Abstract
Male and female offspring can differ in their susceptibility to pre-natal (e.g. egg quality) and post-natal (e.g. sib–sib competition) conditions, and parents can therefore increase their individual fitness by adjusting these maternal effects according to offspring sex. In birds, egg mass and laying/hatching order are the main determinants of offspring viability, but these effects can act differently on each sex. In a previous study, relatively large last-laid (c-)eggs of yellow-legged gulls (Larus michahellis) were more likely to carry a female embryo. This suggests compensatory allocation of maternal resources to daughters from c-eggs, which suffer reduced viability. In the present study, we supplemented yellow-legged gulls with food during the laying period to experimentally test whether their nutritional conditions were responsible for the observed covariation between c-egg sex and mass. As predicted, food supplementation enhanced female c-eggs' mass more than that of male c-eggs. Thus, this experiment indicates that mothers strategically allocated their resources to c-eggs, possibly in order to compensate for the larger susceptibility of daughters to hatching (and laying) order. The results also suggested that mothers decided on resource allocation depending on the sex of already ovulated c-eggs, rather than ovulating ova of either sex depending on food availability.
Keywords: egg size, food availability, laying order, maternal effects, sex allocation
1. Introduction
The expected reproductive value of male and female offspring can vary according to ecological factors that differentially affect the chances that sons and daughters will survive and reproduce (Trivers & Willard 1973; Charnov 1982; Frank 1990). By adjusting their allocation to the production of sons and daughters according to such extrinsic conditions, parents can therefore accrue a natural selection advantage (Charnov 1982; Frank 1990; West et al. 2000; Badyaev et al. 2006).
Offspring of each sex differ in susceptibility to pre- and post-natal maternal effects (e.g. egg size/quality and post-natal environment; West & Sheldon 2002). In birds with altricial offspring, parental strategies of differential allocation to individual offspring are often mediated by asynchronous egg hatching and declining egg mass along the laying sequence, which result in an advantage of offspring hatching from large, early-laid eggs over their siblings (Slagsvold et al. 1984; Magrath 1990; Williams 1994). Because the effects of variation in egg mass and position in the laying/hatching sequence can differ between male and female offspring, adaptive sex allocation is expected to result in a covariation between sex and egg mass or laying/hatching order (see Pike & Petrie 2003; Alonso-Alvarez 2006). Some, mostly correlational, studies have indeed found evidence for biased sex allocation in association with variation in egg size and with laying order (Pike & Petrie 2003; Alonso-Alvarez 2006; Cassey et al. 2006).
Investment in current reproduction may have to be traded against allocation of limiting nutritional resources to competing activities (e.g. self-maintenance; Stearns 1992). The ability of parents to produce large eggs or provide efficient care should therefore vary according to parental general state (Williams 1994; Mousseau & Fox 1998; Christians 2002; McAdam & Boutin 2003; Nager 2006). Furthermore, sex-related variation in offspring size or physiology can result in differences in the production cost entailed by each sex, and parents in relatively poor condition may not be able to afford to produce the most demanding sex (Trivers & Willard 1973; Clutton-Brock et al. 1985; Sheldon & West 2004). Thus, the adaptive sex allocation strategy an individual is expected to pursue will depend on a complex balance between its own state in combination with the differential consequences of maternal effects mediated by condition-dependent egg mass and quality on sons and daughters, as well as by sib–sib competitive environment as determined by laying/hatching order (Bradbury & Griffiths 1999; Nager et al. 2000; Fargallo et al. 2006; Kim & Monaghan 2006).
One way to investigate adaptive patterns of sex allocation is therefore to experimentally manipulate the ecological factors (e.g. food availability) which putatively affect parental decisions on production of offspring of each sex or on sex-specific resource allocation (Bradbury & Blakey 1998; Kilner 1998; Nager et al. 1999, 2000; Rutkowska & Cichon 2002; Arnold et al. 2003; Pike 2005; Perez et al. 2006). If maternal nutritional conditions influence the size of the eggs and food is limiting, experimental food supplementation should enhance egg size and may result in a biased allocation towards the sex which benefits more from developing in large eggs (Martinez-Padilla & Fargallo 2007). Birds, in particular, are ideal models to test for strategic sex allocation depending on maternal state because females, being the heterogametic sex, can achieve ample control over progeny sex (Pike & Petrie 2003). However, to date only very few studies have experimentally analysed sex allocation strategies mediated by variation in mass of individual eggs (Rutkowska & Cichon 2002; Martinez-Padilla & Fargallo 2007).
In the present study of the yellow-legged gull (Larus michahellis), we tested whether food supplementation of the mother affected: (i) egg mass, suggesting that egg quality is constrained by maternal nutritional condition (Kilpi et al. 1996), and (ii) the chance of producing each sex. In this species, clutch size is three eggs (frequency more than 90%), third (c-)eggs are considerably smaller than first (a-) and second (b-)eggs, and hatching is asynchronous, resulting in a viability disadvantage for c-chicks (Rubolini et al. 2009; see Parsons 1970, 1975 for studies of the closely related Larus argentatus). Females are already smaller than males at hatching and suffer larger negative viability effects when hatching last (Saino et al. submitted). We thus focused on the effect of food supplementation on size and sex of c-eggs, because sex-related selection on c-chicks may be more intense. To this goal, on the day of laying of the a-egg, we started a food provisioning protocol whereby some breeding pairs were provided daily with abundant food until clutch completion, whereas others were assigned to a control treatment. Because only 2–3 days elapse between laying of a- and b-eggs, which may be too short an interval to allow mothers to respond to food supplementation, we did not expect any effect of experimental treatment on size and sex of b-eggs. However, we predicted that c-eggs (which are laid 4–5 days after a-eggs) of food-supplemented mothers would be larger than those of control females. In addition, we predicted that in clutches where c-eggs were large, or large relative to a-eggs, they would be more likely to contain a female. This prediction derived from a previous correlational study on the same population where we observed that in clutches where the difference in mass between a- and c-eggs was smaller, c-eggs were more likely to carry a female (Rubolini et al. 2009), suggesting that within-brood egg size variation, as mediated e.g. by variation in maternal condition, could affect sex allocation in this species. Differential sex allocation may occur under two mechanisms: mothers may increase the size of an already ovulated female c-ova (mechanism I); alternatively, under increased food availability mothers may favour maturation of female c-ova (mechanism II). Both mechanisms predict a larger size of female versus male c-eggs, but only mechanism II predicts a female-biased sex ratio.
Differently from several previous food-supplementation experiments, food was provided here after females had started laying. This design has specific advantages, including (i) the results are not confounded by any potential effect of food supplementation on the timing of clutch initiation; (ii) it allows specificity for the effect of nutritional conditions on the test mass of last eggs (c-eggs in this case), which are most commonly the target of brood reduction strategies, because the results are not confounded by any trade-off in resource allocation among the last and the preceding eggs; and (iii) the effect of food supplementation on the covariation between sex and mass of c-eggs is not confounded by complex patterns of sex allocation potentially involving both a- and b-eggs.
2. Material and methods
This experiment was carried out during March–May 2009 in a colony with approximately 500 nests located in the Comacchio lagoon (NE Italy, 44°20′N–12°11′E). We visited the colony daily and weighed newly laid eggs. New nests in one half of the colony were randomly assigned to either a food supplementation or a control treatment. Starting on the day of laying of the a-egg, food-supplemented nests (SUPPL) were provided daily with 300 g of anchovies and 2 smashed, hard-boiled hen eggs (approx. 65 g each) close (less than 40 cm) to the nest (see Bolton et al. 1993). Control nests (CONT) received only one anchovy (approx. 20 g) and a minute (less than 5 g) amount of hard-boiled egg. The food was provided between the hours of 9.00 and 13.00. Laying date of a-eggs did not differ between SUPPL and CONT nests (t95 = 0.24, p = 0.812, difference = 0.23 days). SUPPL and CONT treatments were stopped on the day of laying of the c-egg. To check whether the parents of SUPPL nests were actually consuming the food, we did 28 focal observation sessions of 20 pairs starting immediately after treatment in a given day. In most cases, both members of the focal pair were present in their territory and were observed to actively defend the food source. In 26 cases, the focal SUPPL parents exclusively consumed the food, usually within 1 h from food provisioning, while in two cases adults from neighbouring territories consumed a small fraction of it. We are therefore confident that cases of ‘kleptoparasitism’ by non-focal pairs, which would be practically hard to avoid in gull colonies, were rare and not a major issue in this experiment. It should also be noted that, if anything, the rare occurrence of kleptoparasitism should make our findings conservative.
Food provisioning during laying may have consequences on subsequent incubation behaviour, and thus on egg hatchability. Because variation in incubation behaviour might have different effects on male and female embryos, food supplementation could produce a bias in hatchling sex ratio compared with the control treatment. In order to overcome this possible bias, on the day following that of laying the c-egg, we transferred the entire SUPPL and CONT clutches to nests of unmanipulated synchronous pairs (i.e. pairs that laid their c-egg the same or up to two days before experimental clutches), located in the other half of the colony (see above), and chosen randomly among the most synchronous ones. During the incubation period, some of the foster nests of SUPPL and CONT clutches were flooded following a storm. Experimental eggs from flooded foster nests were dissected to collect a sample of embryo tissues for molecular sexing. However, some experimental eggs were washed out of the foster nests and could no longer be found. Foster nests that were not flooded were visited every day until hatching. A blood sample for molecular sexing was collected from the chicks as soon as they were found hatched. Because we were interested in the effects of food supplementation on mass and sex of c-eggs, in the analyses we only considered the clutches with a c-egg (i.e. 3-egg clutches) where the c-egg could be successfully sexed. Twenty-two (42%) of the 52 CONT clutches and 17 (38%) of the 45 SUPPL clutches that were included in the analyses were collected following the flood, while the others could be left in the foster nest. Thirty CONT and 33 SUPPL clutches could not be considered because no c-egg was laid: (9 CONT; 7 SUPPL clutches), the c-egg disappeared after the flood (7 CONT; 8 SUPPL), or the c-egg disappeared at other stages or was addled/infertile (14 CONT; 18 SUPPL). Molecular sexing was performed according to established protocols (Rubolini et al. 2006; Romano et al. 2008; Saino et al. 2008).
(a). Statistical analyses
We used generalized linear mixed (GLMM) or generalized linear models (GLM) to analyse the effect of experimental treatment on egg mass or interval between laying of consecutive eggs (normal error distribution; identity link-function) or sex (binomial error distribution; logit link-function). In GLMM, clutch identity was always included as a random effect. Where relevant, laying order was included in the models as a three-level fixed effect, and sex or mass of a- and b-eggs as covariates. Bonferroni procedure was applied to reduce the risk of type I errors in statistical tests involving multiple comparisons. Statistical analyses were run using SAS 9.1 and SPSS 13.0 packages. Parameters are reported with their associated standard error.
3. Results
(a). Effect of food supplementation on egg mass
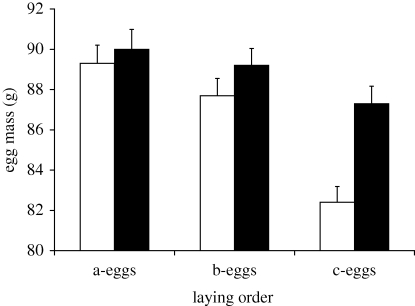
We used data on egg mass and time elapsed between laying of consecutive eggs for 52 CONT and 45 SUPPL 3-egg clutches. As expected, there was no difference in mass between a-eggs of SUPPL or CONT clutches (t95 = 0.51, p = 0.610; figure 1). In a GLMM, egg mass was differentially affected by food supplementation according to laying order (interaction: F2,190 = 9.51, p < 0.001; figure 1). In this analysis, mass of a- or b-eggs did not differ between SUPPL and CONT clutches (pairwise tests: pBonf > 0.99 in both cases), whereas mass of c-eggs was significantly larger in SUPPL than CONT clutches (pBonf = 0.0019; figure 1). Thus, food supplementation enhanced mass of c- but not b-eggs, as expected. In CONT clutches, c-eggs where on average 6.9 g lighter than a-eggs, whereas in SUPPL clutches this difference was reduced to 2.8 g (=41% compared with CONT clutches).
Figure 1.
Mean (s.e.) mass of eggs in the control and food supplemented groups in relation to laying order (white bars, control (n = 52 clutches); black bars, food supplemented (n = 45 clutches)).
A GLM analysis showed that time elapsed between laying of a- or b- and c-eggs did not differ between groups (a–c eggs: CONT = 4.7 (0.13) days; SUPPL = 4.6 (0.13); b–c eggs: CONT = 2.4 (0.10) days; SUPPL = 2.3 (0.07); F1,93 < 0.32, p > 0.570 in both cases) or according to sex of the c-egg (F1,93 < 1.41, p > 0.230 in both cases), and did not depend on the combined effect of treatment and sex of the c-egg (F1,93 < 2.21, p > 0.140 in both cases).
(b). Effect of food supplementation on sex allocation
The proportion of offspring of each sex in a-, b- or c-eggs did not differ between experimental groups (table 1). In addition, the proportion of males did not differ from 0.5 at any of the laying order positions in either of the groups (table 1), despite b- and c-eggs in the CONT group containing approximately twice as many males than females. Variation in egg sex according to laying order was found not to differ between experimental groups in a binomial GLMM (interaction between treatment and laying order: F2,250 = 1.43, p = 0.242). Removing the interaction term revealed no significant variation in sex ratio according to laying order (F2,252 = 1.73, p = 0.179) or treatment (F1,91.6 = 0.26, p = 0.608). Overall, these findings indicate that food supplementation did not alter the sex allocation to eggs of different laying order, and that, in particular, the sex ratio in c-eggs did not differ between groups.
Table 1.
Number of males and females (M/F) in the CONT (n = 52) or SUPPL (n = 45) clutches included in the study where c-eggs could be sexed. χ2 tests of the difference in frequency of males and females in the two groups for all laying order positions are presented. The number of sexed offspring for a- and b-eggs does not sum up to the total number of clutches in either group because of disappearance of the eggs, egg infertility, and hatchling mortality (see also §2). All within treatment-by-laying order Binomial tests for deviation from a 1 : 1 sex ratio were not significant after Bonferroni correction for n = 6 tests.
| control | food supplemented | 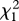 |
p | |
|---|---|---|---|---|
| a-eggs | 17/22 | 21/17 | 1.05 | 0.306 |
| b-eggs | 31/15 | 19/17 | 1.81 | 0.178 |
| c-eggs | 34/18 | 27/18 | 0.30 | 0.584 |
Mass of a-eggs did not vary according to sex in an analysis on the two groups pooled (male eggs: 90.1 (1.03) g, n = 38; female eggs: 89.8 (1.11) g, n = 39; t75 = 0.18, p = 0.854).
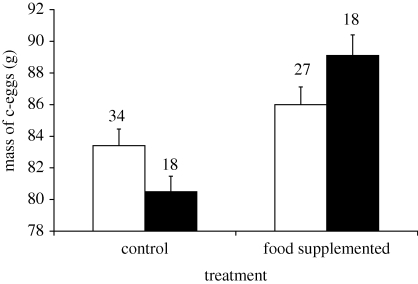
Food supplementation may result in biased sex allocation via two mechanisms (see §1). Mechanism I can be tested by modelling c-egg mass in relation to treatment and offspring sex. Mass of c-eggs was significantly predicted by the interaction between sex and treatment (table 2a). Pairwise comparisons showed that SUPPL female c-eggs were significantly larger than CONT female c-eggs (pBonf < 0.001), whereas no significant difference in mass of male c-eggs emerged between experimental groups (pBonf = 0.437; figure 2). The difference in mass of female c-eggs between the two experimental groups was 8.6 g while that between male eggs was 2.6 g (figure 2). Thus, food supplemented mothers laid c-eggs that were significantly larger than those laid by CONT mothers only when the egg carried a female offspring (table 2a). The interaction between treatment and sex of c-eggs remained significant when we only considered the clutches where all a- and b-eggs were sexed (F1,64 = 4.54, p = 0.037), as well as when we controlled for a-egg mass (reflecting laying performance of the mother before manipulation) and sex of both a- and b-eggs (F1,61 = 4.69, p = 0.034), despite the number of clutches included in this analysis was reduced to 36 CONT and 32 SUPPL clutches.
Table 2.
Linear model of mass of c-eggs or difference in mass between a- and c-eggs in relation to food supplementation treatment and sex of the c-egg; n = 52 CONT and 45 SUPPL c-eggs.
| d.f. | F | p | |
|---|---|---|---|
| (a) mass of c-eggs | |||
| treatment | 1 | 22.38 | <0.001 |
| sex of c-eggs | 1 | 0.003 | 0.957 |
| treatment×sex of c-eggs | 1 | 6.31 | 0.014 |
| error | 93 | ||
| (b) difference in egg mass (a-egg − c-egg) | |||
| treatment | 1 | 13.57 | <0.001 |
| sex of c-eggs | 1 | 0.52 | 0.474 |
| treatment×sex of c-eggs | 1 | 1.29 | 0.259 |
| error | 93 | ||
Figure 2.
Mean (s.e.) mass of c-eggs carrying a male or a female in the control and food supplemented groups. Numbers are sample sizes (white bars, males; black bars, females).
Mechanism II can instead be tested by applying a binomial model to sex of c-eggs in relation to treatment and egg mass. In this model, the interaction between experimental treatment and egg mass significantly predicted the sex of c-eggs (χ21 = 5.74, p = 0.017). According to the coding of sex we used, the coefficient associated with the interaction term (=0.212 (0.088)) indicates that the chances of a c-egg being female increased with egg mass significantly more in SUPPL clutches. No main effect of treatment was found if the interaction term was removed (χ21 = 0.27, p = 0.600; see also table 1).
The same model presented in table 2a was also applied to the difference in mass between the a- and the c-egg (Δmass), rather than mass of the c-egg per se. The interaction between sex of c-eggs and treatment did not significantly predict Δmass (table 2b). However, the difference in Δmass between CONT and SUPPL clutches with a female c-egg (=5.8 g) was larger than that between CONT and SUPPL clutches with a male c-egg (=3.1 g).
4. Discussion
In a previous correlational study of yellow-legged gulls, c-eggs were more likely to carry a female when they were only slightly smaller or even larger than their sibling a-eggs (Rubolini et al. 2009). In the present study, we therefore tested whether the observed covariation between egg sex and mass was mediated by variation in maternal nutritional conditions. Specifically, we tested the prediction that maternal allocation decisions resulted in increased mass of female c-eggs when nutritional constraints on c-egg mass were experimentally lifted by food supplementation. Our main finding was consistent with this prediction, since female c-eggs laid by food supplemented mothers were larger than control female c-eggs, whereas no significant difference was observed between male c-eggs.
At a mechanistic level, covariation between ovum sex and egg mass can arise via two processes. First, mothers may decide to allocate more resources to already ovulated ova of either sex (mechanism I). In the present study, food-supplemented mothers could have allocated more yolk or albumen (or both) to c-eggs only when the c-egg they had already ovulated was female. Alternatively, mothers may decide to develop ova of either sex depending on their nutritional conditions (mechanism II; see Pike 2005). Individual mothers could differ in their ability to profit from food supplementation, and mothers who actually profited by increasing the size of their c-eggs also managed to selectively carry on the development of female rather than male ova. This could be achieved, for example, by interrupting the ongoing development of ova of the ‘wrong’ sex (male) destined to c-eggs and rescuing development of female ova (Emlen 1997; Pike & Petrie 2003; Pike 2005).
Present evidence argues in favour of mechanism I. The duration of the rapid-yolk-development (RYD) phase in yellow-legged gulls is unknown, but in closely related species of similar size is approximately 10 days (Ruiz et al. 2000). Interrupting the development of a male ovum and rescuing a female ovum currently at an earlier stage of development, as envisaged by mechanism II, should result in delayed laying of c-eggs (Emlen 1997). However, there was no hint that the time elapsed between laying of c-eggs and the preceding eggs differed between experimental groups, nor according to the sex of the c-egg or their combined effect. Moreover, the relative frequency of females in c-eggs did not differ between the two experimental groups, whereas mechanism II should have led to female-biased sex ratio of c-eggs in SUPPL compared with CONT clutches. The proportion of males and females among c-eggs was very far from showing any obvious variation between the groups. Thus, lack of support for mechanism II could not simply arise because of low statistical power of tests on sex frequency data. The second prediction, i.e. that female c-eggs were more likely observed as the value of the difference between mass of c-eggs and their sibling a-egg in SUPPL clutches increased, was not strictly fulfilled. Actually, the difference in mass of female c-eggs minus mass of a-eggs between CONT and SUPPL clutches was much larger than that observed for male c-eggs, i.e. in the predicted direction. However, this did not translate into a significant sex by treatment interaction (table 2b). It can be speculated that the analysis of the difference in mass of c- and a-eggs is inherently more affected by random noise. In fact, mass of a- as well as of c-eggs can be affected by random variation in extrinsic conditions (e.g. weather) around laying. Such random effects acting independently on a- and c-eggs would inflate the variance in the difference between mass of a- and c-eggs and blur the statistical effect of the sex by treatment interaction. At a different level, this discrepancy may provide an insight into the function of the observed patterns of maternal sex allocation decisions. When food is plenty, mothers could reduce the mass gap between female but not male c-eggs relative to a-eggs, if the brood size hierarchy around hatching (but also later, see Romano et al. 2008) is the relevant factor influencing condition and viability of female c-chicks. However, the lack of significant sex by treatment interaction (table 2b) would suggest that this is not the case. Alternatively, c-egg mass per se, rather than c-egg mass relative to a-egg mass, could be important in determining viability of daughters compared with sons and thus prompt mothers to invest more in female than male c-eggs. This interpretation would devalue the role of egg mass variation with laying order in determining subsequent sib–sib competition dynamics. Rather, it argues in favour of a direct, differential effect of egg size on offspring of each sex (Williams 1994; but see Krist 2009).
In a previous experimental study of the same gull population (Saino et al. submitted), we found that survival of females but not that of males declines with hatching order. Because hatching order closely mirrors laying order in our model species (e.g. Rubolini et al. 2005; Saino et al. submitted), a straightforward interpretation of the function of laying large female but not male c-eggs is enhancing viability of daughters, which are particularly negatively affected by hatching late.
Thus, our findings are consistent with the hypothesis that individual sex allocation strategies vary according to nutritional conditions during laying, and may function to specifically enhance viability of offspring of the sex which suffers larger viability costs from being in the last position in the laying/hatching sequence.
Acknowledgements
We thank the Parco Regionale del Delta del Po for permission to work in the study area. We are also grateful to all the people who helped during field work.
References
- Alonso-Alvarez C.2006Manipulation of primary sex-ratio: an updated review. Avian Poult. Biol. Rev. 171, 1–20 [Google Scholar]
- Arnold K. E., Griffiths R., Stevens D. J., Orr K. J., Adam A., Houston D. C.2003Subtle manipulation of egg sex ratio in birds. Proc. R. Soc. Lond. B 270, S216–S219 (doi:10.1098/rsbl.2003.0068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A. V., Seaman D. A., Navara K. J., Hill G. E., Mendonca M. T.2006Evolution of sex-biased maternal effects in birds: III. Adjustment of ovulation order can enable sex-specific allocation of hormones, carotenoids, and vitamins. J. Evol. Biol. 19, 1044–1057 (doi:10.1111/j.1420-9101.2006.01106.x) [DOI] [PubMed] [Google Scholar]
- Bolton M., Monaghan P., Houston D. C.1993Proximate determination of clutch size in lesser black-backed gulls: the roles of food supply and body condition. Can. J. Zool. 71, 273–279 (doi:10.1139/z93-039) [Google Scholar]
- Bradbury R. B., Blakey J. K.1998Diet, maternal condition, and offspring sex ratio in the zebra finch, Poephila guttata. Proc. R. Soc. Lond. B 265, 895–899 (doi:10.1098/rspb.1998.0375) [Google Scholar]
- Bradbury R. B., Griffiths R.1999Sex-biased nestling mortality is influenced by hatching asynchrony in the lesser black-backed gull Larus fuscus. J. Avian Biol. 30, 316–322 (doi:10.2307/3677358) [Google Scholar]
- Cassey P., Ewen J. G., Møller A. P.2006Revised evidence for facultative sex ratio adjustment in birds: a correction. Proc. R. Soc. Lond. B 273, 3129–3130 (doi:10.1098/rspb.2006.3628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov E. L.1982The theory of sex allocation Princeton, NJ: Princeton University Press [Google Scholar]
- Christians J. K.2002Avian egg size: variation within species and inflexibility within individuals. Biol. Rev. 77, 1–26 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Albon S. D., Guinness F. E.1985Parental investment and sex differences in juvenile mortality in birds and mammals. Nature 313, 131–133 (doi:10.1038/313131a0) [Google Scholar]
- Emlen S. T.1997When mothers prefer daughters over sons. Trends Ecol. Evol. 12, 291–292 (doi:10.1016/S0169-5347(97)01119-1) [DOI] [PubMed] [Google Scholar]
- Fargallo J. A., Polo V., de Neve L., Martin J., Dávila J. A., Soler M.2006Hatching order and size-dependent mortality in relation to brood sex ratio composition in chinstrap penguins. Behav. Ecol. 17, 772–778 (doi:10.1093/beheco/arl007) [Google Scholar]
- Frank S. A.1990Sex allocation theory for birds and mammals. Annu. Rev. Ecol. Syst. 21, 13–55 (doi:10.1146/annurev.es.21.110190.000305) [Google Scholar]
- Kilner R.1998Primary and secondary sex ratio manipulation by zebra finches. Anim. Behav. 56, 155–164 (doi:10.1006/anbe.1998.0775) [DOI] [PubMed] [Google Scholar]
- Kilpi M., Hillström L., Lindström K.1996Egg-size variation and reproductive success in the herring gull Larus argentatus: adaptive or constrained size of the last egg? Ibis 138, 212–217 (doi:10.1111/j.1474-919X.1996.tb04330.x) [Google Scholar]
- Kim S.-Y., Monaghan P.2006Sex of the first hatched chick influences survival of the brood in the herring gull (Larus argentatus). J. Zool. 270, 116–121 [Google Scholar]
- Krist M.2009Short- and long-term effects of egg size and feeding frequency on offspring quality in the collared flycatcher (Ficedula albicollis). J. Anim. Ecol. 78, 907–918 (doi:10.1111/j.1365-2656.2009.01536.x) [DOI] [PubMed] [Google Scholar]
- Magrath R. D.1990Hatching asynchrony in altricial birds. Biol. Rev. 65, 587–622 (doi:10.1111/j.1469-185X.1990.tb01239.x) [Google Scholar]
- Martinez-Padilla J., Fargallo J.2007Food supply during prelaying period modifies the sex-dependent investment in eggs of Eurasian kestrels. Behav. Ecol. Sociobiol. 61, 1735–1742 (doi:10.1007/s00265-007-0405-3) [Google Scholar]
- McAdam A. G., Boutin S.2003Effects of food abundance on genetic and maternal variation in the growth rate of juvenile red squirrels. J. Evol. Biol. 16, 1249–1256 (doi:10.1046/j.1420-9101.2003.00630.x) [DOI] [PubMed] [Google Scholar]
- Mousseau T. A., Fox C. W.1998Maternal effects as adaptations New York, NY: Oxford University Press [Google Scholar]
- Nager R. G.2006The challenges of making eggs. Ardea 94, 323–346 [Google Scholar]
- Nager R. G., Monaghan P., Griffiths R., Houston D. C., Dawson R.1999Experimental demonstration that offspring sex ratio varies with maternal condition. Proc. Natl Acad. Sci. USA 96, 570–573 (doi:10.1073/pnas.96.2.570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nager R. G., Monaghan P., Houston D. C., Genovart M.2000Parental condition, brood sex ratio and differential young survival: an experimental study in gulls (Larus fuscus). Behav. Ecol. Sociobiol. 48, 452–457 (doi:10.1007/s002650000262) [Google Scholar]
- Parsons J.1970Relationship between egg size and post-hatching chick mortality in the herring gull (Larus argentatus). Nature 228, 1221–1222 (doi:10.1038/2281221a0) [DOI] [PubMed] [Google Scholar]
- Parsons J.1975Asynchronous hatching and chick mortality in the herring gull Larus argentatus. Ibis 117, 517–520 (doi:10.1111/j.1474-919X.1975.tb04247.x) [DOI] [PubMed] [Google Scholar]
- Perez C., Velando A., Dominguez J.2006Parental food conditions affect sex-specific embryo mortality in the yellow-legged gull (Larus michahellis). J. Ornithol. 147, 513–519 (doi:10.1007/s10336-006-0074-4) [Google Scholar]
- Pike T. W.2005Sex ratio manipulation in response to maternal condition in pigeons: evidence for pre-ovulatory follicle selection. Behav. Ecol. Sociobiol. 58, 407–413 (doi:10.1007/s00265-005-0931-9) [Google Scholar]
- Pike T. W., Petrie M.2003Potential mechanisms of avian sex manipulation. Biol. Rev. 78, 553–574 (doi:10.1017/S1464793103006146) [DOI] [PubMed] [Google Scholar]
- Romano M., Caprioli M., Ambrosini R., Rubolini D., Fasola M., Saino N.2008Maternal allocation strategies and differential effects of yolk carotenoids on the phenotype and viability of yellow-legged gull (Larus michahellis) chicks in relation to sex and laying order. J. Evol. Biol. 21, 1626–1640 (doi:10.1111/j.1420-9101.2008.01599.x) [DOI] [PubMed] [Google Scholar]
- Rubolini D., Romano M., Boncoraglio G., Ferrari R. P., Martinelli R., Galeotti P., Fasola M., Saino N.2005Effects of elevated egg corticosterone levels on behavior, growth, and immunity of yellow-legged gull (Larus michahellis) chicks. Horm. Behav. 47, 592–605 (doi:10.1016/j.yhbeh.2005.01.006) [DOI] [PubMed] [Google Scholar]
- Rubolini D., Romano M., Martinelli R., Saino N.2006Effects of elevated yolk testosterone levels on survival, growth and immunity of male and female yellow-legged gull chicks. Behav. Ecol. Sociobiol. 59, 344–352 (doi:10.1007/s00265-005-0057-0) [Google Scholar]
- Rubolini D., Ambrosini R., Romano M., Caprioli M., Fasola M., Bonisoli-Alquati A., Saino N.2009Within-clutch egg size asymmetry covaries with embryo sex in the yellow-legged gull Larus michahellis. Behav. Ecol. Sociobiol. 63, 1809–1819 (doi:10.1007/s00265-009-0808-4) [Google Scholar]
- Ruiz X., Jover L., Pedrocchi V., Oro D., González-Solís J.2000How costly is clutch formation in the Audouin's gull Larus audouinii? J. Avian Biol. 31, 467–575 [Google Scholar]
- Rutkowska J., Cichon M.2002Maternal investment during egg laying and offspring sex: an experimental study of zebra finches. Anim. Behav. 64, 817–822 (doi:10.1006/anbe.2002.1973) [Google Scholar]
- Saino N., Bertacche V., Bonisoli-Alquati A., Romano M., Rubolini D.2008Phenotypic correlates of yolk and plasma carotenoid concentration in yellow-legged gull chicks. Physiol. Biochem. Zool. 81, 211–225 (doi:10.1086/527454) [DOI] [PubMed] [Google Scholar]
- Sheldon B. C., West S. A.2004Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 163, 40–54 (doi:10.1086/381003) [DOI] [PubMed] [Google Scholar]
- Slagsvold T., Andvik J., Rofstad G., Lorentsen O., Husby M.1984On the adaptive value of intraclutch egg-size variation in birds. Auk 101, 685–697 [Google Scholar]
- Stearns S. C.1992The evolution of life histories New York, NY: Oxford University Press [Google Scholar]
- Trivers R. L., Willard D. E.1973Natural selection on parental ability to vary the sex ratio of offspring. Science 179, 90–92 (doi:10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- West S. A., Sheldon B. C.2002Constraints in the evolution of facultative sex ratio adjustment. Science 295, 1685–1688 (doi:10.1126/science.1069043) [DOI] [PubMed] [Google Scholar]
- West S. A., Herre E. A., Sheldon B. C.2000The benefits of allocating sex. Science 290, 288–290 (doi:10.1126/science.290.5490.288) [DOI] [PubMed] [Google Scholar]
- Williams T. D.1994Intraspecific variation in egg size and egg composition: effects on offspring fitness. Biol. Rev. 68, 35–59 [DOI] [PubMed] [Google Scholar]