Abstract
Prey animals can reduce their risk of predation by detecting potential predators before encounters occur. Some animals gain information about nearby predators by eavesdropping on heterospecific alarm calls. Despite having well-developed ears, most lizards do not use vocal information for intraspecific communication, and few studies have shown practical use of the ears in wild lizards. Here, we show that the Madagascan spiny-tailed iguana (Oplurus cuvieri cuvieri) obtains auditory signals for predator detection. The Madagascan spiny-tailed iguana and the Madagascar paradise flycatcher (Terpsiphone mutata) are syntopic inhabitants of the Ampijoroa dry deciduous forest of Madagascar. The iguana and the flycatcher have neither a predator–prey relationship nor resource competition, but they have shared predators such as raptors and snakes. Using playback experiments, we demonstrated that the iguana discriminates mobbing alarm calls of the flycatcher from its songs and then enhances its vigilance behaviour. Our results demonstrate the occurrence of an asymmetrical ecological relationship between the Madagascan spiny-tailed iguana and the paradise flycatcher through eavesdropping on information about the presence of predators. This implies that indirect interspecific interactions through information recognition may be more common than generally thought in an animal community.
Keywords: eavesdropping, heterospecific signal, mobbing alarm call, asymmetrical relationship, Madagascan spiny-tailed iguana
1. Introduction
Animals possess various antipredator behaviours to reduce their risk of predation (Greene 1988; Lima & Dill 1990). In addition to relying on these antipredator behaviours, prey can reduce the risk of predation by detecting potential predators before encounters do occur. It is important for animals to obtain information about the presence of predators as early as possible to increase its survivorship. On the other hand, responding to potential predators under safe situations may be energetically costly (Ydenberg & Dill 1986). Furthermore, unnecessary movement of prey animals may attract predators' attention (Ingle 1968). Therefore, to reduce the cost and risk of predation, precise identification of nearby predators and evaluation of the risk level should be critical for animals.
Auditory signals are able to convey a wealth of detailed information quickly, precisely and over a wide range through combinations of the variety of frequencies and patterns of sound (Halliday & Slater 1983a). Therefore, many vertebrates, especially social mammals and birds, rely greatly on vocalizations to exchange individual and environmental information (Halliday & Slater 1983b). Many species living in groups are known to emit specific alarm calls against nearby predators (Halliday & Slater 1983b). Alarm calls are emitted targeting group members to induce their appropriate antipredator responses to predators (Turner 1973; Klump et al. 1986) or to make predators confused or deter pursuit (Charnov & Krebs 1975; Woodland et al. 1980; see Wheeler 2008 for review). However, these alarm call signals are not necessarily received by only targeted individuals; non-targeted individuals may also receive the signals and obtain information. It has been demonstrated that several mammalian and avian species have an ability to obtain information concerning the presence of nearby predators by eavesdropping on other species' alarm calls (Seyfarth et al. 1980; Seyfarth & Cheney 1990; Rainey et al. 2004; Templeton & Greene 2007; Lea et al. 2008). Such eavesdropping on heterospecific auditory signals has typically been found among species that routinely communicate vocally. Despite the fact that lizards generally have seemingly well-developed ears (Wever 1978), most lizards, except for gekkonids, do not use vocal signals for intraspecific communication (Marcellini 1977; Lopez et al. 1998; Pianka & Vitt 2003; Vercken & Clobert 2008). Accordingly, few studies have shown practical roles of ears in free-living lizards. A recent report showed that the Galapagos marine iguana (Amblyrhynchus cristatus), which does not depend on auditory information for intraspecific communication, eavesdrops on the alarm call of birds and responds with antipredator behaviour (Vitousek et al. 2007). To explore the role of hearing in non-vocal lizards, investigations on interspecific interactions might be a productive approach.
The Ampijoroa dry deciduous forest, which is located in northwestern Madagascar, is a good site for an animal community study, because at least 86 avian, 47 reptilian, 9 amphibian and 20 mammalian species inhabit it sympatrically (Mizuta 2005a; Mori et al. 2006). These terrestrial vertebrates show complex interspecific interaction networks that include predator–prey relationships and resource competition both within and between these classes (Mizuta 2002, 2005b; Mori & Randriamahazo 2002a; Nakamura 2004; Nakamura et al. 2004; Hasegawa et al. 2009). One common lizard in this community is the Madagascan spiny-tailed iguana, Oplurus cuvieri cuvieri. Animals in this genus have well-developed ears (Wever 1978) but do not perform any kind of vocalization. Oplurus cuvieri cuvieri inhabits this site syntopically with another common member of the community, the Madagascar paradise flycatcher, Terpsiphone mutata. This bird communicates vocally and often emits alarm calls while mobbing raptorial predators such as Frances's sparrow hawk, which is a predator common to both the Madagascan spiny-tailed iguana and the Madagascar paradise flycatcher. The flycatcher and the lizard do not have direct ecological interactions such as predator–prey, resource competition or host–parasite interactions. However, we predict that they have another ecological relationship; that is, eavesdropping on the alarm calls of the flycatcher by the iguana. Considering the fact that the lizard and the flycatcher live in close proximity in the forest, the lizard would often have an opportunity to hear alarm calls by the bird, and therefore it is expected that the lizard would have evolved to eavesdrop on the calls. We therefore hypothesized that the Madagascan spiny-tailed iguana uses heterospecific alarm calls to enhance the detectability of nearby predators and reduce the risk of predation.
Here, we experimentally test whether O. c. cuvieri eavesdrops on heterospecific alarm calls. Specifically, we examine whether iguanas distinguish the mobbing alarm calls of the Madagascar paradise flycatchers that were emitted in response to nearby predators from their songs.
2. Material and methods
(a). Study site
Ampijoroa forest is located in Ankarafantsika National Park in northwestern Madagascar (16°15′ S, 46°48′ E). The vegetation consists of a deciduous canopy 10–15 m high and fairly sparse understorey (Jury 2003). Jardin Botanique A (JBA) is a research plot of approximately 25 ha that is located in western Ampijoroa. The forest station, including office facilities and campsites for researchers and ecotourists, and several resident houses, is present approximately 1 km east of JBA. Our main study area consisted of JBA, the forest station of Ampijoroa (approx. 3 ha), trails between them and their immediately surrounding areas.
(b). Animals
Madagascan spiny-tailed iguanas (O. c. cuvieri) are diurnal and widely distributed in western Madagascar (Glaw & Vences 2007). This lizard mainly dwells on tree trunks and on open land (Randriamahazo & Mori 1999). It is a typical ambusher (Mori & Randriamahazo 2002b) and feeds primarily on insects (Randriamahazo 2000). These iguanas appear to communicate with each other primarily through visual and olfactory means, and in particular they often use several types of visual displays for intraspecific communication (Randriamahazo & Mori 1999).
The Madagascar paradise flycatcher is distributed widely throughout Madagascar and on Comoros Islands (Mulder & Ramiarison 2003). Its breeding season lasts from mid-September to late January (Mizuta 2002). It feeds on aerial insects by flycatching and hovering in the middle and lower strata of forests (Hino 1998). These birds make a heterospecific group with other species, such as the Madagascar magpie-robin (Copsychus albospecularis) and the crested drongo (Dicrurus forficatus), and mob their shared predators with alarm calls (R. Ito 2005, unpublished data).
Common possible raptorial predators for the lizard and the bird are Frances's sparrow hawk (Accipiter francesii), Madagascar buzzard (Buteo brachypterus), yellow-billed kite (Milvua aegyptius) and Madagascar harrier hawk (Polyboroides radiatus). These raptors are diurnal and common in the Ampijoroa forest, and prey upon reptiles and small birds (Dee 1986; Langrand 1990; Rene de Roland & Thorstrom 2003; Hasegawa et al. 2009).
(c). Data collection
We conducted experiments between 10:00 and 16:00 from October 2007 to January 2008, when O. c. cuvieri and T. mutata were reproductively active (Mizuta 2000; Randriamahazo & Mori 2001). We searched for lizards by slowly walking along paths and trails. Upon finding a lizard on a tree trunk, we started a playback experiment. We put a Yuasa speaker (YSP-588B) and a Sony digital video camera 3 m from the individual. After setting up the speaker and camera, we played back a CD of two types of 20 s stimuli (see below) to the individual with a Panasonic portable CD player (SL-CT500). To acclimate the lizard to the presence of the observer, we waited for 5 min before the first playback. Playback of stimuli to the same lizard was separated by at least 5 min. The order of playback stimuli was randomized. Lizard behaviours were recorded by the video camera throughout the experiment. During the playbacks, to reduce possible observer's effect, we did not gaze at the subjects directly and stayed motionless behind the video camera as much as possible. If the paradise flycatcher flew into our sight during the experiments, we stopped the playback and did not use the data for analyses. We captured and marked all the lizards after the playback experiments so as not to use the same individuals more than once.
We employed three sources of flycatchers' alarm calls, which were recorded separately when T. mutata mobbed a Frances's sparrow hawk, Madagascar harrier hawk and yellow-billed kite, respectively. There are no obvious differences in alarm calls among predator types (T. Mizuta 2005, personal communication). Songs of T. mutata were selected from three sources, which were collected from three males. Frequency of both alarm calls and songs (fig. S1 in the electronic supplementary material) is expected to be audible to the iguana on the basis of experimental studies of related species (Titus & Frost 1996; Manley 2006; Münchenberg et al. 2008). We used a Sony digital audio player (TCD-D10) to record songs and calls, and edited them using the sound editing software Avisoft-SASlab Light. Mean maximum amplitudes of each stimulus, A-weighting, at 3 m away from the speaker were 73.6 and 74.3 dBA for alarm calls and songs, respectively. Maximum amplitude under natural conditions without playback was 55.5 dBA. We used almost 20 dB higher levels of sounds for playbacks to reduce the effect of background noise. We played back both a mobbing call and a song of flycatchers to each of 35 lizards. To examine possible order effects, 17 lizards were tested with the alarm call first, and the remaining 18 lizards were tested with the song first.
(d). Variables and behavioural responses
Based on the analysis of the digital videos, we classified the behavioural responses of the iguana into the following four minor categories. Immobility: remaining motionless for at least 20 s. Whole body movement: performing locomotory movement from the original place (e.g. running away, climbing up a tree, fleeing into a tree hollow). Anterior body movement: moving the anterior body by the movement of forelegs (e.g. push-up display: anterior body movements up and down in a vertical plane; Jenssen 1977; Leal 1999). Head movement: moving only the head while keeping the body, legs and tail motionless. These four behavioural responses were grouped into two major categories: static (immobility) and active (whole body, anterior body and head movements) responses.
We counted the total number of active responses for each lizard during the 20 s periods immediately before the playback (baseline) and during the 20 s playback periods of each stimulus. To examine the sensitivity of Madagascan spiny-tailed iguanas to each stimulus, we compared the number of active responses between a baseline period and the subsequent playback period, and between alarm call and song playback periods. To test the possible order effects of the playback stimulus, we compared the number of active responses during the baseline period between the first and second stimuli. Because no order effect was detected (§3), we pooled the results of all 35 lizards for the subsequent analyses.
To determine the direction and extent of change in the responses, we compared the number of individuals that exhibited active responses only to alarm calls with the number of individuals responding only to song, using McNemar's test (Zar 2009).
To examine temporal changes of behavioural responses to the alarm calls, we divided the alarm call playback period and its baseline period into four 5 s sections each. For each section, the occurrence of the four types of active responses was recorded, and the proportion of individuals that exhibited each response was compared between the fourth baseline section and each of the playback sections. For the alarm call playback sections, we examined the difference in frequency among the three active response types. The level of statistical significance was set at 0.05, except for the comparisons of the four responses between the fourth baseline section and playback sections, to which Bonferroni correction was applied (0.05/4 = 0.0125).
3. Results
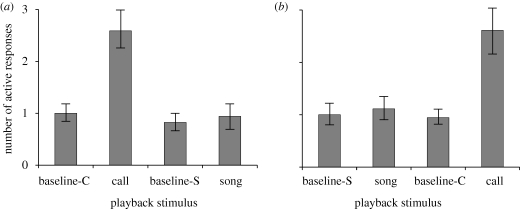
In the cases in which alarm calls were played back first and songs later (n = 17; figure 1a), the number of active responses increased significantly from the baseline period to the alarm call playback period (Wilcoxon's signed-rank test: t = 51, p = 0.002). The number of active responses during the song playback period was not significantly larger than that during the baseline period (t = 4.50, p = 0.827). The lizards responded to the alarm call playback significantly more frequently than to the song playback (t = 62.50, p < 0.001). The number of active responses during the baseline periods was not significantly different between calls and songs (t = 5, p = 0.617), indicating that alarm calls did not affect the number of active responses in the subsequent baseline period for songs.
Figure 1.
Mean number of active-response-exhibiting Madagascan spiny-tailed iguanas in each baseline and playback period. (a) Experiment in which alarm call was played back first and song second. (b) Experiment in which song was played back first and alarm call second. Baseline-C and baseline-S indicate periods preceding alarm call playback and song playback periods, respectively. Error bars represent 1 s.e. See text for detailed descriptions of active responses.
In the cases in which songs were played back first and alarm calls later (n = 18; figure 1b), the number of active responses increased significantly from the baseline period to the alarm call playback period (Wilcoxon's signed-rank test: t = 49, p = 0.009), whereas the number of active responses did not significantly increase from the baseline period to the song playback period (t = 6, p = 0.663). The lizards responded to the alarm call playback significantly more frequently than to the song playback (t = 38.50, p = 0.005). The number of active responses during the baseline periods was not significantly different between songs and calls (t = 0.50, p = 1), indicating that songs did not affect the number of active responses in the subsequent baseline period for alarm calls.
Because no order effect was detected, we pooled the results of all 35 lizards. These combined data also showed that active responses were observed significantly more frequently during the alarm call playback than during the song playback (t = 187, p < 0.001). The number of iguanas that exhibited active responses only to alarm calls was significantly larger than the number responding actively to song alone (McNemar's test: χ2 = 12.07, p < 0.001; table 1).
Table 1.
Comparisons of the number of the spine tailed iguanas that exhibited active and static responses in response to playback sounds. See text for detailed descriptions of active and static responses.
| playback sound | alarm calls |
||
|---|---|---|---|
| response | active | static | |
| songs | active | 18 | 0 |
| static | 14 | 3 | |
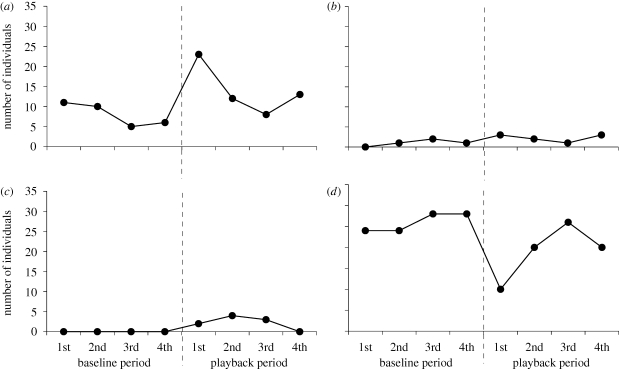
The proportion of individuals that exhibited the four responses changed significantly from the fourth baseline section to the first alarm call playback section (χ2-test: d.f. = 2, χ2 = 25.30, p < 0.0001; figure 2). This change was attributable to the increase of head movement responses and the decrease of immobility. There were no significant differences in the proportion of individuals that exhibited the four responses between the fourth baseline section and the second playback section (Bonferroni correction: χ2 = 7.22, p = 0.027), between the fourth baseline section and the third playback section (χ2 = 2.17, p = 0.339), nor between the fourth baseline section and the fourth playback section (χ2 = 5.01, p = 0.081; figure 2).
Figure 2.
Temporal changes in the number of individuals that exhibited the four behavioural responses: (a) head movement, (b) anterior body movement, (c) whole body movement and (d) immobility. The three active responses (a–c) are not mutually exclusive, but are collectively exclusive of the one static response (d). Dashed lines indicate the commencement of the playback of alarm call.
Because frequencies of anterior and whole body movements during the alarm call playback sections were very low, we combined them and compared it with the frequency of head movement. The frequency of head movements was significantly larger than the combined frequency of anterior and whole body movements (Wilcoxon's signed-rank test: t = 18, p = 0.004; figure 2a,b,c).
4. Discussion
The results demonstrate that Madagascan spiny-tailed iguanas are able to discriminate the alarm calls of flycatchers from their songs. The major response to alarm calls was head movement, suggesting that the iguanas enhanced their vigilance against predators. This indicates that this non-vocal lizard has the ability to perform semantic eavesdropping, in spite of the fact that iguanid species have less-developed ears compared with many other lizard families (Wever 1978; Manley 1990). This provides a clear example of auditory information usage by a non-vocal lizard.
Eavesdropping on heterospecific alarm calls has two major advantages in antipredator strategies. The first advantage relates to the characteristics of hearing. Lizards have the ability to recognize their potential predators based on sight and chemical cues (Bealor & Krekorian 2002; Amo et al. 2006). Sight, however, is directive and lizards need to move their head direction frequently to search for predators visually. In comparison, lizards can obtain auditory information from a wide range of sources and from a greater distance than either visual or olfactory cues in a complex habitat. Thus, there are clear advantages in using auditory cues for predator detection. Second, eavesdropping allows individuals to detect predators in locations that are otherwise difficult to monitor (i.e. aerially). Higher numbers of vigilant individuals increase the likelihood of spotting predators, and heterospecifics may be able to detect predator cues that lizards are unable to notice. Thus, eavesdropping could provide additional information about the presence of predators and might enable the iguana to notice approaching predators more quickly than they would be able to detect predators alone.
Although some birds eavesdrop on variations in heterospecific mobbing alarm calls (Templeton & Greene 2007), birds generally utter similar mobbing alarm calls in response to all predators (Klump & Shalter 1984) and there are no obvious differences in the flycatcher's alarm calls among predator types (T. Mizuta 2005, personal communication). Because of the similarity in mobbing alarm calls, the iguana may not be able to identify predators and evaluate the risk level on the basis of the alarm calls alone. It should be dangerous for prey animals to move before they evaluate their situation precisely, because prey movements can attract predators' attention (Ingle 1968). This may explain why few lizards exhibited intensive antipredator responses such as push-up display or escape, and why most lizards instead exhibited simple head movement, which is considered vigilance behaviour.
Furthermore, the trade-off between cost and benefit would also explain our finding that the major response to the alarm calls was an increase in vigilance behaviours rather than push-up display or escape. Escape behaviour and displays may be energetically costly and result in a loss of time for other activities (Ydenberg & Dill 1986). Therefore, it would be more effective for the lizard to evaluate the situation by first increasing vigilance, and subsequently performing escape or display behaviours only if they are necessary to avoid predation.
In the Ankarafantsika National Park, 86 avian species are found (Mizuta 2005a), and at least 49 of these species are commonly observed (Hasegawa et al. 2009). Some of these birds form mixed-species flocks and use auditory information routinely (Eguchi et al. 1993). We occasionally observed many birds mobbing their predators in the presence of nearby iguanas, suggesting that heterospecifics' mobbing alarm calls would often be accessible to the lizards. Considering the similarity of mobbing alarm calls among bird species (Klump & Shalter 1984), this high availability of alarm calls could have provided an opportunity for evolution of the iguana's ability to use auditory information through eavesdropping, leading to the establishment of an asymmetrical ecological relationship between non-vocal lizards and birds.
Coupled with the evidence that Galapagos marine iguanas also eavesdrop on heterospecific alarm calls (Vitousek et al. 2007), the present study suggests the possibility that eavesdropping on heterospecific auditory information may be a widespread phenomenon in iguanid lizards. Because recent evidence has suggested that hearing in lizards is fairly sensitive, despite the lack of vocal communication in most species (Manley & Köppl 2008), other lizard taxa may also use heterospecific auditory information by similar eavesdropping behaviour. We presume that the asymmetrical ecological relationship between non-vocal lizards and some birds through information recognition may be much more common in animal communities. Further research with other lizard species and taxa will be required to test this possibility.
Acknowledgements
The original idea for this study was inspired during discussions with M. Hasegawa and I. Ikeuchi. We are grateful to M. Nakamura, T. Mizuta, I. Ikeuchi, H. Takahashi, H. Sato, M. Hasegawa, B. Razafimahatratra, T. M. Randriamboavonjy, T. Randrianarisoa, F. D. Hanitrininosy and zoological researchers working in the Ampijoroa forest for their assistance in the field; to T. Mizuta for his technical help in recording and editing birds' songs and calls; and to F. Rakotondraparany and H. Rakotomanana for their help in arranging and conducting this research. We would like to express our gratitude to S. Barribeau and E. Nakajima for helping us to improve the English in this paper. We also thank the staff of Madagascar National Parks for their cooperation in conducting this research. Experimental procedures adhered to the guidelines of Madagascar National Parks. This research was financially supported by a grant-in-aid for the International Scientific Research Program (no. 17405008) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and in part by the Global COE Program (A06) to Kyoto University. The field study was conducted with permission from the Ministry of the Environment and Forests, Madagascar, through Madagascar National Parks.
References
- Amo L., Lopez P., Martin J.2006Can wall lizards combine chemical and visual cues to discriminate predatory from non-predatory snakes inside refuges? Ethology 112, 478–484 (doi:10.1111/j.1439-0310.2005.01170.x) [Google Scholar]
- Bealor M., Krekorian C.2002Chemosensory identification of lizard-eating snakes in the desert iguana, Dipsosaurus dorsalis (Squamata: Iguanidae). J. Herpetol. 36, 9–15 [Google Scholar]
- Charnov E., Krebs J. R.1975The evolution of alarm calls: altruism or manipulation? Am. Nat. 109, 107–112 [Google Scholar]
- Dee T. J.1986The endemic birds of Madagascar Cambridge, UK: International Council for Bird Preservation [Google Scholar]
- Eguchi K., Nagata H., Yamagishi S.1993The mixed-species flocks of birds in a deciduous dry forest of Madagascar. Jpn. J. Ornithol. 42, 27–29 (doi:10.3838/jjo.42.27) [Google Scholar]
- Glaw F., Vences M.2007A field guide to the amphibians and reptiles of Madagascar. Köln, Germany: Vences & Glaw Verlags GbR [Google Scholar]
- Greene H. W.1988Antipredator mechanisms in reptiles. In Defense and life history (eds Gans C., Huey R. B.). Biology of the Reptilia, vol. 16, pp. 1–152 New York, NY: Alan R Liss [Google Scholar]
- Halliday T. R., Slater P. J. B.1983aCauses and effects. Animal behaviour, vol. 1, pp. 1–228 Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- Halliday T. R., Slater P. J. B.1983bCommunication. Animal behaviour, vol. 2, pp. 1–225 Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- Hasegawa M., Mori A., Nakamura M., Mizuta T., Asai S., Ikeuchi I., Rakotomanana H., Okamiya T., Yamagishi S.2009Consequence of interclass competition and predation on the adaptive radiation of lizards and birds in the dry forest of western Madagascar. Ornithol. Sci. 8, 55–66 (doi:10.2326/048.008.0108) [Google Scholar]
- Hino T.1998Mutualistic and commensal organization of avian mixed-species foraging flocks in a forest of western Madagascar. J. Avian Biol. 29, 17–24 (doi:10.2307/3677336) [Google Scholar]
- Ingle D.1968Visual releasers of prey-catching behavior in frogs and toads. Brain Behav. Evol. 1, 500–518 (doi:10.1159/000125522) [Google Scholar]
- Jenssen T.1977Evolution of anoline lizard display behavior. Am. Zool. 17, 203–215 [Google Scholar]
- Jury M. A.2003The climate of Madagascar. In The natural history of Madagascar (eds Goodman S. M., Benstead J. P.), pp. 75–87 Chicago, IL: University of Chicago Press [Google Scholar]
- Klump G., Shalter M.1984Acoustic behavior of birds and mammals in the predator context. 1. Factors affecting the structure of alarm signals. 2. The functional-significance and evolution of alarm signals. Z. Tierpsychol. 66, 189–226 [Google Scholar]
- Klump G. M., Kretzschmar E., Curio E.1986The hearing of an avian predator and its avian prey. Behav. Ecol. Sociobiol. 18, 317–323 (doi:10.1007/BF00299662) [Google Scholar]
- Langrand O.1990Guide to the birds of Madagscar New Haven, CT: Yale University Press [Google Scholar]
- Lea A. J., Barrera J. P., Tom L. M., Blumstein D. T.2008Heterospecific eavesdropping in a nonsocial species. Behav. Ecol. 19, 1041–1046 (doi:10.1093/beheco/arn064) [Google Scholar]
- Leal M.1999Honest signalling during prey–predator interactions in the lizard Anolis cristatellus. Anim. Behav. 58, 521–526 (doi:10.1006/anbe.1999.1181) [DOI] [PubMed] [Google Scholar]
- Lima S. L., Dill L. M.1990Behavioral decisions made under the risk of predation—a review and prospectus. Can. J. Zool. 68, 619–640 (doi:10.1139/z90-092) [Google Scholar]
- Lopez P., Aragon P., Martin J.1998Iberian rock lizards (Lacerta monticola cyreni) assess conspecific information using composite signals from faecal pellets. Ethology 104, 809–820 [Google Scholar]
- Manley G. A.1990Peripheral hearing mechanisms in reptiles and birds. Heidelberg, Germany: Springer [Google Scholar]
- Manley G. A.2006Spontaneous otoacoustic emissions from free-standing stereovillar bundles of ten species of lizard with small papillae. Hear. Res. 212, 33–47 (doi:10.1016/j.heares.2005.10.007) [DOI] [PubMed] [Google Scholar]
- Manley G. A., Köppl C.2008What have lizard ears taught us about auditory physiology? Hear. Res. 238, 3–11 (doi:10.1016/j.heares.2007.09.011) [DOI] [PubMed] [Google Scholar]
- Marcellini D.1977Acoustic and visual-display behavior of gekkonid lizards. Am. Zool. 17, 251–260 [Google Scholar]
- Mizuta T.2000Intrusion into neighboring home range by male Madagascar paradise flycatchers, Terpsiphone mutata: a circumstantial evidence for extra-pair copulation. J. Ethol. 18, 123–126 (doi:10.1007/s101640070011) [Google Scholar]
- Mizuta T.2002Breeding biology of the Madagascar Paradise Flycatcher, Terpsiphone mutata, with special reference to plumage variation in males. Ostrich 73, 67–69 [Google Scholar]
- Mizuta T.2005aThe Ampijoroa Forest, home to the Rufous Vanga. In Social organization of the Rufous Vanga, the ecology of Vangas: birds endemic to Madagascar (ed. Yamagishi S.), pp. 15–40 Kyoto, Japan: Kyoto University Press [Google Scholar]
- Mizuta T.2005bParental care behavior in the monogamous, sexually dimorphic Madagascar Paradise Flycatcher: sex differences and the effect of brood size. Ecol. Res. 20, 547–553 (doi:10.1007/s11284-005-0066-5) [Google Scholar]
- Mori A., Randriamahazo H. J. A. R.2002aLeioheterodon madagascariensis (Madagascar Menarana Snake). Diet. Herpetol. Rev. 33, 57 [Google Scholar]
- Mori A., Randriamahazo H. J. A. R.2002bForaging mode of a Madagascan iguanian lizard, Oplurus cuvieri cuvieri. Afr. J. Ecol. 40, 61–64 (doi:10.1046/j.0141-6707.2001.00340.x) [Google Scholar]
- Mori A., Ikeuchi I., Hasegawa M.2006Herpetofauna of Ampijoroa, Ankarafantsika Strict Nature Reserve, a dry forest in northwestern Madagascar. Herpetol. Natl. Hist. 10, 31–60 [Google Scholar]
- Mulder R. A., Ramiarison R.2003Terpsiphone mutata, Madagascar Paradise Flycatcher, Remaly, Tsingetra, Rengetra, Singetraka, Dadyi, Angetry. In The natural history of Madagascar (eds Goodman S. M., Benstead J. P.), pp. 1134–1138 Chicago, IL: University of Chicago Press [Google Scholar]
- Münchenberg T., Wollenberg K. C., Glaw F., Vences M.2008Molecular phylogeny and geographic variation of Malagasy iguanas (Oplurus and Chalarodon). Amphibia–Reptilia 29, 319–327 [Google Scholar]
- Nakamura M.2004Predation by Eulemur fulvus fulvus on eggs of Ploceus sakalava sakalava (Aves: Ploceidae) in Ankarafantsika, Madagascar. Folia Primatol. 75, 376–378 (doi:10.1159/000081017) [DOI] [PubMed] [Google Scholar]
- Nakamura M., Okamiya T., Yamagishi S.2004The diet of adult and nestling Sickle-billed Vangas Falculea palliata, a species endemic to Madagascar. J. Yamashina Inst. Ornithol. 35, 155–158 [Google Scholar]
- Pianka E. R., Vitt L. J.2003Lizards: windows to the evolution of diversity, pp. 1–346 Berkeley, CA: University of California Press [Google Scholar]
- Rainey H. J., Zuberbühler K., Slater P. J. B.2004Hornbills can distinguish between primate alarm calls. Proc. R. Soc. Lond. B 271, 755–759 (doi:10.1098/rspb.2003.2619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamahazo H. J. A. R.2000Sexual size dimorphism in the lizard Oplurus cuvieri cuvieri (Squamata, Opluridae) from Madagascar. Afr. Zool. 35, 287–293 [Google Scholar]
- Randriamahazo H. J. A. R., Mori A.1999Spatial utilization and social interactions in Oplurus cuvieri cuvieri (Squamata, Opluridae) in Madagascar. Jap. J. Herpetol. 18, 209–217 [Google Scholar]
- Randriamahazo H. J. A. R., Mori A.2001Egg-laying activities and reproductive traits in females of Oplurus cuvieri cuvieri. J. Herpetol. 35, 9 (doi:10.2307/1566110) [Google Scholar]
- Rene de Roland L. A., Thorstrom R.2003Accipiter spp., Goshawk and Sparrowhawks. In The natural history of Madagascar (eds Goodman S. M., Benstead J. P.), pp. 1091–1094 Chicago, IL: University of Chicago Press [Google Scholar]
- Seyfarth R., Cheney D.1990The assessment by vervet monkeys of their own and another species alarm calls. Anim. Behav. 40, 754–764 (doi:10.1016/S0003-3472(05)80704-3) [Google Scholar]
- Seyfarth R. M., Cheney D. L., Marler P.1980Monkey responses to 3 different alarm calls—evidence of predator classification and semantic communication. Science 210, 801–803 (doi:10.1126/science.7433999) [DOI] [PubMed] [Google Scholar]
- Templeton C. N., Greene E.2007Nuthatches eavesdrop on variations in heterospecific chickadee mobbing alarm calls. Proc. Natl Acad. Sci. USA 104, 5479–5482 (doi:10.1073/pnas.0605183104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus T. T., Frost D. R.1996Molecular homology assessment and phylogeny in the lizard family Opluridae (Squamata: Iguania). Mol. Phylogenet. Evol. 6, 49–62 (doi:10.1006/mpev.1996.0057) [DOI] [PubMed] [Google Scholar]
- Turner L. W.1973Vocal and escape responses of Spermophilus beldingi to predators. J. Mammal. 54, 990–993 (doi:10.2307/1379099) [Google Scholar]
- Vercken E., Clobert J.2008The role of colour polymorphism in social encounters among female common lizards. Herpetol. J. 18, 223–230 [Google Scholar]
- Vitousek M. N., Adelman J. S., Gregory N. C., St Clair J. J. H.2007Heterospecific alarm call recognition in a non-vocal reptile. Biol. Lett. 3, 632–634 (doi:10.1098/rsbl.2007.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever E. G.1978The reptile ear: its structure and function, pp. 1–1024 Princeton, NJ: Princeton University Press [Google Scholar]
- Wheeler B.2008Selfish or altruistic? An analysis of alarm call function in wild capuchin monkeys, Cebus apella nigritus. Anim. Behav. 76, 1465–1475 (doi:10.1016/j.anbehav.2008.06.023) [Google Scholar]
- Woodland D. J., Jaafar Z., Knight M. L.1980The pursuit deterrent function of alarm signals. Am. Nat. 115, 748–753 [Google Scholar]
- Ydenberg R. C., Dill L. M.1986The economics of fleeing from predators. Adv. Study Behav. 16, 229–249 (doi:10.1016/S0065-3454(08)60192-8) [Google Scholar]
- Zar J. H.2009Biostatistical analysis, pp. 569–576 Upper Saddle River, NJ: Prentice-Hall [Google Scholar]