in different directions of phenotypic space, and anatomical diversity does not act to constrain the labile nature of allometric patterning. Morphological features to enable efficient processing of food serve to group rodents in allometric space, reflecting the importance of convergent morphology, rather than shared evolutionary history, in the generation of allometric patterns. Our results indicate that the conserved level of morphological integration found among primates cannot simply be extended to all mammals.
Keywords: macroevolution, disparity, cranium, ontogeny, diet, phenotypic covariance structure
1. Introduction
Between species, populations and even sexes, morphological traits exhibit an impressive diversity of scaling relationships with body size. Allometric differentiation among morphological traits is thought to drive the evolution of morphology (Frankino et al. 2005). While allometry has long been a focus of study (Huxley 1932; Reeve & Huxley 1945; Jolicoeur 1963), most work has been directed to the consideration of adult morphologies, and it is only recently that methods have been proposed to provide quantification of the variety of ontogenetic trajectories (Gerber et al. 2008), making possible cladewide comparisons of the role development plays in shaping morphospace occupation and structure.
Stemming from the concept of morphological disparity, a method to quantify the empirical distribution of taxa in morphospace (Foote 1992), and more precisely the associated underlying metrical framework, Gerber et al. (2007, 2008) have recently proposed the notion of ‘allometric disparity’ as a means to quantify the variety of allometric patterns in a clade, which may be displayed in a phenotypic space termed ‘allometric space’. The possibility to compare ontogenetic trajectories derived from the multivariate generalization of allometry, which is the first component of a principal component analysis (PCA) of all traits for a particular species, was initially referred to by Klingenberg & Froese (1991) in part of their study on fish larvae. The ordination method was further explored in heterochrony and allometry studies on water striders (Klingenberg & Spence 1993; Klingenberg 1996a) but a name was not established. Similarly, previous cladewide disparity studies have contrasted adult and juvenile disparity using the same principles but without defining a specific metric (e.g. Zelditch et al. 2003; Eble 2004).
Rodents are exceptional mammals both ecologically and taxonomically. Being the most species-rich group, and encapsulating a phenomenal level of morphological diversity, rodents provide an ideal group in which to study the evolution of allometric patterns. One factor promoting the near worldwide spread of rodents is their opportunistic feeding strategies, reflected in an array of dental (Wood 1955, 1959; Evans et al. 2007), muscular (Becht 1953) and cranial specializations (Hautier et al. 2008). While feeding behaviour has been shown to characterize craniodental morphology among ungulates (Janis 1995; Mendoza et al. 2002) and carnivores (e.g. Van Valkenburgh 1989), few studies have addressed the role of dietary habits in shaping rodent cranial morphology (Samuels 2009).
The hystricognaths contrast to other rodent groups (Wilson & Sánchez-Villagra 2009); this monophyletic clade comprises comparatively few species (less than 13% of rodents; Wilson & Reeder 2005) and yet contains members spanning several orders of magnitude in body size (Nowak 1999) and possessing numerous adaptations to locomotory style (Weisbecker & Schmid 2007). Hystricognaths, restricted in distribution to South America (caviomorphs) and Africa (phiomorphs), possess attributes that contrast sharply with those of the taxonomically diverse muroid clade (1517 species; Carleton & Musser 2005) whose members show a comparatively diminished level of anatomical diversity (Steppan et al. 2004).
In the present study, we provide, to our knowledge, the first exploration of allometric space for rodents. Allometric patterns among muroids and hystricognaths, two major rodent clades with contrasting attributes, are compared. First we assess to what extent alterations in growth dynamics reflect phylogenetic relationships and ecomorphological diversity encapsulated within each clade, and second whether species distribution in morphospace reflects the convergence of modifications to allometries during evolution as a consequence of shared feeding habits.
2. Material and methods
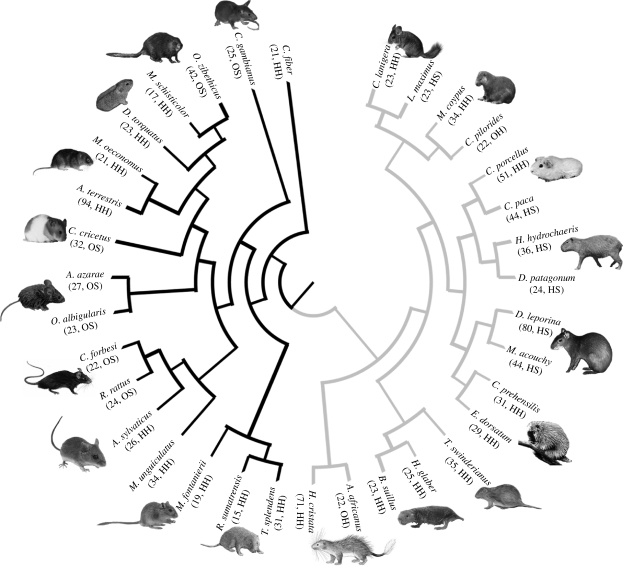
Landmark data were collected from 1113 crania using a Microscribe MX5 Digitizer. Postnatal growth series, including juvenile and adult representatives from the hystricognath (617 specimens) and muroid (496 specimens) clades, were chosen, based upon the availability of adequate sample size, to cover the widest possible phylogenetic breadth and encapsulate an array of ecological and morphological diversity (figure 1; see electronic supplementary material, appendix S1). Landmark data were recorded for 34 species: 17 from each of the two clades. Measurements for 17 cranial traits were derived from landmark data collected for each species, these are: premaxilla ventral length, premaxilla width at maxilla suture, palatine length, palatine width, occipital condyles width, skull length, nasal length, nasal width, frontal midline length, parietal midline length, jugal length, length of dental diastema, maximum interorbital width, basioccipital length, basioccipital width, basisphenoid length and basisphenoid width. Following the multivariate generalization of allometry (Jolicoeur 1963), the first principal component represents the line of best fit to the multivariate data (Pearson 1901), whereby size is considered a latent variable that affects all the original variables simultaneously. Hence, 34 PCAs were performed using log-transformed measurements; one for each species studied. Allometric space was computed for all species from the precept that the distribution of allometric growth can be visualized in the space defined by the normalized vector coefficients (PC1) of the original traits; thus using the 34 originally calculated PC1 vectors as ‘observations’ for a second PCA. The resultant principal component scores for the first and second axes were plotted to examine species distribution in the coefficient space. Each species is represented in allometric space by a single point that summarizes the allometric trajectory for that particular species.
Figure 1.
Phylogenetic relationships among the rodent species included in this study (see text for reference). Branch colours: black, muroids; grey, hystricognaths. Parentheses include number of specimens measured and dietary category (figure 3).
Because each point in allometric space reflects a normalized vector (PC1), it is possible to quantify the distance in allometric space between any two species as an angle between their directions in the space of log-transformed measurements (e.g. Boitard et al. 1982; Gibson et al. 1984; Solignac et al. 1990). If two PC1 vectors x and y are normalized such that x′x = 1 and y′y = 1, then the angle α between the two trajectories is computed as α= arccos(x′y), the arccosine of the inner product of the two vectors (Klingenberg 1996b). To estimate the standard error (s.e.) of the PC1 coefficients, a bootstrap with replacement was performed for 1000 iterations, for each species. In allometric space, similar to morphospace, the spacing of taxa, also referred to as total variance, can be measured to provide a disparity metric. Total variance, computed as the trace of the covariance matrix of allometric patterns, can be quantified using an average angle between trajectories. Since a group of parallel trajectories would have inter-trajectory angles of zero and total variance would be minimal, the average angle metric provides an indication of spacing in state space. Average angle was used to quantify disparity among all species studied, and separately among muroids and hystricognaths. A vector of length p with all coefficients equal to p−1/2 (where p is the number of measured variables), termed the isometric vector, was used to assess allometric trends for given trajectories.
Information on dietary habit was compiled from the literature for all rodents studied. Anderson & Jones (1984) and Nowak (1999) were used for general reference, and for several species further details were obtained from a number of works including Gulotta (1971), Woods (1972), Willner et al. (1983) and Pérez (1992). Each species was assigned to one of four dietary categories based upon food materials that were primarily incorporated into the diet: herbivore hard (HH), herbivore soft (HS), omnivore soft (OS), or omnivore hard (OH) (figure 1). These categories are similar to schemes adopted by Williams & Kay (2001) and Samuels (2009): rodents classified as having a hard diet (HH or OH) consistently incorporated roots, tubers or bark in their diet in contrast to those assigned as having a soft diet (HS or OS), which included species primarily eating fruits, soft leaves and shoots. Canonical variates analysis (CVA) was performed on the PCA scores accounting for 95 per cent of the variance in allometric space. Based here upon the a priori assignment to one of the four dietary groups, CVA analysis produces a set of canonical variate axes that are expressed as linear combinations of the axes of the original multi-dimensional (allometric) space. The canonical axes reflect the maximal ratio of between group to within group distance and thus best separate the dietary groups.
Specimens were measured from the following collections: Naturhistorisches Museum, Basel; Humboldt-Universität zu Berlin—Museum für Naturkunde; Zoological Museum—Natural History Museum of Denmark; Natural History Museum, London; Naturalis, Leiden; Zoologische Staatssammlung, München; Naturhistoriska Riksmuseet, Stockholm; and the Museum and Institute of Zoology—Polish Academy of Sciences, Warsaw.
Evolutionary relationships among rodent species examined here were reconstructed from various studies. For muroids, relationships are based upon the molecular studies of Steppan et al. (2004) and Blanga-Kanfi et al. (2009) and for the arvicolid rodents, Buzan et al. (2008). Relationships among the hystricognaths follow the molecular study of Huchon & Douzery (2001).
3. Results
For the individual PCAs used to construct allometric space, the variance summarized by PC1 ranged across species from 93.1 to 97.3 per cent and for the second component (PC2) variance reduced to between 1.1 and 5.6 per cent across species (electronic supplementary material, table S1). Hence, for the species studied here, the multivariate generalization of simple allometry provides an accurate reflection of growth, with PC1 axes representing the direction of growth in the space of log-transformed measurements. PC1 coefficients were stable, indicated by associated s.e. values ranging between 0.004 and 0.011.
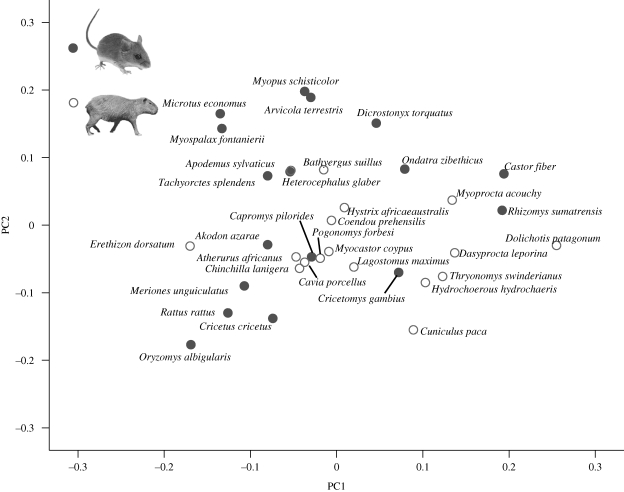
The total variation encompassed within the studied species spans 11 dimensions of space, with the two-dimensional subspace plotted here accounting for the maximal proportion of variance among the allometric trajectories. The first two dimensions of allometric space (figure 2) represent 45.7 per cent of the variation among the species studied, with PC1 summarizing 24.5 per cent and PC2 the remaining 21.2 per cent. Examination of the individual PCAs reveals that allometric coefficients vary among species in relation to the isometric vector (0.242), a feature tying some species into loose clusters in allometric space. For instance, the fossorial rodents Bathyergus suillus, Heterocephalus glaber and Tachyorctes splendens occupy a small cluster, sharing a positive allometry for jugal length. Because the axes in allometric space show the angular deviation from the mean ontogenetic trajectory with respect to the variables associated with the principal component axis, large positive or negative values for a given axis represent large positive or negative deviations in ontogenetic growth, respectively. Thus, species that have positive scores on PC1 are characterized here by a faster than average relative broadening of the palatal region compared with those species with negative scores for this axis, which would indicate a slower than average growth of the palate. PC2 is associated with the relative lengthening of the rostral region (nasal length and premaxilla length), and hence the rostral region lengthens faster than average for species with positive scores on PC2 and slower than average for species with negative scores on this axis.
Figure 2.
Allometric space for 34 rodent species. Clades: closed circles, muroid; open circles, hystricognath.
The occupation of allometric space for the rodent species studied here appears disparate, as indicated by an average angle of 17.1° between PC1 components (range 7.7°–33.1°; electronic supplementary material, table S2). Moreover, species belonging to either the muroid or hystricognath clades do not group exclusively together: each clade occupies an overlapping portion of allometric space. The average angle among PC1 components for hystricognath species is 17.2°, while the trajectories of species belonging to the muroid clade are separated, on average, by 16.9° (electronic supplementary material, tables S3 and S4). The trajectories of pairs comprising one species from each clade are, on average, 17.7° apart from one another. The average angle between hystricognaths is not significantly different from that between muroid species (U = 8.6E03, p = 0.32), and neither clade comprises enough members that are more closely located to one another than they are to members belonging to the other clade to reflect a statistically significant difference (U = 1.6E04, p = 0.15 for muroids; U = 1.9E04, p = 0.2 for hystricognaths). Within the muroid clade, partial relationships are recovered for the arvicolid rodents: species of voles and lemmings included in this study are positioned the furthest along PC2, with the exception of the muskrat, Ondatra zibethicus located away from the upper portion of space. The PCA for O. zibethicus, in contrast to all other species studied, revealed that maximum interorbital width contributed the most to variance along PC1, with this variable exhibiting a positive allometry (0.379 ± 0.004 s.e.; isometric vector = 0.242).
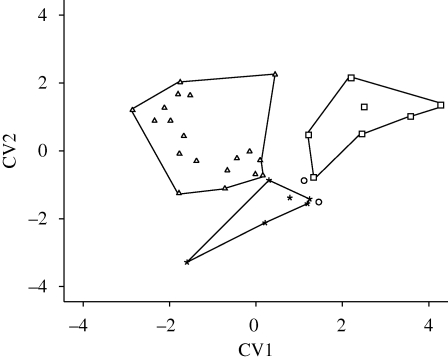
The narrowest angles between trajectories were associated with those species sharing the same dietary habits. Between species classified as HH, the trajectories of Apodemus sylvaticus and Castor fiber were 7.7° apart, and those of A. sylvaticus and Erethizon dorsatum were 8.3° apart. Atherurus atherurus and Capromys pilorides, the two species studied here with an omnivorous diet incorporating hard materials (OH), had an inter-trajectory angle of 8.7° (see electronic supplementary material, tables S5–S7). The first canonical variate (CV1) accounted for 61.6 per cent of variance and separated the herbivores with diets containing predominantly hard material (HH), typically having negative scores on CV1, from those eating mostly soft materials (HS) with positive CV1 scores (figure 3). Minor separation of groups occurred along the second canonical variate axis (CV2), which accounted for an additional 24.4 per cent (figure 3). CVA correctly assigned 94.1 per cent of rodents to their a priori dietary groups, with only two species being misclassified: Chinchilla lanigera was misclassified as being an omnivore (OS) instead of having a soft herbivorous diet (HS) and Akodon azarae, positioned close to zero on both axes, was classified as HH rather than OS.
Figure 3.
CVA analysis of allometric space using predefined dietary habit groups: triangles, herbivore hard (HH); circles, omnivore hard (OH); squares, herbivore soft (HS); asterisks, omnivore soft (OS).
4. Discussion
The developmental basis for the morphological differences observed between species can be compared in an intuitive, effective manner using allometric space. Examination of the evolutionary modifications to growth trajectories through the quantification of the space encapsulating all realizable patterns provides a suitable stage to consider a large-scale comparison of morphological diversity. The overlapping occupation of allometric space by muroids and hystricognaths reveals that anatomical diversity does not act to constrain the labile nature of allometric patterning in rodents.
Since the rodents studied here encapsulate aquatic and semiaquatic, arboreal, fossorial and scansorial forms, spanning three orders of magnitude from the smallest examined species Myopus schisticolor (approx. 26 g) to the largest living rodent the capybara Hydrochoerus hydrochaeris (50–61 kg), the diversity of allometries, exemplified by an average intertrajectory disparity of 17.1°, reflects the complex array of modifications possible and consequent within this diverse clade, whose adaptability has led to unparalleled success during the last 55 Myr of mammalian evolution (Hartenberger 1985; Huchon et al. 2002). The overlapping and continual distribution within allometric space (figure 2) for the rodent species examined here is unexpected. Studies of the topology of form space indicate accessibility in morphospace may be anisotropic (Fontana & Schuster 1998; Stadler et al. 2001), and one would conceive that there might be ‘gaps’ in allometric space that reflect non-functional scaling relationships, and thus species would be more clustered to reflect the adherence to, and selective forces acting for, the expression of particular allometric patterns such that functional size relationships, obtained by the scaling of traits, are maintained irrespective of genetic or environmental variation. Studies of much smaller scope, particularly the consideration of four crustacean species by Boitard et al. (1982) and a study by Klingenberg & Zimmermann (1992) investigating nine species of water striders, indicate taxon distribution in space is not continuous, though comparisons must be considered tentatively because the low number of species studied by these authors make difficult the reliable judgement of how real these gaps are. In the latter study, angles between the ontogenetic trajectories of different species averaged 13.4°, while in their study using 10 species of Antarctic fishes, Klingenberg & Ekau (1996) reported interspecific trajectory angles of between 2.5° and 8.7°, reflecting greater convergence of allometric patterning among these species than for the rodents studied herein. By contrast, range and absolute magnitude of interspecific trajectory angles reported here are comparatively smaller than those documented by Zelditch et al. (2003) between nine piranha species (22.7°–76.5°). Thus, given the present understanding of differences in allometries between species, rodents do not appear to lie outside the current documented range of variability.
Because the methods of this work use the major axis of covariance, the results are of direct relation to the evolution of morphological integration and P matrices, the study of which considers trait associations measured through patterns of trait covariation or correlation (Olson & Miller 1958). In the macroevolutionary theory, disparity and integration potentially reflect the same concept (Eble 2004), namely one of constraint: strong phenotypic integration may constrain the production of novelties and thus disparity, while conversely, comparatively weak integration may make possible the generation of novelties and thus would be associated with an increase in disparity. Further, because functional and developmental constraints are expressed in patterns of integration (e.g. Zelditch et al. 1990), disparity will reflect the possibilities that remain for the functional or developmental differentiation of integrated phenotypes. Our results indicate both muroids and hystricognaths possess similar abilities to evolve in different directions of phenotypic space. While broad cladewide studies of cranial integration are at present lacking for rodents, studies for some other mammals appear to contradict this pattern. Other members of the euarchontoglires clade, particularly primates, have been shown to exhibit conserved patterns of integration, in contrast to the rodents here. Despite a broad range of diversity across the platyrrhine primates, in their extensive analysis Marroig & Cheverud (2001) found a common shared pattern of morphological integration across the whole cranium, independent of phylogenetic history, resulting from common patterns of skull development. Additionally, an earlier study (Cheverud 1982, 1989) indicates Old World monkeys share this constrained pattern with their New World relatives, and work comparing living African apes and humans supports the notion that morphological integration is conserved across the entire order (Ackermann 2002, 2005).
Nevertheless, based on her broad study of cranial integration among carnivorans, Goswami (2006) showed that the conserved level of integration found among primates cannot simply be extended to all mammals. Our results further corroborate this finding and suggest that the similar covariance structure that Steppan (1997) detected among six species of leaf-eared mice (Phyllotis) reflects limited genus level sampling. Moreover, when considering non-mammalian clades, a study of six piranha species (Fink & Zelditch 1996) reported labile levels of integration during ontogeny, suggesting rodents are not unusual in their ability to alter covariance structure during evolution. Similarly, studies that explicitly tested developmental hypotheses in relation to cranial integration have revealed that developmental constraints are transient and flexible in muroid rodents (Zelditch & Carmichael 1989a,b).
Links between dietary habit and dentition, particularly occlusal complexity (Evans et al. 2007), have been found among rodents. Cranial morphology has also been shown to display a significant relationship with differing demands of food processing inherent among varying life habits (Samuels 2009), evidenced here by the successful classification of species to dietary groups based upon their location in allometric space (94.1%; figure 3). The mechanical effort required to process tough plant materials has been linked with the development of a more massive jaw, larger cheek tooth area and a deeper skull and rostrum to provide an increased area for muscle attachment, thus improving efficiency and power during mastication (Satoh 1997; Michaux et al. 2007). Herbivorous rodents studied here occupy a portion of allometric space associated with allometric patterns, reflecting a relative widening of the nasal, lengthening and widening of the premaxilla and widening of the palate (figure 3). However, within this portion of space, relationships between species exhibiting specialized cranial morphologies, such as mole rats, many of whom burrow primarily using their teeth, are not completely preserved. Several mole rats share a positive allometry for the jugal, a feature reflecting the posterior widening of the zygomatic arch to accommodate the masseter muscle, which among non-fossorial rodents passes through an enlarged infraorbital foramen (Moore 1981).
The disparate structure of allometric space indicates that changes in PC slope, which correspond to changes in covariance structure, are common in rodents. The prevalence of slope change among the taxa studied here may be a function of their evolutionary distances from each other. It has been proposed that the more distant the comparison between taxa, in terms of evolutionary relationship, the more likely differences are consequent of slope change rather than changes that conserve the direction of the ontogenetic trajectory (Weston 2003), namely, either an extension or truncation in trajectory (ontogenetic scaling), or a translation in log-transformed space, reflecting a lateral transposition of trajectory; such changes are indicative of an alteration in the duration of growth or a change in prenatal development, respectively. Conservation of direction, through lateral transposition or scaling, is considered to be more easily accomplished and thus more likely to occur during morphological evolution (Creighton & Strauss 1986; Gomez 1992). Further work directed to quantifying the capacity of slope change as a mechanism to generate morphological disparity represents one potentially fruitful future expansion of these methods, considering the evolutionary constraint associated with a conservation of direction and its implications for confounding phylogenetic reconstructions through the promotion of evolutionary convergence.
At a large scale, allometric disparity may be considered a proxy for developmental dynamics; the exploration of allometric space occupation, and its relation with phylogenetic and ecological trends, provides an opportunity to enhance our understanding of factors influencing ontogenetic pathways. The exploration of allometric disparity, and associated correlates, when augmented with cladewide studies of morphological disparity (e.g. Foote 1991), may reveal yet further clues about morphological macroevolution, addressing the question:‘how has the diversity of organic form, living and extinct, come to be?’ (Foote 1997, p.129).
Acknowledgements
Special thanks to Lionel Hautier for thoughtful comments. We also thank all curators for permitting and facilitating access to the specimens in their charge, and Anjali Goswami for discussions. For insightful comments on an earlier version of this paper, we thank P. D. Polly and an anonymous reviewer. L.A.B.W. is supported by a Forschungskredit of Universität Zürich (nr 3771) and thanks the Synthesys project, financed by the European Community Research Infrastructure Action under the FP6 Programme, for grants PL-TAF 4504 and SE-TAF 4869. M.R.S.-V. is supported by the Swiss National Fond (3100A0-116013).
References
- Ackermann R. R.2002Patterns of covariation in the hominoid craniofacial skeleton: implications for paleoanthropological models. J. Hum. Evol. 43, 167–187 (doi:10.1006/jhev.2002.0569) [DOI] [PubMed] [Google Scholar]
- Ackermann R. R.2005Ontogenetic integration of the hominoid face. J. Hum. Evol. 48, 175–197 (doi:10.1016/j.jhevol.2004.11.001) [DOI] [PubMed] [Google Scholar]
- Anderson S., Jones J. K., Jr1984Orders and families of recent mammals of the world New York, NY: John Wiley & Sons [Google Scholar]
- Becht G.1953Comparative biologic-anatomical researches on mastication in some mammals. Proc. Koninklijke Nederlandse Akademie van Wetenschappen Series C 564, 508–526 [Google Scholar]
- Blanga-Kanfi S., Miranda H., Penn O., Pupko T., DeBry R. W., Huchon D.2009Rodent phylogeny revised: analysis of six nuclear genes from all major rodent clades. BMC Evol. Biol. 9, 71 (doi:10.1186/1471-2148-9-71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard M., Lefebvre J., Solignac M.1982Analyse en composantes principales de la variabilité de taille, de croissance et de conformation de espèces du complexe Jaera albrifons (Crustacés Isopodes). Cahiers de Biologie Marine 23, 115–142 [Google Scholar]
- Buzan E. V., Krystufek B., Hänfling B., Hutchinson W. F.2008Mitochondrial phylogeny of Arvicolinae using comprehensive taxonomic sampling yields new insights. Biol. J. Linn. Soc. 94, 825–835 (doi:10.1111/j.1095-8312.2008.01024.x) [Google Scholar]
- Carleton M., Musser G.2005Order Rodentia. In Mammal species of the world (eds Wilson D. E., Reeder D. M.), pp. 745–752 Washington, DC: Smithsonian Institution Press [Google Scholar]
- Cheverud J. M.1982Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution 36, 499–516 (doi:10.2307/2408096) [DOI] [PubMed] [Google Scholar]
- Cheverud J. M.1989A comparative analysis of morphological variation patterns in the papionins. Evolution 43, 1737–1747 (doi:10.2307/2409389) [DOI] [PubMed] [Google Scholar]
- Creighton G. K., Strauss R. E.1986Comparative patterns of growth and development in cricetine rodents and the evolution of ontogeny. Evolution 40, 94–106 (doi:10.2307/2408607) [DOI] [PubMed] [Google Scholar]
- Eble G. J.2004The macroevolution of phenotypic integration. In Phenotypic integration (eds Pigliucci M., Preston K.), pp. 253–273 New York, NY: Oxford University Press [Google Scholar]
- Evans A. R., Wilson G. P., Fortelius M., Jernvall J.2007High-level similarity of dentitions in carnivorans and rodents. Nature 445, 78–81 (doi:10.1038/nature05433) [DOI] [PubMed] [Google Scholar]
- Fink W. L., Zelditch M. L.1996Historical patterns of developmental integration in piranhas. Am. Zool. 36, 61–69 [Google Scholar]
- Fontana W., Schuster P.1998Continuity in evolution: on the nature of transitions. Science 280, 1451–1455 (doi:10.1126/science.280.5368.1451) [DOI] [PubMed] [Google Scholar]
- Foote M.1991Morphologic and taxonomic diversity in a clade's history: the blastoid record and stochastic simulation. Univ. Mich. Mus. Paleontol. Contrib. 28, 101–140 [Google Scholar]
- Foote M.1992Rarefaction analysis of morphological and taxonomic diversity. Paleobiology 19, 185–204 [Google Scholar]
- Foote M.1997The evolution of morphological diversity. Ann. Rev. Ecol. Syst. 28, 129–152 (doi:10.1146/annurev.ecolsys.28.1.129) [Google Scholar]
- Frankino W. A., Zwaan B. J., Stern D. L., Brakefield P. M.2005Natural selection and developmental constraints in the evolution of allometries. Science 307, 718–720 (doi:10.1126/science.1105409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber S., Neige P., Eble G. J.2007Combining ontogenetic and evolutionary scales of morphological disparity: a study of Early Jurassic ammonites. Evol. Dev. 9, 472–482 [DOI] [PubMed] [Google Scholar]
- Gerber S., Eble G. J., Neige P.2008Allometric space and allometric disparity: a developmental perspective in the macroevolutionary analysis of morphological disparity. Evolution 62, 1450–1457 (doi:10.1111/j.1558-5646.2008.00370.x) [DOI] [PubMed] [Google Scholar]
- Gibson A. R., Baker A. J., Moeed A.1984Morphometric variation in introduced populations of the common myna (Acridotheres tristis): an application of the jackknife to principal component analysis. Syst. Zool. 33, 408–421 (doi:10.2307/2413092) [Google Scholar]
- Gomez A. M.1992Primitive and derived patterns of relative growth among species of Lorisidae. J. Hum. Evol. 23, 219–233 (doi:10.1016/S0047-2484(05)80001-6) [Google Scholar]
- Goswami A.2006Morphological integration in the carnivoran skull. Evolution 60, 169–183 [PubMed] [Google Scholar]
- Gulotta E. F.1971Meriones unguiculatus Mamm. Species 3, 5 [Google Scholar]
- Hartenberger J. L.1985The order Rodentia: major questions on their evolutionary origin, relationships and suprafamilial systematics. In Evolutionary relationships among rodents: a multidisciplinary analysis (eds Luckett W. P., Hartenberger J. L.), pp. 1–34 New York, NY: Plenum Press [Google Scholar]
- Hautier L., Michaux J., Marivaux L., Vianey-Liaud M.2008The evolution of the zygomasseteric construction in Rodentia, as revealed by a geometric morphometric analysis of the mandible of Graphiurus (Rodentia, Gliridae). Zool. J. Linn. Soc. 154, 807–821 (doi:10.1111/j.1096-3642.2008.00453.x) [Google Scholar]
- Huchon D., Douzery E. P. J.2001From the Old World to the New World: a molecular chronicle of the phylogeny and biogeography of hystricognath rodents. Mol. Phylogenet. Evol. 20, 238–251 (doi:10.1006/mpev.2001.0961) [DOI] [PubMed] [Google Scholar]
- Huchon D., Madsen O., Sibbald M. J. J. B., Ament K., Stanhope M. J., Catzeflis F., de Jong W. W., Douzery E. J. P.2002Rodent phylogeny and a timescale for the evolution of glires: evidence from an extensive taxon sampling using three nuclear genes. Mol. Biol. Evol. 19, 1053–1065 [DOI] [PubMed] [Google Scholar]
- Huxley J. S.1932Problems of relative growth .London,UK: John Hopkins University Press, Methuen [Google Scholar]
- Janis C. M.1995Correlations between craniodental morphology and feeding behaviour in ungulates: reciprocal illumination between living and fossil taxa. In Functional morphology in vertebrate paleontology (ed. Thomason J. J.), pp. 76–98 Cambridge, UK: Cambridge University Press [Google Scholar]
- Jolicoeur P.1963The multivariate generalization of the allometry equation. Biometrics 19, 497–499 (doi:10.2307/2527939) [Google Scholar]
- Klingenberg C. P.1996aIndividual variation of ontogenies: a longitudinal study of growth and timing. Evolution 50, 2412–2428 (doi:10.2307/2410709) [DOI] [PubMed] [Google Scholar]
- Klingenberg C. P.1996bMultivariate allometry. In Advances in morphometrics (eds Marcus L. F., Corti M., Loy A., Slice D., Naylor G.), pp. 23–49 New York, NY: Plenum Press [Google Scholar]
- Klingenberg C. P., Ekau W.1996A combined morphometric and phylogenetic analysis of an ecomorphological trend: pelagization in Antarctic fishes (Perciformes: Nototheniidae). Biol. J. Linn. Soc. 59, 143–177 (doi:10.1111/j.1095-8312.1996.tb01459.x) [Google Scholar]
- Klingenberg C. P., Froese R.1991A multivariate comparison of allometric growth patterns. Syst. Zool. 40, 410–419 (doi:10.2307/2992236) [Google Scholar]
- Klingenberg C. P., Spence J. R.1993Heterochrony and allometry: lessons from the water strider genus Limnoporus. Evolution 47, 1834–1853 (doi:10.2307/2410225) [DOI] [PubMed] [Google Scholar]
- Klingenberg C. P., Zimmermann M.1992Static, ontogenetic, and evolutionary allometry: a multivariate comparison in nine species of water striders. Am. Nat. 140, 601–620 (doi:10.1086/285430) [Google Scholar]
- Marroig G., Cheverud J. M.2001A comparison of phenotypic variation and covariation patterns and the role of phylogeny, ecology, and ontogeny during cranial evolution of New World monkeys. Evolution 55, 2576–2600 [DOI] [PubMed] [Google Scholar]
- Mendoza M., Janis C. M., Palmqvist P.2002Characterizing complex craniodental patterns related to feeding behaviour in ungulates: a multivariate approach. J. Zool. 258, 223–246 (doi:10.1017/S0952836902001346) [Google Scholar]
- Michaux J., Chevret P., Renaud S.2007Morphological diversity of Old World rats and mice (Rodentia, Muroidae) mandible in relation with phylogeny and adaptation. J. Zool. Syst. Evol. Res. 45, 263–279 (doi:10.1111/j.1439-0469.2006.00390.x) [Google Scholar]
- Moore W. J.1981The mammalian skull Cambridge, UK: Cambridge University Press [Google Scholar]
- Nowak R. M.1999Walker's mammals of the world, 6th edn.Baltimore, MD: John Hopkins University Press [Google Scholar]
- Olson E. C., Miller R. L.1958Morphological integration Chicago, IL: University of Chicago Press [Google Scholar]
- Pearson K.1901On lines and planes of closest fit to systems of points in space. Phil. Mag. 2, 559–572 [Google Scholar]
- Pérez E. M.1992Agouti paca Mamm. Species 9, 7 [Google Scholar]
- Reeve E. C. R., Huxley J. S.1945Some problems in the study of allometric growth. In Essays on growth and form (eds le Gros Clark W. E., Medawar P. B.), pp. 121–156 Oxford, UK: Claredon Press [Google Scholar]
- Samuels J. X.2009Cranial morphology and dietary habits of rodents. Zool. J. Linn. Soc. 156, 864–888 (doi:10.1111/j.1096-3642.2009.00502.x) [Google Scholar]
- Satoh K.1997Comparative functional morphology of mandibular forward movement during mastication of two muroid rodents, Apodemus speciosus (Murinae) and Clethrionomys rufocanus (Arvicolinae). J. Morphol. 231, 131–142 (doi:10.1002/(SICI)1097-4687(199702)231:2<131::AID-JMOR2>3.0.CO;2-H) [DOI] [PubMed] [Google Scholar]
- Solignac M., Cariou M.-L., Wimitzki M.1990Variability, specificity and evolution of growth gradients in the species complex Jaera albifrons (Isopoda, Asellota). Crustaceana 59, 121–145 (doi:10.1163/156854090X00615) [Google Scholar]
- Stadler B. M. R., Stadler P. F., Wagner G. P., Fontana W.2001The topology of the possible: formal spaces underlying patterns of evolutionary change. J. Theor. Biol. 213, 241–274 (doi:10.1006/jtbi.2001.2423) [DOI] [PubMed] [Google Scholar]
- Steppan S. J.1997Phylogenetic analysis of phenotypic covariance structure. II. Reconstructing matrix evolution. Evolution 51, 587–594 (doi:10.2307/2411130) [DOI] [PubMed] [Google Scholar]
- Steppan S. J., Adkins R. M., Anderson J.2004Phylogeny and divergence date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst. Biol. 53, 533–553 (doi:10.1080/10635150490468701) [DOI] [PubMed] [Google Scholar]
- Van Valkenburgh B.1989Carnivore dental adaptations and diet: a study of trophic diversity within guilds. In Carnivore behaviour ecology, and evolution (ed. Gittleman J. L.), pp. 410–436 Ithaca, NY: Cornell University Press [Google Scholar]
- Weisbecker V., Schmid S.2007Autopodial skeletal diversity in hystricognath rodents: functional and phylogenetic aspects. Mamm. Biol. 72, 27–44 (doi:10.1016/j.mambio.2006.03.005) [Google Scholar]
- Weston E. M.2003Evolution of ontogeny in the hippopotamus skull: using allometry to dissect developmental change. Biol. J. Linn. Soc. 80, 625–638 (doi:10.1111/j.1095-8312.2003.00263.x) [Google Scholar]
- Williams S. H., Kay R. F.2001A comparative test of adaptive explanations for hypsodonty in ungulates and rodents. J. Mamm. Evol. 8, 207–229 (doi:10.1023/A:1012231829141) [Google Scholar]
- Willner G. R., Dixon K. R., Chapman J. A.1983Ondatra zibethicus Mamm. Species 141, 8 [Google Scholar]
- Wilson D. E., Reeder D. M.2005Mammal species of the world, 3rd edn Baltimore, MD: John Hopkins University Press [Google Scholar]
- Wilson L. A. B., Sánchez-Villagra M. R.2009Heterochrony and patterns of cranial suture closure in hystricognath rodents. J. Anat. 214, 339–354 (doi:10.1111/j.1469-7580.2008.01031.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. E.1955A revised classification of the rodents. J. Mammal. 36, 165–187 (doi:10.2307/1375874) [Google Scholar]
- Wood A. E.1959Eocene radiation and phylogeny of the rodents. Evolution 13, 354–361 (doi:10.2307/2406112) [Google Scholar]
- Woods C. A.1972Erethizon dorsatum Mamm. Species 29, 1–6 [Google Scholar]
- Zelditch M. L., Carmichael A. C.1989aGrowth and intensity of integration through postnatal growth in the skull of Sigmodon fulviventer. J. Mammal. 70, 477–484 (doi:10.2307/1381419) [Google Scholar]
- Zelditch M. L., Carmichael A. C.1989bOntogenetic variation in patterns of developmental and functional integration in skulls of Sigmodon fulviventer. Evolution 43, 814–824 (doi:10.2307/2409309) [DOI] [PubMed] [Google Scholar]
- Zelditch M. L., Straney D. O., Swiderski D. L., Carmichael A. C.1990Variation in developmental constraints in Sigmodon. Evolution 44, 1738–1747 (doi:10.2307/2409503) [DOI] [PubMed] [Google Scholar]
- Zelditch M. L., Sheets D. H., Fink W. L.2003The ontogenetic dynamics of shape disparity. Paleobiology 29, 139–156 (doi:10.1666/0094-8373(2003)029<0139:TODOSD>2.0.CO;2) [Google Scholar]