Abstract
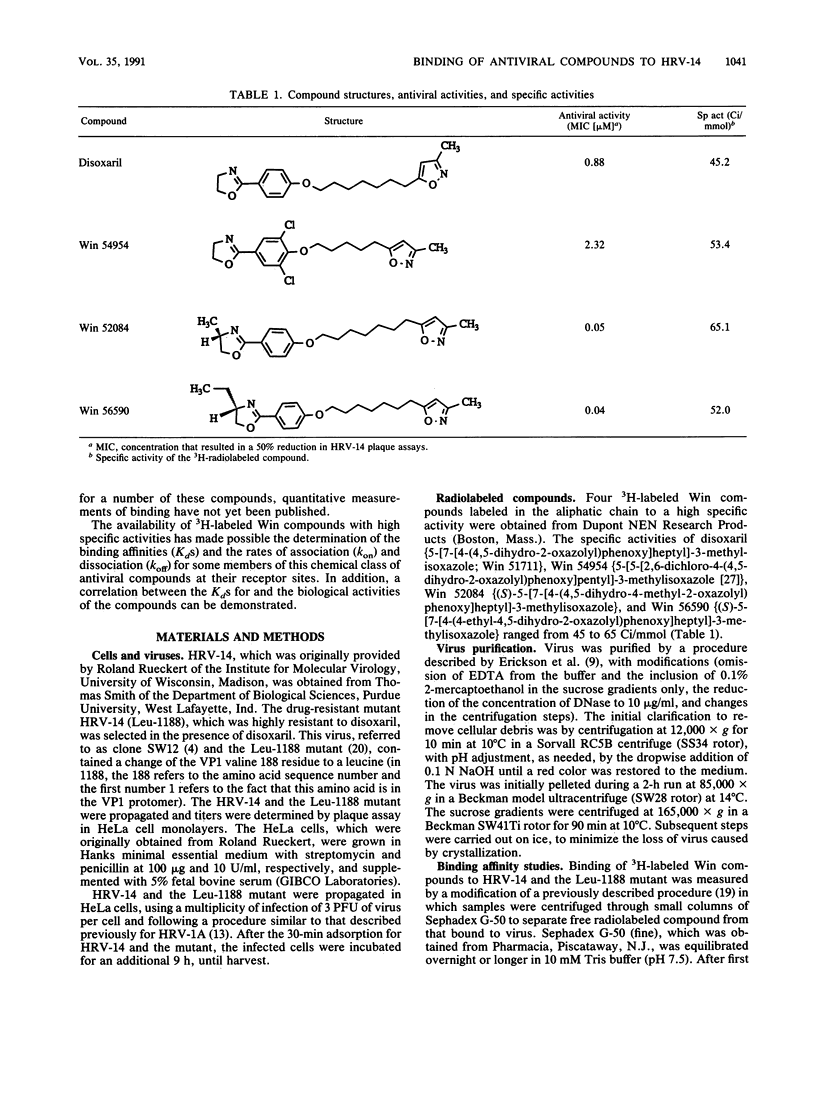
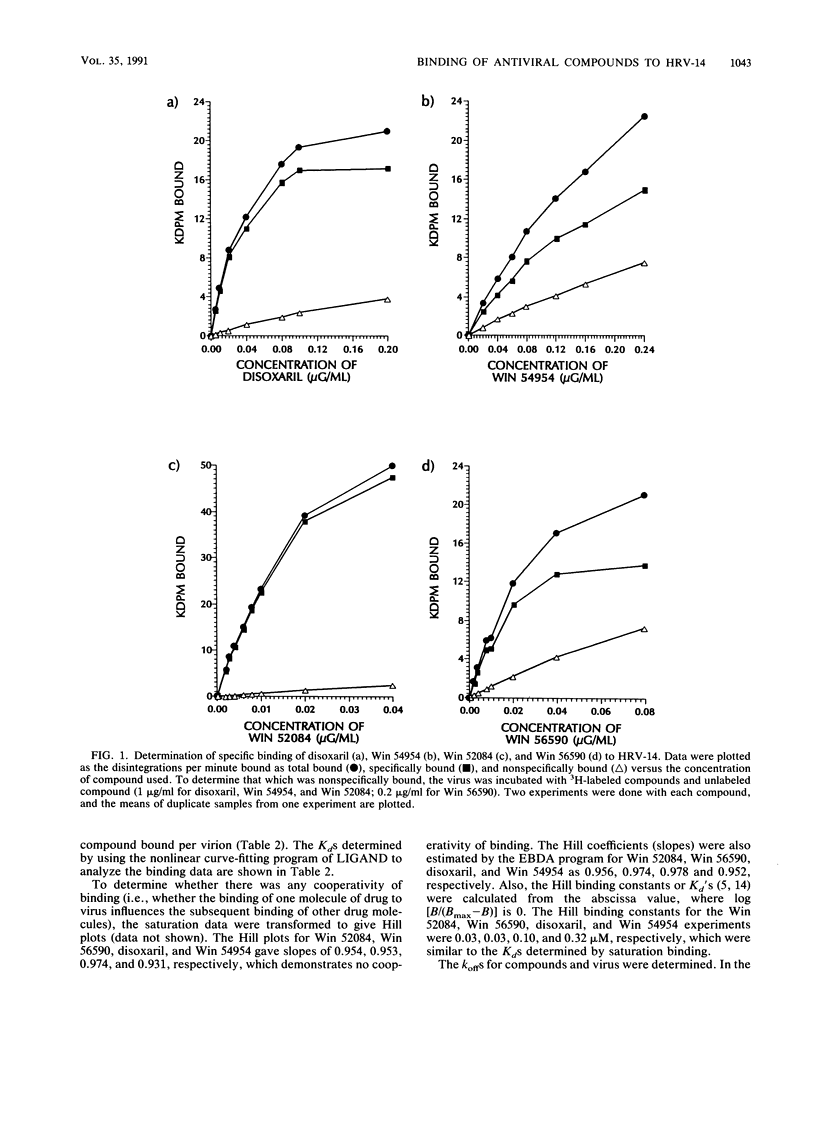
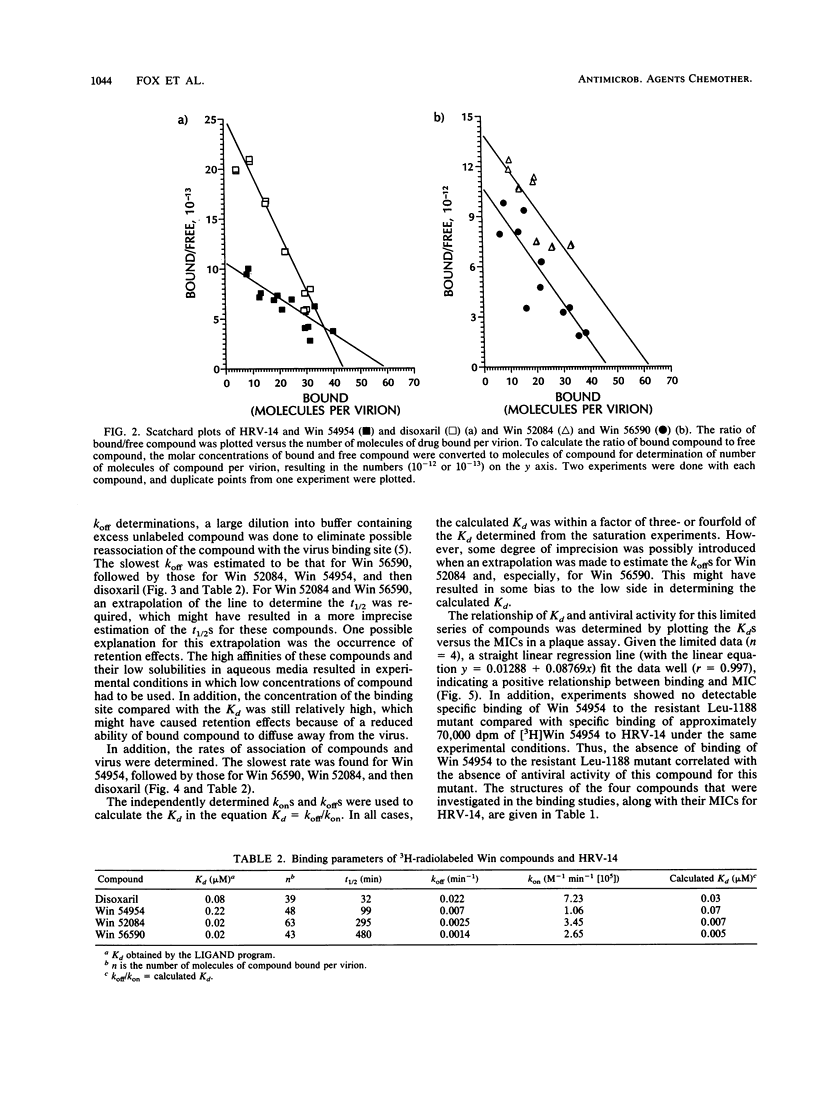
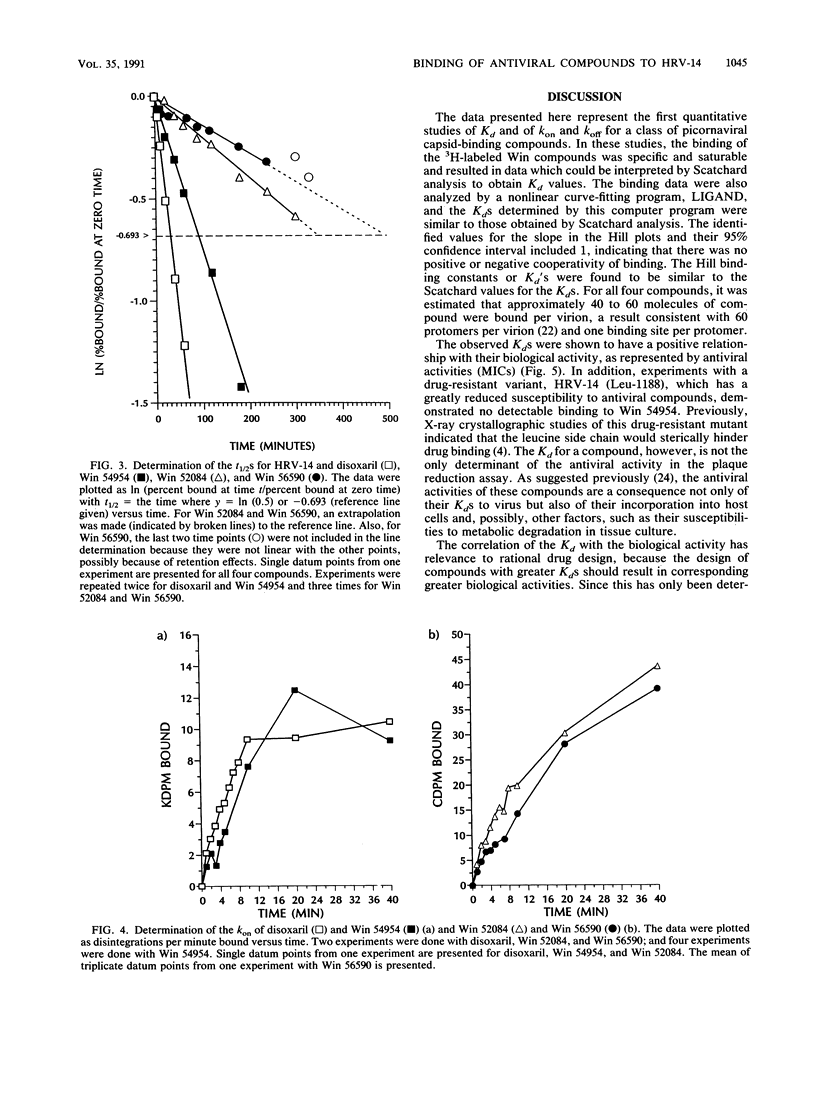
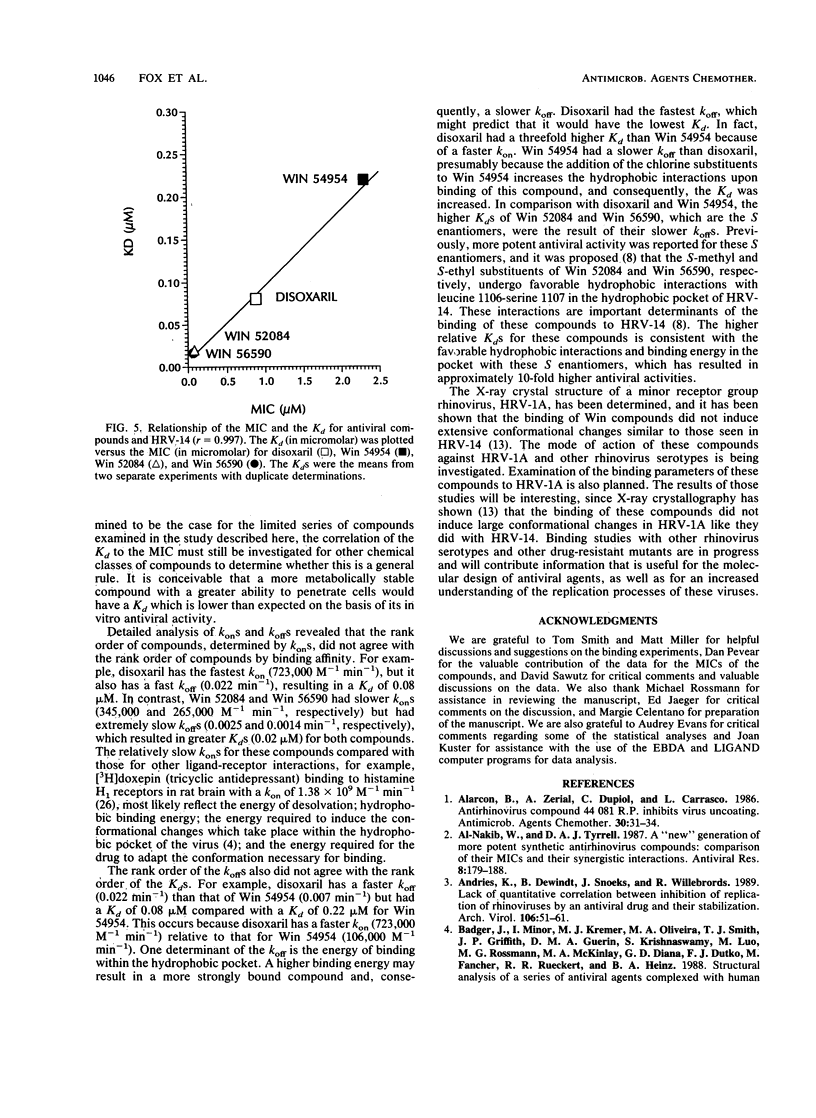
The binding affinities (Kds) and the rates of association and dissociation of members of a chemical class of antiviral compounds at their active sites in human rhinovirus type 14 (HRV-14) were determined. On the basis of analysis by LIGAND, a nonlinear curve-fitting program, of saturation binding experiments with HRV-14, the Kds for Win 52084, Win 56590, disoxaril (Win 51711), and Win 54954 were found to be 0.02, 0.02, 0.08, and 0.22 microM, respectively. The independently determined kinetic rates of association and dissociation resulted in calculated Kd values which were in agreement with the Kd values determined in saturation binding experiments. Scatchard plots of each of four compounds for the binding data indicated that approximately 40 to 60 molecules were bound per HRV-14 virion. Hill plots showed no evidence of cooperativity in binding. Furthermore, the antiviral activities (MICs in plaque reduction assays with HRV-14) for this limited series of compounds (n = 4) correlated well (r = 0.997) with the observed Kds. Likewise, the absence of detectable binding of Win 54954 to the drug-resistant mutant HRV-14 (Leu-1188) corresponded to a lack of antiviral activity. The positive relationship between the antiviral activities and the Kds that were determined may have implications for the molecular design of capsid-binding antirhinovirus drugs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Nakib W., Tyrrell D. A. A 'new' generation of more potent synthetic antirhinovirus compounds: comparison of their MICs and their synergistic interactions. Antiviral Res. 1987 Nov;8(4):179–187. doi: 10.1016/0166-3542(87)90072-6. [DOI] [PubMed] [Google Scholar]
- Alarcon B., Zerial A., Dupiol C., Carrasco L. Antirhinovirus compound 44,081 R.P. inhibits virus uncoating. Antimicrob Agents Chemother. 1986 Jul;30(1):31–34. doi: 10.1128/aac.30.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries K., Dewindt B., Snoeks J., Willebrords R. Lack of quantitative correlation between inhibition of replication of rhinoviruses by an antiviral drug and their stabilization. Arch Virol. 1989;106(1-2):51–61. doi: 10.1007/BF01311037. [DOI] [PubMed] [Google Scholar]
- Chapman M. S., Minor I., Rossmann M. G., Diana G. D., Andries K. Human rhinovirus 14 complexed with antiviral compound R 61837. J Mol Biol. 1991 Feb 5;217(3):455–463. doi: 10.1016/0022-2836(91)90749-v. [DOI] [PubMed] [Google Scholar]
- Diana G. D., Cutcliffe D., Oglesby R. C., Otto M. J., Mallamo J. P., Akullian V., McKinlay M. A. Synthesis and structure-activity studies of some disubstituted phenylisoxazoles against human picornavirus. J Med Chem. 1989 Feb;32(2):450–455. doi: 10.1021/jm00122a027. [DOI] [PubMed] [Google Scholar]
- Diana G. D., Otto M. J., Treasurywala A. M., McKinlay M. A., Oglesby R. C., Maliski E. G., Rossmann M. G., Smith T. J. Enantiomeric effects of homologues of disoxaril on the inhibitory activity against human rhinovirus-14. J Med Chem. 1988 Mar;31(3):540–544. doi: 10.1021/jm00398a009. [DOI] [PubMed] [Google Scholar]
- Erickson J. W., Frankenberger E. A., Rossmann M. G., Fout G. S., Medappa K. C., Rueckert R. R. Crystallization of a common cold virus, human rhinovirus 14: "isomorphism" with poliovirus crystals. Proc Natl Acad Sci U S A. 1983 Feb;80(4):931–934. doi: 10.1073/pnas.80.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. P., Otto M. J., McKinlay M. A. Prevention of rhinovirus and poliovirus uncoating by WIN 51711, a new antiviral drug. Antimicrob Agents Chemother. 1986 Jul;30(1):110–116. doi: 10.1128/aac.30.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz B. A., Rueckert R. R., Shepard D. A., Dutko F. J., McKinlay M. A., Fancher M., Rossmann M. G., Badger J., Smith T. J. Genetic and molecular analyses of spontaneous mutants of human rhinovirus 14 that are resistant to an antiviral compound. J Virol. 1989 Jun;63(6):2476–2485. doi: 10.1128/jvi.63.6.2476-2485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny M. T., Torney H. L., Dulworth J. K. Mechanism of action of the antiviral compound MDL 20,610. Antiviral Res. 1988 Jul;9(4):249–261. doi: 10.1016/0166-3542(88)90056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. S., Smith T. J., Chapman M. S., Rossmann M. C., Pevear D. C., Dutko F. J., Felock P. J., Diana G. D., McKinlay M. A. Crystal structure of human rhinovirus serotype 1A (HRV1A). J Mol Biol. 1989 Nov 5;210(1):91–111. doi: 10.1016/0022-2836(89)90293-3. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. A practical computer-based approach to the analysis of radioligand binding experiments. Comput Programs Biomed. 1983 Aug-Oct;17(1-2):107–113. doi: 10.1016/0010-468x(83)90031-4. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y., Ohsawa C., Aoyama M., Umeda I., Suhara Y., Ishitsuka H. Antivirus agent, Ro 09-0410, binds to rhinovirus specifically and stabilizes the virus conformation. Virology. 1984 Apr 30;134(2):269–276. doi: 10.1016/0042-6822(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y., Shimma N., Ishitsuka H. Comparative studies on the antirhinovirus activity and the mode of action of the rhinovirus capsid binding agents, chalcone amides. Antiviral Res. 1990 Feb;13(2):61–74. doi: 10.1016/0166-3542(90)90022-y. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Pevear D. C., Fancher M. J., Felock P. J., Rossmann M. G., Miller M. S., Diana G., Treasurywala A. M., McKinlay M. A., Dutko F. J. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol. 1989 May;63(5):2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. J Biol Chem. 1989 Sep 5;264(25):14587–14590. [PubMed] [Google Scholar]
- Smith T. J., Kremer M. J., Luo M., Vriend G., Arnold E., Kamer G., Rossmann M. G., McKinlay M. A., Diana G. D., Otto M. J. The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating. Science. 1986 Sep 19;233(4770):1286–1293. doi: 10.1126/science.3018924. [DOI] [PubMed] [Google Scholar]
- Sperber S. J., Hayden F. G. Chemotherapy of rhinovirus colds. Antimicrob Agents Chemother. 1988 Apr;32(4):409–419. doi: 10.1128/aac.32.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. E., Richelson E. High-affinity binding of [3H]doxepin to histamine H1-receptors in rat brain: possible identification of a subclass of histamine H1-receptors. Eur J Pharmacol. 1982 Mar 12;78(3):279–285. doi: 10.1016/0014-2999(82)90029-2. [DOI] [PubMed] [Google Scholar]
- Woods M. G., Diana G. D., Rogge M. C., Otto M. J., Dutko F. J., McKinlay M. A. In vitro and in vivo activities of WIN 54954, a new broad-spectrum antipicornavirus drug. Antimicrob Agents Chemother. 1989 Dec;33(12):2069–2074. doi: 10.1128/aac.33.12.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichhardt H., Otto M. J., McKinlay M. A., Willingmann P., Habermehl K. O. Inhibition of poliovirus uncoating by disoxaril (WIN 51711). Virology. 1987 Sep;160(1):281–285. doi: 10.1016/0042-6822(87)90075-4. [DOI] [PubMed] [Google Scholar]


