Abstract
In the ant species Tetramorium caespitum, communication and foraging patterns rely on group-mass recruitment. Scouts having discovered food recruit nestmates and behave as leaders by guiding groups of recruits to the food location. After a while, a mass recruitment takes place in which foragers follow a chemical trail. Since group recruitment is crucial to the whole foraging process, we investigated whether food characteristics induce a tuning of recruiting stimuli by leaders that act upon the dynamics and size of recruited groups. High sucrose concentration triggers the exit of a higher number of groups that contain twice as many ants and reach the food source twice as fast than towards a weakly concentrated one. Similar trends were found depending on food accessibility: for a cut mealworm, accessibility to haemolymph results in a faster formation of larger groups than for an entire mealworm. These data provide the background for developing a stochastic model accounting for exploitation patterns by group-mass recruiting species. This model demonstrates how the modulations performed by leaders drive the colony to select the most profitable food source among several ones. Our results highlight how a minority of individuals can influence collective decisions in societies based on a distributed leadership.
Keywords: ants, foraging, decision-making, leader, group recruitment
1. Introduction
In eusocial insects—bees, termites or ants—many collective activities, such as foraging, territorial defence or nest building, display complex spatio-temporal patterns that are efficient responses to environmental constraints and opportunities (Hölldobler & Wilson 1990; Seeley 1995). Those collective behaviours require coordination and cooperation among individuals which may differ in their needs and motivation. As such societies can count hundreds or even thousands of nestmates, a centralized decision-making is impossible to achieve since no individual can get an accurate overview of colony needs, nor can interact with all nestmates. Thus, collective decisions are often based on self-organized processes and emerge from numerous interactions among nestmates which are assumed to follow simple decision rules based on local information (Franks & Deneubourg 1997; Camazine et al. 2001; Detrain & Deneubourg 2006). The study of foraging activity has provided several evidences of such decentralized decision-making especially in ants performing mass recruitment (MR). In this later case, successful ants having discovered food lay a chemical trail during their homeward journey that triggers the exit of nestmates and leads them to the food location. Colonies of these mass–recruiting species are able to focus their foraging on the most profitable food resources thanks to a tuning of trail-laying behaviour by individual foragers (Beckers et al. 1993; Detrain et al. 1999; Mailleux et al. 2000, 2003; Portha et al. 2004; Deneubourg et al. 2005; Devigne & Detrain 2006). In this anonymous and democratic decision-making process, all informed foragers participate in the formation of trails without any individual having a heavier weight on collective choices.
In this paper, we study an original mode of recruitment—i.e. the group-mass recruitment (Stradling 1970; Verhaeghe 1978; Biseau et al. 1994; Cerdá et al. 2009). During the first foraging steps, scouts having discovered food perform group recruitment (GR) and behave as leaders. Indeed, the laying of a chemical trail (Attygale & Morgan 1984) needs to be coupled to the physical presence of those leaders that guide recruits and improve their reaching of the food source (Verhaeghe 1978). Once a sufficient number of foragers have reinforced the trail, the initial GR progressively shifts towards a MR in which the trail alone ensures the mobilization of nestmates even in the absence of any leaders. Since leaders are the only ones to own information about food location during the first steps of recruitment, they have de facto a strong influence on collective choices as well as on the global foraging efficiency of ant colonies. Decision-making is then shared only between a subset of individuals and relies on a distributed leadership.
Here, we investigate how leaders of a group mass recruiting ant species, Tetramorium caespitum, act upon collective decisions by tuning their recruitment behaviour according to food quality or to food accessibility. The influence of food quality was tested by offering solutions differing by their sucrose concentration (0.1 M or 1 M). We expect that leaders will increase their recruitment signal according to food energetic content. To test the influence of food accessibility, we offered a mealworm characterized either by a high accessibility (cut mealworm) or by low accessibility to haemolymph (entire mealworm). We predict a higher recruitment towards a cut mealworm since this prey incurs lower handling costs and provides a direct access to haemolymph. Based on those experiments, we built a stochastic model to assess the influence of group leaders on the foraging patterns exhibited by the ant colony.
2. Material and Methods
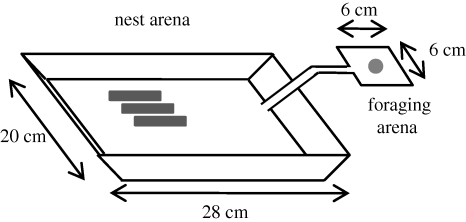
Two T. caespitum ant colonies were collected on rocky slopes of an old quarry at Vaucelles (Belgium). Both were queenless, broodless and contained around 2500 workers. Each colony was reared in the laboratory at a room temperature of 23 ± 2°C with a constant photoperiod of 12 h d−1. The experimental setup consisted of a plastic nest arena (20 × 28 cm) coated with fluon to prevent ants’ escape and connected to a plastic foraging arena (6 × 6 cm) by a cardboard bridge (12 × 1 cm; figure 1). The nest consisted of three test tubes covered with red paper providing obscurity for the ants’ settlement. The two last centimeters of each tube were filled with water to ensure a sufficient humidity inside the nest and was separated from the rest of the tube by a cotton plug. The ants also had access to water and sucrose solution (1 M) ad libitum and were fed with two mealworms (Tenebrio molitor larvae) twice a week.
Figure 1.
Experimental setup.
Before each experiment, colonies were deprived of food for 4 days but kept access to water. After this starvation period, we offered, in the centre of the foraging arena, one of the following food sources: a 600 µl droplet of 1 M sucrose solution, a 600 µl droplet of 0.1 M sucrose solution, a mealworm cut into five pieces or an entire dead mealworm (mean weight + s.d.: 0.20 g ± 0.01 g, n = 20). Then, we recorded during 90 min the behaviour of ants present on the nest arena, the bridge, and the foraging arena. Four replicates were made for each food type and for each colony. We thus performed 32 experiments that were all carried out during spring to avoid any seasonal variation in food preferences. Moreover, ants never received the same food twice consecutively in order to prevent habituation.
To standardize our estimation of group size, we always used the two following criteria to define a group. First, a group should be a single file of recruited ants following a leader. Second, the distance between two consecutive ants within the file should always be lower than 3 cm. Beyond this threshold distance, the ant was not considered as belonging to the group of recruits. Several parameters were measured on video recordings.
— The number of groups that reach the foraging arena.
— The arrival time of these groups on the foraging arena.
— The size of these groups measured at the entrance of the foraging arena.
— The time at which any ant enters or exits the foraging arena.
— The time at which ants switch from GR to MR. This occurs when for the first time a group that was identified at the bridge entrance could not be followed until the bridge end because it melted into the heavy traffic of foragers.
— The probability for a scout to initiate the formation of a group. Since the reaching of the food source is a prerequisite before recruiting nestmates, the probability for a scout to become a leader is given by the number of leaders divided by the total number of fed ants having exited the foraging arena from the start of the experiment until the passage of the ith leader. This probability is a dynamic value which can change in the course of the recruitment and can be calculated after the passage of the 1st, 2nd or ith leader. We choose to calculate this probability for a number of leaders equal to 7. Indeed, this value (i = 7) seems the best trade-off between considering a sufficient number of leaders to get a reliable probability value and focusing our estimate on the beginning of recruitment when groups have not yet melted into MR. This arbitrary number of leaders i = 7 was reached in all cases excepted in three experiments for sucrose 0.1 M, three for the entire mealworm and one for the cut mealworm in which less than seven group leaders were observed.
(a). Model
We developed a stochastic multi-agents model to simulate responses of an ant colony (1000 workers) faced with two food sources offered simultaneously. It allowed us to tune each parameter of GR (based on our experimental values) and to set apart the respective influence of trail-laying foragers and of leaders on collective choices. Moreover, such a stochastic approach takes into account random events that are amplified and could orient collective choices especially when the proportion of leaders that can potentially influence the choice of the colony is very weak (0.1 or 0.03).
In this model, ants are characterized by (i) their location—i.e. inside the nest or at one of the two food sources—and (ii) their food knowledge—i.e. being informed or not (=naive) about the location of a food source.
At time 0, all the ants are naive and stay inside the nest. At each time step, naive ants have a probability Pe to exit the nest that increases with the total concentration (C1 + C2) of both trails leading to food sources (Beckers et al. 1993):
In this equation, the ratio ka/b stands for the probability for an ant to spontaneously exit the nest in the absence of any recruitment trail (C1 + C2=0). We choose the values a = 10, b = 6000 and k = 0.05 so that, at each time step, each ant has a constant probability of 1/12 000 to leave the nest. Thus, for a colony of 1000 workers, a spontaneous exit occurs every 13 s on average. This value fits to our experiments in which the rate of ants leaving the nest and discovering the food source in the absence of recruitment was found to be 0.078 ants per second (linear regression slope of the cumulated number of ants entering the foraging area over time, R2 = 0.67), accounting for a spontaneous arrival on the foraging arena every 12.8 s. Then, as soon as a trail is laid, the probability to exit the nest increases non-linearly with the total concentration of pheromones on both trails (C1 + C2). This nonlinear increase follows a logistic curve in which Pe tends towards the value k = 0.05 which is the maximal exit rate per ant. The exponent value n, that was fixed at n = 2 (as for Lasius niger in Beckers et al. 1993), indicates the sensitivity of ants’ exit response (Pe) to changes in the total concentration of trail pheromones.
Once they have left the nest, naive ants are faced with a choice between sources 1 and 2. For this binary choice, the probability for a naive ant to choose source 1 (and by extension, to choose source 2) is given by the function detailed in Nicolis & Deneubourg (1999):
in which C1 and C2 stand for the concentrations of trails leading, respectively, to food sources 1 and 2. The parameter d corresponds to the intrinsic degree of attraction of an unmarked branch of the bridge: a low d value means that ants are more sensitive to small differences between the two trails. In this function we arbitrarily choose d = 6 which is the estimated value for L. niger (Beckers et al. 1992, 1993). The parameter r determines the level of sensitivity of ants’ response to changes in the intensity of recruiting signals and was fixed at r = 2 as for L. niger species (Beckers et al. 1992, 1993). An r value greater than 1 was chosen in order to account for amplifying processes and collective selection of one food source between two identical ones as experimentally observed in T. caespitum and L. niger species (Pasteels et al. 1987).
Once ants have made a choice, they become informed about the location of the food source they have visited. After a mean feeding time τ, ants come back to the nest. We choose τ = 720 time steps which corresponds to 12 min spent by the ants outside the nest as experimentally observed for ants exploiting a 1 M food source (linear regression, R2 = 0.84). Each returning ant lays a quantity q = 1 of pheromone that reinforces the chemical trail which evaporates at rate fixed at ν = 0.005. Once they enter the nest, these informed ants have a constant probability ε = 0.005 to forget the location of the discovered food source and to rejoin the pool of naive individuals. Alternatively, if informed ants do not forget the location of the discovered food source, they have a probability β = 0.01 to exit the nest and to come back to this food source (an informed ant spend a mean time of 100 s inside the nest). Moreover, informed ants exiting the nest have a probability pl to become a leader by recruiting a group of nestmates of size α and by leading them towards the food location they have memorized.
By using this stochastic model, we investigated how a tuning of pl and α values within the range of experimental data acts upon collective decisions in ants. At the end of each simulation (10 000 time steps), the concentration of each chemical trail is used as an indicator of the exploitation level of each food source.
3. Results
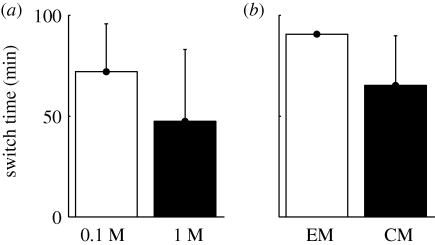
At the beginning of each experiment, groups following leaders can be easily differentiated since the ants’ flow remains low over the bridge. But, as the number of ants reaching the food source increases, a recruitment trail begins to appear and triggers the exit of additional nestmates even in the absence of any leader ant. When such a MR takes place, it becomes impossible to confidently identify groups following leaders since they are mingled within the heavy traffic of mass-recruited foragers. Hence, we took into account only the groups that were formed before the switch from GR to MR. The mean switch times differ for the two different sucrose concentrations and for the two accessibility levels of prey (figure 2).
Figure 2.
Switch time (mean + s.d.) (a) for different concentrations of sucrose solution (white bar, 0.1 M; black bar, 1 M) and (b) for different prey accessibility (white bar, entire mealworm (EM); black bar, cut mealworm (CM)).
The switch appears after 45 min for the 1 M sucrose solution and after 75 min for the 0.1 M one. A high sucrose concentration seems to hasten the occurrence of MR even though this difference is not statistically significant (Mann–Whitney: n1 = 8, n2 = 8, p = 0.17). Likewise, the accessibility of the prey influences the switch time since direct access to haemolymph in cut mealworms results in a quicker occurrence of MR than towards entire prey (Mann–Whitney: n1 = 8, n2 = 8, p = 0.025).
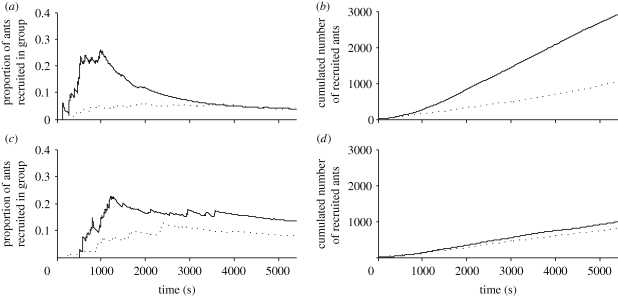
The mean proportion of ants recruited in groups also changes over time for each food type. Figure 3a gives, for each time step and for different sucrose concentrations, the ratio between the cumulated number of ants recruited in groups and the total number of ants having reached the food source. These proportions grow until a maximum value is followed by a plateau or even a decrease that reflects the more important part taken over time by MR. At the beginning of recruitment, the proportion of groups is three times higher when they are recruited towards a 1 M than a 0.1 M sucrose solution. In parallel, a 1 M sucrose solution induces a higher mobilization of foragers whatever the recruitment phase (GR and MR; figure 3b). We find the same trend for prey of different accessibility. In the presence of a cut mealworm, for a similar level of workers’ mobilization, a higher relative number of ants are recruited by leaders than towards an entire prey (figure 3c,d). Hence, the contribution of GR is more important when ants exploit a richer or an easily accessible food source, particularly during the first steps of the recruitment.
Figure 3.
Contribution of group recruitment. Mean proportion of ants recruited in groups (a) towards a sucrose solution (solid line, sucrose 1 M; dotted line, sucrose 0.1 M) or (c) towards a prey (solid line, cut mealworm; dotted line, entire mealworm). Cumulated number of ants entering the foraging arena (b) towards a sucrose solution (solid line, 1 M; dotted line, 0.1 M) or (d) towards a prey (solid line, cut mealworm; dotted line, entire mealworm).
The prevalence of GR at the early stages of foraging led us to investigate how leaders modulate their recruiting behaviour according to food characteristics and thereby influence colony foraging patterns.
(a). Influence of food quality
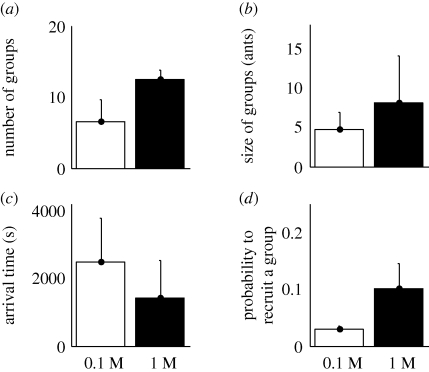
A 1 M sucrose solution triggers a more intense GR, with nearly twice as many groups of recruits exiting the nest than towards a 0.1 M sucrose solution (figure 4a; t-test: n1 = 8, n2 = 8, p < 0.001). The number of ants guided by a leader varies from 1 to 10 workers for the 0.1 M sucrose droplet and from 2 to 25 workers for the 1 M sucrose food source. On average, the number of ants following a leader is significantly larger towards a highly concentrated sucrose solution (figure 4b; Mann–Whitney: n1 = 51, n2 = 99, p = 0.0001). Furthermore, those groups of recruits reach the foraging area twice as early when they are led towards a richer food (figure 4c; Mann–Whitney: n1 = 51, n2 = 99, p < 0.0001). Such an earlier arrival at the 1 M food does not result from an increased walking speed. Instead, it reflects the quicker launching of recruitment within the nest since a higher number of groups are recruited in a shorter time period. On the other hand, as MR towards a 0.1 M droplet appears later, groups are observed over a wider time scale and show a twice as long arrival time.
Figure 4.
Influence of sucrose concentrations (0.1 M and 1 M) on group recruitment. (a) Number of recruited groups. (b) Size of recruited groups. (c) Arrival time of recruited groups on the foraging arena. (d) Probability for a successful scout to recruit a group. All given values are mean + s.d.
As regards the recruiting behaviour of scouts, they show a significantly higher probability to recruit a group of nestmates towards a 1 M sucrose solution than towards a 0.1 M one (figure 4d; t-test: n1 = 5, n2 = 8, p < 0.02). Indeed, 10 per cent of scouts that discover a 1 M droplet recruit a group of nestmates while only 3 per cent do it when recruiting towards a 0.1 M sucrose droplet. Hence, a higher sucrose concentration not only speeds up the global recruitment dynamics, but also influences the individual decision and efficiency of scouts to launch GR.
(b). Influence of food accessibility
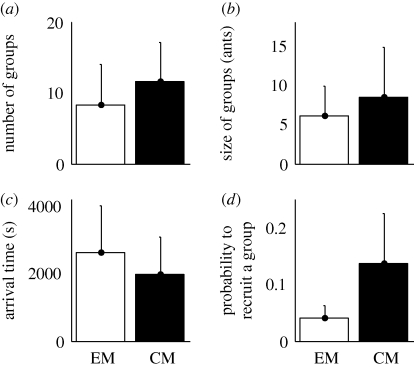
When ants are faced with mealworms, the accessibility to haemolymph does not significantly influence the mean number of recruited groups (figure 5a; t-test: n1 = 8, n2 = 8, p > 0.05). On the other hand, the mean size of groups is significantly larger (figure 5b; Mann–Whitney: n1 = 71, n2 = 92, p = 0.01) and these groups reach the food source earlier when workers are recruited towards a cut prey (figure 5c; Mann–Whitney: n1 = 71, n2 = 92, p < 0.01).
Figure 5.
Influence of prey accessibility (mealworm larva cut in pieces (CM) or entire (EM)). (a) Number of recruited groups. (b) Size of recruited groups. (c) Arrival time of recruited groups on the foraging arena. (d) Probability for a successful scout to recruit a group. All given values are mean + s.d.
As regards the recruiting behaviour of scouts, they show a higher probability to trigger the exit of nestmates and to lead them towards a cut larva (figure 5d; Mann–Whitney: n1 = 5, n2 = 7, p < 0.02). Nearly 15 per cent of scouts lead a group towards a mealworm cut in pieces compared to less than 5 per cent towards an entire prey.
(c). Simulation of collective choices
In our simulations, a colony is assumed to perform group-mass recruitment and to be faced with two food sources (f1 and f2) of the same quality (1 M versus 1 M sucrose solution) or of different quality (0.1 M versus 1 M sucrose solution). For each situation, we performed 5000 simulations and considered that the colony had selected a food source when the trail leading to it accounted for more than 75 per cent of the total pheromone laid by foragers at the end of the simulation.
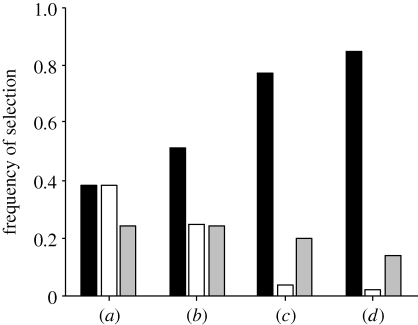
When two identical food sources are presented (f1 = f2 = 1 M sucrose solution), one of the two food sources is selected by the colony in 80 per cent of the simulations (figure 6a). Moreover, as both food sources are identical, the colony selects either the f1 or f2 food source.
Figure 6.
Theoretical results obtained for 5000 simulations illustrating the selection frequency of food sources by a colony faced with: (a) two identical food sources (f1 = f2 = 1 M sucrose solution); (b–d) two different food sources (f1 = 1 M and f2 = 0.1 M), (b) with a tuning of the group size, (c) with a tuning of the probability to recruit a group; and (d) with a tuning of both group size and probability to recruit a group. Black bar, food source 1, f1; white bar, food source 2, f2; grey bar, no selection.
When two food sources of different quality are offered (f1 = 1 M and f2 = 0.1 M), we investigated how a tuning of GR similar to that observed experimentally influence colony foraging responses. As regards the influence of group size on collective choices, we assumed that groups of four recruits are led to the 0.1 M sucrose solution while groups of eight recruits are guided towards the 1 M one. All other parameters were kept equal for both sources. The tuning of group sizes allows the colony to select the more concentrated food source in 50 per cent of cases (figure 6b). We also performed simulations in which we modulated the probability for an ant to become a leader while keeping unchanged the size of the recruited group (eight ants per group). Then, ants returning to the 0.1 M food source have a probability of 0.03 to recruit a group that is threefold lower than the probability of 0.1 to become a group leader towards a 1 M solution. Again, this tuning of the probability to become a leader enables the colony to select the best food source in 75 per cent of the cases (figure 6c). Finally, we investigated colony responses when both the probability to become a leader and the size of recruited groups are tuned. In this case, ants are able to select the best food source with a higher accuracy in 90 per cent of the cases (figure 6d).
4. Discussion
Ant species belonging to Tetramorium genus combine GR and MR when exploiting food sources. Earlier works have shown that there is a tuning of individual trail-laying intensity according to sucrose concentration (Verhaeghe 1978, 1982). Here, we demonstrate that there is also a tuning of GR by T. caespitum leaders during the first steps of food exploitation. Our study provides experimental evidence that groups of recruits are faster to form, larger, and more frequently recruited towards a food droplet with a higher energetic content in sugars. Likewise, when insects are exploited, T. caespitum leaders adjust the intensity of their GR to the accessibility of discovered prey. Towards a cut mealworm of which the haemolymph is accessible, scouts show a higher propensity to recruit large groups of nestmates that are faster to form. Thus, leaders preferentially mobilize nestmates towards food items that require lower handling costs and provide easier access to edible parts.
(a). Role of leaders on foraging choices
Based on those experimental results, we built a model that links the individual tuning of recruitment and the collective choice of a colony faced with two food sources (identical or differing in quality). Our model confirms that a modulation of GR by individual leaders accounts for the selection of the most profitable food at the collective level. Those theoretical predictions are in agreement with previous experiments showing the ability of T. caespitum foragers to counteract an established trail and to reorient their exploitation towards a richer sucrose solution (Pasteels et al. 1987; Beckers et al. 1990). As suggested by these authors, the successive burst of recruits guided by leaders towards a new richer food source could be ‘the necessary impulse to beat an established trail’.
(b). Role of leaders in the emergence of organized patterns
Since ant collective patterns emerge from the multiple interactions between nestmates, they strongly depend on the local density of foragers of which an increase leads to a higher potential for amplification processes and related outcomes (Detrain et al. 1991; Detrain & Deneubourg 2006). In mass recruiting ant species, such an increase of local ant density may result from the laying of an attracting pheromone by scouts while they explore new areas (e.g. in Pheidole pallidula, Detrain et al. 1991; Monomorium pharaonis, Beekman et al. 2001; L. niger, Devigne & Detrain 2006). In group recruiting ant species—like T. caespitum—group leaders achieve the same goal: during the first steps of recruitment, group leading prevails but once a sufficient density of foragers is mobilized, a chemical trail emerges and drains the majority of foragers. The transition from GR to MR depends on the ability of one colony to reach a critical local density of foragers. By tuning the size and frequency of recruited groups, T. caespitum leaders will increase the ant density in the vicinity of more profitable food sources and thereby will fasten and favour the formation of a trail as well as the collective exploitation of those food sites.
(c). Distributed leadership and decision-making
In animal societies, group members have to make collective decisions that best fit their needs and that optimally exploit environmental opportunities. However, the underlying process varies deeply from a despotic decision by one individual to a collegial decision involving all group members (Conradt & Roper 2003, 2005; List 2004; Austen-Smith & Feddersen 2009). The usual case is intermediate as observed for foraging decisions in T. caespitum colonies. Indeed, decision-making is distributed between a subset of leader individuals that are informed about food opportunities and that weigh more heavily on colony choices by recruiting groups of nestmates to a specific food location. Such a distributed leadership not only concerns GR in ants but has been evidenced in other social insects. For instance, in stingless bees, a small subset of individuals—called pilots—recruit groups of nestmates and lead them towards the food source (Aguilar et al. 2005) and thus should exert a similar role to ant leaders on the hive's foraging patterns.
Even though leaders account for a minority of workers taking part in food recruitment, they play a key role in the emergence of collective foraging decisions. Our study has highlighted how a small group of individuals, by a tuning of information transfer, can theoretically lead the colony to select the most profitable food source. Such a distributed leadership between several individuals appears to be a good trade-off between the difficulty for all colony members to get involved in the decision-making process and the risk of a centralized decision taken by one individual not necessarily possessing the most valuable information. A model developed by Couzin et al. (2005) and experimentally validated in human groups (Dyer et al. 2009) shows that a 5 per cent of informed individuals is sufficient to lead a moving group with a good accuracy. Likewise, in social insects, 5–10% of informed nestmates are able to guide the collective choice of swarming honeybees (Jason et al. 2005) or of foraging ants as shown in this study.
Societies relying on a distributed leadership can make decisions in two different ways. First, the respective ‘opinion’ of leaders can be pooled and/or compared until a common decision emerges (Conradt & Roper 2005). The reaching of consensus-based decisions especially matters in the case of nest moving in order to guarantee the cohesion of the community. This may require time in large societies such as for house-hunting honeybees that may dance for three days before reaching unanimity (Seeley & Buhrmann 1999; Leadbeater & Chittka 2007). Other examples can be found in ant colonies selecting a new nest site (Franks et al. 2002), or in groups of baboons choosing a travel direction during the morning departure (Stueckle & Zinner 2008). Second, leaders can behave and recruit congeners on their own without consulting each other or reaching a common agreement. This is the case for T. caespitum during foraging: the majority of workers are recruited towards the best food source without any previous consensus between leaders. At first sight, the absence of consensus is amazing and may seem maladaptive. However, while nest site selection obviously needs unanimity, the situation is quite different in the case of foraging. Indeed, the maintenance of a diversity of ‘opinions’ makes the ant colony more prone to respond adaptively to changes in food availabilities, such flexibility being difficult to achieve by the time-consuming process of consensus building.
Of the various recruitment processes in ants, tandem running is another case of distributed leadership, in which leader individuals guide recruits one by one towards the food source. Such a direct and individualized leading results in a slow but highly accurate transfer of information about food/or nest site opportunities (Richardson et al. 2007). Our study illustrates that group-mass recruitment of T. caespitum seems to be a wonderful intermediate between tandem running and MR. Compared to tandem running, group leaders recruit not only one worker but several nestmates which speeds up information transfer and recruitment towards discovered resources. Moreover, unlike tandem-running, changes in size and occurrence of groups provide group-mass recruiting species with additional ways to fine tune their foraging responses, giving individual leaders more influence. Compared to MR, group leaders improve the accuracy of information transfer about food location and make recruits more likely to reach the food target than through the following of a single chemical trail (Verhaeghe 1978). Moreover, while the chemical trail of mass-recruiting species would evaporate under hot temperatures, the physical presence of leaders coupled to a chemical communication at short range allow GR to persist and remain efficient over a wider range of temperatures and habitats including xeric biotopes (Cerdá et al. 2009).
Acknowledgements
We thank Profs A. Lenoir and J. C. Verhaeghe for their advice and help in collecting ant colonies, Prof. J. L. Deneubourg for fruitful discussions and two anonymous referees for constructive comments and remarks. This study was funded by an FRFC grant (2.4600.09) and a PhD grant from FRIA (Fonds pour la Recherche dans l’Industrie et dans l’Agriculture). C.D. is a senior research associate from the Belgian National Fund for Scientific Research (F.N.R.S.).
References
- Aguilar I., Fonseca A., Biesmeijer C.2005Recruitment and communication of food source location in three species of stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 36, 313–324 (doi:10.1051/apido:2005005) [Google Scholar]
- Attygale A. B., Morgan E. D.1984Identification of trail pheromone of the ant Tetramorium caespitum L. J. Chem. Ecol. 10, 1453–1468 (doi:10.1007/BF00990315) [DOI] [PubMed] [Google Scholar]
- Austen-Smith D., Feddersen T. J.2009Information aggregation and communication in committees. Phil. Trans. R. Soc. B 364, 763–769 (doi:10.1098/rstb.2008.0256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers R., Deneubourg J. L., Goss S., Pasteels J. M.1990Collective decision through food recruitment. Insectes Soc. 37, 258–267 (doi:10.1007/BF02224053) [Google Scholar]
- Beckers R., Deneubourg J. L., Goss S.1992Trails and U-turns in the selection of a path by the ant Lasius niger. J. Theor. Biol. 159, 397–415 (doi:10.1016/S0022-5193(05)80686-1) [Google Scholar]
- Beckers R., Deneubourg J. L., Goss S.1993Modulation of trail laying in the ant Lasius niger (Hymenoptera: Formicidae) and its role in the collective selection of a food source. J. Insect Behav. 6, 751–759 (doi:10.1007/BF01201674) [Google Scholar]
- Beekman M., Sumpter D. J. T., Ratnieks F. L. W.2001Phase transition between disordered and ordered foraging in Pharaoh's ants. Proc. Natl Acad. Sci. USA 98, 9703–9706 (doi:10.1073/pnas.161285298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biseau J. C., de Schuiten M., Pasteels J. M., Deneubourg J. L.1994Respective contributions of leader and trail during recruitment to food in Tetramorium bicarinatum. (Hymenoptera Formicidae). Insectes Soc. 41, 241–254 (doi:10.1007/BF01242295) [Google Scholar]
- Camazine S., Deneubourg J. L., Franks N. R., Sneyd J., Theraulaz G., Bonabeau E.2001Self-organization in biological systems Princeton, NJ: Princeton University Press [Google Scholar]
- Cerdá X., Angulo E., Boulay R., Lenoir A.2009Individual and collective foraging decisions: a field study of worker recruitment in the gypsy ant Aphaenogaster senilis. Behav. Ecol. Sociobiol. 63, 551–562 (doi:10.1007/s00265-008-0690-5) [Google Scholar]
- Conradt L., Roper T. J.2003Group decision-making in animals. Nature 421, 155–158 (doi:10.1038/nature01294) [DOI] [PubMed] [Google Scholar]
- Conradt L., Roper T. J.2005Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456 (doi:10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- Couzin I. D., Krause J., Franks N. R., Levin S. A.2005Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516 (doi:10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- Deneubourg J. L., Nicolis S., Detrain C.2005Optimality of communication in self-organized social behaviour. In Self-organization and evolution in social systems (ed. Hemelrijk C.), pp. 25–35 Cambridge, UK: Cambridge University Press [Google Scholar]
- Detrain C., Deneubourg J. L.2006Self-organized structures in a superorganism: do ants ‘behave’ like molecules? Phys. Life Rev. 3, 162–187 (doi:10.1016/j.plrev.2006.07.001) [Google Scholar]
- Detrain C., Deneubourg J. L., Goss S., Quinet Y.1991Dynamics of collective exploitation in the ant Pheidole pallidula. Psyche 98, 21–32 (doi:10.1155/1991/75196) [Google Scholar]
- Detrain C., Deneubourg J. L., Pasteels J.1999Information processing in social insects Basel, Switzerland: Birkhauser [Google Scholar]
- Devigne C., Detrain C.2006How does food distance influence foraging in the ant Lasius niger: the importance of home-range marking. Insectes Soc. 53, 46–55 (doi:10.1007/s00040-005-0834-9) [Google Scholar]
- Dyer J. R. G., Johansson A., Helbing D., Couzin I. D., Krause J.2009Leadership, consensus decision making and collective behaviour in humans. Phil. Trans. R. Soc. B 364, 781–789 (doi:10.1098/rstb.2008.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. R., Deneubourg J. L.1997Self-organizing nest construction in ants: individual worker behaviour and the nest's dynamics. Anim. Behav. 54, 779–796 (doi:10.1006/anbe.1996.0496) [DOI] [PubMed] [Google Scholar]
- Franks N. R., Pratt S. C., Mallon E. B., Britton N. F., Sumpter J. T.2002Information flow, opinion polling & collective intelligence in house-hunting social insects. Phil. Trans. R. Soc. Lond. B 357, 1567–1583 (doi:10.1098/rstb.2002.1066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölldobler B., Wilson E. O.1990The ants Cambridge, MA: Belknap Press of Harvard University [Google Scholar]
- Jason S., Middendorf M., Beekman M.2005Honeybee swarms: how do scouts guide a swarm of uninformed bees? Anim. Behav. 70, 349–358 (doi:10.1016/j.anbehav.2004.10.018) [Google Scholar]
- Leadbeater E., Chittka L.2007Social learning in insects: from miniature brains to consensus building. Curr. Biol. 17, R703–R713 (doi:10.1016/j.cub.2007.06.01) [DOI] [PubMed] [Google Scholar]
- List C.2004Democracy in animal groups: a political science perspective. Trends Ecol. Evol. 19, 168–169 (doi:10.1016/j.tree.2004.02.04) [DOI] [PubMed] [Google Scholar]
- Mailleux A. C., Detrain C., Deneubourg J. L.2000How do the ants assess food volume? Anim. Behav. 59, 1061–1069 (doi:10.1006/anbe.2000.1396) [DOI] [PubMed] [Google Scholar]
- Mailleux A. C., Deneubourg J. L., Detrain C.2003Regulation of ants’ foraging to resource productivity. Proc. R. Soc. Lond. B. 270, 1609–1616 (doi:10.1098/rspb.2003.2398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolis S., Deneubourg J. L.1999Emerging patterns and food recruitment in ants: an analytical study. J. Theor. Biol. 198, 575–592 (doi:10.1006/jtbi.1999.0934) [DOI] [PubMed] [Google Scholar]
- Pasteels J. M., Deneubourg J. L., Goss S.1987Self-organization mechanisms in ant societies (I): trail recruitment to newly discovered food sources. In From individual to collective behavior in social insects, Experientia supplementum 54 (eds Pasteels J. M., Deneubourg J. L.). Basel, Switzerland: Birkhäuser Verlag [Google Scholar]
- Portha S., Deneubourg J. L., Detrain C.2004How food type and brood influence foraging decisions of Lasius niger scouts. Anim. Behav. 68, 115–122 (doi:10.1016/j.anbehav.2003.10.016) [Google Scholar]
- Richardson T. O., Sleeman P. A., McNamara J. M., Houston A. I., Franks N. R.2007Teaching with evaluation in ants. Curr. Biol. 17, 1520–1526 (doi:10.1016/j.cub.2007.08.032) [DOI] [PubMed] [Google Scholar]
- Seeley T. D.1995The wisdom of the hive: the social physiology of honey bee colonies Cambridge, MA: Harvard University Press [Google Scholar]
- Seeley T. D., Buhrmann S. C.1999Group decision making in swarms of honey bees. Behav. Ecol. Sociobiol. 45, 19–31 (doi:10.1007/s002650050536) [Google Scholar]
- Stradling D. J.1970The estimation of worker ant populations by the mark-release-recapture method: an improved marking technique. J. Anim. Ecol. 39, 575–591 [Google Scholar]
- Stueckle S., Zinner D.2008To follow or not to follow: decision making and leadership during the morning departure in chacma baboons. Anim. Behav. 75, 1995–2004 (doi:10.1016/j.anbehav.2007.12.012) [Google Scholar]
- Verhaeghe J. C.1978Analyse comportementale et modélisation du recrutement d’ouvrières vers une source de nourriture chez Tetramorium caespitum (L.). PhD thesis, Université libre de Bruxelles, Belgium. [Google Scholar]
- Verhaeghe J. C.1982Food recruitment in Tetramorium impurum (Hymenoptera: Formicidae). Insectes Soc. 29, 67–85 (doi:10.1007/BF02224528) [Google Scholar]